Abstract
Phosphodiester linked conjugates of various nucleosides such as d4U, d4T, IdUrd, ddI, ddA, virazole, ara-A and ara-C containing a glucosyl moiety have been described. These compounds were designed to act as prodrugs, where the corresponding 5′-monophosphates may be generated intracellularly. The synthesis of the glycoconjugates was achieved in good yields by condensation of a glucosyl phosphoramidite 7 with nucleosides in the presence of an activating agent. It was demonstrated that the glucose-conjugates improve water solubility of the nucleoside analogues, for example up to 31-fold for ara-A conjugate compared to ara-A alone. The new conjugates were tested for their anti-HIV-1 activity in human lymphocytes. These derivatives offer a convenient design for potential prodrug candidates with the possibility to improve the physicochemical properties and therapeutic activity of nucleoside analogues.
Introduction
Chemically modified nucleosides are widely used as therapeutic agents to treat cancer, fungal, bacterial and viral infections (1–5). Similarly, carbohydrates are of paramount importance in intercellular recognition, bacterial and viral infection processes and inflammation events making them an attractive target for drug development (6–8). Consequently, a conjugate of these two classes of molecules may offer an avenue to design and develop novel therapeutics with improved biological functions. The use of a phosphate ester linkage for conjugation is widely practiced because of its natural occurrence in cellular physiology and as a backbone for DNA and RNA molecules. The culmination of these attributes motivated us to use natural phosphate linkage to conjugate a biologically active nucleoside with a carbohydrate unit anticipating that we could modulate the therapeutic effects of known nucleosides in a favorable manner.
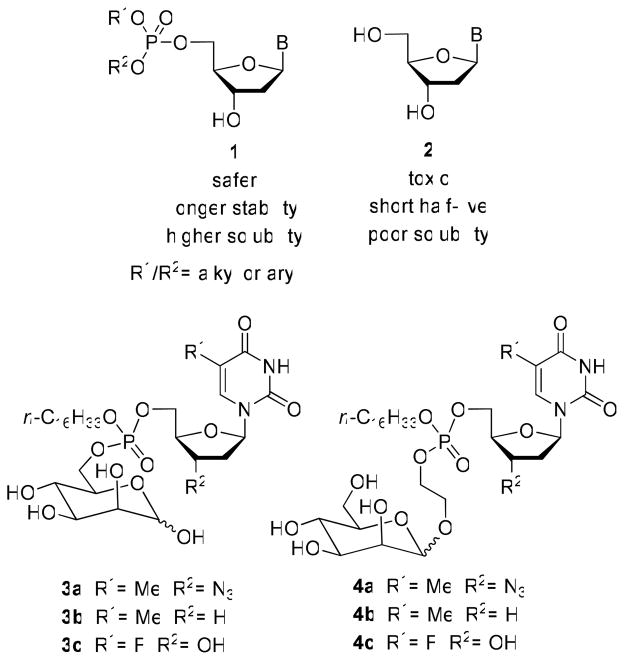
Several modified nucleosides (AZT, d4T, 3TC, ddI, ddC and abacavir) have been approved by the US FDA as anti-HIV drugs. Although these drugs are effective for the treatment of HIV, some of the limitations such as toxicity, short half-life and dependence on cellular enzymes preclude their wider use. In order to overcome these limitations and to improve the therapeutic potential of these nucleosides, several phosphate-based prodrug strategies have been developed (9–16). The prodrug approach appears to be a promising way to improve the anti-HIV activity of the approved nucleosides, reduce their cellular toxicity, enhance cellular uptake and prevent viral resistance (17–19). The 5′-phosphate analogs of nucleosides have been extensively studied as prodrugs. For example, nucleoside glycoconjugate prodrugs (1, Chart 1) are able to penetrate cellular membranes as a result of their higher lipophilicity and are also able to improve the efficacy of antiviral and anticancer nucleoside analogues 2 (20). Phosphate derivatives of AZT (3a,4a), ddT (3b,4b), and FdUrd (3c,4c) with a D-mannose as conjugating moiety were prepared as membrane soluble prodrugs directed towards cells carrying mannosyl receptors (21–22). Previously, glyco-conjugates of oligonucleotides have been reported to demonstrate improved anti-HIV activity (23).
Chart 1.
Prodrugs of Phosphorylated Nucleosides
The main methods described in the literature for the preparation of nucleoside-carbohydrate phosphodiesters are the condensation of protected sugar monophosphates with nucleosides in the presence of an activating agent (21–22, 24) or by coupling of a nucleoside phosphate with a protected glycosyl donor (25). Among the various protocols available for conjugation, the phosphoramidite chemistry offers the best yields (16). Recently, it has been demonstrated that the solid-phase synthesis of dinucleoside and nucleoside-carbohydrate phosphodiester and thiophosphodiester using polymer-bound oxathiophospholanes as the phosphitylating agents is a viable way for the synthesis of conjugates (14).
As a part of our ongoing efforts in the discovery and design of therapeutic nucleoside analogs (26– 28), we embarked on a path to synthesize nucleoside-carbohydrate conjugates linked via the phosphate backbone. We believe that these conjugates will act like prodrugs where the corresponding 5′-monophosphate could be liberated intracellularly by cellular phosphodiesterases. With this objective in sight, we elected to develop conjugation chemistry that will permit the convenient attachment of glucose 6-phosphate (29) to a nucleoside analog of therapeutic value. For this study we have utilized highly efficient phosphoramidite chemistry to create the conjugates of interest. Herein, we describe the synthesis, anti-HIV-1 activity and cytotoxicity of novel glucose-nucleoside conjugates.
Materials and Methods
Candida rugosa lipase (CRL, Type VII, 1410 U/mg) was purchased from Sigma. Pseudomonas cepacia lipase (PSL-C, Amano PS-C II, 1195 U/g) was purchased from Aldrich. Candida antarctica lipase B (CAL-B, Novozym 435, 7300 PLU/g) was a gift from Novo Nordisk Co. Synthesis of α-5 and 6 have been previously described (29). Unless otherwise specified flash chromatography was performed over silica 60 Å (230–400 mesh). 1H-Tetrazole was prepared according to the procedure previously described (30). The nucleosides 8c, 8d, 8f-i, 14, 15, 17–19, 21 and 22 were isolated as amorphous solid upon concentration of the solutions. The melting range of these solid compounds was determined by melting point Gallenkamp apparatus.
Synthesis of 2-cyanoethyl-N,N-diisopropyl-(1,2,3,4-tetra-O-acetyl-α-D-glucopyranoside-6-yl)phosphoramidite (7)
2-Cyanoethyl-N,N,N′,N′-tetraisopropylphosphoramidite (1.1 mL, 3.45 mmol) and pyridinium trifluoroacetate (31) (673 mg, 3.45 mmol) was added to a stirred solution of 6 (1 g, 2.89 mmol) in anhydrous CH2Cl2 (7 mL), and the mixture stirred for 3 h at rt. Upon complete consumption of the starting material (TLC), the solvent was evaporated and the residue purified by flash chromatography using silica 60 Å (32–63 μm). Elution with 33% EtOAc/hexane afforded 7 as clear oil in 91% yield. For the flash chromatography the silica was pretreated with 33% EtOAc/hexane containing 1% Et3N (300 mL) and then with 33% EtOAc/hexane (300 mL). 1H NMR (CDCl3, 600 MHz): δ 1.13 (s, 3H, Me-iPr), 1.14 (s, 3H, Me-iPr), 1.15 (s, 6H, Me-iPr), 1.16 (s, 3H, Me-iPr), 1.17 (s, 6H, Me-iPr), 1.18 (s, 3H, Me-iPr), 1.99 (s, 3H, CH3), 2.00 (s, 6H, CH3), 2.02 (s, 3H, CH3), 2.03 (s, 6H, CH3), 2.14 (s, 3H, CH3), 2.15 (s, 3H, CH3), 2.62 (t, 4H, H2′, 3 JHH 6.4 Hz), 3.60 (m, 6H, H6+ H1″), 3.79 (m, 6H, H1′+H6), 4.05 (m, 2H, H5), 5.03 (m, 2H, H2), 5.09 (t, 1H, H4, 3 JHH 10.1 Hz)), 5.15 (t, 1H, H4, 3 JHH 9.7 Hz), 5.44 (t, 2H, H3, 3 JHH 9.2 Hz), 6.31 (t, 2H, H1, 3 JHH 3.3 Hz). 31P NMR (CDCl3, 242.9 MHz): δ 149.64, 149.91. MS (ESI+, m/z): 549 [(M+H)+, 10%], 571 [(M+Na)+, 100%].
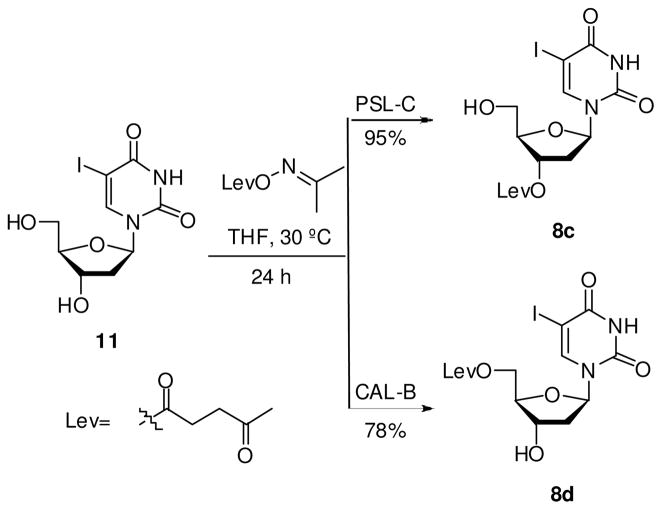
Synthesis of 5-iodo-3′-O-levulinyl-2′-deoxyuridine (8c)
A suspension of 11 (200 mg, 0.56 mmol), O-levulinyl acetonoxime (32) (291 mg, 1.70 mmol), and PSL-C (600 mg) in anhydrous THF (5.7 mL) under nitrogen was stirred at 30 °C and 250 rpm for 24 h. The enzyme was filtered off and washed with CH2Cl2 and THF. The solvents were distilled under vacuum, and the residue was washed with Et2O to afford 8c as white solid in 95% yield. Melting range: 164–166 °C. 1H NMR (MeOH-d4, 300 MHz): δ 2.36 (s, 3H, CH3), 2.52 (m, 2H, H2′), 2.77 (t, 2H, CH2-Lev, 3 JHH 6.7 Hz), 3.01 (t, 2H, CH2-Lev, 3 JHH 6.5 Hz), 3.99 (m, 2H, H5′), 4.29 (m, 1H, H4′), 5.50 (m, 1H, H3′), 6.43 (apparent q, 1H, H1′, 3 JHH 5.8 Hz), 8.72 (s, 1H, H6). MS (ESI+, m/z): 475 [(M+Na)+, 100%].
Synthesis of 5-iodo-5′-O-levulinyl-2′-deoxyuridine (8d)
A suspension of 11 (200 mg, 0.56 mmol), O-levulinyl acetonoxime (32) (291 mg, 1.70 mmol), and CAL-B (200 mg) in anhydrous THF (5.7 mL) under nitrogen was stirred at 30 °C and 250 rpm for 24 h. The enzyme was filtered off, washed with CH2Cl2 and THF, and the solvents distilled under vacuum. The residue was purified by flash chromatography using 5% iPrOH/CH2Cl2 and the resulting solid washed with Et2O to afford 8d as a white solid in 78% yield. Melting range: 140–143 °C. 1H NMR (MeOH-d4, 300 MHz): δ 2.37 (s, 3H, CH3), 2.49 (m, 2H, H2′), 2.87 (t, 2H, CH2-Lev, 3 JHH 6.3 Hz), 3.03 (t, 2H, CH2-Lev, 3 JHH 6.6 Hz), 4.28 (m, 1H, H4′), 4.46 (m, 1H, H5′), 4.55 (m, 2H, H3′+H5′), 6.39 (apparent t, 1H, H1′, 3 JHH 6.5 Hz), 8.72 (s, 1H, H6). MS (ESI+, m/z): 475 [(M+Na)+, 65%].
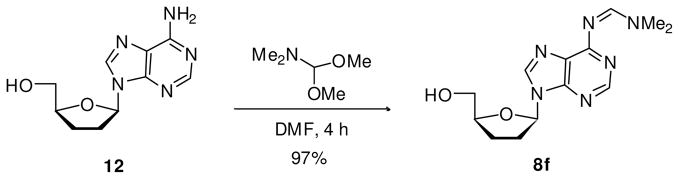
Synthesis of N6-(dimethylamino)methylene-2′,3′-dideoxyadenosine (8f)
A mixture of 12 (149 mg, 0.63 mmol) and N,N-dimethylacetamide dimethyl acetal (824 μL, 6.3 mmol) in anhydrous DMF (3.2 mL) was stirred at rt for 4 h. The mixture was evaporated and the residue purified by flash chromatography using 8% MeOH/CH2Cl2 to afford 8f as white solid in 97% yield. Melting range: 61–64 °C (unstable compound, decompose easily). 1H NMR (MeOH-d4, 300 MHz): δ 2.38 (m, 2H, H2′ ó H3′), 2.72 (m, 2H, H2′ ó H3′), 3.42 (s, 3H, Me), 3.43 (s, 3H, Me), 3.85 (dd, 1H, H5′, |2 JHH| 12.2 Hz, 3 JHH 3.9 Hz), 4.05 (dd, 1H, H5′, |2 JHH| 12.2 Hz, 3 JHH 3.1 Hz), 4.45 (m, 1H, H4′), 6.51 (apparent t, 1H, H1′, 3 JHH 5.6 Hz), 8.59 (s, 1H, H8), 8.70 (s, 1H, H2), 9.07 (s, 1H, HC=N). MS (ESI+, m/z): 291 [(M+H)+, 100%].
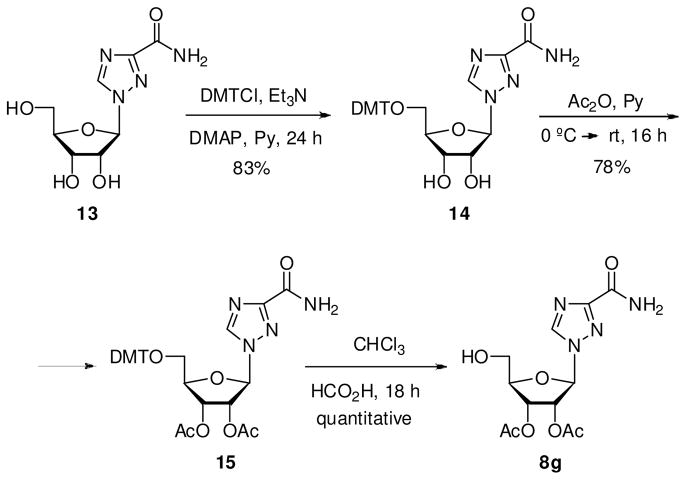
Synthesis of 1-(2′,3′-Di-O-acetyl-β-D-ribofuranosyl)-1H-1,2,4-triazole-3-carboxamide (8g)
To a solution of 15 (295 mg, 0.9 mmol) in chloroform (30 mL) was added formic acid (3–4 drops). After being stirred at rt for 18 h, the mixture was neutralized with 1 N KOH, and the solution was concentrated. The residue was purified by flash chromatography using 1–50% MeOH/CH2Cl2 as elution gradient to yield 8g as white solid in quantitative yield. Melting range: 75–77 °C. 1H NMR (MeOH-d4, 300 MHz): δ 2.29 (s, 3H, CH3), 2.31 (s, 3H, CH3), 2.28 (s, 3H, CH3), 3.92 (dd, 1H, H5′, |2 JHH| 12.5 Hz, 3 JHH 3.7 Hz), 4.03 (dd, 1H, H5′, |2 JHH| 12.5 Hz, 3 JHH 3.1 Hz), 4.52 (q, 1H, H4′, 3 JHH 5.1 Hz), 5.79 (t, 1H, H3′, 3 JHH 5.3 Hz), 5.96 (dd, 1H, H2′, 3 JHH 5.1, 3.9 Hz), 6.43 (d, 1H, H1′, 3 JHH 3.9 Hz), 8.73 (br s, 1H, NH), 9.02 (s, 1H, H5). MS (ESI+, m/z): 351 [(M+Na)+, 90%].
Synthesis of N6-benzoyl-9-(2′,3′-di-O-acetyl-β-D-arabinofuranosyl)adenine (8h)
A 4 M solution of hydrogen chloride in 1,4-dioxane (1.1 mL) was added to a stirred solution of 19 (330 mg, 0.44 mmol) in CH2Cl2 (8.8 mL) at −50 °C. After 15 min, the reaction was quenched by adding a solution of pyridine/MeOH (1:1, v/v) at −50 °C. The mixture was poured into saturated aqueous NaHCO3 solution and extracted with CH2Cl2. The combined organic fractions were dried (Na2SO4) and concentrated under vacuum. The crude was purified by flash chromatography using 1–10% MeOH/CH2Cl2 as elution gradient to yield 8h as white solid in 76% yield. Melting range: 165–167 °C. 1H NMR (MeOH-d4, 300 MHz): δ 1.96 (Me), 2.33 (Me), 4.09 (m, 2H, H5′), 4.38 (m, 1H, H4′), 5.79 (m, 2H, H2′+H3′), 6.89 (d, 1H, H1′, 3 JHH 5.3 Hz), 7.69–7.85 (m, 3H, Hm+Hp), 8.26 (d, 2H, Ho, 3 JHH 8.5 Hz), 8.86 (s, 1H, H8), 8.89 (s, 1H, H2). MS (ESI+, m/z): 478 [(M+Na)+, 100%] and 494 [(M+K)+, 5].
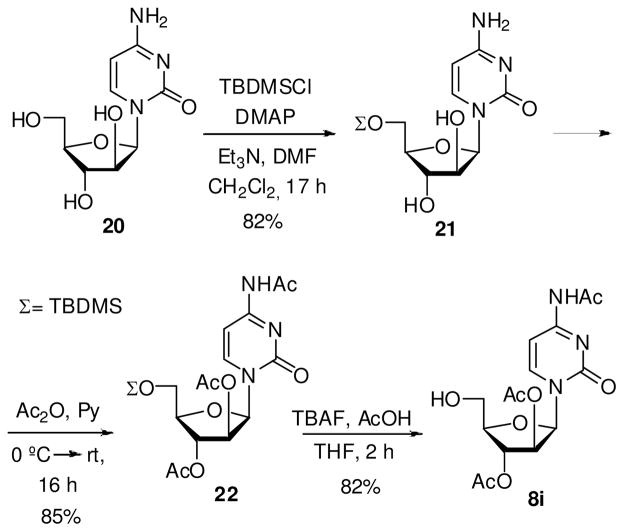
Synthesis of N4-acetyl-1-(2′,3′-di-O-acetyl-β-D-arabinofuranosyl)cytosine (8i)
Acetic acid (57 μL, 1 mmol) and TBAF (2 mL, 1 M in THF, 2 mmol) was added to a solution of 22 (330 mg, 0.68 mmol) in anhydrous THF (6.8 mL), and the reaction was stirred at rt for 2 h. The mixture was concentrated and the residue purified by flash chromatography using 8% MeOH/CH2Cl2 for elution affording 8i as white solid in 82% yield. Melting range: 173–175 °C. 1H NMR (CDCl3, 300 MHz): δ 1.92 (s, 3H, Me), 2.14 (s, 3H, Me), 2.30 (s, 3H, Me), 3.88 (dd, 1H, H5′, |2 JHH| 12.3 Hz, 3 JHH 4.9 Hz), 3.96 (dd, 1H, H5′, |2 JHH| 12.3 Hz, 3 JHH 3.9 Hz), 4.83 (q, 1H, H4′, 3 JHH 4.8 Hz), 5.22 (m, 1H, H3′), 5.58 (dd, 1H, H2′, 3 JHH 3.9, 2.2 Hz), 6.31 (d, 1H, H1′, 3 JHH 4.2 Hz), 7.58 (d, 1H, H5, 3 JHH 7.4 Hz), 8.26 (d, 1H, H6, 4 JHH 7.1 Hz), 9.9 (br s, 1H, NH). MS (ESI+, m/z): 370 [(M+H)+, 5%] and 392 [(M+Na)+, 100].
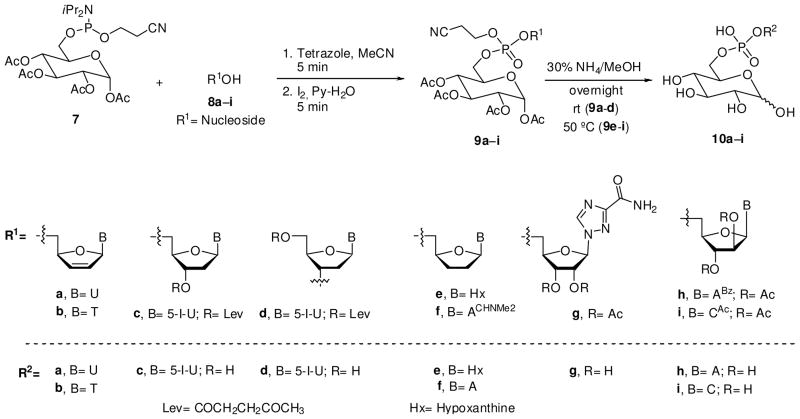
General procedure for the synthesis of phosphotriesters 9a–i
To a stirred solution of nucleoside 8 (1.07 mmol) and 7 (0.72 mmol) in anhydrous MeCN (8 ml) was added 1H-tetrazole (112.7 mg, 1.61 mmol). After the reaction was stirred at rt for 5 min, 5.1 mL of a 1 M solution of I2 in Py/H2O (9:1, v/v) was added to the mixture. After being stirred at rt for 5 min, the mixture was poured into Na2S2O3 (5% aqueous solution) and extracted with CH2Cl2. The combined organic fractions were dried (Na2SO4) and concentrated under vacuum. The crude was purified by flash chromatography using silica 60 Å (32–63 μm) pH 7 and 0–50% MeOH/EtOAc as elution gradient to yield 9 as white hygroscopic foam.
2-Cyanoethyl-(2′,3′-didehydro-2′,3′-dideoxyuridin-5′-yl)-1,2,3,4-tetra-O-acetyl-α-Dglucopyranos-6-yl phosphate (9a)
89% yield. 1H NMR (MeOH-d4, 600 MHz): δ 2.14 (s, 3H, Me), 2.15 (s, 3H, Me), 2.16 (s, 3H, Me), 2.17 (s, 3H, Me), 2.21 (s, 3H, Me), 2.22 (s, 3H, Me), 2.34 (s, 3H, Me), 2.35 (s, 3H, Me), 3.05 (t, 4H, H2‴, 3 JHH 6.1 Hz), 4.31–4.48 (m, 14H, H5″+H6″+H5′+H1‴), 5.23 (m, 4H, H2″+H4′), 5.34 (m, 2H, H4″), 5.61 (m, 2H, H3″), 5.88 (d, 1H, H5, 3 JHH 8.0 Hz), 5.91 (d, 1H, H5, 3 JHH 8.1 Hz), 6.18 (m, 2H, H2′), 6.45 (d, 1H, H1″, 3 JHH 3.7 Hz), 6.47 (d, 1H, H1″, 3 JHH 3.7 Hz), 6.62 (m, 2H, H3′), 7.12 (m, 2H, H1′), 7.72 (d, 1H, H6, 3 JHH 8.1 Hz), 7.74 (d, 1H, H6, 3 JHH 8.1 Hz). 31P NMR (MeOH-d4, 242.9 MHz): δ −2.11, −2.19. MS (ESI+, m/z): 696 [(M+Na)+, 100%].
2-Cyanoethyl-(2′,3′-didehydro-2′,3′-dideoxythymidin-5′-yl)-1,2,3,4-tetra-O-acetyl-α-D-glucopyranos-6-yl phosphate (9b)
88% yield. 1H NMR (MeOH-d4, 600 MHz): δ 2.07 (d, 3H, H7, |4 JHH| 1.1 Hz), 2.08 (d, 3H, H7, |4 JHH| 1.1 Hz), 2.16 (s, 3H, Me), 2.17 (s, 3H, Me), 2.18 (s, 3H, Me), 2.19 (s, 3H, Me), 2.20 (s, 6H, Me), 2.37 (s, 3H, Me), 2.38 (s, 3H, Me), 3.08 (q, 4H, H2‴, 3 JHH 7.2 Hz), 4.36–4.53 (m, 14H, H5″+H6″+H5′+H1‴), 5.25 (m, 4H, H2″+H4′), 5.35 (m, 2H, H4″), 5.65 (m, 2H, H3″), 6.19 (d, 2H, H2′, 3 JHH 6.0 Hz), 6.46 (d, 1H, H1″, 3 JHH 3.8 Hz), 6.49 (d, 1H, H1″, 3 JHH 3.8 Hz), 6.64 (m, 2H, H3′), 7.15 (m, 2H, H1′), 7.58 (d, 1H, H6, |4 JHH| 1.1 Hz), 7.60 (d, 1H, H6, |4 JHH| 1.1 Hz). 31P NMR (MeOH-d4, 242.9 MHz): δ −1.99, −2.11. MS (ESI+, m/z): 710 [(M+Na)+, 100%].
2-Cyanoethyl-(3′-O-levulinyl-5-iodo-2′-deoxyuridin-5′-yl)-1,2,3,4-tetra-O-acetyl-α-D-glucopyranos-6-yl phosphate (9c)
80% yield. 1H NMR (MeOH-d4, 600 MHz): δ 2.18 (s, 6H, Me), 2.19 (s, 3H, Me), 2.20 (s, 6H, Me), 2.21 (s, 3H, Me), 2.26 (s, 3H, Me), 2.37 (s, 3H, Me), 2.38 (s, 6H, Me), 2.64 (m, 4H, H2′), 2.79 (t, 4H, CH2-Lev, 3 JHH 6.2 Hz), 3.04 (t, 4H, CH2-Lev, 3 JHH 6.1 Hz), 3.14 (t, 4H, H2‴, 3 JHH 5.8 Hz), 4.40–4.61 (m, 16H, H5″+H6″+H4′+H5′+H1‴), 5.27–5.50 (m, 4H, H2″+H4″), 5.56 (m, 2H, H3′), 5.65 (t, 2H, H3″, 3 JHH 9.8 Hz), 6.42 (m, 2H, H1′), 6.48 (t, 2H, H1″, 3 JHH 4.1 Hz), 8.24 (s, 1H, H6), 8.31 (s, 1H, H6). 31P NMR (MeOH-d4, 242.9 MHz): δ −1.59, −1.95. MS (ESI+, m/z): 938 [(M+Na)+, 100%].
2-Cyanoethyl-(5′-O-levulinyl-5-iodo-2′-deoxyuridin-3′-yl)-1,2,3,4-tetra-O-acetyl-α-D-glucopyranos-6-yl phosphate (9d)
81% yield. 1H NMR (MeOH-d4, 600 MHz): δ 2.19 (s, 3H, Me), 2.20 (s, 6H, Me), 2.21 (s, 3H, Me), 2.22 (s, 3H, Me), 2.26 (s, 3H, Me), 2.37 (s, 3H, Me), 2.38 (s, 6H, Me), 2.39 (s, 3H, Me), 2.72 (m, 2H, H2′), 2.88 (m, 6H, H2′+CH2-Lev), 3.06 (m, 4H, CH2-Lev), 3.12 (t, 4H, H2‴, 3 JHH 7.1 Hz), 4.40–4.67 (m, 16H, H5″+H6″+H4′+H5′+H1‴), 5.32 (m, 4H, H2″+H3′), 5.42 (m, 2H, H4″), 5.65 (m, 2H, H3″), 6.41 (m, 2H, H1′), 6.50 (d, 1H, H1″, 3 JHH 3.6 Hz), 6.51 (d, 1H, H1″, 3 JHH 3.8 Hz), 8.28 (s, 1H, H6), 8.29 (s, 1H, H6). 31P NMR (MeOH-d4, 242.9 MHz): δ −2.76, −2.99. MS (ESI+, m/z): 938 [(M+Na)+, 100%].
2-Cyanoethyl-(2′,3′-dideoxyinosin-5′-yl)-1,2,3,4-tetra-O-acetyl-α-D-glucopyranos-6-yl phosphate (9e)
80% yield. 1H NMR (MeOH-d4, 600 MHz): δ 2.17 (s, 3H, Me), 2.18 (s, 3H, Me), 2.19 (s, 3H, Me), 2.20 (s, 3H, Me), 2.22 (s, 3H, Me), 2.23 (s, 3H, Me), 2.36 (s, 3H, Me), 2.37 (s, 3H, Me), 2.42 (m, 4H, H3′), 2.80 (m, 4H, H2′), 3.05 (t, 4H, H2‴, 3 JHH 6.1 Hz), 4.30–4.55 (m, 14H, H5″+H6″+H5′+H1‴), 4.62 (m, 2H, H4′), 5.24 (m, 2H, H2″), 5.35 (t, 1H, H4″, 3 JHH 9.8 Hz), 5.37 (t, 1H, H4″, 3 JHH 8.0 Hz), 5.63 (m, 2H, H3″), 6.44 (d, 1H, H1″, 3 JHH 3.7 Hz), 6.47 (d, 1H, H1″, 3 JHH 3.8 Hz), 6.52 (apparent t, 2H, H1′, 3 JHH 5.3 Hz), 8.26 (s, 2H, H2), 8.44 (br s, 2H, H8). 31P NMR (MeOH-d4, 242.9 MHz): δ −1.77, −1.79. MS (ESI+, m/z): 700 [(M+H)+, 100%].
2-Cyanoethyl-[N6-(dimethylamino)methylen-2′,3′-dideoxyadenosin-5′-yl]-1,2,3,4-tetra-O-acetyl- α-D-glucopyranos-6-yl phosphate (9f)
60% yield. 1H NMR (MeOH-d4, 600 MHz): δ 2.17 (s, 3H, Me), 2.18 (s, 3H, Me), 2.19 (s, 3H, Me), 2.20 (s, 3H, Me), 2.22 (s, 3H, Me), 2.23 (s, 3H, Me), 2.36 (s, 3H, Me), 2.37 (s, 3H, Me), 2.45 (m, 4H, H3′), 2.81 (m, 4H, H2′), 3.05 (t, 4H, H2‴, 3 JHH 6.1 Hz), 3.45 (s, 6H, Me-N), 3.48 (s, 6H, Me-N), 4.26–4.72 (m, 16H, H5″+H6″+H4′+H5′+H1‴), 5.21 (m, 2H, H2″), 5.35 (q, 2H, H4″, 3 JHH 9.2 Hz), 5.61 (t, 2H, H3″, 3 JHH 10.2 Hz), 6.42 (d, 1H, H1″, 3 JHH 3.6 Hz), 6.44 (d, 1H, H1″, 3 JHH 3.8 Hz), 6.57 (apparent t, 2H, H1′, 3 JHH 5.9 Hz), 8.62 (s, 2H, H8), 8.67 (s, 2H, H2), 9.28 (s, 1H, HC=N), 9.30 (s, 1H, HC=N). 31P NMR (MeOH-d4, 242.9 MHz): δ −2.18, −2.21. MS (ESI+, m/z): 754 [(M+H)+, 100%].
2-Cyanoethyl-[2′,3′-di-O-acetyl-1′-(3-carbamoyl-1,2,4-triazoyl)-β-D-ribofuranos-5′-yl]-1,2,3,4-tetra-O-acetyl-α-D-glucopyranos-6-yl phosphate (9g)
89% yield. 1H NMR (MeOH-d4, 600 MHz): δ 2.17 (s, 3H, Me), 2.18 (s, 3H, Me), 2.19 (s, 3H, Me), 2.20 (s, 3H, Me), 2.23 (s, 3H, Me), 2.24 (s, 3H, Me), 2.29 (s, 3H, Me), 2.30 (s, 3H, Me), 2.31 (s, 3H, Me), 2.32 (s, 3H, Me), 2.37 (s, 3H, Me), 2.38 (s, 3H, Me), 3.09 (t, 4H, H2‴, 3 JHH 5.9 Hz), 4.36–4.63 (m, 14H, H5″+H6″+H1‴+H5′), 4.71 (m, 2H, H4′), 5.28 (m, 2H, H2″), 5.37 (m, 2H, H4″), 5.64 (m, 2H, H3″), 5.91 (dd, 2H, H3′, 3 JHH 5.7 Hz), 5.97 (t, 1H, H2′, 3 JHH 9.4, 5.1 Hz), 6.49 (m, 4H, H1″+H1′), 8.91 (s, 2H, H5). 31P NMR (MeOH-d4, 242.9 MHz): δ − 2.37, −2.60. MS (ESI+, m/z): 792 [(M+H)+, 90%].
2-Cyanoethyl-[2′,3′-di-O-acetyl-1′-(N6-benzoyladeninyl)-β-D-arabinofuranos-5′-yl]-1,2,3,4-tetra-O-acetyl-α-D-glucopyranos-6-yl phosphate (9h)
70% yield. 1H NMR (CD3CN, 600 MHz): δ 1.86 (s, 3H, Me), 1.87 (s, 3H, Me), 1.97 (s, 3H, Me), 1.98 (s, 3H, Me), 2.01 (s, 3H, Me), 2.02 (s, 3H, Me), 2.06 (s, 3H, Me), 2.07 (s, 3H, Me), 2.17 (s, 3H, Me), 2.18 (s, 3H, Me), 2.19 (s, 3H, Me), 2.83 (q, 4H, H2‴, 3 JHH 5.8 Hz), 4.24 (m, 10H, H5″+H6″+H1‴), 4.45 (m, 6H, H4′+H5′), 5.12 (m, 2H, H2″), 5.21 (q, 2H, H4″, 3 JHH 3.5 Hz), 5.47 (m, 2H, H3″), 5.58 (m, 4H, H3′+H2′), 6.32 (d, 1H, H1″, 3 JHH 3.6 Hz), 6.34 (d, 1H, H1″, 3 JHH 3.6 Hz), 6.74 (d, 2H, H1′, 3 JHH 4.6 Hz), 7.57 (t, 4H, Hm, 3 JHH 7.7 Hz), 7.66 (t, 2H, Hp, 3 JHH 7.3 Hz), 8.06 (d, 4H, Ho, 3 JHH 6.6 Hz), 8.26 (s, 2H, H8), 8.26 (s, 2H, H2), 9.67 (br s, 2H, NH). 31P NMR (CD3CN, 242.9 MHz): δ −1.42, −1.55. MS (ESI+, m/z): 919 [(M+H)+, 100%].
2-Cyanoethyl-[2′,3′-di-O-acetyl-1′-(N4-benzoylcytosinyl)-β-D-arabinofuranos-5′-yl]-1,2,3,4-tetra-O-acetyl-α-D-glucopyranos-6-yl phosphate (9i)
96% yield. 1H NMR (CD3CN, 600 MHz): δ 1.96 (s, 3H, Me), 1.97 (s, 6H, Me), 2.02 (s, 6H, Me), 2.03 (s, 6H, Me), 2.07 (s, 9H, Me), 2.15 (s, 3H, Me), 2.19 (s, 3H, Me), 2.20 (s, 6H, Me), 2.85 (q, 4H, H2‴, 3 JHH 5.7 Hz), 4.11–4.43 (m, 16H, H5″+H6″+H4′+H5′+H1‴), 5.13 (m, 6H, H2″+H4″+H3′), 5.45 (m, 4H, H3″+H2′), 6.27 (d, 1H, H1″, 3 JHH 3.8 Hz), 6.28 (d, 1H, H1″, 3 JHH 3.4Hz), 6.34 (t, 2H, H1′, 3 JHH 3.4 Hz), 7.37 (d, 1H, H5, 3 JHH 7.5 Hz), 7.38 (d, 1H, H5, 3 JHH 7.5 Hz), 8.13 (d, 1H, H6, 3 JHH 7.5 Hz), 8.14 (s, 1H, H6, 3 JHH 7.5 Hz), 9.33 (br s, 2H, NH). 31P NMR (MeOH-d4, 242.9 MHz): δ −2.91, −3.17. MS (ESI+, m/z): 833 [(M+H)+, 100%].
General procedure for the synthesis of phosphodiesters 10a–d
A solution of 9a–d (0.30 mmol) in 6 mL of NH4OH/MeOH (1:1, v/v) was stirred at rt overnight. The solution was concentrated in vacuum and the residue was dissolved in water and treated with DOWEX 50WX8 (H+ form). After stirring for 20 min, the resin was filtered, washed with water, and the filtrate concentrated. The residue was purified by reversed-phase HPLC with a Tracer Excel ODSA column (250 × 10 mm, 5 μm), flow rate 3 mL/min, 0.1% CF3CO2H/H2O (1% MeCN) for elution, to afford 10a–d as hygroscopic white solid.
2′,3′-Didehydro-2′,3′-dideoxyuridine 5′-(α/β-D-glucopyranos-6-yl) phosphate (10a)
47% yield. 1H NMR (D2O, 400 MHz): δ 3.30 (t, 1H, H2″ β anomer, 3 JHH 8.1 Hz), 3.44–4.78 (m, 6H), 4.00 (m, 3H), 4.11 (m, 5H, H5′), 4.68 (d, 1H, H1″ β anomer, 3 JHH 7.9 Hz), 5.16 (m, 2H, H4′), 5.28 (d, 1H, H1″ α anomer, 3 JHH 3.8 Hz), 5.91 (d, 1H, H5, 3 JHH 8.1 Hz), 5.93 (d, 1H, H5, 3 JHH 8.1 Hz), 6.03 (m, 2H, H2′), 6.56 (m, 2H, H3′), 7.01 (m, 2H, H1′), 7.85 (d, 1H, H6, 3 JHH 7.9 Hz), 7.88 (d, 1H, H6, 3 JHH 8.1 Hz). 31P NMR (MeOH-d4, 161.9 MHz): δ 0.64. MS (ESI−, m/z): 451 [(M−H) −, 100%].
2′,3′-Didehydro-2′,3′-dideoxythymidine 5′-(α/β-D-glucopyranos-6-yl) phosphate (10b)
45% yield. 1H NMR (D2O, 600 MHz): δ 1.89 (s, 6H, H7), 3.23 (t, 1H, H2″ β anomer, 3 JHH 8.3 Hz), 3.37–4.52 (m, 5H), 3.68 (m, 1H, 3 JHH 9.8 Hz), 3.87–4.07 (m, 8H), 4.60 (d, 1H, H1″ β anomer, 3 JHH 8.1 Hz), 5.09 (m, 2H, H4′), 5.18 (d, 1H, H1″ α anomer, 3 JHH 3.5 Hz), 5.96 (d, 2H, H2′, 3 JHH 6.3 Hz), 6.48 (m, 2H, H3′), 6.95 (m, 2H, H1′), 7.60 (d, 1H, H6, |4 JHH| 1.2 Hz), 7.61 (d, 1H, H6, |4 JHH| 1.1 Hz). 31P NMR (MeOH-d4, 242.9 MHz): δ 0.38. MS (ESI−, m/z): 465 [(M−H) −, 100%].
2′-Deoxy-5-iodouridine 5′-(α/β-D-glucopyranos-6-yl) phosphate (10c)
40% yield. 1H NMR (D2O, 400 MHz): δ 2.41 (m, 4H, H2′), 3.25 (t, 1H, H2″ β anomer, 3 JHH 8.8 Hz), 3.45–3.74 (m, 6H), 3.94 (m, 1H), 4.07–4.26 (m, 10H), 4.59 (m, 2H, H3′), 4.64 (d, 1H, H1″ β anomer, 3 JHH 7.8 Hz), 5.19 (d, 1H, H1″ α anomer, 3 JHH 3.7 Hz), 6.29 (apparent t, 2H, H1′, 3 JHH 6.9 Hz), 8.27 (s, 1H, H6), 8.28 (s, 1H, H6). 31P NMR (D2O, 161.9 MHz): δ 0.46. MS (ESI−, m/z): 595 [(M−H) −, 100%].
2′-Deoxy-5-iodouridine 3′-(α/β-D-glucopyranos-6-yl) phosphate (10d)
43% yield. 1H NMR (D2O, 400 MHz): δ 2.48 (m, 2H, H2′), 2.67 (m, 2H, H2′), 3.29 (t, 1H, H2″ β anomer, 3 JHH 8.1 Hz), 3.34–4.27 (m, 15H), 4.30 (m, 2H, H4′), 4.70 (d, 1H, H1″ β anomer, 3 JHH 7.9 Hz), 4.81 (m, 2H, H3′), 5.28 (d, 1H, H1″ α anomer, 3 JHH 3.8 Hz), 6.32 (apparent t, 2H, H1′, 3 JHH 6.8 Hz), 8.38 (s, 1H, H6), 8.39 (s, 1H, H6). 31P NMR (D2O, 161.9 MHz): δ 0.42. MS (ESI−, m/z): 595 [(M−H) −, 100%].
General procedure for the synthesis of phosphodiesters 10e–i
A solution of 9e–i (0.30 mmol) in 6 mL of NH4OH/MeOH (1:1, v/v) was stirred overnight at rt (at 50 °C for 10f and 10h). The solution was concentrated in vacuum and the residue was purified by reversed-phase HPLC to afford the ammonium salt of 10e–i as hygroscopic white solid.
2′,3′-Dideoxyinosine 5′-(α/β-D-glucopyranos-6-yl) phosphate (10e)
39% yield. HPLC conditions: Waters XBridge C18 column (150 × 19 mm, 5 μm), flow rate 1.5 mL/min, H2O for elution. 1H NMR (D2O, 400 MHz): δ 2.29 (m, 4H, H3′), 2.65 (m, 4H, H2′), 3.27 (t, 1H, H2″ β anomer, 3 JHH 8.1 Hz), 3.40–3.61 (m, 6H), 3.74 (t, 1H, 3 JHH 9.4 Hz), 3.85–4.44 (m, 8H), 4.54 (m, 2H), 4.65 (d, 1H, H1″ β anomer, 3 JHH 7.9 Hz), 5.22 (d, 1H, H1″ α anomer, 3 JHH 7.9 Hz), 6.43 (dd, 2H, H1′, 3 JHH 6.6, 3.4 Hz), 8.27 (s, 2H, H2), 8.46 (br s, 2H, H8). 31P NMR (MeOH-d4, 161.9 MHz): δ 0.71. MS (ESI−, m/z): 477 [(M−H) −, 100%].
2′,3′-Dideoxyadenosine 5′-(α/β-D-glucopyranos-6-yl) phosphate (10f)
37% yield. HPLC conditions: Waters XBridge C18 column (150 × 19 mm, 5 μm), flow rate 1.5 mL/min, H2O for elution. 1H NMR (D2O, 600 MHz): δ 2.25 (m, 4H, H3′), 2.58 (m, 4H, H2′), 3.23 (t, 1H, H2″ β anomer, 3 JHH 8.1 Hz), 3.40 (m, 6H), 3.73 (m, 4H), 3.95 (m, 3H), 4.1 (m, 2H), 4.49 (m, 2H, H4′), 4.57 (d, 1H, H1″ β anomer, 3 JHH 7.7 Hz), 5.14 (d, 1H, H1″ α anomer, 3 JHH 3.7 Hz), 6.32 (dd, 2H, H1′, 3 JHH 6.6, 3.1 Hz), 8.20 (s, 2H, H8), 8.39 (s, 2H, H2). 31P NMR (MeOH-d4, 242.9 MHz): δ 0.35. MS (ESI−, m/z): 476 [(M−H) −, 100%].
1′-(3-Carbamoyl-1,2,4-triazoyl)-β-D-ribofuranose 5′-(α/β-D-glucopyranos-6-yl) phosphate (10g)
44% yield. HPLC conditions: Mediterránea Sea 18 column (250 × 10 mm, 5 μm), flow rate 1.1 mL/min, H2O for elution. 1H NMR (D2O, 600 MHz): δ 3.17 (t, 1H, H2″ β anomer, 3 JHH 8.1 Hz), 3.36–3.49 (m, 7H), 3.79–4.11 (m, 10H), 4.51 (m, 2H, H3′), 4.55 (d, 1H, H1″ β anomer, 3 JHH 8.1 Hz), 4.62 (m, 2H, H2′), 5.11 (d, 1H, H1″ α anomer, 3 JHH 3.5 Hz), 6.01 (d, 2H, H1′, 3 JHH 3.5 Hz), 8.73 (s, 2H, H5). 31P NMR (D2O, 242.9 MHz): δ 0.50. MS (ESI−, m/z): 485 [(M−H) −, 100 %].
1′-Adeninyl-β-D-arabinofuranose 5′-(α/β-D-glucopyranos-6-yl) phosphate (10h)
35% yield. HPLC conditions: Waters XBridge C18 column (150 × 19 mm, 5 μm), flow rate 1.5 mL/min, H2O for elution. 1H NMR (D2O, 600 MHz): δ 3.29 (t, 1H, H2″ β anomer, 3 JHH 8.1 Hz), 3.42–3.65 (m, 6H), 3.90 (m, 1H), 4.04 (m, 2H), 4.13 (m, 1H), 4.25 (m, 6H), 4.52 (m, 2H, H3′), 4.65 (d, 1H, H1″ β anomer, 3 JHH 8.4 Hz), 4.68 (m, 2H, H2′), 5.22 (d, 1H, H1″ α anomer, 3 JHH 3.7 Hz), 6.49 (dd, 2H, H1′, 3 JHH 6.49 Hz, |4 JHH| 1.6 Hz), 8.31 (s, 2H, H8), 8.48 (s, 2H, H2). 31P NMR (D2O, 242.9 MHz): δ 0.23. MS (ESI−, m/z): 508 [(MH) −, 100 %].
1′-Cytosinyl-β-D-arabinofuranose 5′-(α/β-D-glucopyranos-6-yl) phosphate (10i)
49% yield. HPLC conditions: Mediterránea Sea 18 column (250 × 10 mm, 5 μm), flow rate 1.1 mL/min, H2O for elution. 1H NMR (D2O, 400 MHz): δ 3.28 (t, 1H, H2″ β anomer, 3 JHH 8.1 Hz), 3.49–3.61 (m, 5H), 3.74 (t, 1H, 3 JHH 9.4 Hz), 4.05–4.29 (m, 12H), 4.53 (m, 2H, H2′), 4.66 (d, 1H, H1″ β anomer, 3 JHH 8.0 Hz), 5.24 (d, 1H, H1″ α anomer, 3 JHH 3.7 Hz), 6.27 (d, 2H, H1′, 3 JHH 5.4 Hz), 6.30 (d, 1H, H5, 3 JHH 7.8 Hz), 6.32 (d, 1H, H5, 3 JHH 7.8 Hz), 8.17 (d, 1H, H6, 3 JHH 8.1 Hz), 8.18 (d, 1H, H6, 3 JHH 8.1 Hz). 31P NMR (D2O, 161.9 MHz): δ 0.41. MS (ESI−, m/z): 484 [(M−H) −, 100%].
Synthesis of 1-(5′-O-dimethoxytrityl-β-D-ribofuranosyl)-1,2,4-triazole-3-carboxamide (14)
4,4′-Dimethoxytrityl chloride (417 mg, 1,23 mmol), Et3N (171 μL, 1.23 mmol) and DMAP (catalytic amount) are added to a solution of 13 (150 mg, 0.62 mmol) in anhydrous pyridine (3.1 mL), and the reaction is stirred at rt during 24 h. The mixture was poured into NaHCO3 (5% aqueous solution) and extracted with EtOAc. The combined organic fractions were dried (Na2SO4) and concentrated under vacuum. The crude was purified by flash chromatography using 2% MeOH/CH2Cl2 for elution to yield 14 as yellow solid in 83% yield. Melting range: 111–113 °C. 1H NMR (MeOH-d4, 300 MHz): δ 3.48 (m, 2H, H5′), 3.86 (s, 6H, OMe), 4.39 (m, 1H, H4′), 4.65 (t, 1H, H2′ ó H3′, 3 JHH 5.6 Hz), 4.85 (t, 1H, H2′ ó H3′, 3 JHH 3.4 Hz), 6.13 (d, 1H, H1′, 3 JHH 3.1 Hz), 6.91 (d, 4H, Hm, 3 JHH 8.9 Hz), 7.35 (m, 7H, Hm+Ho+Hp), 7.53 (d, 2H, Ho, 3 JHH 8.2 Hz), 8.82 (s, 1H, H5). MS (ESI+, m/z): 569 [(M+Na)+, 100%].
Synthesis of 1-(2′,3′-di-O-acetyl-5′-O-dimethoxytrityl-β-D-ribofuranosyl)-1,2,4-triazole-3-carboxamide (15)
To a solution of 14 (404 mg, 0.74 mmol) in anhydrous pyridine (5.2 mL) at 0 °C was added acetic anhydride (350 μl, 3.7 mmol). After being stirred at rt during 16 h, the mixture was poured into saturated aqueous NaHCO3 solution and extracted with CH2Cl2. The combined organic fractions were dried (Na2SO4) and concentrated under vacuum. The crude was purified by flash chromatography using 1% MeOH/CH2Cl2 for elution to yield 15 as white solid in 78% yield. Melting range: 99–101 °C. 1H NMR (MeOH-d4, 300 MHz): δ 2.24 (s, 3H, CH3), 2.28 (s, 3H, CH3), 3.48 (m, 1H, H5′), 3.60 (dd, 1H, H5′, |2 JHH| 11.9, 3 JHH 3.2 Hz), 3.92 (s, 6H, OMe), 4.53 (q, 1H, H4′, 3 JHH 3.9 Hz), 5.89 (t, 1H, H3′, 3 JHH 5.3 Hz), 6.20 (dd, 1H, H2′, 3 JHH 5.1, 3.9 Hz), 6.43 (d, 1H, H1′, 3 JHH 3.8 Hz), 6.97 (d, 4H, Hm, 3 JHH 8.8 Hz), 7.38 (m, 7H, Hm+Ho+Hp), 7.58 (d, 2H, Ho, 3 JHH 8.3 Hz), 8.75 (br s, 1H, NH), 8.98 (s, 1H, H5). MS (ESI+, m/z): 653 [(M+Na)+, 100%].
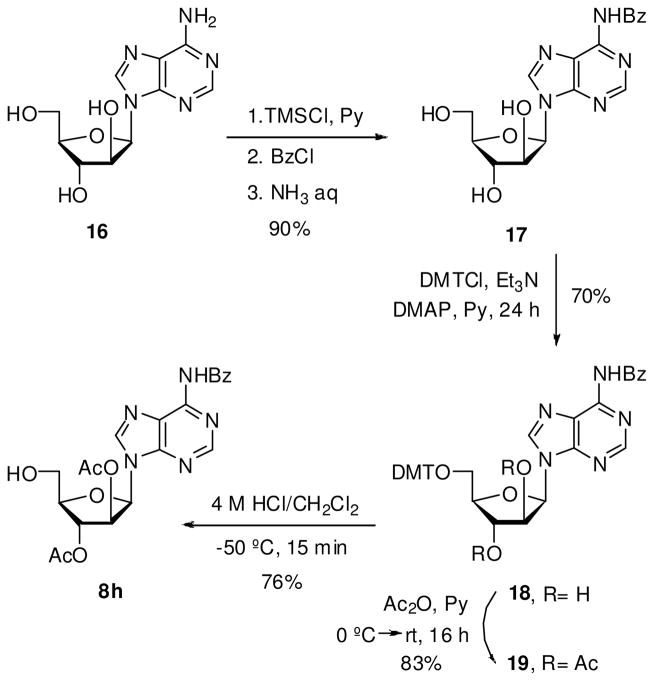
Synthesis of 9-(β-D-arabinofuranosyl)-N6-benzoyladenine (17)
Compound 16 was evaporated with anhydrous pyridine (3 × 10 mL). To a solution of 16 (400 mg, 1.5 mmol) in anhydrous pyridine (7.5 mL) was added trimethylsilyl chloride (1.4 mL, 11.2 mmol). Upon complete consumption of the starting material, benzoyl chloride (870 μL, 7.5 mmol) was added and the reaction was stirred at rt for 2 h. The mixture was then cooled at 0 °C and 2 mL of water was added. After 5 min, 3 mL of aqueous ammonia was added and the mixture was stirred at this temperature for 30 min. The reaction was concentrated and the residue washed with water and EtOAc to give 17 as white solid in 90% yield. Melting range: 185–187 °C. 1H NMR (DMSO-d6, 300 MHz): δ 3.68 (m, 2H, H5′), 3.83 (q, 1H, H4′, 3 JHH 4.8 Hz), 4.23 (m, 2H, H2′+H3′), 5.15 (s, 1H, OH), 5.61 (d, 1H, OH, |4 JHH| 3.9 Hz), 5.74 (d, 1H, OH, |4 JHH| 5.2 Hz), 6.42 (d, 1H, H1′, 3 JHH 5.1 Hz), 7.57 (m, 3H, Hm+Hp), 8.04 (d, 1H, Ho, 3 JHH 8.4 Hz), 8.53 (s, 1H, H8), 8.73 (s, 1H, H2). MS (ESI+, m/z): 372 [(M+H)+, 100%].
Synthesis of N6-benzoyl-9-(5′-O-dimethoxytrityl-β-D-arabinofuranosyl)adenine (18)
A similar procedure as that described for 14 afforded 18 as yellow solid in 70% yield. Melting range: 147–149 °C. 1H NMR (MeOH-d4, 300 MHz): δ 3.68 (m, 2H, H5′), 3.88 (s, 6H, MeO), 4.29 (m, 1H, H4′), 4.57 (m, 2H, H2′+H3′), 6.73 (d, 1H, H1′, 3 JHH 4.1 Hz), 6.96 (d, 4H, Hm, 3 JHH 8.2 Hz ), 7.33–7.77 (m, 12H, Hm+Ho+Hp), 8.21 (d, 2H, Ho, 3 JHH 7.2 Hz), 8.67 (s, 1H, H8), 8.82 (s, 1H, H2). MS (ESI+, m/z): 696 [(M+Na)+, 100%].
Synthesis of N6-benzoyl-9-(2′,3′-di-O-acetyl-5′-O-dimethoxytrityl-β-D-arabinofuranosyl)–adenine (19)
A similar procedure as that described for 15 afforded 19 as white solid in 83% yield. Melting range: 88–90 °C. Flash chromatography was performed using 2% MeOH/CH2Cl2 for elution. 1H NMR (MeOH-d4, 300 MHz): δ 1.76 (s, 3H, Me), 2.27 (s, 3H, Me), 3.71 (m, 2H, H5′), 3.94 (s, 6H, OMe), 4.49 (q, 1H, H4′, 3 JHH 4.1 Hz), 5.75 (dd, 1H, H3′, 3 JHH 5.4, 4.3 Hz), 5.91 (dd, 1H, H2′, 3 JHH 5.3, 4.2 Hz), 6.85 (d, 1H, H1′, 3 JHH 5.4 Hz), 7.03 (d, 4H, Hm, 3 JHH 8.9 Hz ), 7.37–7.65 (m, 12H, Hm+Hp+Ho), 7.82 (d, 2H, Ho, 3 JHH 7.2 Hz), 8.62 (s, 1H, H8), 8.77 (s, 1H, H2). MS (ESI+, m/z): 779 [(M+Na)+, 100%].
Synthesis of 1-(5′-O-tert-butyldimetylsilyl-β-D-arabinofuranosyl)cytosine (21)
tert-Butyldimetylsilyl chloride (342 mg, 2.3 mmol), anhydrous Et3N (287 μl, 2.2 mmol), and DMAP (38 mg, 0.15 mmol) were added to a solution of 20 (500 mg, 2.1 mmol) in anhydrous DMF (10.3 mL) and anhydrous CH2Cl2 (5.2 ml). The mixture was stirred at room temperature during 17 h. Next, the solvents were concentrated, and the residue purified by flash chromatography using 10% MeOH/CH2Cl2 for elution affording 21 as white solid in 82% yield. Melting range: 162–164 °C. 1H NMR (MeOH-d4, 300 MHz): δ 0.16 (s, 3H, Me-Si), 0.17 (s, 3H, Me-Si), 0.95 (s, 9H, Me3C), 4.12 (m, 3H, H4′+H5′), 4.33 (m, 1H, H2′ ó H3′), 4.46 (m, 1H, H2′ ó H3′), 5.93 (d, 1H, H5, 3 JHH 7.5 Hz), 6.16 (d, 1H, H1′, 3 JHH 3.7 Hz), 7.85 (d, 1H, H6, 4 JHH 7.5 Hz). MS (ESI+, m/z): 358 [(M+H)+, 100%].
Synthesis of N4-acetyl-1-(2′,3′-di-O-acetyl-5′-O-tert-butyldimetylsilyl-β-D-arabinofuranosyl)–cytosine (22)
A similar procedure as that described for 15, except that 10 equivalents of acetic anhydride were used, afforded 22 as white solid in 85% yield. Melting range: 229–231 °C. Flash chromatography was performed using 50–100% EtOAc/hexane as elution gradient. 1H NMR (CDCl3, 300 MHz): δ 0.12 (s, 3H, Me-Si), 0.13 (s, 3H, Me-Si), 0.94 (s, 9H, Me3C), 1.93 (s, 3H, Me), 2.11 (s, 3H, Me), 2.30 (s, 3H, Me), 3.90 (dd, 1H, H5′, |2 JHH| 10.7 Hz, 3 JHH 4.1 Hz), 3.94 (dd, 1H, H5′, |2 JHH| 11.3 Hz, 3 JHH 3.9 Hz), 4.06 (q, 1H, H4′, 3 JHH 3.9 Hz), 5.28 (dd, 1H, H3′, 3 JHH 4.5, 3.4 Hz), 5.58 (dd, 1H, H2′, 3 JHH 4.5, 2.5 Hz), 6.35 (d, 1H, H1′, 3 JHH 4.7 Hz), 7.44 (d, 1H, H5, 3 JHH 7.5 Hz), 8.12 (d, 1H, H6, 4 JHH 7.5 Hz). MS (ESI+, m/z): 484 [(M+H)+, 100%].
Solubility assays
The experiments were carried out taking 1 or 2 mg of the corresponding compound and then adding consecutive amounts of water with a micropipette. After each addition a vigorous shaken of the solution was done. Then, the solution was checked for insoluble material with a magnifying glass.
Antiviral assays
The procedures for the antiviral assays in human peripheral blood mononuclear (PBM) cells were reported previously (45). Briefly, uninfected phytohemagglutinin (PHA)-stimulated human PBM cells were infected with HIV-1LAI [about 63,000 disintegrations of reverse transcriptase (RT) activity per min per 107 cells per 10 mL of medium]. The nucleoside analogs or prodrugs were then added to duplicate or triplicate cultures. Uninfected and untreated PBM cells were grown in parallel at equivalent cell concentrations as controls. The cultures were maintained in a humidified 5% CO2-95% air incubator at 37 °C for 6 days after infection, at which point all cultures were sampled for supernatant RT activity. The supernatant was clarified, and the viral particles were then pelleted at 40,000 rpm for 30 min by using a rotor and suspended in virus-disrupting buffer. The RT assay was performed in 96-well microdilution plates by using (rA)n.(T)12–18 as the template primer. The RT results were expressed in disintegrations per minute per millilitre of originally clarified supernatant.
Cytotoxicity assays
The nucleoside analogs and prodrugs were evaluated for their potential toxic effects on uninfected PHA-stimulated human PBM cells and also in CEM (lymphoblastoid) and Vero (African green monkey kidney) cells as described previously (46). PBM cells were obtained from whole blood of healthy HIV-1 and hepatitis B virus-seronegative volunteers and collected by single-step Ficoll-Hypaque discontinuous gradient centrifugation. CEM (CEM-CCRF) cells were a T-lymphoblastoid cell line that was obtained from the American Type Culture Collection, Rockville, MD. The CEM cells were maintained in RPMI 1640 medium supplemented with 20% heat-inactivated fetal bovine serum, penicillin (100 U/mL), and streptomycin (100 μg/mL). The PBM and CEM cells were cultured with and without drug for 6 days, at which time portions were counted for cell proliferation and viability by the trypan blue exclusion method. Only the effects on cell growth are reported, since these correlated well with cell viability. The toxicity of the compounds in Vero cells was assessed after 3 days of treatment with a hemacytometer.
Results and Discussion
We envisioned phosphoramidite 7 as the key building block for the synthesis of the target nucleoside-carbohydrate prodrugs for several reasons. First, the phosphoramidite chemistry offers excellent yield during solution-phase coupling of various nucleosides (33). Second, phosphoramidite compounds are reasonably stable and easy to handle during synthesis. Third, the glucose-amidite 7 would be an ideal molecule offering a versatile conjugation unit for attachment of glucose to a wide range of nucleosides.
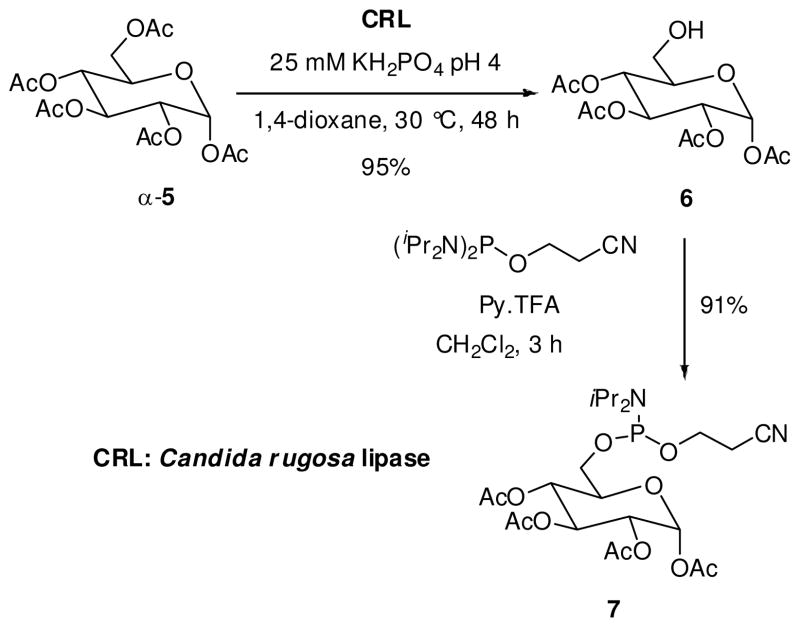
A major challenge faced by carbohydrate or nucleoside chemists is to orchestrate selective transformation of hydroxyl groups with similar reactivity in a single molecule. Gratifyingly, enzyme-catalyzed reactions have provided selective modifications with high selectivity and efficiency (34). The synthesis of glucosyl phophoramidite 7 was accomplished in few steps via a regioselective enzymatic hydrolysis of 1,2,3,4,6-penta-O-acetyl-α-D-glucopyranose (5, Scheme 1).
Scheme 1.
Synthesis of D-glucose phosphoramidite
Commercial Candida rugosa lipase (CRL) was found to be an efficient catalyst for both regio- and chemoselective deacetylation of the primary hydroxyl group in the peracetylated α-D-glucose, furnishing the 6-OH derivative 6 in excellent yield after crystallization from diethyl ether/n-hexane (4:1) (29). The synthesis of the phosphoramidite 7 was carried out by phosphitylation of 6 with 2-cyanoethyl-N,N,N′,N′-tetraisopropyl phosphoramidite and pyridinium trifluoroacetate as activator in high yield (31). The stability of phosphoramidite 7 was determined both in solution and as neat product. A solution of 7 in CDCl3 was found to be stable for six months (by 31P NMR). Similarly, neat amidite 7 was stored for 18 months in the freezer (−24 °C) under nitrogen without any noticeable degradation.
The conjugation of glucosyl phosphoramidite 7 was practiced with a model nucleoside 8a (d4U). Following standard amidite coupling conditions 7 was first activated with 1H-tetrazole and the resulting intermediate was oxidized in situ using an iodine/pyridine/water solution to give the phosphotriester derivative 9a in 89% isolated yield after flash chromatography. As expected, the presence of P-diastereoisomers, due to the phosphorus stereocenter, was apparent from two signals at −2.11 and 2.19 ppm in the 31P NMR spectrum. Next, the acetyl groups and the cyanoethyl group were removed simultaneously from 9a by treatment with a 1:1 (v/v) mixture of aqueous ammonia and MeOH. The residue was purified on a DEAE Sephadex-A25 column with a linear gradient of triethylammonium hydrogen carbonate buffer (0.1–1.0 M) and the product lyophilized. However, this purification protocol resulted in lower purity of the product with a significant amount of TEA salt. Alternatively, the crude residue was mixed with a Dowex 50WX8 hydrogen form exchange resin and, after filtration, the free acid was subjected to reverse phase HPLC purification with a tracer excel 120 ODS-A column using H2O (0.1% TFA, 1% MeCN) for elution. This technique allows isolation of the phosphodiester 10a in high purity and 47% yield. The two α/β-anomeric products corresponding to the glycosyl unit were observed in the 1H NMR spectrum.
Having established an efficient protocol for the glucose-nucleoside conjugate, we were ready to synthesize the glucose-conjugates of seven biologically active nucleosides (d4T, ddI, IdUrd, ddA, virazole, ara-A and ara-C) that we selected for this study. Since d4T has only one reactive site, the synthesis of conjugate 10b was easily accomplished from 8b in two convenient steps (Scheme 2). Pronucleotide 10b was purified by HPLC and the structure was elucidated by NMR spectroscopy.
Scheme 2.
Synthesis of Nucleosyl D-Glucopyranosyl Phosphodiesters
For the synthesis of the IdUrd-glucose phosphodiester derivative 10c, coupling of sugar phosphoramidite 7 with the 5′-hydroxyl group of the parent nucleoside required the masking of the 3′-hydroxyl group. The selective protection of the 3′-secondary alcohol in 11 was accomplished with acetonoxime levulinate catalyzed by Pseudomonas cepacia lipase (PSL-C) in THF at 30 °C (Scheme 3). Under these conditions, facile regioselective 3′-O-acylation of 11 was observed within 24 h furnishing exclusively the 3′-O-levulinyl derivative 8c in 95% yield. Importantly, pure 3′-O-Lev-IdUrd 8c was isolated via precipitation without a need for column chromatography.
Scheme 3.
Enzymatic Synthesis of 3′- and 5′-O-Lev-IdUrd.
The protected nucleoside 8c was coupled with phosphoramidite 7 according to the procedure summarized in Scheme 2. After oxidation, the coupled product was purified by column chromatography to afford the phosphotriester 9c in 80% yield. Next, the 3′-O-levulinyl group was cleaved by treatment of 9c with 1 M hydrazine hydrate in pyridine-acetic acid (3:2 v/v) at room temperature without affecting the phosphate protecting group, thereby establishing the orthogonality of these two protecting groups. However, this reaction resulted in a lower yield due to the troublesome purification of the final product. Therefore, 9c was first deprotected at the phosphate group with Et3N-MeCN (1:1, v/v) to furnish the free phosphate in 90% yield, and then the levulinyl group was removed leading to compound 10c. Nevertheless, the yield did not improve. Finally, the phosphodiester derivative 10c was obtained in satisfactory yield by treatment of 9c with a 1:1 (v/v) mixture of aqueous ammonia and MeOH (35). These conditions allowed deprotection of both the cyanoethyl- and levulinyl groups simultaneously furnishing 10c in 40% yield after HPLC purification.
In order to determine the relationship between biological activity and site of conjugation, we decided to synthesize a 3′-O-conjugate prodrug 10d starting with IdUrd. Preparation of the 5′-O-protected IdUrd derivative 8d was performed with acetonoxime levulinate catalyzed by Candida antarctica lipase (CAL-B) (Scheme 3). Acylation takes place with high selectivity at the 5′-hydroxyl group providing 8d in 78% yield. Coupling of phosphoroamidite 7 and nucleoside 8d followed by in-situ oxidation and cleavage of the blocking groups, yielded glucoconjugate 10d with 43% overall yield.
Next, glucoconjugates of nucleosides having purine bases were synthesized. Synthesis of glucosyl-ddI phosphodiester 10e was accomplished in similar manner as described above. Condensation of 7 and 8e with 1H-tetrazole and iodine oxidation gave phosphotriester 9e in 80% yield. To release the free phosphate, 9e was treated with NH4OH and the crude product was purified by HPLC (see Experimental Part) in 39% yield.
In order to synthesize the glucose-conjugate of ddA (12, Scheme 4), it is essential that N 6-amino group is masked appropriately avoiding the interference with highly reactive amidite 7. Conventional protection of the N 6-amino group in 12 following the Jones protocol (36) resulted in low yields, perhaps due to facile depurination of ddA. Therefore, formamidine was chosen as the protecting group for amino group that is installed under neutral conditions following the protocol described by Minamoto (37).
Scheme 4.
Synthesis of formamidine base protected ddA
Treatment of ddA (12) with DMF-dimethyl acetal generated the dimethylaminomethyleneamino compound 8f in quantitative yield. Coupling of 1H-tetrazole activated phosphoramidite 7 with 8f, followed by the oxidation of the resulting phosphite triester afforded the desired phosphotriester 9f in 60% yield. The modest yield of the coupling and oxidation steps can be explained in terms of possible side reactions. The formamidine group is more labile than the benzoyl group and degradation of the formamidine into formamide was observed (37). Subsequently, the phosphate moiety, the acetyl groups and the formamidine group were simultaneously cleaved by aqueous ammonium at 50 °C, which gave the phosphodiester 10f as its ammonium salt. Purification of 10f was achieved by HPLC (see Experimental Part).
Prior to the conjugation of amidite 7 to the antiviral drug virazole 13, masking of the 2′- and 3′-hydroxyl groups was essential. Our first attempt was based on simultaneous protection of 2′- and 3′-hydroxyl groups of 13 as an acetal using acetone, copper (II) sulphate and sulphuric acid resulted in less than 40% yield of the desired product. The yield could not be improved when 13 was treated with 2,2-dimethoxypropane in acetone. Next, we attempted the acylation of the secondary hydroxyl groups of 13 with acetonoxime levulinate in the presence of Pseudomonas cepacia (PSL-C) (38–40). Unfortunately, mixture of acylated products and low conversions (<10%) were observed after 4 days at 50 °C. The conversion of 13 was improved by employing vinyl acetate as the acylating agent. However, 3′,5′-di-O-acetyl nucleoside was obtained as the major product, in addition to the 2′,5′-di-O-acetyl and the triacetyl derivatives. Additionally, we attempted the selective enzymatic hydrolysis of peracylated virazole anticipating the formation of 8g (41). Multiple enzymes (Candida antarctica lipase B, porcine pancreas lipase, subtilisine) were employed but we could not achieve the desired regioselectivity.
These failures directed our attention to a more traditional three-step approach described in Scheme 5. Selective protection of the primary hydroxyl group was easily achieved by treatment of virazole (13) with dimethoxytrityl chloride, Et3N, and DMAP in pyridine. Product 14 was then subjected to acetylation with acetic anhydride in pyridine to provide 2′,3′-di-O-acetyl-5′-O-DMT nucleoside 15 in 78% yield. Deprotection of the 5′-hydroxyl group by formic acid in chloroform led to the desired precursor 8g in quantitative yield. The protected nucleoside 8g was coupled as described above and the resulting phosphotriester 9g was deprotected with a 1:1 (v/v) mixture of aqueous ammonia and MeOH. Phosphodiester 10g was purified by reverse phase HPLC on a C18 column.
Scheme 5.
Synthesis of virazole nucleoside precursor
Vidarabine is an ara-nucleoside drug with wide range of antiviral activity. However, vidarabine is toxic and metabolically unstable (rapid deamination). Therefore, 5′-conjugates of vidarabine have been synthesized as prodrugs (42). In this vein, we decided to extend our study toward synthesis of glycoconjugate 10h. First, ara-adenosine (16) was N-benzoylated following the transient Jones protocol to furnish N6-benzoyl-ara-adenosine (17) in 90% yield. Transformation of 17 into 19 proceeded well yielding the corresponding 2′,3′-di-O-acetyl-5′-O-DMT derivative. Traditional deprotection of dimethoxytrityl group of 19 with formic acid in CHCl3 resulted in low yield and formation of depurinated products. Milder deprotection of 19 was accomplished with 4 M HCl in CH2Cl2 at −50 °C
Coupling of phosphoramidite 7 with protected ara-adenosine 8h and subsequent oxidation gave the compound 9h in 70% yield after column chromatography. Deprotection of 9h by aqueous ammonium at 50 °C and purification of the reaction product by reverse phase HPLC led to phosphodiester 10h.
Ara-cytidine is yet another drug that is poorly soluble in water and prodrug approaches have been utilized to improve the oral bioavailability (43–44). We believe that synthesis of a glucose-conjugate 10i would offer increased solubility for 20 in water. Preparation of the protected ara-cytidine precursor 8i was accomplished as depicted in Scheme 7. The primary hydroxyl group in 20 was protected as silyl ether by reaction with tert-butyldimethylsilyl chloride, Et3N, and DMAP in DMF and CH2Cl2 to afford 21 in 82% yield. Peracetylation was then performed using acetic anhydride in pyridine furnishing protected 22. Subsequent treatment with tetrabutylammonium fluoride removed the TBDMS group and afforded the 2′,3′-protected nucleoside 8i in 57% overall yield from ara-cytidine. The phosphotriester 9i was prepared in 96% isolated yield by coupling of 7 and 8i followed by in situ oxidation. Finally, compound 10i was purified by reverse phase HPLC. Based on their NMR data, all conjugates 10a-i were prepared in furanose form.
Scheme 7.
Synthesis of ara-cytidine precursor
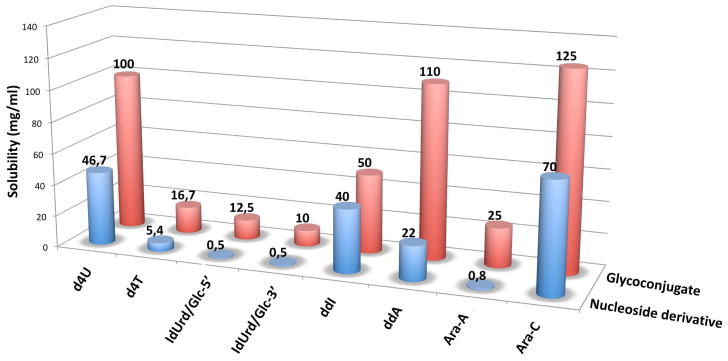
In order to determine the impact of conjugating the glucose moiety onto the nucleosides, we compared the solubility of prodrugs 10a–i with unprotected nucleosides (Figure 1). Solubility assays revealed that all conjugates 10a–i prepared in this study were more soluble in water than the parent nucleosides. It is noteworthy that the phosphodiester of ara-adenosine 10h was found to be 31-times more soluble in water than the ara-adenosine. Also, the 3′-O- and 5′-O-conjugate derivatives of IdUrd 10c and 10d displayed markedly increased solubility in water (25- and 20-times more soluble, respectively). More importantly, poorly soluble nucleoside derivatives IdUrd and ara-A showed significant improvement in water solubility after conjugation with glucose. Clearly, such carbohydrate conjugates may lead to improved oral bioavailability of the drugs that lack water solubility.
Figure 1.
Comparative water solubility data of free nucleosides and prodrugs 10a-i.
Biological Evaluation
Antiviral assays
Nine glucose-conjugates 10a-i were tested against HIV-1 with AZT (zidovudine) used as a positive control. All the anti-HIV assay was performed in human PBM cells (27). We also included the unprotected starting materials used for the synthesis of conjugates and a representative commercial sample of the same product in the assay as additional controls. A stock solution (40 mM) of the conjugates 10a-i and various controls were prepared in sterile DMSO and then diluted to the desired concentration in growth media.
An overall summary of the data expressed as the effective concentration required for inhibition of viral replication by 50% (EC50) and 90% (EC90) is summarized in Table 1. The anti-HIV activity of the conjugates tested were in the order of 10b > 10f > 10i > 10g > 10e > 10h > 10c. The traditional anti-HIV nucleosides d4T and ddI after conjugation showed moderate activity indicating that the potency of 10b and 10e could be attributed to the free nucleosides. Similar pattern of anti-HIV activity was observed with ddA before and after conjugation. The improved activity of ddA conjugate 10f compared to the conjugate of ddI 10e may be due to the efficient deamination of 10f to liberate ddI intracellularly. Furthermore, both virazole conjugate 10g and ara-A conjugate 10h exhibited better activity than their nucleoside counterpart, thus indicating that the glucose-conjugates are possibly a good design for developing prodrugs. Most importantly, the conjugation of glucose to the nucleosides via a phosphate linkage did not abolish the antiviral activity of the parent nucleosides. The retention of modest anti-HIV activity exhibited by the new conjugates is encouraging and warrants further studies.
Table 1.
Effects of various nucleosides and their conjugates 10a-h against HIV-1 and their cytotoxicity assays.
| Analog | Nucleoside/Conjugate (source) | Anti-HIV-1 activity in PBM cellsa |
Cytotoxicity (IC50 [μM])b |
|||
|---|---|---|---|---|---|---|
| EC50 [μM] | EC90 [μM] | PBM cells | CEM cells | VERO cells | ||
| AZT | (commercial) | 0.0054 | 0.050 | > 100 | 14.3 | 56.0 |
| d4U | (synthesized) | > 100 | > 100 | > 100 | 55.9 | > 100 |
| 10a | Glc-d4U | > 100 | > 100 | > 100 | > 100 | > 100 |
| d4T | (commercial) | 0.073 | 0.37 | |||
| d4T | (synthesized) | 0.069 | 0.37 | > 100 | > 100 | > 100 |
| 10b | Glc-d4T | 0.34 | 2.0 | > 100 | 76.8 | > 100 |
| IdUrd | (commercial) | 65.7 | > 100 | |||
| IdUrd | (synthesized) | 86.5 | > 100 | > 100 | 2.7 | > 100 |
| 10c | Glc-5′-IdUrd | 32.4 | > 100 | > 100 | 2.3 | > 100 |
| 10d | Glc-3′-IdUrd | > 100 | > 100 | > 100 | 76.0 | > 100 |
| ddI | (commercial) | 0.32 | 2.8 | |||
| ddI | (synthesized) | 0.06 | 0.76 | > 100 | > 100 | > 100 |
| 10e | Glc-ddI | 15.5 | > 100 | > 100 | 22.7 | > 100 |
| ddA | (synthesized) | 0.59 | 3.8 | > 100 | > 100 | > 100 |
| 10f | Glc-ddA | 1.1 | 9.9 | 87.6 | 13.2 | 73.2 |
| Virazole | (commercial) | 77.5 | > 100 | |||
| Virazole | (synthesized) | 85.9 | > 100 | > 100 | 53.0 | > 100 |
| 10g | Glc-Virazole | 12.8 | 39.1 | > 100 | 17.6 | > 100 |
| Ara-A | (commercial) | > 100 | > 100 | |||
| Ara-A | (synthesized) | > 100 | > 100 | 26.0 | 26.2 | > 100 |
| 10h | Glc-Ara-A | 19.5 | > 100 | > 100 | 18.9 | > 100 |
| Ara-C | (synthesized) | 4.9 | 11.9 | 57.0 | < 0.1 | ND |
| 10i | Glc-Ara-C | 5.8 | 14.0 | 17.5 | < 1.0 | < 1.0 |
Cytotoxicity assays
The new conjugates were evaluated for their potential toxic effects using three different cell lines. First, the PBM cells were selected because of their slow growth and also being host cells for the anti-HIV assay. Second, we included lymphocytic CEM cells due to their quick replication and its common use for anti-HIV-1 assays. Third, Vero cells were utilized due to their rapid growth and being anchored on the surface. The screening of new conjugates in three cell types will enable us to distinguish the observed anti-HIV-1 activity from the toxicity. Among various conjugates tested, the most potent anti-HIV-1 compound was 10b with EC50 of 0.34 μM demonstrating no cytotoxicity in PBM and Vero cells and marginal IC50 of 76.8 μM in CEM cells. On the other hand, the ddA conjugate 10f was found to be relatively more cytotoxic in all three cell lines. Overall the new conjugates were found to be somewhat more cytotoxic in CEM cells compared to the PBM and Vero cells.
In summary, the anti-HIV-1 and cytotoxicity assays with conjugates 10a-i reported herein confirm that the modification of various nucleosides with glucose-phosphate-conjugate did not completely abolish the original activity of the parent nucleosides. No significant toxicity was observed for the conjugates tested in this study with the exception of ara-C, which was found to be more cytotoxic when in the form of glyco-conjugate than as a simple nucleoside.
Conclusions
Glycoconjugates of biologically active nucleoside analogs have been prepared as potential prodrugs, since they can be cleaved intracellularly to the corresponding nucleosides and nucleotides. We have shown that phosphoramidite approach is a convenient method that allows synthesis of stable glycosyl-nucleoside phosphodiester compounds. We demonstrated that the glucosyl 6-phosphoramidite 7 is easily accessible in high yield through a regioselective chemoenzymatic strategy. Furthermore, we established that the phosphodiesters of d4T, IdUrd, ddI, ddA, virazole, ara-A and ara-C exhibited higher solubility in water compared to their parent nucleosides. These water soluble conjugates of nucleosides may improve their oral bioavailability and increase the intestinal absorption as reported for other carbohydrate-conjugates (47–48). The anti-HIV-1 data is encouraging and expected to offer new opportunities in designing therapeutic nucleosides via a straightforward chemical conjugation approach. We anticipate that the amidite 7 will find wider applications for other therapeutic agents that require enhanced water solubility.
Supplementary Material
Scheme 6.
Synthesis of ara-adenosine precursor
Acknowledgments
Financial support of this work by the Spanish Ministerio de Educación y Ciencia (MEC) (Project MEC-CTQ-2007-61126) is gratefully acknowledged. RFS is supported in part by NIH grants 5P30-AI-50409 (CFAR), 5R37-AI-025899, 5R37-AI-041980 and by the Department of Veterans Affairs.
Footnotes
Dedicated to Professor Francisco Palacios on the occasion of his 60th birthday.
Supporting Information Available: 1H, 13C NMR spectral data. The level of purity is indicated by the inclusion of copies of 1H and 13C NMR spectra. In addition, some 2D NMR experiments are shown, which were used to assign the peaks. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.De Clercq E. Recent highlights in the development of new antiviral drugs. Curr Opin Microbiol. 2005;8:552–560. doi: 10.1016/j.mib.2005.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schinazi RF, Liotta DC. Frontiers in Nucleoside and Nucleic Acids. IHL Press; Tucker, Georgia: 2004. [Google Scholar]
- 3.Rachakonda S, Cartee L. Challenges in Antimicrobial Drug Discovery and the Potential of Nucleoside Antibiotics. Curr Med Chem. 2004;11:775–793. doi: 10.2174/0929867043455774. [DOI] [PubMed] [Google Scholar]
- 4.De Clercq E. Antiviral drugs in current clinical use. J Clin Vir. 2004;30:115–133. doi: 10.1016/j.jcv.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 5.Chu CK. Recent Advances in Nucleosides: Chemistry and Chemotherapy. Elsevier Science; New York: 2002. [Google Scholar]
- 6.Dove A. The Bittersweet Promise of Glycobiology. Nat Biotechnol. 2001;19:913–917. doi: 10.1038/nbt1001-913. [DOI] [PubMed] [Google Scholar]
- 7.Dwek RA. Glycobiology: Toward Understanding the Function of Sugars. Chem Rev. 1996;96:683–720. doi: 10.1021/cr940283b. [DOI] [PubMed] [Google Scholar]
- 8.Varki A. Biological Roles of Oligosaccharides: all of the Theories are correct. Glycobiology. 1993;3:97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peterson LW, McKenna CE. Prodrug approaches to improving the oral absorption of antiviral nucleotide analogues. Expert Opin Drug Deliv. 2009;6:405–420. doi: 10.1517/17425240902824808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jessen HJ, Schulz T, Balzarini J, Meier C. Bioreversible Protection of Nucleoside Diphosphates. Angew Chem Int Ed. 2008;47:8719–8722. doi: 10.1002/anie.200803100. [DOI] [PubMed] [Google Scholar]
- 11.Wendicke S, Warnecke S, Meier C. Efficient Synthesis of Nucleoside Diphosphate Glycopyranoses. Angew Chem Int Ed. 2008;47:1500–1502. doi: 10.1002/anie.200703237. [DOI] [PubMed] [Google Scholar]
- 12.Gold H, van Delft P, Meeuwenoord N, Codée JDC, Filippov DV, Eggink G, Overkleeft HS, van der Marel GA. Synthesis of Sugar Nucleotides by Application of Phosphoramidites. J Org Chem. 2008;73:9458–9469. doi: 10.1021/jo802021t. [DOI] [PubMed] [Google Scholar]
- 13.Timmons SC, Jakeman DL. Stereoselective Chemical Synthesis of Sugar Nucleotides via Direct Displacement of Acylated Glycosyl Bromides. Org Lett. 2007;9:1227–1230. doi: 10.1021/ol063068d. [DOI] [PubMed] [Google Scholar]
- 14.Ahmadibeni Y, Parang K. Solid-Phase Synthesis of Dinucleoside and Nucleoside-Carbohydrate phosphodiesters and Thiophosphodiesters. J Org Chem. 2006;71:6693–6696. doi: 10.1021/jo0611115. [DOI] [PubMed] [Google Scholar]
- 15.Xiao Q, Ju Y, Zhao Y. A Convenient Route to Symmetric Phosphate, Phosphorothioate, and Phosphoroselenoate of AZT and d4T. Synth Commun. 2003;33:4157–4162. [Google Scholar]
- 16.Schlienger N, Périgaud C, Gosselin G, Imbach JL. Synthesis and Studies of Mononucleoside Glucosyl Phosphotriester Derivates. J Org Chem. 1997;62:7216–7221. doi: 10.1021/jo9706638. [DOI] [PubMed] [Google Scholar]
- 17.Herdewijn P. Nucleosiode Prodrugs and Delivery Strategies. In: Beaucage SL, Bergstrom DE, Glick GD, Jones RA, editors. Current Protocols in Nucleic Acid Chemistry. Chapter 15. John Wiley & Sons; New York: 2007. pp. 15.0.1–15.0.4. [Google Scholar]
- 18.Meier C, Renze J, Ducho C, Balzarini J. Curr Top Med Chem. 2002;2:1111–1121. doi: 10.2174/1568026023393183. [DOI] [PubMed] [Google Scholar]
- 19.Wagner CR, Iyer VV, McIntee EJ. Pronucleotides: toward the in vivo delivery of antiviral and anticancer nucleotides. Med Res Rev. 2000;20:417–451. doi: 10.1002/1098-1128(200011)20:6<417::aid-med1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 20.Parang K, Wiebe LI, Knaus EE. Novel Approaches for Designing 5′-O-Ester Prodrugs of 3′-Azido-2′,3′-dideoxythymidine (AZT) Curr Med Chem. 2000;7:995–1039. doi: 10.2174/0929867003374372. [DOI] [PubMed] [Google Scholar]
- 21.Henin Y, Gouyette C, Schwartz O, Debouzy JC, Neumann JM, Huynh-Dinh T. Lipophilic Glycosyl Phosphotriester Derivatives of AZT: Synthesis, NMR Transmembrane Transport Study and Antiviral Activity. J Med Chem. 1991;34:1830–1837. doi: 10.1021/jm00110a011. [DOI] [PubMed] [Google Scholar]
- 22.Gouyette C, Neumann JM, Fauve R, Huynh-Dinh T. 6- and 1-Substituted Mannosyl Phosphotriesters as Lipophilic Macrophage-Targeted Carriers of Antiviral Nucleosides. Tetrahedron Lett. 1989;30:6019–6022. [Google Scholar]
- 23.D’Onofrio J, Petraccone L, Martino L, Di Fabio G, Iadonisi A, Balzarini J, Giancola C, Montesarchio D. Synthesis, Biophysical Characterization, and Anti-HIV Activity of Glyco-Conjugated G-Quadruplex-Forming Oligonucleotides. Bioconjugate Chem. 2008;19:607–616. doi: 10.1021/bc7003395. [DOI] [PubMed] [Google Scholar]
- 24.Neumann JM, Herve M, Debouzy JC, Guerra FI, Gouyette C, Dupraz B, Huynh-Dinh T. Synthesis and transmembrane transport studies by NMR of a glucosyl phospholipid of thymidine. J Am Chem Soc. 1989;111:4270–4277. [Google Scholar]
- 25.Salam MA, Behrman EJ. Synthesis of nucleoside 5′-(β-D-glucopyranosyl monophosphates) by the sugar ortho ester route. Carbohydr Res. 1982;102:139–146. [Google Scholar]
- 26.Martínez-Montero S, Fernández S, Rodríguez-Pérez T, Sanghvi YS, Wen K, Gotor V, Ferrero M. Improved Synthesis and Isolation of 2′-O-Methyladenosine: Effective and Scalable Enzymatic Separation of 2′/3′-O-Methyladenosine Regioisomers. Eur J Org Chem. 2009:3265–3271. [Google Scholar]
- 27.Díaz-Rodríguez A, Sanghvi YS, Fernández S, Schinazi RF, Theodorakis EA, Ferrero M, Gotor V. Synthesis and Anti-HIV Activity of Conformationally Restricted Bicyclic Hexahydroisobenzofuran Nucleoside Analogs. Org Biomol Chem. 2009;7:1415–1423. doi: 10.1039/b818707j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lavandera I, Fernández S, Magdalena J, Ferrero M, Kazlauskas RJ, Gotor V. An Inverse Substrate Orientation for the Regioselective Acylation of 3′,5′-Diaminonucleosides Catalyzed by Candida antarctica lipase B? Org Biomol Chem. 2005;6:1381–1390. doi: 10.1002/cbic.200400422. [DOI] [PubMed] [Google Scholar]
- 29.Rodríguez-Pérez T, Lavandera I, Fernández S, Sanghvi YS, Ferrero M, Gotor V. Novel and Efficient Chemoenzymatic Synthesis of D-Glucose-6-Phosphate and Molecular Modeling Studies on the Selective Biocatalysis. Eur J Org Chem. 2007:2769–2778. [Google Scholar]
- 30.Bronisz R. Synthesis and structure of {[Zn(1,2-di(1,2,3,4-tetrazol-2-yl)ethane)3](ClO4)2}n. The first coordination polymer based on 2-substituted tetrazole. Inorg Chim Acta. 2002;340:215–220. [Google Scholar]
- 31.Sanghvi YS, Guo Z, Pfundheller HM, Converso A. Improved Process for the Preparation of Nucleosidic Phosphoramidites Using a Safer and Cheaper Activator. Org Proc Res Dev. 2000;4:175–181. [Google Scholar]
- 32.Lavandera I, García J, Fernández S, Ferrero M, Gotor V, Sanghvi YS. Enzymatic Regioselective Levulinylation of 2′-Deoxyribonucleosides and 2′-O-Methylribonucleosides. In: Beaucage SL, Bergstrom DE, Glick GD, Jones RA, editors. Current Protocols in Nucleic Acid Chemistry. Chapter 2. John Wiley & Sons; New York: 2005. pp. 2.11.1–2.11.36. O-Levulinyl acetonoxime is now commercially available from Sai Life Sciences ( www.sailifesciences.com) [DOI] [PubMed] [Google Scholar]
- 33.Beaucage SL, Iyer RP. Advances in the Synthesis of Oligonucleotides by the Phosphoramidite Approach. Tetrahedron. 1992;48:2223–2311. [Google Scholar]
- 34.Ferrero M, Gotor V. Biocatalytic Selective Modifications of Conventional Nucleosides, Carbocyclic Nucleosides, and C-Nucleosides. Chem Rev. 2000;100:4319–4348. doi: 10.1021/cr000446y. [DOI] [PubMed] [Google Scholar]
- 35.Reese CB, Yan H. An approach to the desulfurization of oligonucleotide phosphorothioates. Tetrahedron Lett. 2003;44:2501–2504. [Google Scholar]
- 36.Ti GS, Gaffney BL, Jones RA. Transient protection: Efficient one-flask synthesis of protected deoxynucleosides. J Am Chem Soc. 1982;104:1316–1319. [Google Scholar]
- 37.Minamoto K, Fujiki Y, Shiomi N, Uda Y, Sasaki T. Systematic General Synthesis of Purine 8,5′-Imino and Substituted Imino Cyclonucleosides. J Chem Soc, Perkin Trans. 1985;1:2337–2346. [Google Scholar]
- 38.Lavandera I, Fernández S, Magdalena J, Ferrero M, Jarjap H, Savile CK, Kazlauskas RJ, Gotor V. Molecular Basis for the Unusual Regioselectivity of Lipase from Pseudomonas cepacia toward the Secondary Alcohol of 2′-Deoxynucleosides. ChemBioChem. 2006;7:693–698. doi: 10.1002/cbic.200500451. [DOI] [PubMed] [Google Scholar]
- 39.García J, Fernández S, Ferrero M, Sanghvi YS, Gotor V. Study of regioselective enzymatic acylation of β-L-2′-deoxynucleosides: application to resolution of β-D/L-2′-deoxynucleoside racemic mixtures. Org Lett. 2004;6:3759–3762. doi: 10.1021/ol048502v. [DOI] [PubMed] [Google Scholar]
- 40.García J, Fernández S, Ferrero M, Sanghvi YS, Gotor V. Novel Enzymatic Synthesis of Levulinyl Protected Nucleosides useful for Solution Phase Synthesis of Oligonucleotides. Tetrahedron: Asymmetry. 2003;14:3533–3540. [Google Scholar]
- 41.García J, Fernández S, Ferrero M, Sanghvi YS, Gotor V. Building-Blocks for the Solution Phase Synthesis of Oligonucleotides: Regioselective Hydrolysis of 3′,5′-di-O-Levulinylnucleosides Using an Enzymatic Approach. J Org Chem. 2002;67:4513–4519. doi: 10.1021/jo020080k. [DOI] [PubMed] [Google Scholar]
- 42.Shen W, Kim JS, Mitchell S, Kish P, Kijek P, Hilfinger J. 5′-O-D-Valyl Ara A, a Potential Prodrug for Improving Oral Bioavailability of the Antiviral Agent Vidarabine. Nucleosides Nucleotides Nucleic Acids. 2009;28:43–55. doi: 10.1080/15257770802581757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wechter WJ, Gish DT, Greig ME, Gray GD, Moxley TE, Kuentzel SL, Gray LG, Gibbons AJ, Griffin RL, Neil GL. Orally active derivatives of ara-cytidine. J Med Chem. 1976;19:1013–1017. doi: 10.1021/jm00230a007. [DOI] [PubMed] [Google Scholar]
- 44.Hong CI, Kirisits AJ, Nechaev A, Buchheit DJ, West CR. Synthesis and antitumor activity of 1-β-D-arabinofuranosylcytosine and cytidine conjugates of thioether lipids. J Med Chem. 1990;33:1380–1386. doi: 10.1021/jm00167a016. [DOI] [PubMed] [Google Scholar]
- 45.Schinazi RF, Sommadossi JP, Saalmann V, Cannon DL, Xie MW, Hart GC, Smith GA, Hahn EF. Activity of 3′-azido-3′-deoxythymidine nucleotide dimers in primary lymphocytes infected with human immunodeficiency virus type 1. Antimicrob Agents Chemother. 1990;34:1061–1067. doi: 10.1128/aac.34.6.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stuyver LJ, Lostia S, Adams M, Mathew J, Pai BS, Grier J, Tharnish P, Choi Y, Chong Y, Choo H, Chu CK, Otto MJ, Schinazi RF. Antiviral activities and cellular toxicities of modified 2′,3′-dideoxy-2′,3′-didehydrocytidine analogues. Antimicrob Agents Chemother. 2002;46:3854–3860. doi: 10.1128/AAC.46.12.3854-3860.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hollman PCH, van Trijp JMP, Buysman MNCP, van der Gaag MS, Mengelers MJB, de Vries JHM, Katan MB. Relative bioavailability of the antioxidant flavonoid quercetin from various foods in man. FEBS Lett. 1997;418:152–156. doi: 10.1016/s0014-5793(97)01367-7. [DOI] [PubMed] [Google Scholar]
- 48.Kaminaga Y, Nagatsu A, Akiyama T, Sugimoto N, Yamazaki T, Maitani T, Mizukami H. Production of unnatural glucosides of curcumin with drastically enhanced water solubility by cell suspension cultures of Catharanthus roseus. FEBS Lett. 2003;555:311–316. doi: 10.1016/s0014-5793(03)01265-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.