Abstract
The utility of live attenuated vaccines for controlling HIV epidemics is being debated. Live attenuated HIV vaccines (LAHVs) could be extremely effective in protecting against infection with wild-type strains, but may not be completely safe as the attenuated strain could cause AIDS in some vaccinated individuals. We present a theoretical framework for evaluating the consequences of the tradeoff between vaccine efficacy (in terms of preventing new infections with wild-type strains) and safety (in terms of vaccine-induced AIDS deaths). We use our framework to predict, for Zimbabwe and Thailand, the epidemiological impact of 1,000 different (specified by efficacy and safety characteristics) LAHVs. We predict that paradoxically: (i) in Zimbabwe (where transmission is high) LAHVs would significantly decrease the AIDS death rate, but (ii) in Thailand (where transmission is low) exactly the same vaccines (in terms of efficacy and safety characteristics) would increase the AIDS death rate. Our results imply that a threshold transmission rate exists that determines whether any given LAHV has a beneficial or a detrimental impact. We also determine the vaccine perversity point, which is defined in terms of the fraction of vaccinated individuals who progress to AIDS as a result of the vaccine strain. Vaccination with any LAHV that causes more than 5% of vaccinated individuals to progress to AIDS in 25 years would, even 50 years later, lead to perversity (i.e., increase the annual AIDS death rate) in Thailand; these same vaccines would lead to decreases in the annual AIDS death rate in Zimbabwe.
Live attenuated vaccines (due to their high protective efficacy, low cost, and simple immunization schedules) have been used successfully to control smallpox, polio, and measles epidemics (1, 2). Preliminary attempts have been made to develop a live attenuated HIV vaccine (LAHV) (3–6); however, the debate about the potential utility of developing LAHVs has been controversial, mainly due to concerns that have arisen about vaccine safety (3, 4, 7–9). The major safety concern is that even a very attenuated strain of HIV (if used as a LAHV) could actually cause AIDS in some fraction of vaccinated individuals (4, 5). Live attenuated polio vaccine has caused some cases of paralytic poliomyelitis (10), and any live attenuated vaccine has the potential to cause disease. Studies in macaques of the closely related simian immunodeficiency virus have shown that live attenuated strains can be highly effective in protecting against infection with the wild-type strain (4, 11), but that some macaques develop AIDS as a result of the attenuated strain (5, 12). Concern about the potential safety of LAHVs has been heightened by a recent report of the natural history of HIV infection in a cohort of gay men in Australia (13). Some of these men were infected with a naturally attenuated strain of HIV. Long-term follow-up studies of this cohort have shown that this strain of HIV, although attenuated, can cause immune deterioration (13). The design of a LAHV therefore presents a formidable challenge.
The efficacy and safety of LAHVs will be determined by phase III clinical trials; however, the safety effects of any LAHV will need to be understood at the epidemic level before any candidate vaccine can be considered as a potential epidemic control agent. Here we present a mathematical model that provides a theoretical framework that can be used for LAHVs to predict the tradeoff between vaccine efficacy (in terms of preventing new infections with wild-type HIV) and vaccine safety effects (in terms of vaccine-induced AIDS deaths) at the epidemic level. We evaluate the consequences of this tradeoff by using our model to predict the potential epidemiological impact of vaccination with a variety of LAHVs in Thailand and Zimbabwe. Specifically, we use our model to address two research questions: (i) could vaccination with a LAHV lead to the eradication of the wild-type strain of HIV? and (ii) what impact would a mass vaccination campaign with a LAHV have on the annual AIDS death rate? We discuss the implications of our results for vaccine design, phase III clinical trials, and epidemic control strategies.
Mathematical models of HIV vaccines have been used: (i) to calculate vaccine efficacy and vaccination coverage levels for eradicating HIV (using a prophylactic non-LAHV) (14–16), (ii) to design phase III trials (17–19), (iii) to evaluate the effect of changes in risk behavior on vaccination campaigns (15, 20), and (iv) to determine the degree of vaccine-induced cross-immunity that would be necessary to control multiple subtypes (20–22). These analyses (14–22) have evaluated the consequences of vaccines that do not have the potential to cause AIDS. Here, we present a theoretical framework that enables us to model the effects of a mass vaccination campaign that uses a LAHV. We use our model to explicitly quantify the tradeoff between the potential efficacy (in terms of reducing the number of new infections with the wild-type strain) and the safety effects (in terms of vaccine-induced AIDS deaths) of a LAHV. We predict the potential epidemiological impact of mass vaccination with a variety of LAHVs (with different efficacy and safety characteristics) in two countries with very different HIV epidemics: Zimbabwe (where the current HIV prevalence is 25%) (http://www.unaids.org/hivaidsinfo/documents.html) and Thailand (where the current HIV prevalence is 2%) (http://www.unaids.org/hivaidsinfo/documents.html).
Methods
Mathematical Model of Mass Vaccination with a LAHV.
Our model tracks the temporal dynamics of individuals in five groups: susceptible individuals (X), unvaccinated individuals infected with wild-type HIV (YW), individuals uninfected with the wild-type strain but infected with the vaccine strain (either by vaccination or transmission of the vaccine strain) (YV), individuals dually infected with the vaccine and the wild-type strain (YVW), and individuals with AIDS (A). Our model is specified by five coupled ordinary differential equations (see Appendix). A flow diagram of the model is given in Fig. 4, which is published as supplemental material on the PNAS web site, www.pnas.org; the five colored boxes represent the five groups (i.e., state variables) and the black arrows show the processes that move individuals from one state to another state. Our modeling framework allows us to analyze the epidemiological consequences of cross-interactions among strains; similar frameworks have been used to analyze multiple strain dynamics for myxoma, influenza, and tuberculosis (23–28) or to evaluate the effects of vaccination and cross-immunity (29).
We model vaccine efficacy by assuming that a LAHV can have two beneficial effects: (i) a degree effect (i.e., the vaccine induces some degree of protection against infection with wild-type strains), and (ii) a therapeutic effect (i.e., the vaccine reduces the wild-type disease progression rate in dually infected individuals). Dually infected individuals (YVW) are individuals that are vaccinated (i.e., they are originally YV individuals), but subsequently become infected with the wild-type strain. We assume that these dually infected individuals (YVW) (due to the presence of the vaccine strain) may have a lower viral load than unvaccinated HIV-infected individuals (YW). Reductions in viral load will decrease the disease progression rate, thus YVW individuals (in comparison with YW individuals) will have a slower disease progression rate (30, 31). We model the safety effect of a LAHV by assuming that the vaccine strain can cause a fraction of the vaccinated individuals (who remain uninfected with the wild-type strains) to slowly progress to AIDS. We also include the possibility that the vaccine strain could be transmitted from vaccinated individuals (YV) to susceptible individuals (X).
Initial Conditions.
To set the initial conditions for our analyses we used our model equations (assuming no vaccination), Latin hypercube sampling methodology, and parameter estimates based on probability density functions (pdfs) to estimate the current annual AIDS death rate in Thailand and Zimbabwe. We used the following pdfs: average survival time with AIDS (1/μA) (pdf: 9 months - 1 year - 18 months) (32, 33), average infectiousness (i.e., transmission probability) of wild-type HIV per sexual partnership (βW) (pdf: 0.05 to 0.1 to 0.2) (34), and average disease progression rate to AIDS due to infection with wild-type strains (vW) (pdf: range from 50% progression to AIDS in 7.5 years to 50% progression in 10 years) (33, 35–37). To obtain country-specific estimates we used current estimates of the population size of sexually active adults [Zimbabwe 5,560,000 (http://www.unaids.org/hivaidsinfo/documents.html) and Thailand 34,433,000 (http://www.unaids.org/hivaidsinfo/documents.html)] and estimates of the average sexually active lifespan (31 years in Zimbabwe (http://www.unaids.org/hivaidsinfo/documents.html) and 49 years in Thailand (http://www.unaids.org/hivaidsinfo/documents.html). The parameter c (average rate of acquiring new sex partners) was derived as a dependent variable. We compared our estimates with reported data on the current annual AIDS death rate in Zimbabwe and Thailand.
Uncertainty Analysis of Analytical Equilibrium Results.
We used our model and conducted an uncertainty analysis of the endemic equilibria to predict whether mass vaccination with a LAHV could eradicate wild-type HIV in Zimbabwe and/or Thailand. To make these predictions we analyzed our model to obtain analytical expressions for the endemic equilibria states (see supplemental Appendix, which is published on the PNAS web site). These analyses revealed that if the vaccination coverage level (p) was greater than zero then either a coexistence endemic state would be reached (where both the wild-type HIV strain and the LAHV strain would be present), or the wild-type strains would be eradicated and only the LAHV strain would reach an endemic state. We derived analytical expressions for these two possible endemic equilibria (i.e., coexistence or wild-type eradication). Then we used uncertainty analysis (based on Latin hypercube sampling, which is a type of stratified Monte Carlo sampling scheme; refs. 38–43) to obtain 1,000 different sets of parameter estimates to define the characteristics of 1,000 different mass vaccination campaigns. Mass vaccination campaigns were defined in terms of vaccine efficacy (in terms of ψ and g), vaccine safety (vV), and vaccination coverage level (p); each symbol specifies a parameter in the model, see Appendix for precise definitions of each parameter. The pdfs that we used for ψ, g, vV, and p are described in the next section. We then determined for each of these 1,0000 parameter sets (by using the derived analytical expressions for the endemic equilibria) whether they would stabilize at either the coexistence or the wild-type eradication state. We derived country-specific results by using the appropriate demographic parameters for Zimbabwe and Thailand. For further details see the supplemental Appendix.
Prediction Methodology: Time-Dependent Uncertainty Analysis.
To predict the potential impact of LAHVs on the annual AIDS death rate, we analyzed our model by using time-dependent uncertainty analyses based on Latin hypercube sampling. Previously we have used this analysis to make predictions for other infectious disease models (38–43); details are given elsewhere (38–43). By using uncertainty analysis we were able to use pdfs, rather than single values, to specify parameter estimates. For each analysis, each parameter was assigned 1,000 different values sampled from the specified pdf; values were chosen by dividing the pdf for each parameter into 1,000 equi-probable intervals, and then sampling the pdf (randomly and without replacement) 1,000 times. The pdf for each parameter was sampled independently. We explored the potential effects of LAHVs with a fairly wide range of efficacy and safety characteristics. This methodology enabled us to generate (for each country) 1,000 different simulations; where each simulation had a different vaccination coverage level and a different LAHV (as specified by the efficacy and safety characteristics). For each simulation we assumed that a mass vaccination campaign (with a coverage level of p) would be initiated at time 0 and a fraction (p) of new susceptibles would be vaccinated annually.
We predicted the effects of mass vaccination campaigns (with follow-up programs) that would vaccinate anywhere from 80% to 95% of susceptibles (range of pdf: 0.95 > p > 0.8). Only a LAHV that has performed extremely well in terms of efficacy in phase III trials is likely to be licensed. Therefore we assumed that the LAHVs used in our vaccination campaigns would have fairly high efficacy characteristics. To specify the pdf for LAHV efficacy we assumed that: (i) the “degree” of vaccine-induced protection (ψ) against infection with the wild-type strains was between 50% and 95% (range of pdf: 0.95 > ψ > 0.5), and that (ii) the “therapeutic” effect of the vaccine (δ) in dually infected individuals reduced the wild-type disease progression rate from 1- to 10-fold (range of pdf: 1 > δ > 0.1). The long-term risks of LAHV (in terms of the risk of developing AIDS due to the vaccine strain) might not be apparent in short-term phase III trials as disease progression rates are slow, therefore we evaluated the consequences of a wide range of risks. Risk was defined in terms of the vaccine-induced disease progression rate (vV); we evaluated the consequences of vaccines that could cause anywhere from 1% to 10% (with a most likely value of 5%) of the vaccinated individuals to progress to AIDS in 25 years. These vaccine-induced disease progression rates (pdf: minimum 1%, peak 5%, maximum 10% in 25 years) translated into a range in the annual progression rate to AIDS of 5 to 40 individuals per 10,000 vaccinated individuals. Finally, we assumed that, although the vaccine strain could be transmitted, the infectiousness of the vaccine strain was 10- to 1,000-fold (with a peak at 100) less infectious than the wild-type strain (range of pdf: 0.10 > α > 0.001).
Results
The current annual AIDS death rate in Zimbabwe is 1,987 per 100,000 individuals (http://www.unaids.org/hivaidsinfo/documents.html), and in Thailand it is 159 per 100,000 individuals (http://www.unaids.org/hivaidsinfo/documents.html). The median values from our estimates for the initial conditions for our analyses closely matched the reported data (predicted median estimate for Zimbabwe was 1,982 per 100,000, and predicted median estimate for Thailand was 176 per 100,000).
Predictions: Could a LAHV Eradicate Wild-Type HIV?
All of our 1,000 predictions (for Thailand and Zimbabwe) gave the same
result: the wild-type strain of HIV would be eradicated, and the
prevalence of the vaccine strain would rise to an endemic steady state.
Hence, our results revealed that mass vaccination with a LAHV
eventually would result in the replacement of the virulent pathogen
(wild-type HIV) with the relatively avirulent pathogen (the LAHV
strain). Further calculations revealed that at this new endemic steady
state, the vaccine strain could be eradicated simply by stopping
vaccination, because the basic reproductive number
(R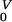 ) of the LAHV strain (which
is calculated from our model) is significantly less than one.
) of the LAHV strain (which
is calculated from our model) is significantly less than one.
Predictions: What Effect Would a LAHV Have on the Annual AIDS Death Rate?
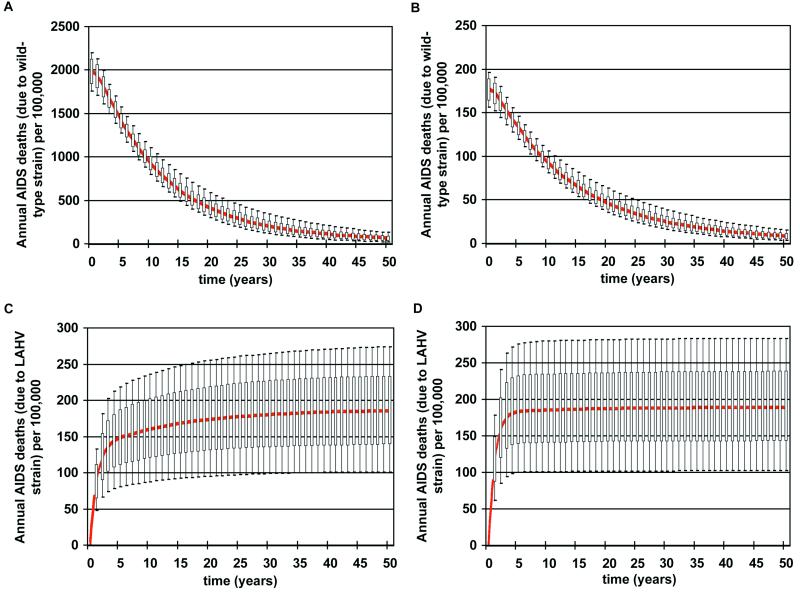
We then determined the time frame for eradication. Time-dependent uncertainty analysis of our model revealed that eradication of wild-type HIV would take several decades in both countries [Fig. 1A (Zimbabwe) and Fig. 1B (Thailand)]. The current annual AIDS death rate in Zimbabwe (Fig. 1A) is significantly higher than in Thailand (Fig. 1B). However, in both countries AIDS deaths (due to infection with wild-type strains of HIV) decreased quickly and dramatically because vaccination coverage level was high and the LAHVs were extremely effective in blocking transmission [Fig. 1A (Zimbabwe) and Fig. 1B (Thailand)]. The annual AIDS death rate (due to the vaccine) in Thailand and Zimbabwe reached the same endemic level [Fig. 1C (Zimbabwe) and Fig. 1D (Thailand)].
Figure 1.
Predictions of the epidemiological impact of 1,000 different LAHVs. Annual AIDS death rates are plotted in the form of box plots; the median values are shown in red. Uncertainty analysis predictions (1,000 simulations for each country) of the annual AIDS death rates (per 100,000 individuals) that are the result of infection with the wild-type strain of HIV are plotted for Zimbabwe (A) and Thailand (B). Uncertainty analysis predictions (1,000 simulations for each country) of the annual AIDS death rates (per 100,000 individuals) that are the result of the LAHV strain are plotted for Zimbabwe (C) and Thailand (D).
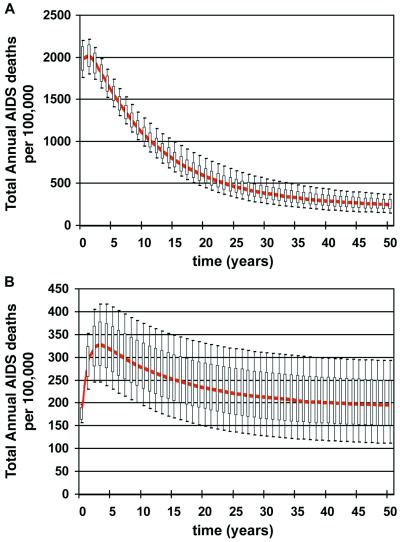
Our analyses revealed the epidemic-level consequences of both the efficacy (Fig. 1 A and B) and the safety (Fig. 1 C and D) effects of LAHVs. We then combined these efficacy and safety predictions to calculate whether the net effect (in terms of the total annual AIDS death rate) of vaccination with a LAHV would be beneficial or detrimental. The total annual AIDS death rate = AIDS deaths due to wild-type strains of HIV + AIDS deaths that are vaccine-induced. The net effect of vaccination in Zimbabwe was always very beneficial; the total annual AIDS death rate decreased substantially over time for all of the 1,000 simulations (Fig. 2A). In contrast, in Thailand the total annual AIDS death rate actually increased over time in the majority of the simulations (Fig. 2B); thus the net effect of vaccination in this country was often detrimental.
Figure 2.
Predictions of the epidemiological impact of 1,000 different LAHVs. Annual AIDS death rates are plotted in the form of box plots; the median values are shown in red. Uncertainty analysis predictions (1,000 simulations for each country) of the total annual AIDS death rates (per 100,000 individuals) are plotted for Zimbabwe (A) and Thailand (B). The total annual AIDS death rate equals the annual AIDS deaths that are caused by the wild-type strain plus the annual AIDS deaths that are caused by the vaccine strain.
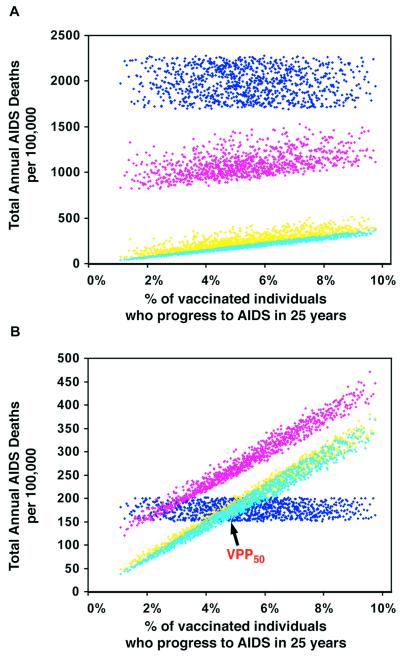
Determining the Impact of Vaccine Safety on the Total Annual AIDS Death Rate.
We quantified the impact of vaccine safety (defined in terms of the percentage of vaccinated individuals who would progress to AIDS as a result of the vaccine strain) on increasing the total annual AIDS death rate. In Zimbabwe the total annual AIDS death rate decreased over time in all of the 1,000 simulations; the predicted prevaccination (i.e., the current) annual AIDS death rates are shown by the dark blue data in Fig. 3A. At any given time point after mass vaccination campaigns were begun, the total annual AIDS death rate decreased as vaccine safety increased; results are shown after 10 years (pink data), 50 years (yellow data), and 200 years (light blue data) of vaccination in Fig. 3A. In contrast, in Thailand after vaccination campaigns were begun the total annual AIDS death rate actually increased over time in some of the simulations (Fig. 3B); the predicted prevaccination (i.e., the current) annual AIDS death rates are shown by the dark blue data in Fig. 3B. Almost all of the 1,000 different LAHVs led to an initial increase in the total annual AIDS death rate in Thailand, as can be seen from the pink data (which are predicted data after 10 years of vaccination) in Fig. 3B. However, after several decades, some of the vaccines had increased (whereas others had decreased) the total annual AIDS death rate in Thailand; the outcome depended on vaccine safety (Fig. 3B).
Figure 3.
Time-dependent uncertainty analysis predictions showing the effect of vaccine safety (in terms of the percentage of the vaccinated individuals that progress to disease in 25 years as a result of vaccination with a LAHV) on the total annual AIDS death rates per 100,000; 1,000 predictions are shown for Zimbabwe (A) and Thailand (B) at each time point. Dark blue data show the initial conditions prevaccination (which is time 0); these initial conditions are model estimates of the current annual AIDS death rates in these countries. Pink data are predicted data 10 years after vaccination. Yellow data are predicted data 50 years after vaccination, and the light blue data are predicted data 200 years after vaccination. The VPP at 50 years (VPP50) for Thailand is shown.
LAHVs have beneficial epidemic-level effects (by reducing transmission) and also detrimental effects (by causing vaccine-induced AIDS deaths). We used our predicted data to determine the vaccine perversity point (VPP) for Thailand (Fig. 3B); the VPP is a threshold defined in terms of the vaccine safety level. The VPP is defined (at any specified time) to be the vaccine-induced disease progression rate at which the beneficial epidemic-level effects caused by the vaccine equal the detrimental epidemic-level effects caused by the vaccine; thus at the VPP the net impact on the total annual AIDS death rate is zero. Almost all of the 1,000 different LAHVs led to an initial increase in the total annual AIDS death rate in Thailand, hence the VPP (at 10 years) occurs at approximately 2% (which is the point at which the pink data cross the dark blue data in Fig. 3B). At 50 years, the value of the VPP in Thailand is approximately 5% (i.e., a vaccine-induced disease progression rate of 5% in 25 years); this VPP (VPP50) occurs at the point at which the yellow data cross the dark blue data in Fig. 3B. LAHVs that had a faster vaccine-induced disease progression rate than the VPP50 caused more harm than good in Thailand (even 50 years later) as they increased the total annual AIDS death rate (Fig. 3B). Conversely, LAHVs that had a slower vaccine-induced disease progression rate than the VPP50 caused more good than harm in Thailand as they decreased (after several decades) the total annual AIDS death rate (Fig. 3B). Mass vaccination with any LAHV that caused more than 5% of vaccinated individuals to progress to AIDS in 25 years led to perversity (i.e., an increase in the annual AIDS death rate) in Thailand (Fig. 3B), but not in Zimbabwe (Fig. 3A). There is no VPP for Zimbabwe (at any time point) (Fig. 3A), because all of the LAHVs that we “tested” in that country led to a decrease in the annual AIDS death rate (i.e., all of the predicted data for Zimbabwe, at every time point, lay below the dark blue data at time 0; Fig. 3A).
Discussion
Our theoretical framework enables us to conduct a “biological risk-benefit” analysis for any particular LAHV for any specified HIV epidemic. We evaluated the potential epidemiological consequences of 1,000 different LAHVs, where the vaccines differed on the basis of efficacy and safety characteristics. Vaccination campaigns with exactly the same LAHVs may prove beneficial in one country (as we showed for Zimbabwe), but could be detrimental in another (as we showed for Thailand). These results may seem paradoxical, because in both countries we used LAHVs that had exactly the same efficacy and safety characteristics. We obtained these apparently paradoxical results, because the effectiveness of LAHVs (unlike the effectiveness of prophylactic non-LAHV) is determined not only by the efficacy and safety characteristics of the vaccine, but also by the transmission rate. The transmission rate determines (for any given LAHV) the tradeoff between the vaccine's efficacy and safety characteristics. Hence the transmission rate determines whether the vaccine has a beneficial or a detrimental net impact (where net impact is defined in terms of the total annual AIDS death rate). In Zimbabwe the transmission rate is high, thus the benefits of reducing transmission (i.e., high efficacy) outweigh the safety consequences. In Thailand the transmission rate is fairly low, thus the safety consequences can outweigh the benefits of reducing transmission. Our results imply that a threshold transmission rate (TTR) exists. If the transmission rate is greater than the TTR, then the efficacy effect of a LAHV outweighs the safety effect. Conversely, if the transmission rate is less than the TTR, then the safety effect outweighs the efficacy effect. Our model can be used to determine the exact value of the TTR for any specified vaccine efficacy and safety level.
Our results bring to light several issues that need to be considered regarding the utility of LAHVs as potential epidemic control agents. First, our results indicate that LAHVs may only be useful for controlling HIV in countries where there are very high transmission rates (i.e., where the transmission rate is significantly greater than the TTR). Second, our results imply that if LAHVs are to be used then it will be necessary to decide on multiple goals for epidemic control strategies (44) rather than simply to consider only eradication. For both Zimbabwe and Thailand our analyses show that vaccination with a fairly effective LAHV could lead to the eradication of wild-type HIV; however, on the way to eradication the total annual AIDS death rate could increase in Thailand. It is obviously unacceptable to achieve eradication at the price of an increased AIDS death rate. Thus, our results suggest that it may be necessary (if LAHVs are to be used as epidemic control agents) to design fairly complex vaccination strategies that can achieve both eradication of wild-type HIV and a significant reduction in the annual AIDS death rate. Optimal control theory could be used to design more complex vaccination strategies that can simultaneously attain multiple goals. Such an approach would entail specifying an objective function to maximize the benefits and minimize the costs of vaccination. This methodology has been used previously for designing optimal treatment strategies for HIV-infected individuals on chemotherapy (45). Third, our results imply that it will be necessary to implement large-scale surveillance systems to determine when wild-type strains of HIV have been eradicated; after wild-type HIV has been eradicated all of the AIDS deaths would be the result of the vaccine strain.
Our results also have important practical implications for vaccine design, and the implementation of phase III clinical trials. Our model can be used (as we have shown) to determine the VPP for a LAHV used in any particular geographic region. Our results show that only LAHVs that induce almost no disease should be considered for use, particularly in low incidence areas. It may be possible to develop a very safe LAHV, but it may only be possible at a cost to efficacy; increasing the attenuation of vaccine strains (in the simian immunodeficiency virus model) tends to increase vaccine safety, but to decrease efficacy (3, 4, 7). We have quantified the extremely high safety standards that must be met before LAHV can be considered safe as epidemic control agents. Very large numbers of participants will need to be monitored for many years to determine whether such high levels of vaccine safety have been attained in a phase III clinical trial. We have determined that the net effect of any LAHV is very dependent on the transmission rate. Thus it will be necessary to conduct many phase III clinical trials of any candidate LAHV in a variety of geographic locations where there is a wide variety of transmission rates. Testing LAHVs could involve conducting many more phase III trials than the testing of prophylactic non-LAHV. If phase III trials of LAHVs are conducted in only high transmission areas then these vaccines could look very beneficial. However, if these vaccines were then used to control epidemics where there is only moderate or low transmission then these vaccines could increase the severity of the epidemic.
It appears unlikely that the HIV pandemic will be controlled—particularly in Africa—unless effective vaccines are developed (36, 46). If prophylactic non-LAHV are used they will reduce transmission and produce only beneficial epidemic-level effects; perverse effects will only occur if risky behavior increases (15, 20). The effectiveness of high efficacy prophylactic non-LAHV can be assessed by evaluating our predictions for the annual AIDS death rate and considering only the efficacy effects of LAHVs [Fig. 3A (Zimbabwe) and Fig. 3B (Thailand)]. These vaccines would eradicate HIV in both Thailand and Zimbabwe without producing any perverse effects, unless risky behavior increased. Unfortunately, it is very difficult to develop such vaccines. It is possible that LAHVs may be the most effective HIV vaccines that are developed.
We have shown that LAHVs potentially could be very effective as epidemic control agents, but that LAHVs should have an extremely low risk of causing AIDS in vaccinated individuals. In addition, there are several other safety concerns that need to be addressed when considering LAHVs. For example, a LAHV may cause insertional mutagenesis, or the LAHV strain could increase in pathogenicity by recombining with a superinfecting wild-type strain. Our theoretical framework can be expanded to evaluate the consequences of these additional safety concerns. Clearly, there are many ethical issues that need to be debated when considering LAHVs as epidemic control agents. We have shown that while effective LAHVs could eradicate wild-type HIV, these vaccines could also (in countries where the incidence rate is low) increase the severity of the HIV epidemic. It is therefore possible that LAVHs may only be considered for use in developing countries (where incidence rates are high), but not in developed countries (where incidence rates are low). However, any mass vaccination campaign based on LAVHs will need to be designed by considering the HIV pandemic rather than simply a country-specific epidemic, because country-specific HIV epidemics are linked. Thus it appears likely that the discussion of the development and the potential utility of LAHVs for control of the HIV pandemic will become even more controversial.
Supplementary Material
Acknowledgments
We are grateful to Chuck Daley, Holly Gaff, Frits van Griensven, Mojgan Haddad, Penny Hitchcock, Rod Hoff, Jim O. Kahn, Tom Lietman, Mike McCune, and Kristin Swanson for their insightful comments. S.M.B. is also very grateful to Dan, Jake, and Nelson Freimer for their input. We gratefully acknowledge the financial support of the National Institutes of Health/National Institute of Allergy and Infectious Diseases Grant RO1AI41935 (S.M.B. and K.K.), the Australian National Council on AIDS and Related Diseases (J.M.), and the Macfarlane Burnet Centre for Medical Research (J.M.).
Abbreviations
- LAHV
live attenuated HIV vaccine
probability density function
- VPP
vaccine perversity point
- TTR
threshold transmission rate
Appendix
The mathematical model is specified by the following five ordinary differential equations:
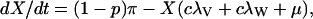 |
1 |
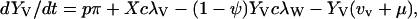 |
2 |
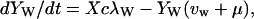 |
3 |
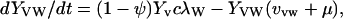 |
4 |
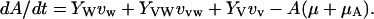 |
5 |
λ specifies the per-capita risk of infection with either the vaccine strain (λV) or the wild-type strain (λW). λ is calculated as the product of the probability of selecting an infectious sex partner and the infectiousness of HIV per sexual partnership (β), which differs for the wild-type strain (βW) and the vaccine strain (βV). Only YV individuals can transmit the LAHV strain; the parameter α specifies the degree to which the vaccine strain is less infectious than the wild-type strain (hence βV = α βW). We assume that dually infected individuals (YVW) can only transmit the wild-type strain; LAHVs may reduce the infectiousness of the wild-type strain in these dually infected individuals by a factor g, but in our current analyses we set g = 1.0. Hence, λV = βV (YV/NSA) and λW = βW [(YW + gYVW)/NSA]; NSA specifies the size of the sexually active population and is equal to X + YW + YVW + YV.
The remaining parameter definitions are: p = the fraction of new susceptibles vaccinated, π = the number of new susceptibles that join the sexually active population per unit time, c = the average rate of acquiring new sex partners, 1/μ = the average period of acquisition of new sex partners, 1/μA = the average survival time with AIDS, ψ = the degree of protection that the LAHV provides against infection with the wild-type strain, v = the progression rate to AIDS in individuals infected with the LAHV strain (vv), the wild-type strain (vw), or both strains (vvw), 1/μA = the average survival time from AIDS to death. The disease progression rates are related by the expression vvw = δ vw; where δ specifies the vaccine-induced degree of reduction in the wild-type disease progression rate.
References
- 1.Mims C A. The Pathogenesis of Infectious Diseases. 3rd Ed. San Diego: Academic; 1987. pp. 303–321. [Google Scholar]
- 2.Melnick J A. In: Vaccines. 2nd Ed. Plotkin S A, Mortimer E A, Saunders W B, editors. San Diego: Academic; 1994. pp. 155–204. [Google Scholar]
- 3.Desrosiers R C. Nat Med. 1998;4:982. doi: 10.1038/1949. [DOI] [PubMed] [Google Scholar]
- 4.Almond N, Stott J. Immunol Lett. 1999;66:167–170. doi: 10.1016/s0165-2478(98)00153-9. [DOI] [PubMed] [Google Scholar]
- 5.Baba T W, Liska V, Khimani A H, Ray N B, Dailey P J, Penninck D, Bronson R, Greene M F, McClure H M, Martin L N, et al. Nat Med. 1999;5:194–203. doi: 10.1038/5557. [DOI] [PubMed] [Google Scholar]
- 6.Geretti A M. Rev Med Virol. 1999;9:57–67. doi: 10.1002/(sici)1099-1654(199901/03)9:1<57::aid-rmv237>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 7.Johnson P. Nat Med. 1999;5:154–155. doi: 10.1038/5515. [DOI] [PubMed] [Google Scholar]
- 8.Murphy-Corb M. Nat Med. 1997;3:17–18. doi: 10.1038/nm0197-17. [DOI] [PubMed] [Google Scholar]
- 9.Mills, J., Desrosiers, R., Rud, E. & Almond, N. (2001) AIDS Res. Hum. Retroviruses, in press. [DOI] [PubMed]
- 10.Blume S, Geesink I. Science. 2000;288:1593–1594. doi: 10.1126/science.288.5471.1593. [DOI] [PubMed] [Google Scholar]
- 11.Johnson R P, Desrosiers R C. Curr Opin Immunol. 1998;10:436–439. doi: 10.1016/s0952-7915(98)80118-0. [DOI] [PubMed] [Google Scholar]
- 12.Berkhout B, Verhoef K, van Wamel J L, Back N K. J Virol. 1999;73:1138–1145. doi: 10.1128/jvi.73.2.1138-1145.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Learmont J C, Geczy A F, Mills J, Ashton L J, Raynes-Greenow C H, Garsia R J, Dyer W B, McIntyre L, Oelrichs R B, Rhodes D I. N Engl J Med. 1999;340:1715–1722. doi: 10.1056/NEJM199906033402203. [DOI] [PubMed] [Google Scholar]
- 14.McLean A R, Blower S M. Proc R Soc London Ser B. 1993;253:9–13. doi: 10.1098/rspb.1993.0075. [DOI] [PubMed] [Google Scholar]
- 15.Blower S M, McLean A R. Science. 1994;265:1451–1454. doi: 10.1126/science.8073289. [DOI] [PubMed] [Google Scholar]
- 16.McLean A R, Blower S M. Trends Microbiol. 1995;3:458–463. doi: 10.1016/s0966-842x(00)89010-1. [DOI] [PubMed] [Google Scholar]
- 17.Longini I M, Datta S, Halloran M E. J Acquired Immune Defic Syndr. 1996;13:440–447. doi: 10.1097/00042560-199612150-00007. [DOI] [PubMed] [Google Scholar]
- 18.Longini I M, Halloran M E. Appl Stat. 1996;45:165–173. [Google Scholar]
- 19.Longini I M, Sagatelian K, Rida W N, Halloran M E. Stat Med. 1998;17:1121–1136. doi: 10.1002/(sici)1097-0258(19980530)17:10<1121::aid-sim824>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 20.Blower S M, Koelle K, Mills J. In: Quantitative Evaluation of HIV Prevention Programs. Kaplan E H, Brookmeyer R, editors. New Haven, CT: Yale Univ. Press; 2001. , in press. [Google Scholar]
- 21.Porco T C, Blower S M. Interfaces. 1998;28:167–190. [Google Scholar]
- 22.Porco T C, Blower S M. Math Popul Stud. 2000;8:205–229. [Google Scholar]
- 23.Levin S A, Pimentel D. Am Nat. 1981;117:308–315. [Google Scholar]
- 24.Andreasen V, Lin J, Levin S A. J Math Biol. 1997;35:825–842. doi: 10.1007/s002850050079. [DOI] [PubMed] [Google Scholar]
- 25.Stilianakis N I, Perelson A S, Hayden F G. J Infect Dis. 1998;177:863–873. doi: 10.1086/515246. [DOI] [PubMed] [Google Scholar]
- 26.Mena-Lorca J, Velasco-Hernandez J X, Castillo-Chavez C. Inst Math Appl J Math Appl Med Biol. 1999;16:307–317. [PubMed] [Google Scholar]
- 27.Castillo-Chavez C, Feng Z. J Math Biol. 1997;35:629–656. doi: 10.1007/s002850050069. [DOI] [PubMed] [Google Scholar]
- 28.Blower S M, Small P M, Hopewell P. Science. 1996;273:497–500. doi: 10.1126/science.273.5274.497. [DOI] [PubMed] [Google Scholar]
- 29.McLean A R. Proc R Soc London Ser B. 1995;261:389–393. [Google Scholar]
- 30.Cranage M P, Sharpe S A, Whatmore A M, Polyanskaya N, Norley S, Cook N, Leech S, Dennis M J, Hall G A. J Gen Virol. 1998;79:1935–1944. doi: 10.1099/0022-1317-79-8-1935. [DOI] [PubMed] [Google Scholar]
- 31.Robinson H L, Montefiori D C, Johnson R P, Manson K H, Kalish M L, Lifson J D, Rizvi T A, Lu S, Hu S L, Mazzara G P. Nat Med. 1999;5:526–534. doi: 10.1038/8406. [DOI] [PubMed] [Google Scholar]
- 32.Grant A D, Djomand G, DeCock K M. AIDS. 1997;11, Suppl. B:S43–S54. [PubMed] [Google Scholar]
- 33.Collaborative Group on AIDS Incubation and HIV Survival including the CASCADE EU Concerted Action. Lancet. 2000;355:1131–1137. [PubMed] [Google Scholar]
- 34.Grant R M, Wiley J A, Winkelstein W. J Infect Dis. 1998;156:189–193. doi: 10.1093/infdis/156.1.189. [DOI] [PubMed] [Google Scholar]
- 35.Munoz A, Sabin C A, Phillips A N. AIDS. 1997;11, Suppl. A:S69–S76. [PubMed] [Google Scholar]
- 36.Heilman C A, Baltimore D. Nat Med. 1998;4:532–534. doi: 10.1038/nm0598supp-532. [DOI] [PubMed] [Google Scholar]
- 37.Malamba S S, Morgan D, Clayton T, Mayanja B, Okongo M, Whitworth J. AIDS. 1999;13:2555–2562. doi: 10.1097/00002030-199912240-00009. [DOI] [PubMed] [Google Scholar]
- 38.Blower S M, Hartel D, Dowlatabadi H, May R M, Anderson R M. Philos Trans R Soc London B. 1991;321:171–187. doi: 10.1098/rstb.1991.0006. [DOI] [PubMed] [Google Scholar]
- 39.Blower S M, Dowlatabadi H. Int Stat Rev. 1994;62:229–243. [Google Scholar]
- 40.Blower S M, McLean A R, Porco T C, Small P M, Hopewell P C, Sanchez M A, Moss A R. Nat Med. 1995;1:815–821. doi: 10.1038/nm0895-815. [DOI] [PubMed] [Google Scholar]
- 41.Lietman T C, Porco T C, Dawson C, Blower S M. Nat Med. 1999;5:572–576. doi: 10.1038/8451. [DOI] [PubMed] [Google Scholar]
- 42.Blower S M, Porco T C, Darby G. Nat Med. 1998;4:673–678. doi: 10.1038/nm0698-673. [DOI] [PubMed] [Google Scholar]
- 43.Blower S M, Gershengorn H B, Grant R M. Science. 2000;287:650–654. doi: 10.1126/science.287.5453.650. [DOI] [PubMed] [Google Scholar]
- 44.Blower S M, Gerberding J L G. J Mol Med. 1998;76:624–636. doi: 10.1007/s001090050260. [DOI] [PubMed] [Google Scholar]
- 45.Kirschner D, Lenhart S, Serbin S. J Math Biol. 1997;35:775–792. doi: 10.1007/s002850050076. [DOI] [PubMed] [Google Scholar]
- 46.Letvin N L. Science. 1998;280:1875–1880. doi: 10.1126/science.280.5371.1875. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.