Abstract
Estradiol (E2), a female sex hormone, has important biological functions. Human pancreas-specific protein disulfide isomerase (PDIp), a protein folding catalyst, was recently found to be able to bind E2. Here we report the characterization of its E2-binding site by using biochemical methods coupled with molecular modeling tools. Analysis of various truncated PDIp proteins showed that the b-b′ fragment contains an intact E2-binding site that has the same binding affinity as the full-length PDIp protein, with apparent Kd values of approximately 170 nM. Computational modeling and docking analyses revealed that the E2-binding site in the b-b′ fragment is located in a hydrophobic pocket composed mainly of the b′ domain and partially of the b domain. The hydrogen bond, formed between the 3-hydroxyl group of E2 (donor) and PDIp’s His278 (acceptor) is indispensable for its binding. By contrast, the 17β-hydroxyl group of E2 is of negligible importance for E2 binding. This binding model was jointly confirmed by a series of experiments, such as selective mutation of the binding site amino acid residues and selective modification of the ligand structures.
Keywords: PDI, PDIp, estrogen receptor, pancreas, E2, estrogen-binding protein
Estradiol (E2), an important endogenous female sex hormone, exerts a wide array of biological functions in various target organs or tissues in a woman’s body, such as the development of reproductive organs and secondary sex characteristics. Many of the well-known physiological functions of E2 are mediated by the genomic actions of the estrogen receptor (ER) α and β (1), which are transcription factors that can initiate the expression of various target genes. In addition, the non-genomic actions of ERs have also been suggested to play a role in mediating E2-induced rapid signal transduction in certain systems (2–3).
Besides ERs, a few other proteins have also been found to have the ability to bind endogenous estrogens, serving as important modulators of the biological actions of these female hormones. For instance, the sex hormone binding globulin (SHBG), a well-known estrogen-binding protein present in large quantity in circulation, can profoundly modulate the bioavailability of free circulating estrogens, subsequently altering the tissue and intracellular levels of estrogens and their hormonal activity in various target sites (4–5). Protein disulfide isomerase (PDI), a well-known protein folding catalyst for disulfide bond formation (6–7), can also bind estrogens (8–10) and modulate estrogen level and hormonal actions in human breast cancer cells (11). Recently, we reported, for the first time, that the human pancreas-specific PDI homolog (PDIp), which is highly expressed in pancreatic acinar cells (12–13) and has both disulfide isomerase (12, 14) and chaperone activities (14–15), is another intracellular E2-binding protein that can modulate estrogen actions in mammalian cells (16). In light of these earlier observations, and also given the fact that these two intracellular proteins are present at unusually high levels in certain human tissues or cells (e.g., the pancreatic PDIp accounts for approximately 0.1% of the total cellular protein (16)), it was speculated that PDI and PDIp may function as important intracellular E2-storage proteins in these cells (9, 11, 16). In addition, the recent observation showing the co-presence of PDIp with ERα and ERβ in the pancreas of rodents (16) also points to the possibility that PDIp may be a viable modulator of the actions of endogenous estrogens in this non-classical target tissue.
At present, the E2-binding site structures of human PDI and PDIp are not known, although a few earlier studies have suggested that their peptide-binding sites may overlap with their E2-binding sites (9, 15, 17). Elucidation of the estrogen-binding site structures of these intracellular proteins is of considerable interest because it will aid in the identification of potential xenobiotics that may be able to alter estrogen action by modulating the estrogen-binding activity of these intracellular proteins. The main purpose of our present study, therefore, was to delineate the structural basis of human PDIp’s E2-binding activity. Through a combined use of computational modeling analysis, site-directed mutagenesis and selective ligand modifications, we located the PDIp’s E2-binding site to a hydrophobic pocket between the b′ and b domains. In addition, we have also built the PDIp-E2 binding model, and have identified a hydrogen bond formed between PDIp-His278 and the 3-hydroxyl group of E2 to be essential for their binding interaction.
MATERIALS AND METHODS
Chemicals, reagents, cell lines and tissues
E2 and its structural analogs were purchased from Steraloids (Newport, RI). [3H]E2 (specific activity of 110 Ci/mmol) was obtained from Perkin Elmer (Waltham, MA). Mastoparan (Ile-Asn-Leu-Lys-Ala-Leu-Ala-Ala-Leu-Ala-Lys-Lys-Ile-Leu) was obtained from Sigma-Aldrich (St. Louis, MO). All other chemicals and reagents used in this study were of analytical grade or higher. The mouse anti-PDIp antiserum (1:2500 dilution for Western blotting) was raised in our laboratory (16). The monkey kidney cos-7 cells were obtained from the ATCC (Manassas, VA), and cultured in the DMEM medium supplemented with 10% fetal bovine serum (FBS).
Plasmid construction and protein purification
Human PDIp (its cDNA clone was obtained from ATCC, catalog no. 6706839) was cloned into the pET-19b vector at the sites of 5′-NdeI/XhoI-3′ without a signal peptide and also cloned into the pcDNA3.1 vector with a signal peptide at the sites of 5′-HindIII/XhoI-3′. The plasmids for the expression of histidine-tagged truncated PDIp proteins (a-b, b-b′, b′-x-a′-c fragments) were constructed by cloning the corresponding cDNA sequences into a modified pET-19b vector as described earlier (16). Purification of the recombinant histidine-tagged proteins expressed in E. coli was carried out as described earlier (14, 16). The PDIp b, b′, x-a′-c fragments were cloned into a modified pGEX-4T-1 vector containing the NdeI sites at 5′-NdeI/SalI-3′ and expressed as the GST-tagged fusion proteins. After expression in E. coli cells these proteins were purified using the Glutathione Sepharose 4 Fast Flow (GE health). Site-directed mutagenesis was performed using the Phusion® Site-Directed Mutagenesis Kit from New England Biolabs according to the instructions of the manufacturers.
[3H]E2-binding assay for purified PDIp proteins
Desalting was employed to separate the free [3H]E2 and protein-bound [3H]E2 as described earlier (16). Proteins were incubated with [3H]E2 (typically at a 4.5-nM final concentration) in 10 mM sodium phosphate buffer (pH 7.4) at 4°C overnight. The incubation mixture (100 μL) was subjected to desalting using the PD miniTrap G-25 columns (from GE health) pre-equilibrated with 10 mM sodium phosphate buffer (pH 7.4, 0.15 M NaCl). Eluted fractions from 0.5 to 1.15 mL were collected and mixed with 3 ml of the scintillation cocktail (Fisher Scientific, Pittsburgh, PA) for radioactivity measurement on a Beta Counter (Perkin Elmer). For saturation experiments, increasing concentrations of [3H]E2 were present during the incubation. The nonspecific binding was determined in the presence of excess non-radiolabeled E2 (10 μM).
Computational homology modeling
Since the x-ray structures of human PDIp b-b′ fragment is not available at present, the human PDI b-b′ domain structure (PDB code: 2k18) (18) was thus used as a template to build the homology structural model for human PDIp b-b′ fragment. Protein sequence alignment shows that the sequence identity between the human PDIp b-b′ fragment and human PDI b-b′ fragment is 38% (data are presented in the results section). The Homology Modeling module of Insight II (Accelrys, Inc., San Diego, CA) was used to generate the 3-D structure model for the human PDIp b-b′ fragment. The side-chain rotamers were manually optimized to minimize the intramolecular bump and then the protein structure was further optimized by the Discover module of Insight II.
Binding site determination and molecular docking
The E2-binding site(s) on the homology structural model of the human PDIp b-b′ fragment were determined by using the Active-Site-Search function in the Binding-Site module of Insight II. The site-open-size parameter was set at 5 Å and the site-cut-off-size parameter was set at 150 Å3. We defined the binding pocket with amino acid residues within 5 Å around the candidate binding site. Simulated Annealing docking method in the Affinity module was used to dock E2 into the candidate binding pocket. Water molecules were excluded and side chains in the binding site were allowed to move in the docking analysis. One hundred docking modes were calculated and the ones with the lowest binding energy were chosen for further minimization.
RESULTS AND DISCUSSION
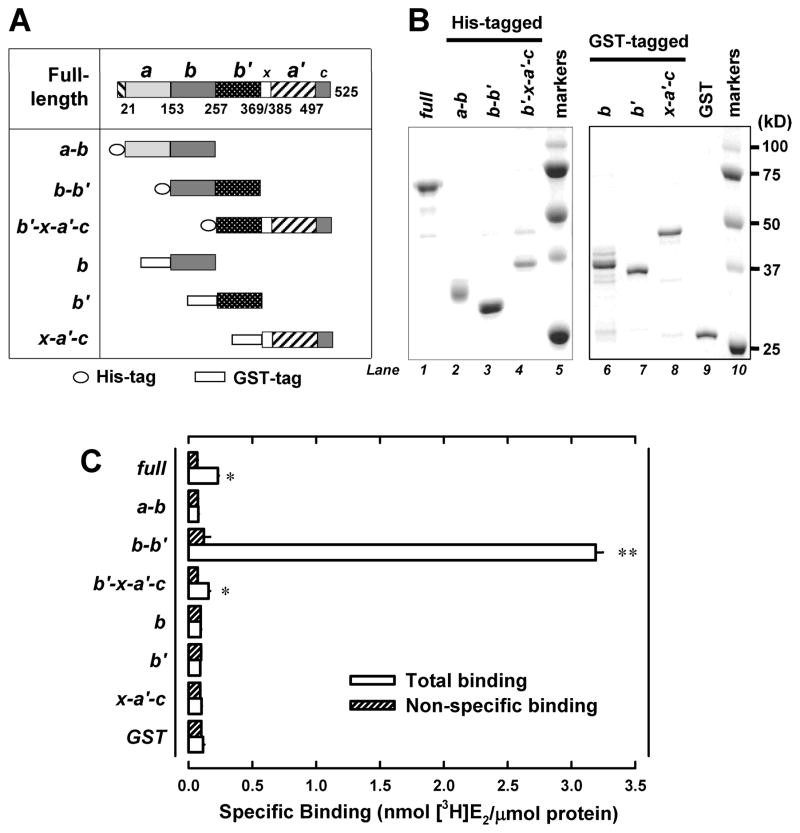
As depicted in Figure 1A, the human PDIp protein is a large protein composed of four thioredoxin-like domains, a, b, b′ and a′, plus a small linker region x between b′ and a′ and a C-terminal acidic extension c. To experimentally locate the E2-binding site in this protein, we designed three truncated human PDIp fragments (namely, a-b, b-b′, and b′-x-a′c, as depicted in Figure 1A), with a His-tag attached to their N-termini for the convenience of purification. Since these three fragments cover the full length of the PDIp protein with each fragment containing two neighboring main domains (i.e., a-b, b-b′, and b′-a′), theoretically they would allow us to determine whether the E2-binding site is only associated with any of the individual domains or jointly formed by any of the two neighboring domains. These three PDIp fragments as designed above were selectively over-expressed in E. coli cells and purified (left part in Figure 1B), and then subjected to in vitro analysis of their [3H]E2-binding ability. The results (Figure 1C) showed that the b-b′ fragment has a distinct, high specific binding activity for [3H]E2, although a weak binding activity was also detected for the b′-x-a′-c fragment. No binding activity was detected for the a-b fragment when it was assayed at an equivalent molar protein concentration under the same conditions. These observations were repeated by using proteins prepared from over four independent experiments, and the b-b′ fragment was consistently found to have a very high E2-binding activity. Accordingly, these results suggest that the E2-binding site is not associated with each individual domain, but it is associated with the b-b′ domain complex.
Figure 1. Human PDIp b-b′ fragment contains the E2-binding site.
A. Domain organization of the human PDIp protein (based on Q13087 in the UniProtKB database). Domain boundaries of human PDIp are determined by sequence alignment of PDIp with PDI, whose domain boundaries are described earlier (28). Fragments a-b, b-b′ and b′-x- a′-c were constructed as histidine-tagged fusion proteins. Fragment b, b′ and x-a′-c were constructed as GST-tagged fusion proteins. B. SDS-PAGE analysis of three histidine-tagged PDIp fragments (left part) and three GST-tagged fragments (right part), which were selectively expressed in E. coli cells and then purified using chromatography. C. The binding of [3H]E2 by each of the purified PDIp fragments (at a final concentration of 0.5 μM) after incubation with 4.5 nM [3H]E2 in 10 mM sodium phosphate buffer (pH 7.4) in the absence or presence of 10 μM cold E2. Each value is the mean ± S.D. of triplicate determinations. * P < 0.05, ** P < 0.01, compared to the corresponding non-specific binding. In other experiments, P values were also < 0.05 (for the b′-x-a′-c domain) or < 0.01 (for the b-b′ domain), respectively.
To further verify the above observations, next we selectively expressed the single b and b′ domains for testing of their individual E2-binding activity. In our initial experiments, we also adopted the strategy of attaching a His-tag to the b and b′ single domain fragments. Unfortunately, the yield of the b and b′ single domain proteins (with a His-tag) was very low when they were individually expressed in E. coli cells, likely due to their much smaller sizes and lower stability. After we modified the strategy by attaching a GST tag to the N-termini of the b and b′ fragments, the problem of low protein yield was solved. The b and b′ single domain fragments (with a GST-tag) were purified (Figure 1B), and then subjected to analysis of their ability to bind [3H]E2. However, the protein fragment that contained either b or b′ single domain did not have an appreciable [3H]E2-binding activity (Figure 1C). The experiment was repeated three times, and each time the same observations were made. For comparison, we have also prepared the single a′ domain (in the form of x-a′-c), and this fragment also did not have an appreciable [3H]E2-bidning activity (Figure 1C).
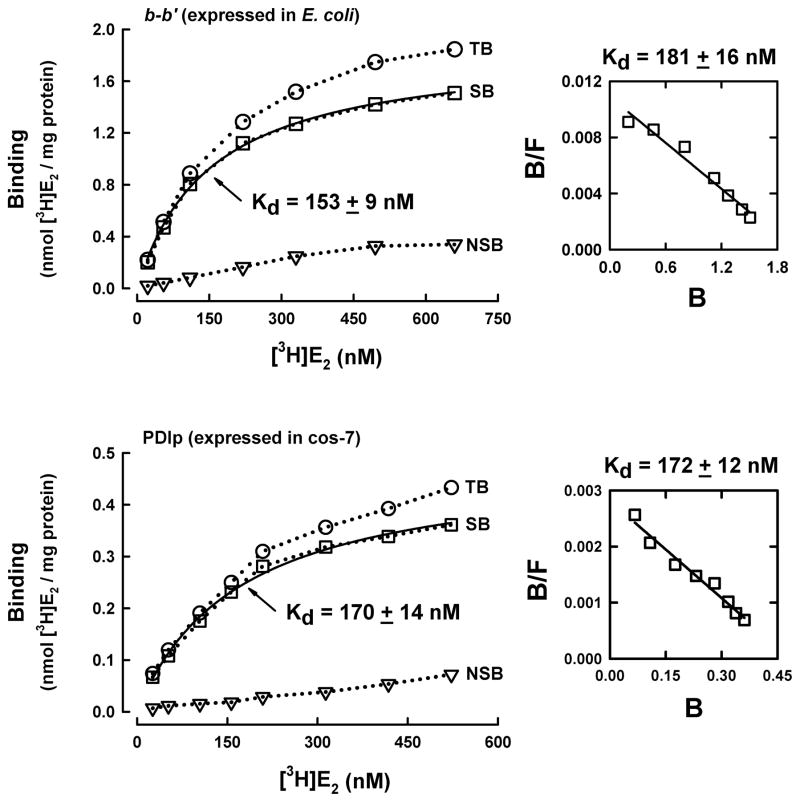
Using the purified b-b′ fragment, next we determined its E2-binding affinity (i.e., the Kd value) when different concentrations (1–650 nM) of the radiolabeled E2 were tested as the binding ligand. Analysis of the binding curve pattern as well as the Scatchard plot suggests that the PDIp b-b′ fragment displays the single binding site kinetics, with an apparent Kd value of 153 – 181 nM (Figure 2A). To verify that the human PDIp only has a single E2 binding site, we also determined the [3H]E2-binding affinity of the full-length PDIp that was selectively over-expressed in the cos-7 mammalian cells. The preparation and purification of this protein is summarized in Figure S1. Similarly, analysis of the binding curve pattern and Scatchard plot shows that the recombinant full-length human PDIp protein also exhibits the single site binding kinetics, with apparent Kd value of approximately 170 nMn (Figure 2B), which is very similar to the apparent Kd value of the purified recombinant b-b′ fragment. Notably, the Kd value of the full-length PDIp purified from E.coli cells was approximately 1500 nM (16), which is much higher than the Kd values for the b-b′ fragment purified from E.coli cells and the full-length PDIp purified from mammalian cells. We suspect that the lower E2-binding affinity of the full-length PDIp expressed in E.coli cells might be due to impact folding of the protein. Taken together, these observations suggest that the human PDIp protein only has a single binding site for E2, and the binding site is located in its b-b′ fragment.
Figure 2. Determination of the dissociation constant (Kd) of the human b-b′ fragment and the full-length human PDIp for E2.
The total binding (TB) of [3H]E2 by the b-b′ fragment or the full-length PDIp protein (at 20 μg/ml final concentration) was determined in the presence of increasing concentrations of [3H]E2 (22 to 660 nM) in 10 mM sodium phosphate buffer (pH 7.4). The non-specific binding (NSB) was determined in the presence of excess non-radiolabeled E2 (10 μM). Total binding (TB) subtracted by NSB gives rise to specific binding (SB). The binding curve (left part) was obtained using curve regression analysis (hyperbola model) of the SigmaPlot software. The corresponding Scatchard plot was shown in the right part of each panel. Panel A is the data obtained with the purified recombinant PDIp b-b′ fragment expressed in E.coli cells. Panel B is the data for the purified human PDIp protein selectively expressed in cos-7 cells (the purification procedures are described in Supplementary Data File and shown in Figure S1). Each value is the mean of duplicate measurements.
Next we sought to predict the three-dimensional (3-D) E2-binding pocket structure of PDIp b-b′fragment by using computational modeling analysis. To achieve this goal, we first built the 3-D structure of the b-b′ fragment by using the homology modeling approach according to the known structure of the b-b′ fragment of the human PDI protein (18), which shares 38% sequence homology with the human PDIp (as shown Figure S2A). Based on the homology structural model of human PDIp b-b′ fragment, we noticed that its backbone structure is very similar to that of the human PDI b-b′ fragment (Figure S2B). The most notable differences between these two structures are the β5 and the starting region of α4, where PDIp has four more amino acid residues. The other small differences between these two structures are in the α3′ region, due to difference in their amino acid compositions.
To predict the E2-binding pocket in the b-b′ fragment, we used the Insight II modeling program to calculate the size of the cavities present in the protein structure. Three cavities were found in the b-b′ fragment (Figure S2C): cavity I is located between the b and b′ domains, i.e., it is formed jointly by these two domains (cavity size = 376 Å3). In comparison, cavity II is solely formed by the b domain (size = 207 Å3), and cavity III is solely formed by the b′ domain (size = 129 Å3). Based on the experimental data which showed that the single b or b′ domain does not have an intact E2-binding site whereas the b-b′ domain complex has the binding site, these experimental data were considered as the scientific basis for excluding cavities II and III for further consideration as viable E2-binding sites. By contrast, cavity I matches the experimental findings, i.e., the binding pocket is formed by both b and b′ domains but not by individual domain present alone. Here it should also be noted that cavity I also has an optimal size that would enable it to function as a low affinity E2-binding site. Earlier studies (19-21) showed that the volumes of the E2-binding pockets of human ERα and ERβ are 266 and 275 Å3, respectively. The fact that the size of cavity I (376 Å3) is slightly larger than the E2-binding pockets of human ERs suggests that E2 likely will bind more loosely inside the PDIp’s binding pocket than inside the binding pockets of ERs, which agrees well with the known differences in their E2 binding affinities. On the other hand, the much smaller sizes of cavities I and II than those of the human ERs further suggest that they are unlikely to be viable E2-binding sites.
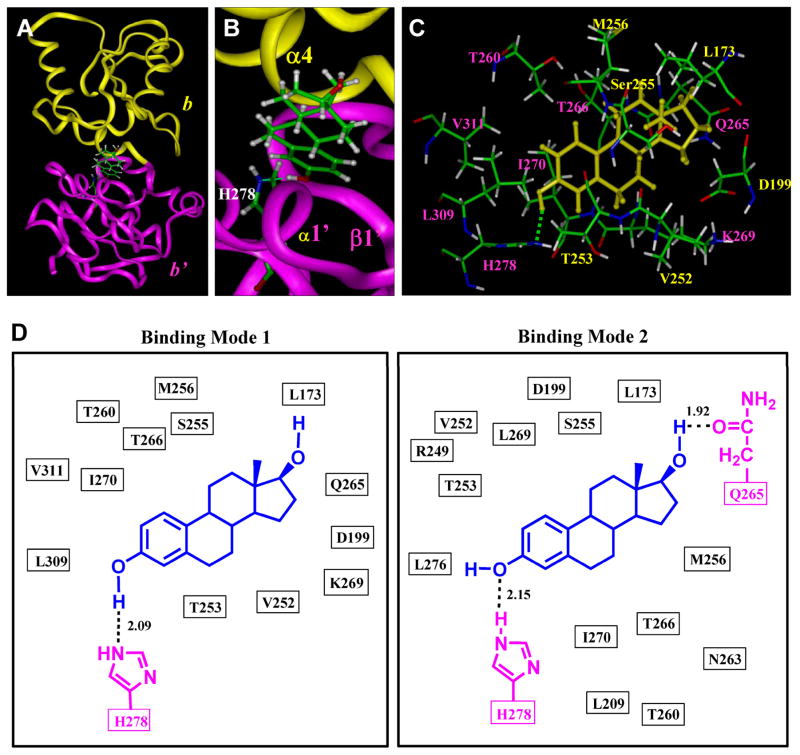
Next, we used the Affinity module of InsightII to dock E2 into cavity I. The overall E2-PDIp binding interaction is shown in Figure 3A and 3B, and the amino acid residues surrounding the binding pocket are shown in Figure 3C. Two candidate E2-binding modes are suggested (Figure 3D). In both binding modes, E2 has a very similar overall orientation and positioning inside the binding pocket, and hydrogen bonds are formed between E2 and PDIp b-b′ fragment. In mode I (Figure 3D), one hydrogen bond is formed between the 3-hydroxyl group of E2 and the nitrogen atom of PDIp-His278. In mode II (Figure 3D), two hydrogen bonds are formed: one between the 3-hydroxyl group of E2 and PDIp-His278 and the other one between the 17-hydroxyl group of E2 and PDIp-Gln265. To experimentally test the binding modes as suggested by our computational docking analysis, we first sought to determine whether the two identified amino acid residues, His278 and Gln265, are indeed involved in the binding interactions between PDIp and E2 through forming hydrogen bonds. To do so, we introduced point mutations to probe whether changes in these two amino acid residues would alter PDIp’s E2-binding activity.
Figure 3. Docking analysis of the binding mode of E2 inside human PDIp b-b′ fragment.
A. Overview of the docking result (mode I) of E2 binding inside the PDIp b-b′ fragment. E2 and H278 are shown in the ball-and-stick format and colored according to atoms. The protein structure is shown in ribbon. Yellow colored region denotes the b domain and magenta colored region denotes the b′ domain. B. A close-up view of the docking result of the E2-PDIp binding model, showing that a hydrogen bond (by green dash) is formed between the 3-hydroxyl group of E2 (a hydrogen bond donor) and PDIp′s His278 (a hydrogen bond acceptor). C. Interaction of E2 with amino acid residues in the binding pocket (mode I). Labeling of amino acid residues is shown in yellow for the b domain and in magenta for the b′ domain. E2 molecule is colored in yellow. Amino acids are shown in the ball-and-stick format and colored according to atoms, i.e., green for carbon, red for oxygen, white for hydrogen, and blue for nitrogen. D. Plots of the docking results of E2 binding with PDIp b-b′ fragment in mode I and II. The distance is in angstroms. E2 is colored in blue and H278, Q265 are in magenta.
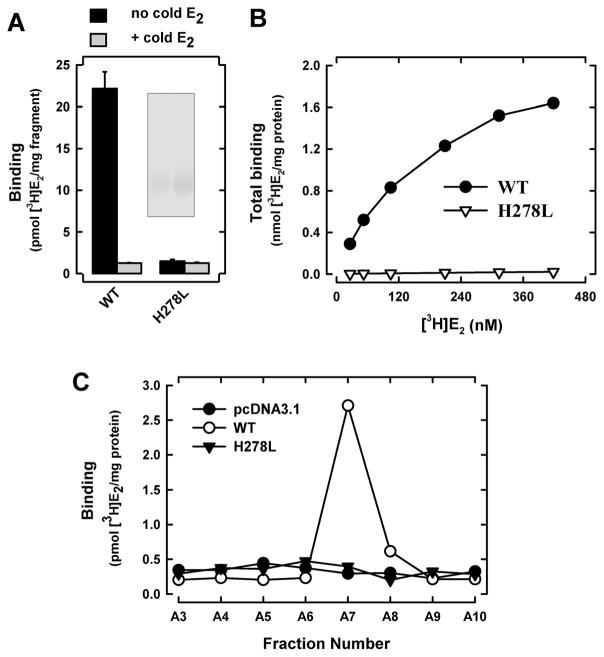
We first chose to mutate His278 to leucine (H278L). According to earlier studies (22–23), the side-chain length of leucine is similar to that of histidine but it does not contain electro-negative atoms that are necessary for the formation of hydrogen bonds. As shown in Figure 4A, the purified H278L b-b′ fragment completely lost [3H]E2-binding activity. Saturation binding assay further confirmed that this mutant fragment did not have an appreciable [3H]E2-binding activity even when very high concentrations of [3H]E2 were present (Figure 4B). Similarly, we also created the H278L mutation in the full-length PDIp, which was over-expressed in mammalian cells and isolated by SEC (Figure S3). Assay of the [3H]E2 binding activity of each of the SEC fractions showed that the PDIp H278L mutant protein does not have an appreciable [3H]E2-binding activity (Figure 4C). Taken together, these data show that PDIp’s His278 is indispensable for its binding interaction with E2.
Figure 4. H278L mutant protein lacks E2-binding activity.
A. The total [3H]E2 binding by purified PDIp b-b′ fragments (wild-type and H278L mutant proteins, purified from E. coli cells) after incubation with 4.5 nM [3H]E2 in the absence or presence of excess cold E2 (10 μM) in 10 mM sodium phosphate buffer. Inset shows the SDS-PAGE analysis of the purified proteins. B. The total [3H]E2 binding by PDIp b-b′ fragments (at the 20-μg/ml final concentration, wild-type protein and the H278L mutant protein) was determined in the presence of increasing concentrations of [3H]E2 (26 to 420 nM) in 10 mM sodium phosphate buffer (pH 7.4). C. The total [3H]E2 binding by SEC fractions of cos-7 cell lysates containing the expressed full-length wild-type PDIp or the H278L mutant protein (see Figure S4). The final concentration of [3H]E2 in these incubations was 4.5 nM. Each value is the mean of duplicate determinations.
Using the same approach, we also mutated Gln265 to leucine (Q265L). As suggested by the binding mode II (Figure 3D), Gln265 may form a hydrogen bond with the 17-hydroxyl group of E2. [3H]E2 binding assays showed that the purified Q265L b-b′ fragment (over-expressed in E. coli cells, Figure S4A) and the full-length PDIp Q265L mutant protein (over-expressed in cos-7 cells, Figure S4B) each display similar [3H]E2-binding activity as the corresponding wild-type proteins. The apparent Kd value for Q265L b-b′ fragment was found to be 185 ± 8 nM (Figure S4C), which is nearly the same as that of the wild-type b-b′ fragment. In addition, we have also prepared the double mutant protein Q265L/H278L for testing its [3H]E2-binding activity. As expected, this double mutant protein does not have any [3H]E2-binding activity. Collectively, these observations suggest that PDIp’s Gln265 may either form a non-essential, weak hydrogen bond with the 17-hydroxyl group of E2 (as suggested by the binding mode II, Figure 3D), or it may not form a viable hydrogen bond at all (as suggested by the binding mode I, Figure 3D). In addition, we performed the in silico analysis (using Insight II) to predict the effect of point mutations (H278L and Q265L) on the structures of the PDIp b-b′ fragment. No appreciable differences were observed between the wild-type fragment and these two mutant fragments in terms of their secondary structures (which were also predicted by Jpred 3, http://www.compbio.dundee.ac.uk/www-jpred/) and tertiary structures (data not shown).
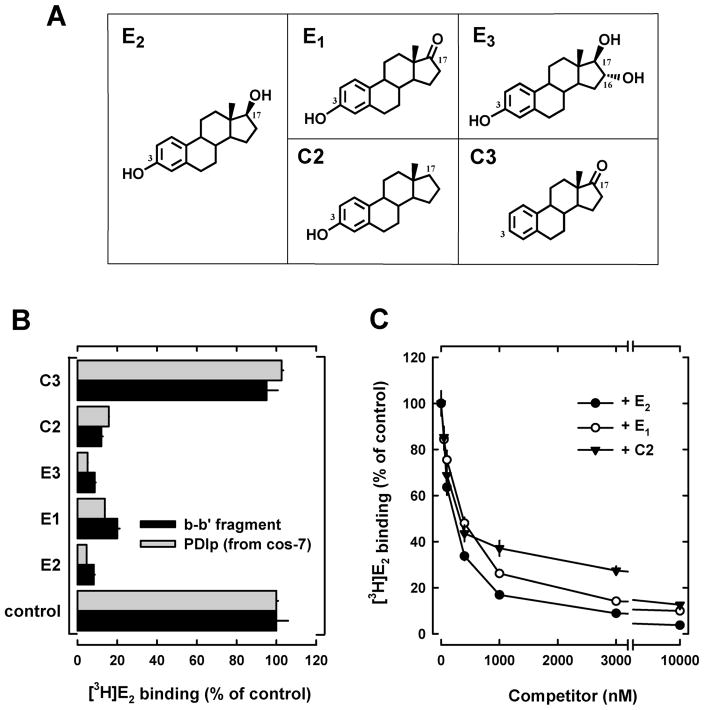
To provide further experimental support for the conclusions of the mutagenesis studies, we employed an alternative approach by using a number of E2 derivatives that share the same core structure as E2 but with their C-3 or C-17 hydroxyl group selectively modified such that they cannot form the same type of hydrogen bonds with PDIp as does E2. In these experiments, both purified b-b′ fragment (expressed in E. coli cells) and purified full-length recombinant human PDIp (expressed in cos-7 cells) were used to assay their relative binding activity by measuring their ability to compete off the binding of [3H]E2. We found that E1, E3, and C2, all of which contain an intact 3-hydroxyl group but differ in their 17-hydroxyl group (structures shown in Figure 5A), could efficiently compete with [3H]E2 for binding to the b-b′ fragment or the full-length PDIp (Figure 5B). There is no significant difference between the IC50 values of E2, E1 and C2 (Figure 5C). In contrast, C3, which lacks the 3-hydroxyl group (Figure 5A), displayed no appreciable binding activity (Figure 5B). These observations provide further support for the suggestion that the 3-hydroxyl group of E2 is essential for the binding interaction with PDIp by forming hydrogen bond(s), whereas the 17-hydroxyl group of E2 is not important. These results are in agreement with the observations made with the point mutation studies as described above.
Figure 5. Relative binding activity of PDIp for several E2 derivatives.
A. Chemical structures of E2 and several of its analogs used in this study. B. Relative binding of [3H]E2 by recombinant PDIp b-b′ fragment (at 20 μg/ml final concentration, purified from E.coli cells) (see Figure 1B) or by purified recombinant full-length PDIp protein expressed in cos-7 cells (at 20 μg/ml final concentration, see Figure S1). Protein was incubated with 4.5 nM [3H]E2 in the absence or presence of 10 μM E2 or its analogs in sodium phosphate buffer (10 mM, pH 7.4). C. Relative [3H]E2 binding by the PDIp b-b′ fragment purified from E. coli cells after incubation with 4.5 nM [3H]E2 in 10 mM sodium phosphate buffer (pH 7.4) in the absence (control, set as 100%) or presence of increasing concentrations of E2, E1 and C2. Each value is the mean ± S.D. of triplicate determinations.
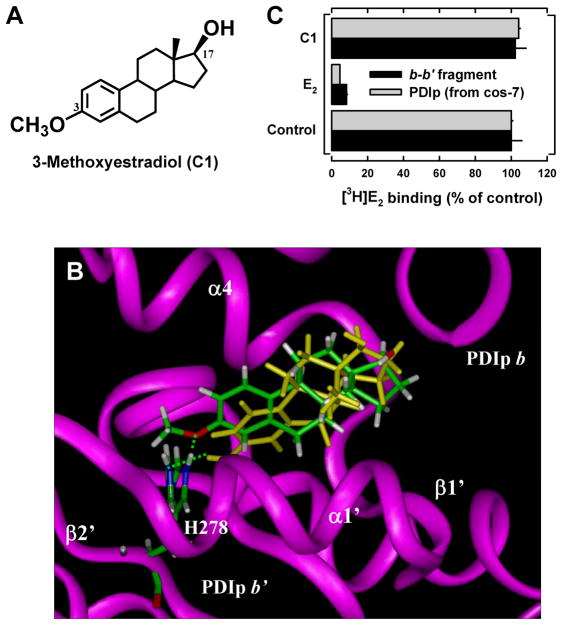
Notably, while the two binding modes suggested by the docking studies are very similar in their overall structure and binding interaction, there are also some notable subtle differences. In mode I (Figure 3D), the 3-hydroxyl group of E2 is a hydrogen bond donor and the nitrogen atom of PDIp-His278 is a hydrogen bond acceptor. In mode II, the 3-hydroxyl group of E2 serves as a hydrogen bond acceptor and PDIp-His278 as a hydrogen bond donor in the formation of a hydrogen bond. Based on our earlier experience, it is almost certain that only one of the modes is the preferred binding mode. Based on the known difference in the strength of the OH—N hydrogen bond (6.9 kcal/mol) vs. the O—HN hydrogen bond (1.9 kcal/mol) (24), it is predicted that the binding mode I, which contains the stronger OH—N hydrogen bond, would be the preferred binding mode over mode II, which contains the weaker O—HN hydrogen bond. To provide experimental evidence for this prediction, we chose to determine the binding activity of 3-methoxyestradiol (structure shown in Figure 6A) for PDIp. Theoretically, the oxygen atom in the 3-methoxy group of 3-methoxyestradiol will only be able to serve as a hydrogen bond acceptor but not a hydrogen bond donor in forming a hydrogen bond (i.e., it can only bind to PDIp in mode I but not in mode II). Computational docking analysis showed that 3-methoxyestradiol can still bind inside the pocket in a similar way as does E2, because there is enough space around His278 to fully accommodate the methoxy group (Figure 6B). As expected, the computational binding model showed that 3-methoxyestradiol (serving as a hydrogen bond acceptor) still can form a hydrogen bond with His278 (serving as a hydrogen bond donor). However, in the binding assay, we found that 3-methoxyestradiol has no appreciable binding activity for the b-b′ fragment or the full-length PDIp (Figure 6C). This experimental observation suggests that the hydrogen bond formed between 3-methoxyestradiol and His278 likely is either extremely weak, or more likely, it is not formed at all. Taken together, it is concluded that E2 binds to PDIp by forming a hydrogen bond between the 3-hydroxyl group of E2 and PDIp-His278 according to mode I, but not mode II (Figure 3D).
Figure 6. Docking analysis of the interaction of PDIp with E2 analog C1.
A. Chemical structure of 3-methoxyestradiol (C1). B. Docking analysis of the binding modes of E2 and C1 in the binding pocket of the PDIp b-b′ fragment. Protein structure is shown in ribbon and colored in magenta. E2, C1 and His278 are shown in the ball-and-stick format. C1 and His 278 are colored according to atoms and E2 is colored yellow. Green dashes denote hydrogen bonds. α-Helics and β-sheets are labeled according to Figure S2A. C. Relative [3H]E2 binding by recombinant PDIp b-b′ fragment or by purified recombinant full-length PDIp protein in the absence or presence of 10 μM E2 or its C1 analog. Each value is the mean ± S.D. of triplicate determinations.
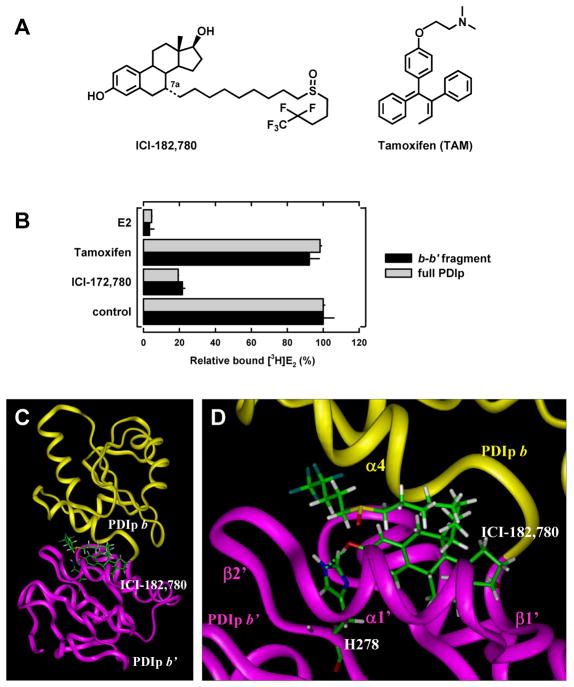
The results of this study also provide a good explanation for our early observation that while PDIp has no appreciable binding affinity for tamoxifen, it still retains considerable binding affinity for ICI-172,780 (16) (also see Figure 7B). It is known that tamoxifen does not have a phenolic OH group in its structure (see Figure 7A), and thus it cannot form the necessary hydrogen bond with His278 of PDIp. In comparison, ICI-172,780 has the same core steroid structure as E2 (see Figure 7D). Our docking analysis showed that ICI-172,780 can still be bound inside PDIp’s binding pocket in a similar way as E2 (Figure 7C, 7D), although its long side chain at the C-7 position slightly interferes with the binding, which is consistent with its lower binding affinity (Figure 7B).
Figure 7. Docking analysis of the interaction of PDIp with ICI-172,780.
A. Chemical structures of ICI-172,780 and tamoxifen. B. Relative [3H]E2 binding in the absence or presence of excess E2, tamoxifen or ICI compound (10 μM) by the recombinant PDIp b-b′ fragment (at 20 μg/ml final concentration, purified from E.coli cells) or by the recombinant full-length PDIp protein (at 20 μg/ml final concentration, purified from cos-7 cells). These proteins were incubated with 4.5 nM [3H]E2 in sodium phosphate buffer (10 mM, pH 7.4). Each data point is the mean ± S.D. of triplicate determinations. C. The docking results showing the binding interaction of ICI-172,780 with the PDIp b-b′ fragment. D. A close-up view of the docking result of ICI-172,780 binding in PDIp b-b′ fragment, showing that a hydrogen bond (represented by the green dashed line) was formed between the 3-hydroxyl group of ICI-172,780 (hydrogen bond donor) and PDIp-His278 (hydrogen bond acceptor). The protein structure is shown in ribbon. Yellow-colored regions denote the b domain and magenta-colored regions denote the b′ domain. ICI-172,780 and His278 are shown in ball-and-stick format and colored according to atoms. α-Helics and β-sheets are labeled according to Figure S2A.
Based on the sequence and structural similarities between their b-b′ fragments (Figure S2A, S2B), it is suggested that PDI and PDIp may share a similar overall E2-binding pocket, although additional experimental evidence is needed to verify this possibility. Despite their potential structural similarity, it is of interest to note that the affinity of PDI for E2 (around 1500 nM, as reported earlier (9–10)) is considerably lower than that of PDIp. The lower binding affinity of PDI for E2 was also reflected in another study which showed that the peptide-binding activity of PDI is markedly less sensitive to the competitive inhibition by E2 compared to the peptide-binding activity of PDIp (15).
Notably, it is well known that the 3-hydroxyl group of E2 plays an essential role in its binding interaction with human ERα and ERβ by forming hydrogen bonds (19, 25–26). The structural model developed in the present study offers a good explanation for the experimental observation that the human PDIp has a much lower E2-binding affinity than human ERs, for the following reasons: (i) PDIp forms only one hydrogen bond with the 3-hydroxyl group of E2, whereas human ERs form two hydrogen bonds with this hydroxyl group. (ii) Whereas the 17β-hydroxyl group of E2 plays an important role in its interaction with human ERα or ERβ (25), its role in the interaction with human PDIp appears to be of minimal importance or simply nonexistent. (iii) The E2-binding site of PDIp is significantly larger than those of human ERα and ERβ, i.e., it is less compact, which would suggest a relatively loser binding interaction. (iv) For both ERα and ERβ, almost all amino acid residues in their binding pockets, except those that form hydrogen bonds with E2, are hydrophobic residues, which provide stronger hydrophobic interactions with the four aliphatic rings of E2. However, in the case of PDIp, some polar residues (Thr253, Thr260, Thr266, Ser225 and Gln265 shown in Figure 3C, 3D) are also present in the binding pocket, which may reduce the hydrophobic interactions with E2. These notable differences distinguish human ERs from PDIp in their binding interactions with E2.
Lastly, it is of note that observations made in the present study may also shed light on understanding the binding interaction of PDIp with its substrate peptides, which was shown to be inhibited by E2 and its analogs (15, 17). In support of this suggestion, we found that mastoparan, a peptide that can bind to PDIp and its binding can be inhibited by E2 as reported in an early study (15), can inhibit PDIp’s [3H]E2-binding activity (Figure S5). Therefore, the reciprocal inhibition of PDIp’s ligand binding activity between E2 and mastoparan suggests that they share the same or partially overlapping binding site in PDIp. Interestingly, an earlier study reported that the specificity of the peptide substrates during their interaction with PDIp is associated with the tyrosine and/or tryptophan residues in the peptide substrates (27). Since these two residues contain a free phenolic hydroxyl group and phenolic amine group, respectively, it is tempting to suggest that the presence of these amino acid residues may serve as hydrogen bond donors in forming hydrogen bonds with PDIp-His278 during PDIp-peptide interaction, in a similar way as observed for the 3-hydroxyl phenolic group in E2. This possibility merits further investigation.
In conclusion, the human PDIp has a single E2-binding site (apparent Kd of approximately 170 nM), and its E2-binding site is located in a hydrophobic pocket largely composed of the b′ domain and partially of the b domain. The hydrogen bond formed between the 3-hydroxyl group of E2 (hydrogen bond donor) and PDIp-His278 (hydrogen bond acceptor) is indispensable for E2 binding.
Supplementary Material
Acknowledgments
Funding information: This study was supported, in part, by a grant from the NIH (CA97109). Some of the instruments employed in this study are part of the COBRE core facility that is supported by the NIH Grant P20RR021940 from the National Center for Research Resources.
We would like to thank Dr. Grace Liejun Guo in our department for recommending us to use the gel filtration system in her laboratory for the purification of the recombinant PDIp.
Abbreviations used
- PDI
protein disulfide isomerase
- PDIp
pancreas-specific PDI homolog
- ERα and ERβ
estrogen receptor α and β, respectively
- E2
estradiol
- SEC
size-exclusion chromatography
Footnotes
SUPPORTING INFORMATION AVAILABLE
Figures S1 and S3 depicting the isolation of wild type and H278L PDIp proteins, respectively, Figure S2 providing homology modeling result of the b-b′ fragment, and Figure S4 and S5 showing E2-binding of Q265L mutant protein and the inhibition effect of peptide on E2-binding, respectively. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Ciocca DR, Roig LM. Estrogen receptors in human nontarget tissues: biological and clinical implications. Endocr Rev. 1995;16:35–62. doi: 10.1210/edrv-16-1-35. [DOI] [PubMed] [Google Scholar]
- 2.Razandi M, Pedram A, Merchenthaler I, Greene GL, Levin ER. Plasma membrane estrogen receptors exist and functions as dimers. Mol Endocrinol. 2004;18:2854–2865. doi: 10.1210/me.2004-0115. [DOI] [PubMed] [Google Scholar]
- 3.Li L, Haynes MP, Bender JR. Plasma membrane localization and function of the estrogen receptor alpha variant (ER46) in human endothelial cells. Proc Natl Acad Sci U S A. 2003;100:4807–4812. doi: 10.1073/pnas.0831079100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fortunati N, Catalano MG. Sex hormone-binding globulin (SHBG) and estradiol cross-talk in breast cancer cells. Horm Metab Res. 2006;38:236–240. doi: 10.1055/s-2006-925337. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Graubard BI, Klebanoff MA, Ronckers C, Stanczyk FZ, Longnecker MP, McGlynn KA. Maternal hormone levels among populations at high and low risk of testicular germ cell cancer. Br J Cancer. 2005;92:1787–1793. doi: 10.1038/sj.bjc.6602545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hatahet F, Ruddock LW. Protein disulfide isomerase: a critical evaluation of its function in disulfide bond formation. Antioxid Redox Signal. 2009;11:2807–2850. doi: 10.1089/ars.2009.2466. [DOI] [PubMed] [Google Scholar]
- 7.Wilkinson B, Gilbert HF. Protein disulfide isomerase. Biochim Biophys Acta. 2004;1699:35–44. doi: 10.1016/j.bbapap.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 8.Tsibris JC, Hunt LT, Ballejo G, Barker WC, Toney LJ, Spellacy WN. Selective inhibition of protein disulfide isomerase by estrogens. J Biol Chem. 1989;264:13967–13970. [PubMed] [Google Scholar]
- 9.Primm TP, Gilbert HF. Hormone binding by protein disulfide isomerase, a high capacity hormone reservoir of the endoplasmic reticulum. J Biol Chem. 2001;276:281–286. doi: 10.1074/jbc.M007670200. [DOI] [PubMed] [Google Scholar]
- 10.Hiroi T, Okada K, Imaoka S, Osada M, Funae Y. Bisphenol A binds to protein disulfide isomerase and inhibits its enzymatic and hormone-binding activities. Endocrinology. 2006;147:2773–2780. doi: 10.1210/en.2005-1235. [DOI] [PubMed] [Google Scholar]
- 11.Fu X, Wang P, Zhu BT. Protein disulfide isomerase is a multifunctional regulator of estrogenic status in target cells. J Steroid Biochem Mol Biol. 2008;112:127–137. doi: 10.1016/j.jsbmb.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Desilva MG, Lu J, Donadel G, Modi WS, Xie H, Notkins AL, Lan MS. Characterization and chromosomal localization of a new protein disulfide isomerase, PDIp, highly expressed in human pancreas. DNA Cell Biol. 1996;15:9–16. doi: 10.1089/dna.1996.15.9. [DOI] [PubMed] [Google Scholar]
- 13.Desilva MG, Notkins AL, Lan MS. Molecular characterization of a pancreas-specific protein disulfide isomerase, PDIp. DNA Cell Biol. 1997;16:269–274. doi: 10.1089/dna.1997.16.269. [DOI] [PubMed] [Google Scholar]
- 14.Fu XM, Zhu BT. Human pancreas-specific protein disulfide-isomerase (PDIp) can function as a chaperone independently of its enzymatic activity by forming stable complexes with denatured substrate proteins. Biochem J. 2010;429:157–169. doi: 10.1042/BJ20091954. [DOI] [PubMed] [Google Scholar]
- 15.Klappa P, Stromer T, Zimmermann R, Ruddock LW, Freedman RB. A pancreas-specific glycosylated protein disulphide-isomerase binds to misfolded proteins and peptides with an interaction inhibited by oestrogens. Eur J Biochem. 1998;254:63–69. doi: 10.1046/j.1432-1327.1998.2540063.x. [DOI] [PubMed] [Google Scholar]
- 16.Fu X, Zhu BT. Human pancreas-specific protein disulfide isomerase homolog (PDIp) is an intracellular estrogen-binding protein that modulates estrogen levels and actions in target cells. J Steroid Biochem Mol Biol. 2009;115:20–29. doi: 10.1016/j.jsbmb.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klappa P, Freedman RB, Langenbuch M, Lan MS, Robinson GK, Ruddock LW. The pancreas-specific protein disulphide-isomerase PDIp interacts with a hydroxyaryl group in ligands. Biochem J. 2001;354:553–559. doi: 10.1042/0264-6021:3540553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denisov AY, Maattanen P, Dabrowski C, Kozlov G, Thomas DY, Gehring K. Solution structure of the bb′ domains of human protein disulfide isomerase. FEBS J. 2009;276:1440–1449. doi: 10.1111/j.1742-4658.2009.06884.x. [DOI] [PubMed] [Google Scholar]
- 19.Brzozowski AM, Pike AC, Dauter Z, Hubbard RE, Bonn T, Engstrom O, Ohman L, Greene GL, Gustafsson JA, Carlquist M. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature. 1997;389:753–758. doi: 10.1038/39645. [DOI] [PubMed] [Google Scholar]
- 20.Manas ES, Unwalla RJ, Xu ZB, Malamas MS, Miller CP, Harris HA, Hsiao C, Akopian T, Hum WT, Malakian K, Wolfrom S, Bapat A, Bhat RA, Stahl ML, Somers WS, Alvarez JC. Structure-based design of estrogen receptor-beta selective ligands. J Am Chem Soc. 2004;126:15106–15119. doi: 10.1021/ja047633o. [DOI] [PubMed] [Google Scholar]
- 21.Manas ES, Xu ZB, Unwalla RJ, Somers WS. Understanding the selectivity of genistein for human estrogen receptor-beta using X-ray crystallography and computational methods. Structure. 2004;12:2197–2207. doi: 10.1016/j.str.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 22.Birukou I, Schweers RL, Olson JS. Distal histidine stabilizes bound O2 and acts as a gate for ligand entry in both subunits of adult human hemoglobin. J Biol Chem. 2010;285:8840–8854. doi: 10.1074/jbc.M109.053934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshiro M, Jun F, Susumu K, Kenji O, Yuji S, Yasuo H, Kazuyuki M. Site-Directed Mutagenesis of His-176 and Glu-177 in Pseudomonas aeruginosa Alkaline Protease: Effect on Catalytic Activity. J Ferment Bioeng. 1997;84:588–590. [Google Scholar]
- 24.Emsley J. Very Strong Hydrogen Bonds. Chemical Society Reviews. 1980;9:91–124. [Google Scholar]
- 25.Zhu BT, Han GZ, Shim JY, Wen Y, Jiang XR. Quantitative structure-activity relationship of various endogenous estrogen metabolites for human estrogen receptor alpha and beta subtypes: Insights into the structural determinants favoring a differential subtype binding. Endocrinology. 2006;147:4132–4150. doi: 10.1210/en.2006-0113. [DOI] [PubMed] [Google Scholar]
- 26.Gabbard RB, Segaloff A. Structure-activity relationships of estrogens. Effects of 14-dehydrogenation and axial methyl groups at C-7, C-9 and C-11. Steroids. 1983;41:791–805. doi: 10.1016/0039-128x(83)90054-5. [DOI] [PubMed] [Google Scholar]
- 27.Ruddock LW, Freedman RB, Klappa P. Specificity in substrate binding by protein folding catalysts: tyrosine and tryptophan residues are the recognition motifs for the binding of peptides to the pancreas-specific protein disulfide isomerase PDIp. Protein Sci. 2000;9:758–764. doi: 10.1110/ps.9.4.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian G, Xiang S, Noiva R, Lennarz WJ, Schindelin H. The crystal structure of yeast protein disulfide isomerase suggests cooperativity between its active sites. Cell. 2006;124:61–73. doi: 10.1016/j.cell.2005.10.044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.