Abstract
Although nicotine is generally considered to be the main psychoactive component of tobacco, growing evidence highlights the importance of non-nicotine compounds in smoking reinforcement. Monoamine oxidase (MAO) inhibition is a major consequence of smoking and MAO inhibitors, such as tranylcypromine, increase nicotine reinforcement. Tranylcypromine has multiple pharmacological effects, increasing monoamine release for a few hours immediately after its administration and blocking MAO activity for several days. To assess the relative role of these two actions, adult male rats were tested in consecutive daily 3-hr sessions for self-administration of nicotine (3μg/kg/inj, i.v.) either 20 hr or 1hr following administration of tranylcypromine (3 mg/kg). Both paradigms were shown to produce highly significant inhibition of MAO activity. However, whereas animals readily acquired self-administration when pretreated with tranylcypromine 1hr prior to testing, they did not with the longer pretreatment interval. Such animals did immediately acquire nicotine self-administration when the tranylcypromine pretreatment interval was switched to 1hr prior to testing on day 4, indicating that an acute effect of the MAO inhibitor was responsible for enhanced nicotine reinforcement. Several lines of evidence implicate serotonin (5-HT) as the mediator of this enhancement: (1) Tranyclypromine-enhanced nicotine reinforcement was blocked by the 5-HT2 receptor antagonists, ritanserin and ketanserin; (2) parachloroamphetamine (PCA), a 5-HT releaser, also enhanced nicotine self-administration in animals in which MAO activity was inhibited; (3) pretreatment with tranylcypromine increased PCA-induced 5-HT overflow in the nucleus accumbens. These findings suggest that MAO inhibition enhances serotonergic transmission, which serves a critical role in the reinforcing effects of nicotine.
Keywords: nicotine, self-administration, MAOI, parachloroamphetamine, 5-HT2A, microdialysis
INTRODUCTION
Tobacco is one of the most abused drugs, with nicotine being recognized as the main psychoactive component (Harvey et al., 2004). However, over 4000 compounds have been identified in tobacco smoke (Report, 1989), some of which are known to inhibit monoamine oxidase (MAO), an enzyme responsible for the breakdown of monoamines. Indeed, MAO-A and -B activities have been shown to be inhibited in smokers by about 40 to 50% when compared to non-smokers (Fowler et al., 1996a, b, 2003). Experimentally, it has been shown that rodents pretreated with tranylcypromine, an irreversible MAO inhibitor (MAOI), exhibit enhancement of nicotine-induced behaviors, including locomotor activity, locomotor sensitization and self-administration (Guillem et al., 2005; Villégier et al., 2003, 2006, 2007). Tranylcypromine, as with most drugs used to mimic experimentally tobacco smoke-induced MAO inhibition, is an irreversible MAO inhibitor that increases monoamine release for a few hours immediately after its administration and blocks MAO activity for several days (Guillem et al., 2005; Villégier et al., 2006). Studies have usually assessed MAO activities in parallel to responses to nicotine, especially in nicotine self-administration studies (Villégier et al., 2007; Lotfipour et al, submitted), showing a potential correlation between the degree of MAO activity blockade and the level of responding for nicotine self-administration. However, parachloroamphetamine (PCA), a serotonin (5-HT) releaser, has been shown to reproduce the effects of tranylcypromine in facilitating nicotine-induced locomotor activity (Villégier et al., 2006), suggesting that the direct monoamine releasing effect of tranylcypromine may be sufficient to increase nicotine’s effects. These findings support the concept that both the MAOI effect and the direct 5-HT releasing effect of tranylcypromine may play a key role to enhance nicotine effects.
The aim of the present study was to assess the relative role of these two tranylcypromine actions in the enhancement of nicotine reinforcement, as measured using the self-administration paradigm. This question was addressed using a small nicotine dose (3 μg/kg/inj, i.v.) in the absence of non-nicotine related cues. The dose of nicotine used is normally too small to support self-administration. To differentiate between short- and long-term pharmacological effects, tranylcypromine was administered either 1hr or 20hr prior to consecutive daily 3-hr nicotine self-administration sessions. The role of 5-HT in MAOI enhancement of nicotine self-administration was further evaluated by examining the effects of selective receptor antagonists and of the releaser, PCA. Neurochemical studies were also conducted to evaluate underlying mechanisms. The data support the concept that both MAO inhibition and acute release of 5-HT contribute to tranylcypromine enhancement of nicotine self-administration.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats were obtained at postnatal day P60 (Charles River). After surgery, animals were single-housed and maintained on a 12-hr light/dark cycle (lights on at 07:00 am) with food and water ad libitum. Tests were performed during the light part of the light-dark cycle. Animals were housed in an AAALAC-accredited vivarium maintained by UCI Laboratory Animal Resources personnel. Experimental procedures were performed in compliance with NIH Guide for Care and Use of Laboratory Animals (NIH No 85-23, rev. 1985) and approved by the UCI Institutional Animal Care and Use Committee. All efforts were made to minimize animal suffering and their number.
Self-administration
Catheter
The catheter consisted of Silastic tubing (0.03 cm ID × 0.064 cm OD) attached to a guide cannula (Plastic Products, Inc., C313G-5up). The guide cannula was embedded in cranioplastic cement with Marlex (see Manzardo et al., 2002).
Surgery
Surgery commenced at age P68. Animals were anesthetized with Equithesin (0.3 ml/100 g, i.p.) and a chronic catheter was implanted into the external jugular vein (see Belluzzi et al., 2005). The catheters were flushed daily with 0.2 ml heparinized saline solution (0.6 ml of 1000 units/ml heparin in 30 ml saline). Prior to testing and after the final test session, propofol (0.2 ml) was injected through the catheter to test its patency as indicated by rapid anesthesia. Data were discarded from animals not demonstrating rapid anesthesia.
Self-Administration
Four days after surgery, rats were tested in self-administration chambers with two nose-poke holes (See Belluzzi et al., 2005). No preliminary nose-poke training or other reinforcement was used. All rats were naïve to the operant chambers and to any reinforcer before the first self-administration session. A 10-ml syringe was mounted in an infusion pump and connected by polyethylene tubing to a feed-through swivel. The house light remained on during the experiment and no non-nicotine cues were programmed. Each nose poke at the reinforced hole induced a 1.1-sec 20-μl infusion of nicotine (3 μg/kg/inj) or saline and was followed by a 60-sec time-out period during which responses were not counted. To control for nonspecific activating effects of drugs, non-reinforced nose-pokes at a second adjacent hole were counted. They had no programmed consequences and were followed by a 60-sec time-out period. Reinforced (R) and non-reinforced (NR) holes were arbitrarily assigned.
Acquisition of nicotine self-administration 1hr or 20hr after tranylcypromine pretreatment
Tests for acquisition of self-administration consisted of five consecutive daily 3-hr sessions with nicotine (3 μg/kg/inj/i.v.). One hour before each session, rats received a tranylcypromine pretreatment (3 mg/kg, i.p.) for the tra1hr pretreated group (one group, n=10), or a saline injection for the saline group (one group, n=8) while injections were performed 20 hrs prior to each session for the tra20hr pretreated group (one group, n=7).
Switch experiment
The three first sessions
Independent groups of animals were pretreated with either tranylcypromine (3 mg/kg, i.p.) (tra20hr pretreated group, 19 groups, n=5–11), or saline (1.5 ml/kg, i.p.) (saline pretreated group, 2 groups, n=5) 20hr prior to three consecutive daily 3-hr sessions of nicotine self-administration. The fourth day was the test day on which a treatment switch occurred. For each test described below, rats from one test came from the same batch and were tested in a single experiment. For each experiment, data of the three first sessions were checked so that the groups displayed the same level of response to the R and NR holes. Then, animals were randomly designed to the groups and corrections were made to counterbalance the groups when it was necessary.
The fourth session
Tra1hr switch experiment
On the 4th day, one tra20hr and one saline pretreated groups were pretreated with tranylcypromine (3 mg/kg, i.p.) 1hr prior to the nicotine self-administration session. Two tra20hr pretreated groups were tested for self-administration of saline (20 μl/inj, i.v.) 1 hr following tranylcypromine (3 mg/kg, i.p.) pretreatment and of nicotine (3 μg/kg/inj, i.v.) 1 hr following saline (3 ml/kg, i.p.) pretreatment, respectively.
Tranylcypromine 1hr dose-response
Four independent tra20hr pretreated groups were tested on the fourth day for nicotine self-administration 1 hr after tranylcypromine (0, 0.75, 1.5 or 3 mg/kg, i.p.) pretreatment.
Effect of ritanserin and ketanserin
Five independent Tra20hr pretreated rats were pretreated with tranylcypromine (3 mg/kg, i.p.) and tested 1 hr later for nicotine self-administration. The involvement of 5-HT2 receptors was tested by administering ritanserin (1 or 5 mg/kg, s.c.), ketanserin (1 or 5 mg/kg, s.c.) or vehicle in five independent groups 15 min prior to the beginning of the self-administration session.
PCA-switch experiment
One Tra20hr and one saline pretreated groups were tested for nicotine self-administration 1 hr following PCA (1 mg/kg, i.p.) pretreatment. Two tra20hr pretreated groups were tested for saline self-administration 1 hr after PCA pretreatment and nicotine self-administration 1 hr after saline pretreatment, respectively.
PCA dose-response
Four independent tra20hr pretreated groups were tested for nicotine self-administration 1 hr after PCA (0, 0.5, 1 or 1.5 mg/kg, i.p.) pretreatment.
MAO activity measurement
Experimental groups
To assess MAO activity in tra20hr and saline pretreated groups, nine independent groups (n=4/group) received 1, 3 or 5 daily injections of tranylcypromine (3 mg/kg, i.p.) or saline timed so that the last injection occurred either 20 or 1 hr prior to sacrifice. To assess MAO activity on test day for the PCA-switch group and microdialysis experiment, two groups received three daily tranylcypromine (n=4) or saline (n=4) injections timed so that the last injection occurred 44 hr prior to sacrifice.
MAO assay
Brains were removed and immediately homogenized in 8 ml of 50 mM sodium phosphate buffer (PB), pH7.4. Homogenates were centrifuged (600g, 10 min, 4°C), and the resulting supernatants were diluted with PB (20 mg tissue/ml). Protein content was analyzed using a Bradford assay and enzymatic analysis of MAO-A and -B activities using a radiochemical assay, modified from established procedures (Hauptmann and Shih, 2001). Briefly, 100 μl tissue aliquots were incubated in 1 ml PB buffer, pH 7.4, with [14C]serotonin (5-HT) or [14C]phenylethylamine (PEA) for measurement of MAO-A and B activities, respectively, supplemented with addition of unlabeled ligand. Blanks were prepared by omitting brain homogenate. Reactions were terminated by cooling on ice and adding 6N HCl. The deaminated products were extracted using benzene/ethyl acetate for [14C]5-HT and toluene for [14C]PEA and vortexed for 30 sec. Tubes were centrifuged (1400 × g) and the organic layer was removed and added to Safety-Solve scintillation fluid for scintillation counting.
Microdialysis
Animals were implanted with both an intravenous catheter and a cranial guide cannula, using a stereotaxic frame (Kopf Instruments, Tujunga, CA, USA) (Villégier et al., 2007). A chronic guide cannula (20 gauge; CMA/Microdialysis AB, Stockholm, Sweden) was implanted 2.0 mm above the NAc Shell (antero-posterior, +2.0 mm; medio-lateral, ±1.2 mm; dorso-ventral, 5.8 mm) (Paxinos and Watson, 1986). The guide cannula was fixed to the skull with acrylic dental cement maintained by skull screws, and sealed with a dummy cannula.
Microdialysis procedure
In vivo microdialysis was performed in two groups of rats that received three daily tranylcypromine (3 mg/kg, i.p., n=6) or saline (n=5) pretreatments. Microdialysis was scheduled 44 hr following the last injection to correspond to the interval between the last tranylcypromine pretreatment and behavioral testing following PCA treatment. The difference between microdialysis and self-administration conditions is that rats were not exposed to nicotine and were not introduced to the operant chamber. Data collection times were chosen to parallel behavioral experiments such that they occurred in the time period analogous to peak self-administration.
On the experimental day, the dummy cannula was replaced with a 2 mm microdialysis probe (CMA/12). The quality of probes was tested in vitro before the experiment with an average recovery of 10.7% ± 1.0%, n=16. Microdialysis was carried out under a free-moving condition, with the probe continuously perfused with artificial cerebrospinal fluid (CMA Microdialysis N. Chelmsford MA, USA) at a flow rate of 1.1 μl/min delivered by a microinfusion pump (CMA/100 microdialysis, N. Chelmsford MA, USA). After a 4-hr equilibration period, samples were collected every 20 min. After 60 min, rats received an injection of saline (1.5 ml/kg, i.p.) and samples were collected for 60 min. Then rats received an injection with PCA (1 mg/kg, i.p.) and samples were collected for another 200 min. The position of microdialysis probes was verified histologically (Paxinos and Watson, 1986).
High performance liquid chromatography with electrochemical detection
Microdialysate samples were injected by an ESA 542 refrigerated autosampler onto a 150 × 3.2 mm ODS C18 column (ESA Inc., Chelmsford MA) connected to an ESA 580 HPLC pump. 5-HT and dopamine (DA) levels were determined by an electrochemical ESA 5600 detector connected to a 5014B Microdialysis Cell (ESA, Chelmsford, MA) with the dominant potential set to 320 mV. The detection limit was 500 fg. Measurements were analyzed using CoulArray for Windows32 Software 2.0 (ESA Inc., Chelmsford, MA, USA) using standard curves (Sigma-Aldrich, St. Louis, MO). Data were expressed as pg/20 μl, unadjusted for recovery. PCA-induced changes in 5-HT and DA were compared to 5-HT and DA levels obtained following saline injection.
Statistics
Results presented are means ± SEM of data obtained with 5 to 13 animals per group. Data were analyzed using three-way ANOVA (for day × R/NR × treatment interval), two-way ANOVA (for treatment interval × R/NR or R/NR × treatment dose with repeated measures on R/NR and treatment dose × day with repeated measures on day) and one-way ANOVA (for treatment, R/NR and treatment interval). Significant effects were tested separately with ANOVAs and Bonferroni- or Dunnett’s-corrected post-hoc comparisons. Day and R/NR responding were treated as within-subject factors, and pharmacological treatments were analyzed as between-subjects factors. All statistical analyses were performed using SYSTAT 10 statistical software. Statistical significance was set at p < 0.05.
Materials
(−) Nicotine hydrogen tartrate, tranylcypromine hydrochloride, PCA, ketanserin tartrate and ritanserin were purchased from Sigma-Aldrich. Doses are expressed as salts, except for nicotine which is expressed as base. The products were dissolved in saline (NaCl, 0.9%), except for ketanserin and ritanserin, which were dissolved in saline <0.5% DMSO. pH was adjusted to 7.4 prior to injection.
5-HT and DA standards were ordered from ESA, (Chelmsford, MA) at a concentration of 1 mg/ml. Dilutions of the stock solutions were made with MD-TM mobile phase.
RESULTS
MAO activity and acquisition of nicotine self-administration following tra1hr and tra20hr treatments
MAO activity (Fig. 1a)
Figure 1. Effect of the tra1hr and tra20 hr pretreatments on MAO activity and nicotine self-administration.
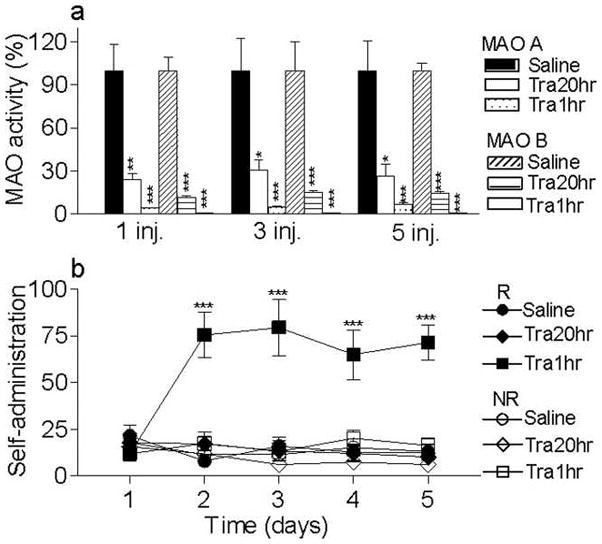
(a) MAO A and B activities were assessed in rats that received 1, 3 or 5 consecutive saline or tranylcypromine injections scheduled so that the last injection occurred 1 or 20 hours prior to the sacrifice. *p<0.05 **p<0.01 ***p<0.001, significantly different from saline treated group. (b) Naive animals were tested in 5 daily 3-h self-administration sessions on an FR 1 reinforcement schedule with nicotine (3 μg/kg/inj in 20 μl, i.v.) available. The mean (± SEM) total responses are plotted daily for each treatment group. Either 20hr or 1hr prior to each self-administration session, rats received an injection of tranylcypromine (3 mg/kg, i.p.) (Tra20hr and Tra1hr) or of saline. Responses at the reinforced and non reinforced holes are presented (R and NR). ***p<0.001, significantly different from R Tra20hr and Saline and from NR Tra1hr. n = 5 to 9/group.
As shown in Figure 1a, MAO activity was significantly affected by tranylcypromine treatment (F1,36 = 185.1, p < 0.001). However, there was no significant effect of pretreatment interval (F1,36= 1,790, p = 0.189), treatment number (F2,36 = 0,01, p = 0.987), or interactions of tranylcypromine treatment × interval (F1,36 = 1,8, p = 0.189), tranylcypromine treatment × day (F2,36 = 0.01, p = 0.987), interval × day (F2,36 = 0,01, p = 0.989) or tranylcypromine treatment × interval × day (F2,36 = 0,01, p = 0.989). Both MAO A and B activities were significantly decreased following tranylcypromine treatment (p < 0.02). However, there was no significant difference in the effect of tranylcypromine treatment on MAO A and B (p > 0.148).
Nicotine self-administration (Fig. 1b)
As shown in Figure 1b, self-administration scores were significantly affected by the tranylcypromine pretreatment interval (F1,22 = 29.7, p < 0.001), with significant interactions of day × pretreatment interval (F4,19 = 12.2, p < 0.001), R/NR × pretreatment interval (F1,22 = 10.2, p = 0.004) and day × R/NR × pretreatment interval (F4,19 = 14,2, p < 0.001). When animals were pretreated with tranylcypromine 1 hr before testing, they acquired nicotine self-administration, as defined by a significant difference between R and NR responding (F1,9 = 13.9, p = 0.005). There was a significant day × R/NR interaction (F4,36 = 11.1, p < 0.001), with significantly higher responding at the reinforced hole than the non-reinforced on days 2 to 5 (p < 0.001). Moreover responding for nicotine was significantly affected by day (F4,36 = 15.6 p < 0.001), with significantly higher responses on days 2 to 5 as compared to day 1 (p < 0.002). In contrast, pretreatment with tranylcypromine 20 hr prior to the test did not result in acquisition of nicotine self-administration, as shown by the absence of an effect of R/NR (F1,6 = 2,3, p = 0.177) or day × R/NR interaction (F4,24 = 0.4, p = 0.830). This group responded significantly less for nicotine than when tranylcypromine was given 1hr prior to the self-administration session (significance on day 2 to 5, p < 0.001). Since MAO activity was significantly inhibited in the tra20hr group, these findings indicate that acute effects of tranyclypromine administration in the tra1hr group were responsible for enhancement of nicotine self-administration.
Acute tranylcypromine enhances nicotine self-administration via 5-HT2A receptor activation (Fig. 2)
Figure 2. Tra1hr switch experiment promotes a 5-HT2A dependent nicotine self-administration.
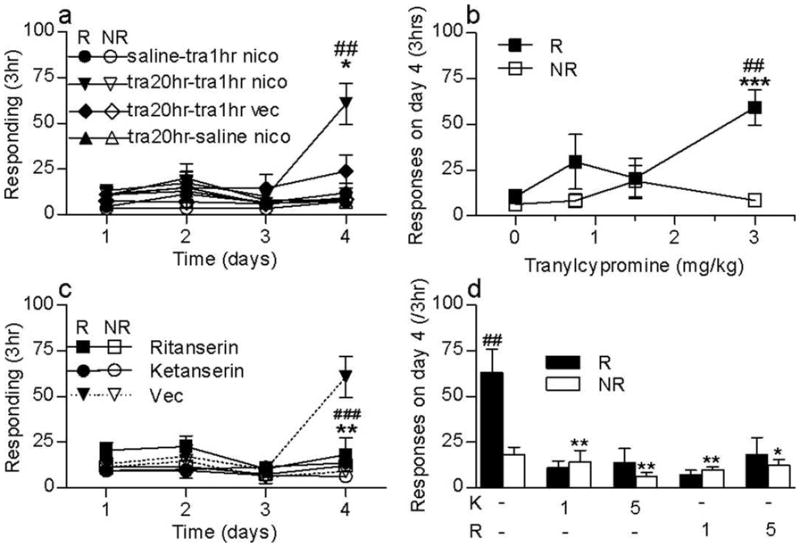
(a) Tra1hr switch experiment. Independent groups of naive animals were tested in 3 daily 3-h self-administration sessions on an FR 1 reinforcement schedule with nicotine (3 μg/kg/inj in 20 μl, i.v.) available 20 hr following tranylcypromine (3 mg/kg, i.p.) or saline (circles; saline-tra1hr-nico) pretreatment. Saline (1.5 ml/kg, i.p.) (up triangles; tra20hr-saline-nico) or tranylcypromine 3 mg/kg i.p. were given one hour before self-administration on test day. On test day, nicotine (3 μg/kg/inj in 20 μl) (down triangles, tra20hr-tra1hr-nico) or saline (diamonds; tra20hr-tra-saline) was available i.v. Reinforced (R) and non reinforced (NR) responses are presented. ##p<0.01 versus R saline; ***p<0.05 versus NR tranylcypromine 3 mg/kg; n = 5–12/group. (b) Tranylcypromine dose-response. Responding on day 4 are presented for each tranylcypromine dose 0.75, 1.5 or 3 mg/kg i.p. (filled squares for reinforced responding and open squares for non-reinforced responding). No nicotine self-administration was obtained following saline pretreatment whereas tranylcypromine 3 mg/kg significantly increased responses for nicotine. ##p<0.01 versus R saline; ***p<0.001 versus NR tranylcypromine 3mg/kg; n = 5–12/group. (c) (d) Effect of ketanserin and ritanserin on tra1hr-enhancement of nicotine self-administration. On test day, tra20hr pretreated rats receive an injection with vehicle (Vec), ketanserin (1 or 5 mg/kg, i.p.) or ritanserin (1 or 5 mg/kg, i.p.) prior to tra1hr pretreatment and were tested for nicotine self-administration. (c) Results are presented on the whole session for ritanserin and ketanserin (5mg/kg) ###p<0.001 R ketanserin versus R Vec; **p<0.01 R ritanserin versus R Vec (d) R and NR responding are presented for the fourth session for ketanserin and ritenserin 1 and 5 mg/kg. ##p<0.01 versus NR vehicle, **p<0.01 versus R vehicle. n = 5–9/group.
Animals pretreated with tranylcypromine 20hr prior to test on days 1–3 showed significant self-administration behavior on day 4 when tranylcypromine was given 1hr prior to the test session (Fig. 2a). A significant group difference was seen on day 4 (F3,22 = 7.6, p = 0.001) when these animals were compared to three other control groups. Post-hoc Dunnett-corrected comparison showed that responding of these animals at the reinforced hole on day 4 was significantly higher than reinforced responding in the other groups (p<0.03) (Fig 2a). There was no significant difference in nonreinforced responding. It is important to note that tranylcypromine administration 1hr prior to test did not enhance nicotine self-administration in animals pretreated on days 1–3 with saline. Thus, prior inhibition of MAO activity was critical for acute tranylcypromine enhancement of nicotine self-administration. The enhancement of reinforced responding by acute tranylcypromine pretreatment was also dependent on the availability of i.v. nicotine, since the behavior was not observed when nicotine was replaced with saline.
Tranylcypromine dose-response (Fig. 2b)
Of the three different tranylcypromine doses tested 1hr prior to the fourth session, only the 3 mg/kg dose significantly increased nicotine self-administration (Fig. 2b). Analysis of responses on test day showed a significant effect of tranylcypromine dose (F3,23 = 5.0, p = 0.008). Post-hoc Bonferroni-corrected t-test showed that reinforced responding following 3 mg/kg tranylcypromine was significantly higher than responding following saline (p = 0.006). There was also a significant reinforced/non-reinforced effect (F1,23 = 10.2 p = 0.004) and a significant reinforced/non-reinforced × tranylcypromine dose interaction (F3,23 = 5.4, p = 0.0059), with reinforced responding being significantly higher than non-reinforced responding (p < 0.001).
Effect of ketanserin and ritanserin on acute tranylcypromine enhancement of nicotine self-administration (Fig. 2c and d)
Acute tranycypromine enhancement of nicotine self-administration was blocked by pretreatment with the 5-HT2A receptor antagonists, ritanserin (5 mg/kg) and ketanserin (5 mg/kg; Fig. 2c). Overall responding on day 4 was significantly influenced by antagonist pretreatment (F5,34 = 9.1 p < 0.001), with reinforced responses significantly reduced in ritanserin and ketanserin treatment groups as compared to vehicle control (p = 0.007 and p = 0.001, respectively). As shown in Figure 2d, both low (1 mg/kg, s.c.) and medium (5 mg/kg, s.c.) doses of ketanserin and ritanserin significantly influenced nicotine self-administration (F4,25 = 6.3, p = 0.001). Post-hoc Dunnett adjusted t-tests showed that each antagonist treatment significantly decreased reinforced responding (p < 0.01), but did not modify responding at the nonreinforced hole (p = 0.5).
PCA substitution promotes a 5-HT-dependent enhancement of nicotine self-administration (Fig. 3)
Figure 3. PCA-switch experiment promotes a 5-HT release-dependent nicotine self-administration.
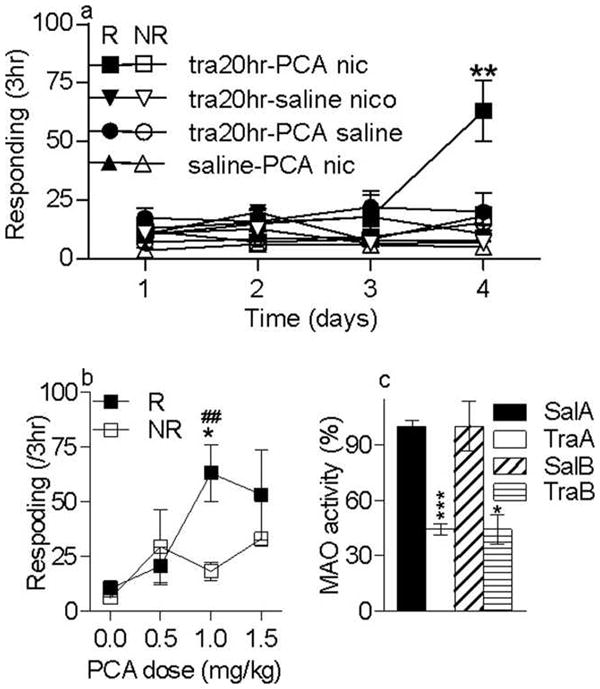
(a) Rats received an injection of either saline (1.5 ml/kg, i.p.) (saline PCA-nic) or tranylcypromine (3 mg/kg,i.p.) 20 hr prior to the three first self-administration sessions. On day 4 rats received PCA 1 hr prior to the self-administration session. Two other independant tra20hr pretreated group were tested for saline i.v. self-administration following PCA (tra20hr-PCA-sal) and for nicotine self-administration following saline (tra20hr-saline-nico). **p<0.01 versus NR and versus R and the saline PCA-nic group. n = 5–11/group. (b) PCA dose-response. On test day independent groups received three different doses of PCA (0.5, 1 or 1.5 mg/kg ip) or saline. Responses on day 4 at both the reinforced and non-reinforced holes are presented. *p<0.05 versus R saline; ##p<0.01 versus NR PCA 1mg/kg. n = 5–11/group. (c) MAO activity. Effect of tra20hr pretreatment given on the three first self-administration sessions on MAO-A and -B activities measured on day 4. Rats received 3 daily tranylcypromine (3 mg/kg, i.p.) (TraA and TraB) injections timed so that the last injection occurred 44 hours before sacrifices. The saline group (SalA and SalB) received 3 injections similarly timed. Tranylcypromine significantly reduced MAO activity. *p<0.05 and ***p<0.001 versus the corresponding Sal group. n = 4/group.
Animals pretreated with tranylcypromine 20hr prior to test on days 1–3 showed significant self-administration behavior on day 4 when treated with the 5-HT releaser, PCA (1 mg/kg), 1hr prior to the test session (Fig. 3a). A significant group difference was seen on day 4 (F3,19 = 5.5, p = 0.006) when these animals were compared to three other control groups. Post-hoc Dunnett-corrected comparison showed that responding of these animals at the reinforced hole on day 4 was significantly higher than reinforced responding in the other groups (p < 0.01). Furthermore reinforced responding was significantly higher than nonreinforced responding (p < 0.01). As shown by the other control groups, acute PCA administration did not enhance nicotine self-administration in animals pretreated on days 1–3 with saline. Thus, prior inhibition of MAO activity was critical for acute PCA enhancement of nicotine self-administration. The enhancement of reinforced responding by acute PCA pretreatment was also dependent on the availability of i.v. nicotine, since the behavior was not observed when nicotine was replaced with saline.
PCA dose response (Fig. 3b)
When three different PCA doses were tested in separate groups of animals, only the 1 mg/kg PCA pretreatment significantly increased nicotine self-administration (Fig. 3b). Analysis of responses on the test day showed a significant effect of PCA dose (F3,22 = 4.3, p = 0.015). Post-hoc Dunnet-corrected t-test comparison showed that reinforced responding was significantly higher in animals pretreated with 1 mg/kg PCA than in the vehicle pretreated group (p = 0.02). Moreover there was a significant R/NR effect (F1,22 = 4.5, p = 0.04) and a significant R/NR × PCA dose interaction (F3,22 = 3.5, p = 0.03), with reinforced responding significantly higher than nonreinforced responding only in animals pretreated with the 1 mg/kg PCA dose (p = 0.006).
MAO activity (Fig. 3c)
Three daily tranylcypromine (3mg/kg) pretreatments significantly decreased MAO activity, as measured 44 hrs following the last tranylcypromine injection (−55.76%, F1,6 = 178.8, p < 0.0001 and −55.95%, F1,6 = 13.1, p = 0.01, for MAO A and B, respectively) (Fig. 3c).
Enhanced PCA-induced 5-HT release in tranylcypromine pretreated rats (Fig. 4)
Figure 4.
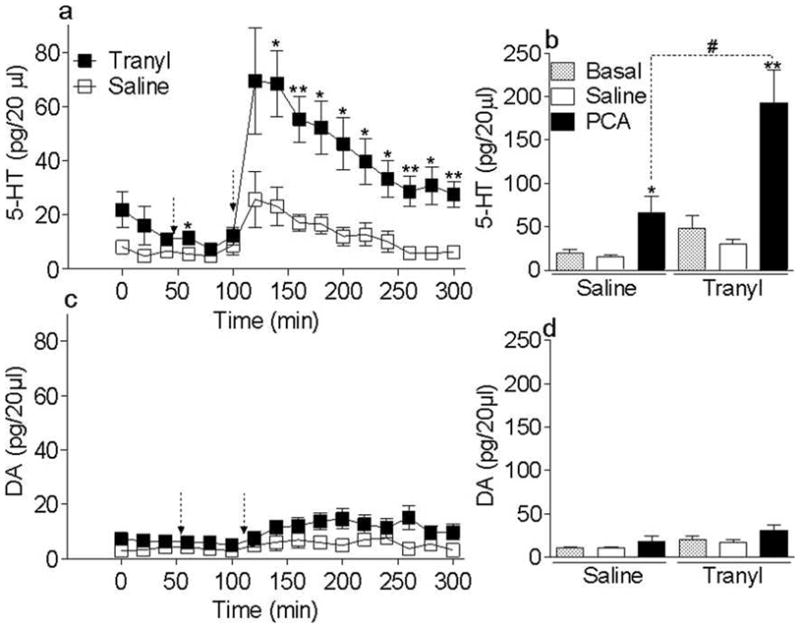
(a) (b) Time course of the effect of PCA on extracellular 5-HT and DA levels in the NAc in tra20hr and saline chronically pretreated rats. Animals were pretreated for three days with either tranylcypromine (3 mg/kg, i.p.) or saline (1.5 ml/kg, i.p.). Forty four hours after the third injection (at T=0), dialysate samples were collected every twenty minutes for measurement of extracellular levels of 5-HT and DA in the NAc shell. At T=60 min, rats received a saline (1.5 ml/kg, i.p.) (first arrow) and samples were collected for one hour. At T=120 min, rats were given PCA (1 mg/kg, i.p.) (second arrow) and samples were collected for 200 min. Extracellular 5-HT and DA levels are expressed as the mean ± SEM in pg/20 μl; *p<0.05, **p<0.01 tra20hr pretreated versus saline pretreated groups. n = 5–6/group.
(b) (d) Increased extracellular 5-HT and DA levels following PCA in tranylcypromine chronically treated rats. Acute PCA-induced changes in extracellular 5-HT and DA levels measured on 200 min following saline and PCA injection, are expressed as the mean ± SEM in pg/60 μL. *p<0.05, **p<0.01 PCA versus saline and #p<0.05 Tra20hr-PCA versus Saline-PCA pretreatment. n = 5–6/group.
The time course of the effects of PCA on extracellular 5-HT levels in the NAc shell is shown in Figure 4a. PCA-induced extracellular 5-HT levels in the tranylcypromine pretreated group were significantly higher than in the saline pretreated group, as indicated by significant effects of tranylcypromine pretreatment dose (F1,9 = 8.0, p = 0.02), PCA dose (F1,9 = 26.0, p < 0.001) and a significant PCA dose × tranylcypromine dose interaction (F1,9 = 7.9, p = 0.02) (Fig. 4b). Tranylcypromine pretreated rats did not show different basal or saline-induced extracellular 5-HT levels as compared to saline pretreated controls (p = 0.106 and p = 0.1, respectively). PCA increased extracellular 5-HT levels in both saline and tranylcypromine pretreated groups (p = 0.03 and p = 0.006, respectively). However, PCA-induced 5-HT overflow was significantly higher in tranylcypromine pretreated rats than in saline pretreated controls (p = 0.02; Fig. 4b).
The time course of the effects of PCA on extracellular DA levels in the NAc shell is shown in Figure 4c. PCA-induced extracellular DA levels in the tranylcypromine pretreated group were not different when compared to saline pretreated group. Although there was a significant effect of PCA dose (F2,18 = 5.8, p = 0.01), there was no significant effect of tranylcypromine pretreatment dose (p > 0.1, Fig;4c and d).
DISCUSSION
The first finding of this study is that there is not a strict link between MAO inhibition and response for nicotine self-administration
Inhibition of MAO obtained with tranylcypromine, an irreversible and non-specific MAOI, depends rather on the amount of time since the last injection than on the number of injections. Indeed, MAO inhibitions reached 96%, 79.5% and 55.5%, respectively, 1, 20 and 44 hr following the last tranylcypromine injection (Villégier et al., 2007). In this case, the 96% and 79.5% MAO activity blockade were not shown to be significantly different but were associated with a 471% increase of nicotine self-administration or no self-administration, respectively. These results suggest that a correlation between MAO inhibition and increase in self-administration does not apply for the very low doses of nicotine.
The second finding of this study is that the reinforcing effect of nicotine depends more on the enhancement of serotonergic transmission, than on the level of MAO activity blockade
We have shown in the switch experiment, that, only in repeated tra20hr pretreated rats, tra1hr or PCA promoted self-administration on the test day. Then, we show that a combination of both repeated and acute effects of tranylcypromine seem required to support the reinforcing effects of nicotine and that both components involve 5-HT.
5-HT2A serotonergic transmission can facilitate nicotine self-administration
When the test day treatment was saline, for animals pretreated with tra20hr on the three first sessions, rats did not exhibit self-administration while MAO activity was blocked by 55.5%. However, when the test day treatment was tra1hr, they exhibited a +687% increase of nicotine self-administration, that was totally blocked by ketanserin and ritenserin, two 5-HT2 receptor antagonists, and MAO activity was blocked by 99%. Finally, when PCA, a serotonin releaser, was injected in place of tra1hr on test day, nicotine rewarding properties were increased to the same extend, while MAO activity blockade was not further decreased when compared to acute saline injected group (55.5 %). These results indicate that the 55.5% MAO activity blockade alone is not likely to support nicotine rewarding properties. However, the PCA-induced 5-HT releasing effect and the direct MA releasing effect of tranylcypromine are likely to underlie a consistent 5-HT release, and 5-HT2A transmission stimulation (Villégier et al., 2006, 2007) that appear to be the key mechanism to promote nicotine reinforcing property. It is worth noting that ketanserin and ritanserin present a very high affinity for 5HT2 receptor sites (5-HT2A/C and 5-HT2A/2B/2C, respectively) (Ruiu et al., 2000). Our data are consistent with previous work showing a significant reduction of nicotine self-administration following ketanserin (2 mg/kg) treatment (Levin et al., 2008), the role of 5-HT in the NAc to support tranylcypromine enhancement of nicotine-induced locomotor activity (Villégier et al., 2006) and more generally, MDMA-stimulant effects and morphine-rewarding properties (Baumann et al., 2008; Weitemier and Murphy, 2009).
Repeated tranylcypromine treatment is associated to serotonergic hyper-excitability
When pretreatment given on the three first sessions was saline, rats injected with tra1hr or PCA on test day, displayed a 99% and 0% MAO activity blockade, respectively, and did not exhibit self-administration. However, when pretreatment given on the first sessions was tra20hr, rats displayed a 99% and 55.5% MAO activity blockade, respectively, and did exhibit significant nicotine self-administration. These results indicate a permissive effect of repeated tra20hr pretreatment, for acute tra1hr and PCA injections to promote nicotine-reinforcing effects on test day. This effect may simply be due to the 55.5% MAO activity blockade. However, this explanation does not seem adequate as a 99% MAO activity blockade can be induced following an acute tra1hr treatment given on test day, but is not associated with behavioral response if rats were given saline pretreatment on the first sessions. According to the kinetic of the microdialysis curve, the immediate increase of extracellular 5-HT levels following PCA injection observed in tra20hr pretreated rats when compared to saline pretreated rats, is more likely to come from a higher 5-HT release than from an inhibition of 5-HT catabolism. For this reason, we believe that 3 days continuous MAO activity blockade or repeated MA releasing action, or the association of both might be at the origin of a sensitization process, resulting in hyper-excitability of serotonergic transmission.
This mechanism is in accordance with previous findings showing that PCA-induced neurotoxicity, an effect related to PCA ability to release monoamines (Brodkin et al., 1993), is potentiated by prior pretreatment by pargyline, a MAO inhibitor (Benmansour and Brunswick, 1994). Moreover, Lanteri et al. (2009) showed similar results and hypothesized that the enhanced 5-HT release at the synaptic junction lead to 5-HT1A autoreceptor internalization, a process at the origin of an increased serotonergic transmission.
Anatomy of 5-HT transmission involved in tranylcypromine enhancement of nicotine self-administration
The present study shows that an increased 5-HT transmission in the NAc and the subsequent activation of 5-HT2 receptors plays a permissive role in the expression of tranylcypromine and nicotine-induced reward.
Anatomically, 5-HT2A/2C receptors are important in controlling basal ganglia output (Nowak et al., 2006) and have been identified in several key areas, including the ventral tegmental area, NAc and prefrontal cortex in rats (Szucs et al., 2005). Auclair et al. showed that a bilateral injection of SR46349B, a specific 5-HT2A antagonist, into the ventral tegmental area hampered psychostimulant- and opiates-induced locomotor activity and DA release in the NAc, while bilateral injection into the medial prefrontal cortex was ineffective (Auclair et al., 2004a, b). If one assumes that the same circuitry mediates rewarding and stimulant processes, these data suggest that the 5-HT2A receptors implicated in nicotine self-administration may be preferentially located in the ventral tegmental area.
Here, we show an increased serotonergic transmission in the NAc, however, other teams have shown 5-HT release in the prefrontal cortex induced by systemic PCA (Sharp et al., 1986) and amplified 5-HT release in the prefrontal cortex following repeated treatments with addictive substances (Tassin et al., 2008).
A complex interaction between nicotine and serotonin tone
Nicotine is commonly considered to be a monoamine releaser (Summers and Giacobini, 1995; Summers et al., 1996) that increases serotonergic neuron firing (Li et al., 1998; Marubio et al., 1999; Olausson et al., 2001a, b, 2002). This increased release of 5-HT is, however, transient. Indeed, an immediate inhibitory retro-control blocking the firing of serotonergic raphe neurons through the stimulation of somato-dendritic 5-HT1A receptors has been described (Li et al., 1998; Mihailescu et al., 1998; Engberg et al., 2000; Balfour and Ridley, 2000; Ma et al., 2005). This may explain why injection of nicotine alone transiently decreases the extracellular 5-HT levels in the ventral striatum (Villégier et al., 2006).
The nicotine dose used here is very small and 10-fold less than that used in most studies (Shoaib et al., 1997; Guillem et al., 2005). Relative to cigarettes, it represents the nicotine concentration present in the body after one puff of inhalation of tobacco smoke (Benowitz and Jacob, 1990). We can hypothesize that at very low doses, nicotine’s effect on 5-HT release is very weak and almost entirely dependent on the effect of tranylcypromine and PCA to enhance 5-HT tone and compensate for the indirect inhibitory effect of nicotine on serotonergic cells.
Interestingly, higher doses of nicotine (10 μg/kg, i.v.) may release 5-HT to a sufficient threshold to induce reward as self-administration is induced 20 hrs following tranylcypromine, i.e. with no requirement of tranylcypromine monoamine releasing effect (Villégier et al., 2007). Although in other studies, animal self-administer nicotine in the absence of any pretreatment after several weeks of exposure (Guillem et al., 2005; Shoaib et al., 1997), the association with tranylcypromine pretreatment induces nicotine self-administration in a matter of days in the present study. This scenario is more likely to model tobacco’s effects in humans, as smoking experience of very light smokers is primarily associated with positive, i.e. acute pharmacological effects of nicotine, rather than negative (withdrawal avoidance, mood states,…) reinforcement (Coggins et al., 2009).
Reciprocally, the impact of serotonergic transmission on nicotine-induced reinforcing effects does not appear simple, as shown by our present finding of the ineffectiveness of high PCA doses (1.5 mg/kg) to enhance nicotine self-administration. Similarly, both 5-HT2A and/or 5-HT2C receptor agonists and antagonists can reduce discriminative and rewarding effects of nicotine (Grottick et al., 2001; Batman et al., 2005; Zaniewska et al., 2007). For these reasons Levin et al. (2008) proposed that an optimal serotonergic tone supports nicotine self-administration and that either increases or decreases from this tone reduces nicotine self-administration. While it remains unclear whether this optimal serotonergic tone is induced by tobacco, an increase of extracellular 5-HT levels probably results from the reduction of MAO activity measured in the brain of smokers. Serotonin release may also be induced by stress or conditional cues (Fulford and Marsden, 2007). Nicotine has been tested in several studies as an enhancer of responding for cues and reciprocally, cues are considered as consolidating responding for nicotine (Olausson et al., 2004a; b; Brunzell et al., 2006). This could explain how stress and cues associated with drug intake may lead to increased tobacco consumption in human and nicotine self-administration in rodent. However, this point will deserve further investigations as, in the present study, our concern was to assess the reinforcing properties of nicotine, and not its addictive properties. Thus, to avoid any associative process, we designed a protocol with no cue associated with responding for nicotine.
Conclusion
Our study shows that experimentally, hyper-excitability of serotonergic transmission is obtained following chronic MAO inhibition with tranylcypromine. This hyper-excitability, probably mediated through 5-HT2A transmission, may be a key factor responsible for the increased reinforcing effects of low doses of nicotine. Thus, serotonergic tone may be involved in individual vulnerability to tobacco dependence and may also explain the possible mechanism of action of interacting chemicals contained in tobacco smoke. Finally, according to our present findings, 5-HT transmission may be an interesting therapeutic target to improve long term abstinence in smokers.
Acknowledgments
This work was supported by a fellowship from PHS Grant DA19138 and DA21267. Experiments comply with the current laws of the country in which they were performed. All experimental procedures were performed in compliance with NIH Guide for Care and Use of Laboratory Animals (NIH No 85-23, rev. 1985) and approved by the UCI Institutional Animal Care and Use Committee.
References
- Auclair A, Blanc G, Glowinski J, Tassin JP. Role of serotonin 2A receptors in the D-amphetamine-induced release of dopamine: comparison with previous data on alpha1b-adrenergic receptors. J Neurochem. 2004a;91:318–26. doi: 10.1111/j.1471-4159.2004.02714.x. [DOI] [PubMed] [Google Scholar]
- Auclair A, Drouin C, Cotecchia S, Glowinski J, Tassin JP. 5-HT2A and alpha1b-adrenergic receptors entirely mediate dopamine release, locomotor response and behavioural sensitization to opiates and psychostimulants. Eur J Neurosci. 2004b;20:3073–84. doi: 10.1111/j.1460-9568.2004.03805.x. [DOI] [PubMed] [Google Scholar]
- Balfour DJ, Ridley DL. The effects of nicotine on neural pathways implicated in depression: a factor in nicotine addiction? Pharmacol Biochem Behav. 2000;66:79–85. doi: 10.1016/s0091-3057(00)00205-7. [DOI] [PubMed] [Google Scholar]
- Batman AM, Munzar P, Beardsley PM. Attenuation of nicotine’s discriminative stimulus effects in rats and its locomotor activity effects in mice by serotonergic 5-HT2A/2C receptor agonists. Psychopharmacology (Berl) 2005;179:393–401. doi: 10.1007/s00213-004-2035-z. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Rothman RB. Locomotor stimulation produced by 3,4-methylenedioxymethamphetamine (MDMA) is correlated with dialysate levels of serotonin and dopamine in rat brain. Pharmacol Biochem Behav. 2008;90:208–17. doi: 10.1016/j.pbb.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluzzi JD, Wang R, Leslie FM. Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology. 2005;30:705–12. doi: 10.1038/sj.npp.1300586. [DOI] [PubMed] [Google Scholar]
- Benmansour S, Brunswick DJ. The MAO-B inhibitor deprenyl, but not the MAO-A inhibitor clorgyline, potentiates the neurotoxicity of p-chloroamphetamine. Brain Res. 1994;650:305–12. doi: 10.1016/0006-8993(94)91796-5. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P., 3rd . III, Pharmacokinetics, metabolism, and pharmacodynamics of nicotine. In: Wonnacott RMS, Stolerman IP, editors. Nicotine Pharmacology: Molecular, Cellular, and Behavioral Aspects. Oxford University Press; Oxford: 1990. pp. 112–151. [Google Scholar]
- Brodkin J, Malyala A, Nash JF. Effect of acute monoamine depletion on 3,4-methylenedioxymethamphetamine-induced neurotoxicity. Pharmacol Biochem Behav. 1993;45:647–53. doi: 10.1016/0091-3057(93)90520-4. [DOI] [PubMed] [Google Scholar]
- Brunzell DH, Chang JR, Schneider B, Olausson P, Taylor JR, Picciotto MR. beta2-Subunit-containing nicotinic acetylcholine receptors are involved in nicotine-induced increases in conditioned reinforcement but not progressive ratio responding for food in C57BL/6 mice. Psychopharmacology (Berl) 2006;184:328–38. doi: 10.1007/s00213-005-0099-z. [DOI] [PubMed] [Google Scholar]
- Coggins CR, Murrelle EL, Carchman RA, Heidbrederc C. Light and intermittent cigarette smokers: a review (1989–2009) Psychopharmacology. 2009;207:343–363. doi: 10.1007/s00213-009-1675-4. [DOI] [PubMed] [Google Scholar]
- Engberg G, Erhardt S, Sharp T, Hajós M. Nicotine inhibits firing activity of dorsal raphe 5-HT neurones in vivo. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:41–5. doi: 10.1007/s002100000252. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Logan J, Wang GJ, Volkow ND. Monoamine oxidase and cigarette smoking. Neurotoxicology. 2003;24:75–82. doi: 10.1016/s0161-813x(02)00109-2. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Wang GJ, Pappas N, Logan J, MacGregor R, Alexoff D, Shea C, Schlyer D, Wolf AP, Warner D, Zezulkova I, Cilento R. Inhibition of monoamine oxidase B in the brains of smokers. Nature. 1996a;379:733–6. doi: 10.1038/379733a0. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Wang GJ, Pappas N, Logan J, Shea C, Alexoff D, MacGregor RR, Schlyer DJ, Zezulkova I, Wolf AP. Brain monoamine oxidase A inhibition in cigarette smokers. Proc Natl Acad Sci U S A. 1996b;93:14065–9. doi: 10.1073/pnas.93.24.14065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulford AJ, Marsden CA. An intact dopaminergic system is required for context conditioned release of 5-HT in the nucleus accumbens of postweaning isolation reared rats. Neuroscience. 2007;149:392–400. doi: 10.1016/j.neuroscience.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Grottick AJ, Corrigall WA, Higgins GA. Activation of 5-HT(2C) receptors reduces the locomotor and rewarding effects of nicotine. Psychopharmacology (Berl) 2001;157:292–8. doi: 10.1007/s002130100801. [DOI] [PubMed] [Google Scholar]
- Guillem K, Vouillac C, Azar MR, Parsons LH, Koob GF, Cador M, Stinus L. Monoamine oxidase inhibition dramatically increases the motivation to self-administer nicotine in rats. J Neurosci. 2005;25:8593–600. doi: 10.1523/JNEUROSCI.2139-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey DM, Yasar S, Heishman SJ, Panlilio LV, Henningfield JE, Goldberg SR. Nicotine serves as an effective reinforcer of intravenous drug-taking behavior in human cigarette smokers. Psychopharmacology (Berl) 2004;175:134–42. doi: 10.1007/s00213-004-1818-6. [DOI] [PubMed] [Google Scholar]
- Hauptmann N, Shih JC. 2-Naphthylamine, a compound found in cigarette smoke, decreases both monoamine oxidase A and B catalytic activity. Life Sci. 2001;68:1231–41. doi: 10.1016/s0024-3205(00)01022-5. [DOI] [PubMed] [Google Scholar]
- Lanteri C, Hernández Vallejo SJ, Salomon L, Doucet EL, Godeheu G, Torrens Y, Houades V, Tassin JP. Inhibition of monoamine oxidases desensitizes 5-HT1A autoreceptors and allows nicotine to induce a neurochemical and behavioral sensitization. J Neurosci. 2009;29:987–97. doi: 10.1523/JNEUROSCI.3315-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Slade S, Johnson M, Petro A, Horton K, Williams P, Rezvani AH, Rose JE. Ketanserin, a 5-HT2 receptor antagonist, decreases nicotine self-administration in rats. Eur J Pharmacol. 2008;600:93–97. doi: 10.1016/j.ejphar.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Rainnie DG, McCarley RW, Greene RW. Presynaptic nicotinic receptors facilitate monoaminergic transmission. J Neurosci. 1998;18:1904–12. doi: 10.1523/JNEUROSCI.18-05-01904.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Strecker RE, McKenna JT, Thakkar MM, McCarley RW, Tao R. Effects on serotonin of (-)nicotine and dimethylphenylpiperazinium in the dorsal raphe and nucleus accumbens of freely behaving rats. Neuroscience. 2005;135:949–58. doi: 10.1016/j.neuroscience.2005.06.074. [DOI] [PubMed] [Google Scholar]
- Manzardo AM, Stein L, Belluzzi JD. Rats prefer cocaine over nicotine in a two-lever self-administration choice test. Brain Res. 2002;924:10–9. doi: 10.1016/s0006-8993(01)03215-2. [DOI] [PubMed] [Google Scholar]
- Marubio LM, del Mar Arroyo-Jimenez M, Cordero-Erausquin M, Léna C, Le Novère N, de Kerchove d’Exaerde A, Huchet M, Damaj MI, Changeux JP. Reduced antinociception in mice lacking neuronal nicotinic receptor subunits. Nature. 1999;398:805–10. doi: 10.1038/19756. [DOI] [PubMed] [Google Scholar]
- Mihailescu S, Palomero-Rivero M, Meade-Huerta P, Maza-Flores A, Drucker-Colín R. Effects of nicotine and mecamylamine on rat dorsal raphe neurons. Eur J Pharmacol. 1998;360:31–6. doi: 10.1016/s0014-2999(98)00658-x. [DOI] [PubMed] [Google Scholar]
- Nowak P, Szczerbak G, Biedka I, Drosik M, Kostrzewa RM, Brus R. Effect of ketanserin and amphetamine on nigrostriatal neurotransmission and reactive oxygen species in Parkinsonian rats. In vivo microdialysis study. J Physiol Pharmacol. 2006;57:583–97. [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Taylor JR. Nicotine enhances responding with conditioned reinforcement. Psychopharmacology (Berl) 2004a;171:173–8. doi: 10.1007/s00213-003-1575-y. [DOI] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Taylor JR. Repeated nicotine exposure enhances responding with conditioned reinforcement. Psychopharmacology (Berl) 2004b;173:98–104. doi: 10.1007/s00213-003-1702-9. [DOI] [PubMed] [Google Scholar]
- Olausson P, Akesson P, Petersson A, Engel JA, Söderpalm B. Behavioral and neurochemical consequences of repeated nicotine treatment in the serotonin-depleted rat. Psychopharmacology (Berl) 2001a;155:348–61. doi: 10.1007/s002130100710. [DOI] [PubMed] [Google Scholar]
- Olausson P, Akesson P, Engel JA, Söderpalm B. Effects of 5-HT1A and 5-HT2 receptor agonists on the behavioral and neurochemical consequences of repeated nicotine treatment. Eur J Pharmacol. 2001b;420:45–54. doi: 10.1016/s0014-2999(01)00939-6. [DOI] [PubMed] [Google Scholar]
- Olausson P, Engel JA, Söderpalm B. Involvement of serotonin in nicotine dependence: processes relevant to positive and negative regulation of drug intake. Pharmacol Biochem Behav. 2002;71:757–71. doi: 10.1016/s0091-3057(01)00673-6. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. Reducing the Health Consequences of Smoking. Chapter 2. New York: Academic Report USGs; 1986. The rat brain in stereotaxic coordinates; pp. 79–92. 1989. [Google Scholar]
- Ruiu S, Marchese G, Saba PL, Gessa GL, Pani L. The 5-HT2 antagonist ritanserin blocks dopamine re-uptake in the rat frontal cortex. Molecular Psychiatry. 2000;5:673–677. doi: 10.1038/sj.mp.4000804. [DOI] [PubMed] [Google Scholar]
- Sharp T, Zetterstrom T, Christmanson L, Ungerstedt U. p-Chloroamphetamine releases both serotonin and dopamine into rat brain dialysates in vivo. Neurosci Lett. 1986;72:320–4. doi: 10.1016/0304-3940(86)90534-3. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Schindler CW, Goldberg SR. Nicotine self-administration in rats: strain and nicotine pre-exposure effects on acquisition. Psychopharmacology (Berl) 1997;129:35–43. doi: 10.1007/s002130050159. [DOI] [PubMed] [Google Scholar]
- Summers KL, Giacobini E. Effects of local and repeated systemic administration of ()nicotine on extracellular levels of acetylcholine, norepinephrine, dopamine, and serotonin in rat cortex. Neurochem Res. 1995;20:753–9. doi: 10.1007/BF01705545. [DOI] [PubMed] [Google Scholar]
- Summers KL, Lippiello P, Giacobini E. A microdialysis study of the effects of the nicotinic agonist RJR-2403 on cortical release of acetylcholine and biogenic amines. Neurochem Res. 1996;21:1181–6. doi: 10.1007/BF02532393. [DOI] [PubMed] [Google Scholar]
- Szucs RP, Frankel PS, McMahon LR, Cunningham KA. Relationship of cocaine-induced c-Fos expression to behaviors and the role of serotonin 5-HT2A receptors in cocaine-induced c-Fos expression. Behav Neurosci. 2005;119:1173–83. doi: 10.1037/0735-7044.119.5.1173. [DOI] [PubMed] [Google Scholar]
- Tassin JP. Uncoupling between noradrenergic and serotonergic neurons as a molecular basis of stable changes in behavior induced by repeated drugs of abuse. Biochem Pharmacol. 2008;75:85–97. doi: 10.1016/j.bcp.2007.06.038. [DOI] [PubMed] [Google Scholar]
- Villégier AS, Blanc G, Glowinski J, Tassin JP. Transient behavioral sensitization to nicotine becomes long-lasting with monoamine oxidases inhibitors. Pharmacol Biochem Behav. 2003;76(2):267–74. doi: 10.1016/s0091-3057(03)00223-5. [DOI] [PubMed] [Google Scholar]
- Villégier AS, Lotfipour S, McQuown SC, Belluzzi JD, Leslie FM. Tranylcypromine enhancement of nicotine self-administration. Neuropharmacology. 2007;52(6):1415–25. doi: 10.1016/j.neuropharm.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Villégier AS, Salomon L, Granon S, Changeux JP, Belluzzi JD, Leslie FM, Tassin JP. Monoamine Oxidase Inhibitors Allow Locomotor and Rewarding Responses to Nicotine. Neuropsychopharmacology. 2006;31(8):1704–13. doi: 10.1038/sj.npp.1300987. [DOI] [PubMed] [Google Scholar]
- Weitemier AZ, Murphy NP. Accumbal dopamine and serotonin activity throughout acquisition and expression of place conditioning correlative relationships with preference and aversion. Eur J Neurosci. 2009;29:1015–26. doi: 10.1111/j.1460-9568.2009.06652.x. [DOI] [PubMed] [Google Scholar]
- Zaniewska M, McCreary AC, Przegaliński E, Filip M. Effects of the serotonin 5-HT2A and 5-HT2C receptor ligands on the discriminative stimulus effects of nicotine in rats. Eur J Pharmacol. 2007;571:156–65. doi: 10.1016/j.ejphar.2007.05.067. [DOI] [PubMed] [Google Scholar]


