Abstract
Insulin-like growth factor II (IGF-II) plays a pivotal role in fetal and cancer development by signaling through the IGF-I and insulin receptors and activating the estrogen signaling cascade. We previously showed that precursor IGF-II (proIGF-II, the predominant form expressed in cancer) and not mature IGF-II (mIGF-II) blocks resveratrol (RSV) (a phytoalexin/anticancer agent)-induced cell death in MCF-7 cells. We hypothesize that proIGF-II regulates antiapoptotic proteins and/or the mitochondria to inhibit RSV actions and promote cell survival. This study examines the effect of mIGF-II and proIGF-II on survivin expression and mitochondrial polarization in response to RSV. RSV inhibits survivin expression and stimulates mitochondrial depolarization, caspase 7 activation and cell death. These effects were completely blocked by the addition of proIGF-II. RSV treatment had no effect on transfected MCF-7 cells constitutively expressing proIGF-II, while IGF-II siRNA transfection decreased survivin levels. Our results provide new insights for the potential use of proIGF-II as target for new anticancer therapies.
Keywords: Insulin-like growth factor II, survivin, resveratrol, MCF-7, breast cancer, mitochondria
Introduction
The insulin-like growth factor II (IGF-II) promotes proliferation, inhibits apoptosis and stimulates angiogenesis and transformation of breast cancer cells (Perdue et al. 1991; De León et al. 1992; Yang et al. 1996; Yee and Lee 2000). The IGF-II gene encodes a single transcript, which is translated into the precursor IGF-II (proIGF-II) resulting in a 156 amino acid protein, including an 89 amino acid E-domain. ProIGF-II is converted to mIGF-II intracellularly by sequential cleavage after amino acids 104 and 87. Differential glycosylation and cleavage within the E-domain can therefore result in multiple proIGF-II isoforms (Bell et al. 1984; Gowan et al. 1987; Daughaday and Trivedi 1992). ProIGF-II is expressed in tumors of different origin and it is more potent than mIGF-II in stimulating thymidine incorporation in NIH-3T3 cells (Yang et al. 1996). ProIGF-II has been shown to be more potent than mIGF-II in stimulating dermal fibroblasts derived from 12-week old fetuses, and in stimulating interleukin-3-dependent granulocyte-macrophage colony-forming cell production from peripheral blood cells (Gowan et al. 1987; Schwartz et al. 1990), although Perdue et al. (1991) attributed the acidic nature of proIGF-II (secondary to glycosylation) as a potential modulator of proIGF-II efficiency in coupling this mitogen binding affinity to the induction of autophosphorylation of the IGF-IR, and found that at saturating concentrations both IGF-II forms had similar potencies. Of note, Yang et al. (1996) showed that unglycosylated proIGF-II was 10 times more potent than the glycosylated proIGF-II or mIGF-II in inducing thymidine incorporation in IGF-IR-expressing NIH3T3 cells.
IGF-II mitogenic effects are mediated through the IGF-IR and IR-A, both members of the same tyrosine kinase receptor family (Bowers et al. 2000). Furthermore, the transcription of IGF-II is regulated by estrogen and IGF-II binding to the IGF-IR phosphorylates and activates the membrane estrogen receptor (ERα) (Yee and Lee 2000). Thus, IGF-II can stimulate estrogen regulated genes including itself by activating the ER in the cell membrane. Also, of particular interest in breast cancer is the regulation of IGF-II by prolactin, WT-1, Wnt, phosphatase and tensin homolog (PTEN), Integrins and p53 (Werneretal. 1993; Zhangetal. 1996; Goel et al. 2000; Kang-Park et al. 2003). Wild-type p53 inhibits IGF-II whereas mutated p53 stimulates IGF-II expression (Zhang et al. 1996; Lee et al. 2003). Similarly, wild-type p53, but not mutant p53, represses survivin expression at both the mRNA and protein levels (Mirza et al. 2000). The diverse regulation of IGF-II by all these factors shows that it plays a critical role in breast development, differentiation and homeostasis.
We recently demonstrated that resveratrol (RSV) regulates IGF-II gene expression in a dose-dependent manner in Michigan Cancer Foundation (MCF)-7 and T47D breast cancer cells. High RSV concentration (10−4 M) inhibited IGF-II gene expression, proIGF-II secretion and induced cell death (Vyas et al. 2005). RSV is a phytoestrogen that exhibits potent cancer chemopreventive activity. RSV exerts its inhibitory effect on cancer by regulating diverse cellular events associated with tumor initiation, promotion and progression (Bhat and Pezzuto 2002). At present, the precise mechanisms of RSV anti-tumorigenic or chemopreventive activities are not completely understood (Jang et al. 1997; Dong 2003). Since RSV regulates IGF-II and IGF-II inhibits apoptosis, we hypothesized that RSV anticancer effects are, in part, directly associated with its inhibitory effect on IGF-II. Furthermore, since we demonstrated that constitutively expressed IGF-II blocked cell death induced by RSV we also hypothesized that IGF-II must regulate pro-survival proteins and/or inhibit proapoptotic proteins.
Survivin is a member of the inhibitor of apoptosis (IAPs) family. Its overexpression and intracellular organelle localization is associated with inhibition of cell death initiated by both the extrinsic and intrinsic apoptotic pathways and regulation of mitosis. Preferential nuclear localization of survivin in tumors has been linked to a favorable prognosis in breast cancer (Kennedy et al. 2003). Survivin role during mitosis involves regulation of microtubule function and formation of a normal bipolar apparatus (Schwartz et al. 1993; Fortugno et al. 2002; Altieri 2004; Dohi et al. 2004). Survivin, like IGF-II, is strongly and diffusely expressed in embryonic and fetal organs and it is overexpressed in a variety of tumors including breast carcinomas. Thus, our study is aimed at determining the differential effect of mIGF-II and proIGF-II on survivin expression and regulation of subcellular localization, mitochondrial membrane depolarization and cleavage/activation of caspase 7 in RSV treated breast cancer cells.
Materials and methods
Cell culture
MCF-7 breast carcinoma cell line was obtained from the American Type Culture Collection. MCF-7 cells were maintained in a 5% CO2 incubator at 37°C, using Dulbecco’s modified Eagle’s medium (DMEM)/F12 media (Cellgro) supplemented with 10 ml of 5000 units penicillin/streptomycin (100 units/ml penicillin and 100 units/ml streptomycin sulfate, Cellgro), 4 mM L-glutamine (Cellgro), 3 μg/ml β-amphotericin, and 5% fetal bovine serum (Hyclone). Recombinant human precursor IGF-II (proIGF-II, aa 1-156, non-glycosylated) and recombinant mIGF-II were purchased from GroPep (Adelaide, Australia) and PeproTech (Rocky Hill, NJ, USA), respectively. RSV was purchased from Sigma Chemical Co. (St Louis, MO, USA) and dissolved in dimethyl sulfoxide (DMSO, Fischer Scientific, Pittsburgh, PA, USA). Cell lysates from RSV treated cells cell lysates (CL) were collected (6 and 9 h), centrifuged (800 rpm for 5 min) and kept frozen (−20°C) until assayed.
Transfection of MCF-7 cells with IGF-II sense cDNA
The human IGF-II (cDNA) was kindly provided by Dr Graeme I Bell (Howard Hughes Medical Institute, University of Chicago, Chicago, IL, USA). The sense precursor IGF-II was generated by PCR and cloned into the HindIII-Xba I site of the pRc/Cytomegalovirus vector (CMV) vector (Invitrogen, San Diego, CA, USA) as previously detailed (DeLeon et al. 1999).
Transfection of MCF-7 cells was carried out by the liposome method (DOTAP, Boehringer). G418 (Geneticin, 560 μg/ml) was used to maintain stable transfectants. Stable transfectants were cloned by limiting dilution in 96 well plates.
SiRNA transfection
MCF-7 cells were seeded at a density of 5 × 105 cells/well in six well plates. Five picomoles siRNA (Santa Cruz Biotechnology, CA, USA) were added to the cells followed by 24 h incubation at 37°C in a 5% CO2 incubator. Cells were treated with RSV (10−4) and IGF-II (mIGF-II, proIGF-II) for 9 h. Cell lysates were prepared using the complete Lysis-M kit (Roche Applied Science, Germany), according to manufacturer protocol and stored at −20°C until assayed.
Western blot analysis
Total protein (60 μg) of cell lysates or subcellular compartment fractions [membrane/organelle (F2) and nucleus (F3)] obtained by ProteoExtractR Subcellular Proteome Extraction Kit (Calbiochem) were collected after 6 and 9 h of RSV treatment and used to load polyacrylamide-SDS gradient gels (4–12%), transferred to a Polyvinyllidene Difluoride (PVDF) membrane (Invitrogen, Carlsbad, CA, USA) using a X-Cell SureLockR electrophoretic Transfer module (Invitrogen). Protein concentration was measured using the Coomassie Plus Protein Assay Reagent™ (Pierce Biotechnology, Rockford, IL, USA). PVDF membranes were blocked with 2% Bovine Serum Albumin (BSA) IgG free (Sigma Chemical Co.) in phosphate buffered saline (PBS)/0.05% Tween for 2 h. Membranes were then incubated with anti-IGF-II monoclonal antibody (Amano), anti-survivin monoclonal antibody (Santa Cruz Biotechnology), or anti-caspase 7 (BD Biosciences), and incubated at 4°C overnight. The blots were also probed with β-actin (whole cell lysates) or IGF-I antibodies (Sigma Chemical Co.) and used as a protein loading control (organelles and nuclear fractions). Specific markers were used to verify each cell fraction; nucleus (LEGDF, nuclear pore), organelles (VDAC, MnSOD, COX-2 and COX-4). To address loading we added human recombinant IGF-I peptide (which was added at equimolar concentration in F2, F3 cell fractions before aliquoting the samples), as loading control for F2 and F3. After 3 × 10 min washes in PBS/0.05% Tween, the corresponding biotinylated secondary antibodies (1:1000, Amersham, Arlington Heights, IL, USA) were added to the membranes (1 h at RT), followed by 3 × 10 min washes and incubation with HRP complexes (1:1000 Amersham, Arlington Heights). Protein visualization was achieved by using enhanced chemiluminescence (ECL) and autoradiography with Hyperfilm ECL film (Amersham, Arlington Heights). The signals on the x-ray films were quantified using ChemiImager™ 4000 (Alpha Innotech Corporation, San Leandro, CA, USA).
Real time PCR
One step SYBR real-time RT-Polymerase Chain Reaction (PCR) was performed to assess survivin expression in RSV (10−4) treated cells at 3 and 6 h, using the primers survivin-forward (TCA AGG ACC ACC GCA TCT CTA C-3′0 and survivin-reverse (TGA AGC AGA AGA AAC ACT GGG C-3′). PCR amplifications were performed using the iCycler (BIO-RAD). Reactions were performed in a mixture consisting of a 50 μl volume solution containing 1X SYBR Green supermix PCR buffer (BIO-RAD; 100 mM KCl, 6 mM MgCl2, 40 mM Tris–HCL, PH 8.4, .4 mM of each dNTP [dATP, dCTP, dGTP and dTTP], iTaq DNA polymerase 50 U/ml, SYBR Green I, 20 mM Fluorescein) 300 nM of each primer, 0.25 U/ml Multi-Scribe Reverse Transcriptase (Promega) and 0.4 U/ml Rnase Inhibitor (Promega). The RT-PCR protocol starts with 30 min at 42°C for the RT. Prior to the PCR step iTaq DNA polymerase activation at 95°C for 10 min was performed. Followed by 30 s denaturation at 95 °C, 15 s annealing at 57°C and 1.5 min elongation at 72°C for 40 cycles. Fluorescence was detected at the end of every 72°C extension phase. To exclude the contamination of unspecific PCR products such as primer dimers, melting curve analysis was applied to all final PCR products after the cycling protocol.
Mitochondrial membrane potential assessment using JC-1 dye
JC-1 was used for the investigation of mitochondrial membrane depolarization that occurs in the initial stages of apoptosis. JC-1 that fluoresces in the FL-1 is indicative of depolarized Δψ. The JC-1 assay measures the red/green fluorescence in a quantitative approach using the fluorescence-activated cell sorter (FACS). MCF-7 cells were cultured to an optimal density of 5 × 105 in 25T flasks and treated with RSV (10−4 M). Cells were detached and centrifuged at 800 g for 5 min at room temperature. Freshly prepared JC-1 working solution (5 μg/ml) was added to each pellet, followed by incubation 15 min at 37°C in a CO2 incubator. Cells were resuspended in 1 × Assay Buffer and analyzed immediately by flow cytometry using the CellQuest program (Becton Dickinson, CA, USA).
Caspase activation assay
MCF-7 cells (1 × 104 cells/well) were seeded in 96 well plates and incubated overnight at 37°C. The following day RSV (10−4 M) alone or in combination with IGF-II (mature or precursor forms, 100 ng/ml) was added to the cells for 6 or 9 h, followed by addition of 50 μl of caspase assay buffer (150 mM HEPES, 450 mM NaCl, 150 mM KCl, 30 mM MgCl2, 1.2 mM EGTA, 1.5% Nonidet P40, 0.3% CHAPS, 30% sucrose, 150 μM Asp-Glu-Val-Asp 7 Flamino-4-methyl coumarin (DEVD-AMC), 30 mM DTT, 3.0 mM PMSF). Caspase activity was measured by reading proteolytically released fluorochrome from the DEVD-AMC substrate using a plate reading fluorometer with an excitation at 360 nm and an emission at 460 nm.
Statistical analysis
★Values are expressed as the mean ± SEM of three or more replicate experiments. Statistical differences between mean values were determined by one-way ANOVA, SPSS 11.0 software (SPSS, Inc., Chicago, IL, USA). A level of p < 0.05 was considered significant.
Results
RSV and/or IGF-II effects on survivin protein levels
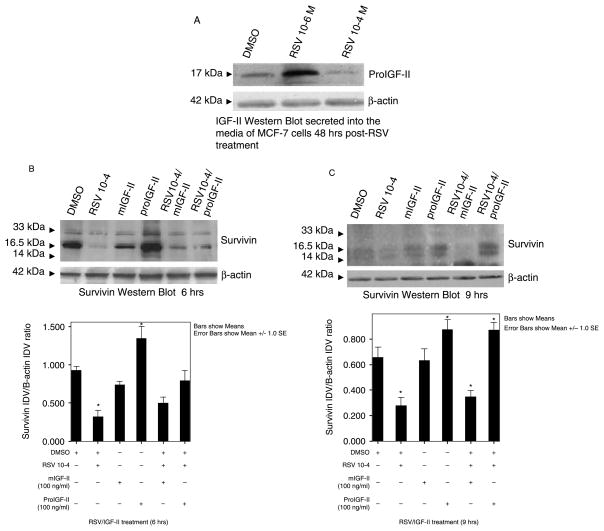
Our initial studies demonstrated that RSV has a biphasic effect on IGF-II regulation. Figure 1(A) shows an IGF-II Western blot, depicting a concentration—dependent regulation of IGF-II by RSV-treated cells. RSV 10−6 M stimulates proIGF-II while RSV 10−4 M inhibits proIGF-II levels in the media of MCF-7 cells. Figure 1 (panels (B),(C)) shows RSV (10−4 M)-induced inhibition of survivin protein levels [70 and 60% as compared to vehicle (DMSO) cells] 6 and 9 h post-treatment (16.5 kDa bands). Noteworthy, we found that treatment with proIGF-II alone (100 ng/ml) was associated with a significant and rapid increase (40%) in survivin protein levels when compared to vehicle and mIGF-II. Furthermore, addition of recombinant proIGF-II to RSV (10−4) treated cells blocked RSV-induced inhibition of survivin protein levels 6 and 9 h post-treatment. M-IGF-II partially blocked RSV-inhibitory effect 6 h post-treatment, but not at 9 h. Figure 1(B),(C)(lower panels) shows bar graphs of densitometric analysis of survivin densitometry units [integrated density units] normalized to β-actin densitometry units on three separate experiments. Please note that although only one Western blot is depicted as a representative experiment, the bar graphs represent three separate experiments done in triplicate (three Western blots per experiment).
Figure 1.
Western blot of IGF-II (A) and survivin (B),(C) from MCF-7 cells treated with RSV (10−4 M) and/or IGF-II (mature and precursor forms, 100 ng/ml). (A) Western blot of proIGF-II secretion at 48 h after RSV treatment. The 17 kDa band represents proIGF-II and is the only IGF-II form secreted by MCF-7 cells. Panels (B),(C) represent survivin Western blots (whole cell lysates) 6 and 9 h post-treatment. The 16.5 kDa band represents survivin monomer, while the 33 kDa bands represent survivin dimers. β-actin was used as loading control (42 kDa). Lower panels (B),(C) show a bar graph of survivin data normalized to β-actin and presented as the mean ± SE of three separate experiments. Asterisks indicate values significantly different from vehicle (DMSO-only) or RSV-treated groups (* p < 0.05; ** p < 0.05, respectively).
IGF-II siRNA effect on survivin protein expression
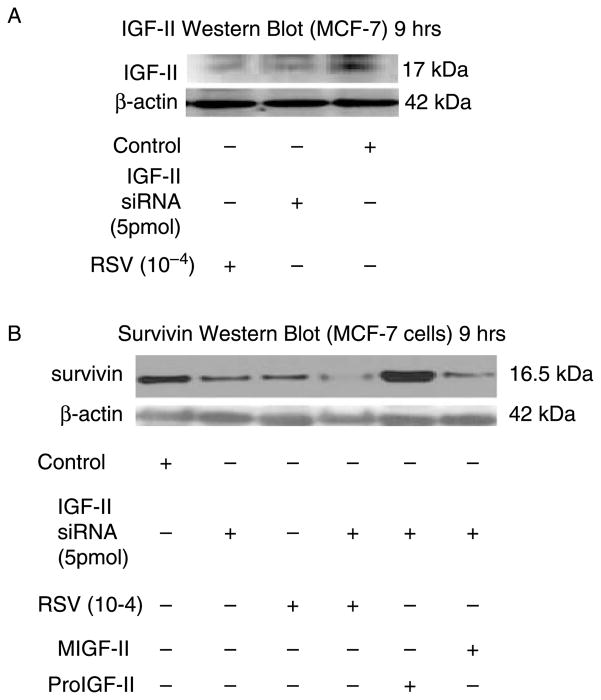
To confirm that RSV-inhibitory effect on survivin is mediated by proIGF-II, we transfected MCF-7 cells with IGF-II siRNA. A 70% decrease in IGF-II protein is seen in IGF-II siRNA transfected cells, while RSV (10−4) induced an 80% decrease in IGF-II levels (Figure 2(A)). Figure 2(B) shows survivin Western blot of IGF-II siRNA-transfected cells; a 60% decrease in survivin is seen in IGF-II siRNA-transfected and RSV (10−4) treated MCF-7 cells. Of note, addition of proIGF-II (but not mIGF-II) overcame the inhibitory effect induced by IGF-II siRNA treatment. Thus, proIGF-II enhances survivin in the presence of RSV because proIGF-II stimulates survivin mRNA and protein demonstrating that RSV inhibitory effect on survivin is due to proIGF-II inhibition.
Figure 2.
Western blot of IGF-II and survivin following IGF-II siRNA treatment in MCF-7 cells. (A) Western blot of proIGF-II secretion 9 h after RSV and IGF-II siRNA treatment. The 17 kDa band represents proIGF-II and is the only IGF-II form secreted by MCF-7 cells. (B) shows IGF-II siRNA and RSV effects on survivin protein levels, in the presence or absence of recombinant IGF-II (mature or precursor forms, 100 ng/ml).
Resveratrol effect on survivin protein levels in MCF-7 cells transfected with proIGF-II cDNA
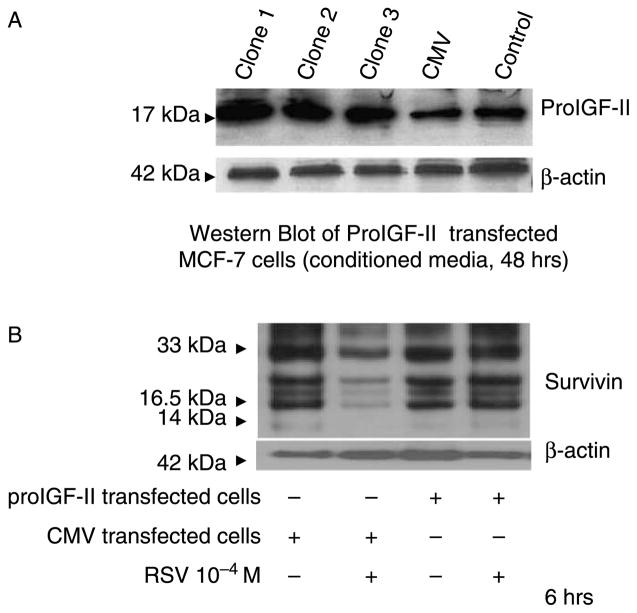
To further confirm the stimulatory effect of proIGF-II on survivin protein levels, we transfected MCF-7 cells with either an empty vector (CMV) or a vector containing proIGF-II cDNA. Figure 3(A) shows Western blot of proIGF-II secreted into the media of three different clones transfected with proIGF-II cDNA, 48 h post-treatment. As shown, a marked increase in proIGF-II (no mIGF-II was detected) secreted into the media of MCF-7 cells is observed, indicating that no post-translational cleavage of proIGF-II to mIGF-II occurs; Members of the proprotein convertase family mediate the processing of proIGF-II, and has found to be abnormal in many tumors types including breast cancer. Next, survivin protein levels were assessed by Western blot analysis 6 h after RSV (10−4) treatment. As seen on Figure 3(B), RSV treatment did not decrease survivin protein levels (16.5 kDa) in MCF-7 cells expressing proIGF-II. Noteworthy, proIGF-II transfected cells alone or treated with RSV (10−4) were associated with an increase of survivin dimers (33 kDa) 6 h post-treatment when compared to cells transfected with the vector alone, suggesting the possibility that proIGF-II is an important regulator of survivin dimerization.
Figure 3.
Western blot of IGF-II (A) and survivin (B),(D) from precursor IGF-II transfected MCF-7 cells. Panel (A) Western blot of proIGF-II secretion 48 h post-collection. Panel (B) shows a Western blot of survivin from CMV-only transfected (control cells) or proIGF-II transfected MCF-7 cells treated with RSV. Panels (C),(D) represents survivin Western blots of MCF-7 cells treated with RSV (10−4 M), MCF-7 cells transfected with an empty vector (CMV) were used as control group 6 and 9 h post-treatment. The 16.5 kDa band represents survivin monomer, while the 33 kDa bands represent survivin dimers. β-actin was used as loading control (42 kDa).
Resveratrol and/or IGF-II effects on survivin mRNA levels
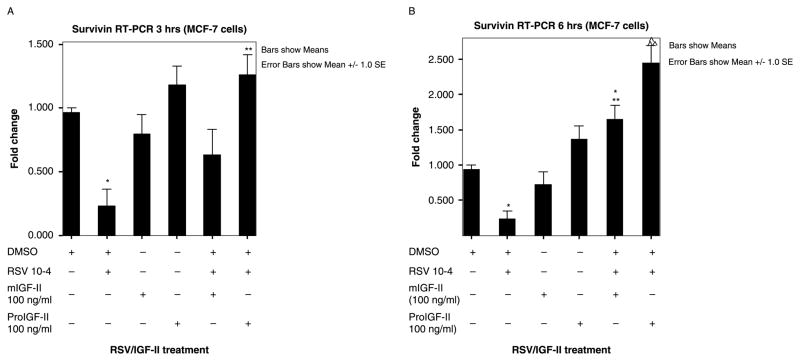
RSV (10−4) effect on survivin gene expression was assessed by real-time PCR. Figure 4(A),(B) shows bar graphs of fold change in survivin mRNA normalized to Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA on three separate experiments done in triplicate. Because survivin protein levels changed at 6 and 9 h, we reasoned that mRNA changes may occur earlier and chose to analyze mRNA levels at 3 and 6 h post-treatment. RSV-treated MCF-7 cells were also incubated in the presence of 100 ng/ml of either mIGF-II or proIGF-II. As seen on Figure 4 (panels (A),(B)) RSV (10−4) induced a significant decrease (3 and 2 folds, respectively) in survivin mRNA levels 3 and 6 h after treatment. Treatment of MCF-7 cells with mIGF-II alone kept survivin mRNA levels comparable to Vehicle-only (DMSO) group at all treatment times, while treatment with proIGF-II alone induced a 0.5 and 1 fold increase in survivin mRNA levels 3 and 6 h post-treatment when compared to DMSO-only group. Noteworthy, proIGF-II not only blocked RSV inhibition of survivin mRNA levels, but also induced 2 folds increase in survivin mRNA 3 and 6 h post-treatment when added to RSV-treated cells (with a corresponding increase in survivin protein levels); while mIGF-II blocked RSV-inhibition and increased survivin mRNA (1 fold) only 6 h post-treatment, interestingly, this effect did not translate into increased survivin protein levels. In summary, RSV-induced downregulation of IGF-II expression has an inhibitory effect on survivin mRNA and protein levels; exogenously added proIGF-II was more potent than mIGF-II in blocking RSV effect.
Figure 4.
RSV (10−4 M) and/or IGF-II (mature and precursor forms, 100 ng/ml) effect on survivin gene expression in MCF-7 cells and assessed by real-time PCR (RT-PCR). Panels (A),(B) represents survivin gene expression expressed as fold increase/decrease relative to corresponding control groups 3 and 6 h post-treatment. GAPDH was used as internal control. Asterisks and ^ indicate values significantly different from vehicle (DMSO-only) or RSV 10−4 groups (* p < 0.05 or ^ p < 0.01; ** p < 0.05, or ^^ p < 0.01, respectively).
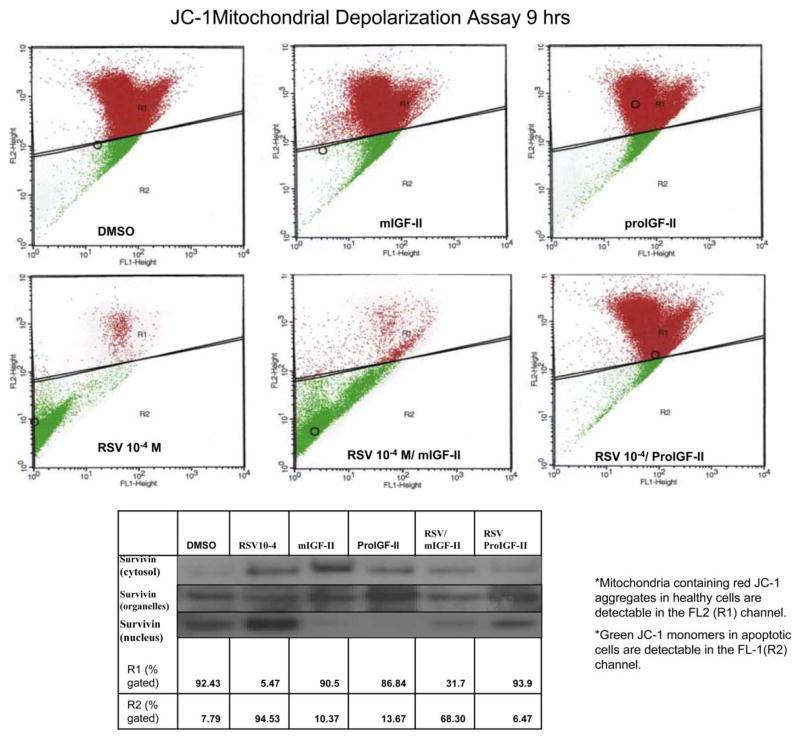
Effects of RSV and/or IGF-II on mitochondrial membrane polarization and survivin subcellular localization (organelles and nuclear fractions)
Mitochondrial membrane depolarization was assessed by using the JC-1 dye uptake by the mitochondria. As seen on Figure 5 RSV 10−4 induced a significant increase on mitochondrial membrane depolarization 9 h after treatment. Addition of mIGF-II or proIGF-II alone maintained mitochondrial membrane potential comparable to vehicle (DMSO) groups. Noteworthy, Addition of proIGF-II to RSV treated cells maintained mitochondrial Δψ similar to the vehicle (DMSO) group at all treatment times, while mIGF-II was associated with a decrease in mitochondrial Δψ when compared to vehicle or proIGF-II/RSV 10−4-treated cells. Figure 5 (lower panel), shows survivin Western blot from cytosol (F1), organelles (F2) and nucleus (F3) fractions, where in proIGF-II-alone treated cells, survivin localized predominantly in the cytosol and organelles fractions (F1 and F2), while in RSV-treated cells survivin is seen in the cytosol and nuclear fractions (F1 and F3); mIGF-II-alone treated cells show survivin predominantly in the cytosolic fraction (F1).
Figure 5.
RSV (10−4 M) and/or IGF-II (mature and precursor forms, 100 ng/ml) effect on MCF-7 mitochondrial membrane potential (Δψ) and Western blots of survivin subcellular localization (organelles and nuclear fractions). Mitochondrial membrane depolarization was used as an indicator of apoptotic cell death and assessed by potential-dependent lipophilic cation JC-1(5 μg/ml) at 6 and 9 h post-treatment. The JC-1 assay measured the red/green fluorescence in a quantitative approach using the FACS. The FL-1 channel associates with membrane depolarization and indicates apoptotic cell death.
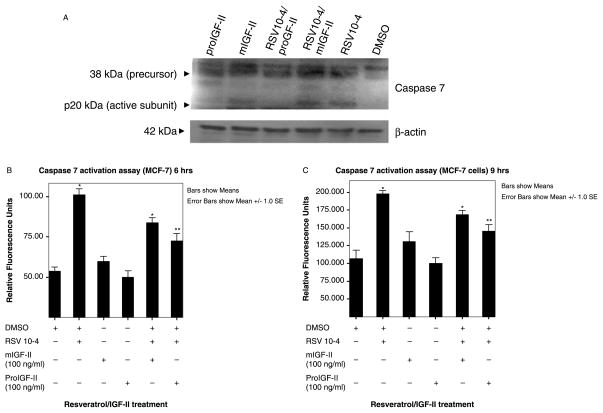
Effect of RSV and/or IGF-II on caspase-7 activation
To further confirm our results, we measured caspase 7 enzymatic activity (since MCF-7 are caspase-3 deficient cells) by a caspase activation assay and caspase 7 cleavage by Western blot analysis. Figure 6(A) shows a caspase 7 Western blot. RSV induced cleavage of caspase 7, an effect blocked by addition of proIGF-II, but not mIGF-II. Figure 6(B) shows relative fluorescence units (RFU) induced by proteolytically released fluorochrome from the DEVD-AMC substrate after RSV (10−4) treatment, in the presence or absence of IGF-II (mature and precursor forms). Of note, addition of proIGF-II to RSV-treated cells induced a statistically significant decrease (p < 0.01) in caspase 7 activity 6 and 9 h post-treatment, while mIGF-II did not (p > 0.05), when compared to RSV (10−4) treated groups.
Figure 6.
Western blot of caspase 7 cleavage and caspase 7 activation assay in MCF-7 cells treated with RSV (10−4 M) and/or IGF-II (mature and precursor forms, 100 ng/ml). Panel (A) shows Western blot of caspase 7 cleavage 6 h post-treatment with RSV 10−4 and/or IGF-II (mature or precursor forms, 100 ng/ml). Panels (B),(C) show caspase 7 activation assay data represented as RFU 6, and 9 h post-treatment. Asterisks indicate values significantly different from vehicle (DMSO-only) (*p < 0.05) or RSV-treated groups (**p < 0.05).
Discussion
Understanding the molecular mechanisms involved in breast cancer cell proliferation and progression to a hormone-independent state are critical for designing new potential targeted therapies. In this respect, we have previously shown that the IGF-II is regulated by the phytoestrogen RSV in a concentration-dependent manner in the ER (+) MCF-7 and T47D breast cancer cells (Vyas et al. 2005). Since no IGF-I mRNA (assessed by RT-PCR) or protein expression was observed, we concluded that IGF-II was a key target in the induced cell death observed with RSV treatment. In our present study, we provide evidence demonstrating that IGF-II blocked RSV inhibitory effects on breast cancer cells, in part, by regulating the IAPs family member survivin. Furthermore, we found that mature and precursor IGF-II forms induced different levels of survivin gene expression and differentially regulated survivin subcellular localization in MCF-7 breast cancer cells.
Our results showed that RSV induced a significant decrease in survivin mRNA and cytosolic protein levels, while addition of proIGF-II alone significantly increased survivin. proIGF-II (not mIGF-II) blocked RSV (10−4) inhibitory effect on survivin protein levels. Although mIGF-II was able to maintain survivin mRNA levels comparable to control in RSV-treated cells, this effect did not translate in an increase in survivin protein levels. To our knowledge, this constitutes the first evidence showing differential effects of IGF-II isoforms on regulation of specific cell survival associated target genes. An interesting possibility is that increased survivin protein is a result of increased survivin mRNA stability of more efficient translation in cells treated with proIGF-II. Recent studies using DU145 prostate cancer cells showed that IGF-I was able to modulate survivin levels by promoting stabilization and translation of a survivin mRNA pool through activation of the mTOR pathway (Vaira et al. 2007).
The difference between precursor and mIGF-II effects on survivin expression shown in the present study is of great significance, due to the fact that both, proIGF-II and mIGF-II bind the IGF-IR with the same affinity. Nevertheless, Yang et al. (1996) showed that proIGF-II was 10 times more potent than mIGF-II in stimulating thymidine incorporation into NIH-3T3 cells. Furthermore, Schwartz et al. (1993) showed that proIGF-II was more potent than mIGF-II in augmenting IL-3-induced expansion of GM-CFC in serum-deprived peripheral blood cells. Of note, mIGF-II binding to the IGF-IR (in contrast to proIGF-II) causes a significant downregulation of the IGF-IR in Caco-2 human colon cancer cells (Kim et al. 2005). Similarly, binding of mIGF-II to the IGF-IR may cause downregulation of this receptor in breast cancer cells resulting in less receptor activation and decreased stimulation, contributing to the significant enhanced effect of proIGF-II and causing the differential effects observed between the IGF-II isoforms. Another interesting possibility, presently under evaluation in our laboratory, focus on differential binding affinity and/or induction of phosphorylation between mIGF-II and proIGF-II binding to the IR-A. Thus, the differential effects of proIGF-II and mIGF-II may represent a combined effect of differential signaling through the IGF-IR and IR-A.
Survivin, like proIGF-II, is unique among other IAPs because it is selectively expressed during development and in most common human cancers but it is not expressed in normal adult tissues. Re-expression of these proteins in cancer plays a significant role in apoptosis inhibition and tumor progression (Tanaka et al. 2000).
An important aspect that should be taken into consideration is represented by different subcellular pools of survivin (Mahotka et al. 2002; Altieri 2004; Vischioni et al. 2004; Li et al. 2005; Sah et al. 2006). Although the biological basis between survivin localization and prognosis remains to be established, it is possible that survivin compartmentalization might reflect different functions of this antiapoptotic protein in relation to protection from apoptosis. Survivin post-translational modifications could differentially affect its subcellular localization; if nuclear survivin cannot associate with p34, an essential step in apoptosis inhibition, it may actually induce apoptosis, an observation in agreement with our study where RSV-treatment induced not only survivin down-regulation, but also nuclear localization. This may explain, in part, why different patterns of survivin localization are seen in different tumor types and may contribute to understanding the different prognostic implications of cytoplasmic and nuclear survivin. Increasing the cytoplasmic concentration of survivin by active nuclear export may promote survivin’s antiapoptotic function; thus, preferential nuclear localization of survivin in tumors is linked to a favorable prognosis in breast (Kennedy et al. 2003) and colon cancer (Knauer et al. 2007).
Of note, a recent study by Ceballos-Cancino et al. (2007) reported that survivin is a key regulator of Smac/DIABLO differential release from mitochondria during apoptosis induced by DNA damage. Increased survivin protein levels delayed Smac/DIABLO release by direct interaction, possibly by a size-exclusion mechanism. Thus, the differential effects of proIGF-II and mIGF-II on mitochondrial membrane polarization status and caspase activation might be linked to an increase in mitochondrial survivin pool, blocking Smac/DIABLO release in proIGF-II-treated cells. Furthermore, proIGF-II not only associated with survivin localization in the cytosol and organelles fractions, but also increased survivin dimerization. Although the mechanism of dimer formation is not well elucidated, crystallographic studies have demonstrated that survivin dimer formation bolsters protein-protein interactions (Verdecia et al. 2000). The Baculoviral Inhibitors of apoptosis Repeats (BIR) domains in the survivin dimers seem to play an important role in the recruitment of proteins to the microtubule organizing centers, to protect it from proteolysis.
In summary, we propose that proIGF-II exerts a coordinated pleiotropic effect to inhibit apoptosis that results in chemoresistance and cell survival. Thus, we conclude that proIGF-II effects in the mitochondria and its regulation of survivin expression and sub-cellular localization is a novel mechanism of cancer cell survival and chemoresistance.
Acknowledgments
This research was supported by 5P20 MD001632, and NIGMS 2R25GM060507-5.
References
- Altieri D. Molecular circuits of apoptosis regulation and cell division control: The survivin paradigm. J Cell Biochem. 2004;92:656–663. doi: 10.1002/jcb.20140. [DOI] [PubMed] [Google Scholar]
- Bell GI, Merryweather JP, Sanchez-Pescador R, Stempien MM, Priestley L, Scott J. Sequence of a cDNA clone encoding human preproinsulin growth factor II. Nature. 1984;310:775–777. doi: 10.1038/310775a0. [DOI] [PubMed] [Google Scholar]
- Bhat KP, Pezzuto JM. Cancer chemopreventive activity of resveratrol. Ann NY Acad Sci. 2002;957:210–229. doi: 10.1111/j.1749-6632.2002.tb02918.x. [DOI] [PubMed] [Google Scholar]
- Bowers JL, Tyulmenkov VV, Jernigan SC, Klinge CM. Resveratrol acts as a mixed agonist/antagonist for estrogen receptors α and β. Endocrinology. 2000;141(10):3657–3667. doi: 10.1210/endo.141.10.7721. [DOI] [PubMed] [Google Scholar]
- Ceballos-Cancino G, Espinosa M, Maldonado V, Melendez-Zajgla J. Regulation of mitochondrial Smac/DIABLO-selective release by survivin. Oncogene. 2007;26(54):7569–7575. doi: 10.1038/sj.onc.1210560. [DOI] [PubMed] [Google Scholar]
- Daughaday WH, Trivedi B. Heterogeneity of serum peptides with immunoreactivity detected by a radioimmunoassay for proinsulin-like growth factor II E-domain. J Clin Endocrinol Metab. 1992;75:641–645. doi: 10.1210/jcem.75.2.1379260. [DOI] [PubMed] [Google Scholar]
- De León DD, Wilson DM, Powers M, Rosenfeld RG. Effects of insulin-like growth factors and IGF receptor antibody on the proliferation of human breast cancer cells. Growth Factors. 1992;6(4):327–336. doi: 10.3109/08977199209021544. [DOI] [PubMed] [Google Scholar]
- DeLeon DD, Issa N, Nainani S, Asmerom Y. Reversal of cathepsin D routing modulation in MCF-7 breast cancer cells expressing antisesnse IGF-II. Horm Metab Res. 1999;31:142–147. doi: 10.1055/s-2007-978712. [DOI] [PubMed] [Google Scholar]
- Dohi T, Beltrami E, Wall NR, Plescia J, Altieri DC. Mitochondrial survivin inhibits apoptosis and promotes tumorogenesis. J Clin Invest. 2004;114(8):1117–1127. doi: 10.1172/JCI22222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z. Molecular mechanism of the chemopreventive effect of resveratrol. Mutat Res. 2003;523–524:145–150. doi: 10.1016/s0027-5107(02)00330-5. [DOI] [PubMed] [Google Scholar]
- Fortugno P, Wall NR, Giodini A, O’Connor DS, Plescia J, Padgett KM. Survivin exists in immunochemically distinct subcellular pools and is involved in spindle microtubule function. J Cell Sci. 2002;115:575–585. doi: 10.1242/jcs.115.3.575. [DOI] [PubMed] [Google Scholar]
- Goel HL, Moro L, King M, Teider N, Centrella M, McCarthy TL, Holgado-Madruga M, Wong AJ, Marra E, Languino LR. Beta 1 integrins modulate cell adhesion by regulating IGF-II levels in the microenvironment. Cancer Res. 2000;66(1):331–342. doi: 10.1158/0008-5472.CAN-05-2588. [DOI] [PubMed] [Google Scholar]
- Gowan LK, Hampton B, Hill DJ, Schlueter RJ, Perdue JF. Purification and characterization of a unique high molecular weight form of insulin-like growth factor II. Endocrinology. 1987;121:2050–2055. doi: 10.1210/endo-121-2-449. [DOI] [PubMed] [Google Scholar]
- Jang M, Cai L, Udeani GO, Showing KV, Thomas CF, Beecher CW, Fong HH. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275(5297):218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- Kang-Park S, Lee YI, Lee YI. PTEN modulates IGF-II mediated signaling; the protein phosphatase activity of PTEN downregulates IGF-II expression in hepatoma cells. FEBS Lett. 2003;545(2–3):203–208. doi: 10.1016/s0014-5793(03)00535-0. [DOI] [PubMed] [Google Scholar]
- Kennedy SM, O’Driscoll L, Purcell R, Fitz-Simons N, McDermott EW, Hill AD, O’Higgins NJ, Parkinson M, Linehan R, Clynes M. Prognostic importance of survivin in breast cancer. Br J Cancer. 2003;88:1077–1083. doi: 10.1038/sj.bjc.6600776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Holthuizen PE, Kim J, Park JH. Overexpression of mIGF-II leads to growth arrest in Caco-2 human colon cancer cells. Growth Horm IGF Res. 2005;15(1):64–71. doi: 10.1016/j.ghir.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Knauer SK, Kramer OH, Knosel T, Engels K, Rodel F, Kovacs AF, Dietmaier W, Klein-Hitpass L, Habtemichael N, Schweitzer A, Brieger J, Rodel C, Mann W, Petersen I, Heinzel T, Stauber RH. Nuclear export is essential for the tumor promoting activity of survivin. FASEB J. 2007;21:207–216. doi: 10.1096/fj.06-5741com. [DOI] [PubMed] [Google Scholar]
- Lee Y, Han YH, Lee SY, Lee YI, Park SK, Moon HB. Activation of IGF-II signaling by mutant type p53: Physiological implications for potentiation of IGF-II signaling by p53 mutant. Mol Cell Endocrinol. 2003;203:51–63. doi: 10.1016/s0303-7207(03)00117-5. [DOI] [PubMed] [Google Scholar]
- Li F, Yang J, Rammath N, Javle MM, Tan D. Nuclear or cytoplasmic expression of survivin: What is the significance? Int J Cancer. 2005;114:509–512. doi: 10.1002/ijc.20768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahotka C, Liebmann J, Wenzel M, Suschek CV, Schmitt M, Gabbert HE. Differential subcellular localization of functionally divergent survivin splice variants. Cell Death Differ. 2002;9:1334–1342. doi: 10.1038/sj.cdd.4401091. [DOI] [PubMed] [Google Scholar]
- Mirza A, McGuirk M, Hockenberry TN, Wu Q, Ashar H, Black S. Human survivin is negatively regulated by wild type p53 and participates in p53 dependent apoptotic pathway. Oncogene. 2000;21:2613–2622. doi: 10.1038/sj.onc.1205353. [DOI] [PubMed] [Google Scholar]
- Perdue JF, LeBon TR, Kato J, Hampton B, Fujita-Yamaguchi Y. Binding specificities and transducing function of the different molecular weight forms of IGF-II on IGF-IR. Endocrinology. 1991;129(6):3101–3108. doi: 10.1210/endo-129-6-3101. [DOI] [PubMed] [Google Scholar]
- Sah NK, Khan Z, Khan GJ, Bisen PS. Structural, functional and therapeutic biology of survivin. Cancer Lett. 2006;244(2):164–171. doi: 10.1016/j.canlet.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Schwartz GN, Szabo JM, Hudgins WR, Perdue JF. Two forms of insulin-like growth factor II enhanced granulopoiesis in serum-free liquid cultures of peripheral blood. Exp Hematol. 1990;18:610. [PubMed] [Google Scholar]
- Schwartz GN, Hudgins WR, Perdue JF. Glycosylated insulin-like growth factor II promoted expansion of granulocyte-macrophage colony-forming cells in serum-deprived liquid cultures of human peripheral blood cells. Exp Hematol. 1993;21(11):1447–1454. [PubMed] [Google Scholar]
- Tanaka K, Iwamoto S, Gon G, Nohara T, Iwamoto M, Tanigawa N. Expression of survivin and its relationship to loss of apoptosis in breast carcinomas. Clin Cancer Res. 2000;6:127–134. [PubMed] [Google Scholar]
- Vaira V, Lee CW, Goel HL, Bosari S, Languino LR, Altieri DC. Regulation of survivin expression by IGF-1/mTOR signaling. Oncogene. 2007;26:2678–2684. doi: 10.1038/sj.onc.1210094. [DOI] [PubMed] [Google Scholar]
- Verdecia MA, Huang H, Dutil E, Kaiser DA, Hunter T, Noel JP. Structure of human anti-apoptotic protein survivin reveals a dimeric arrangement. Nat Struct Biol. 2000;7(7):602–608. doi: 10.1038/76838. [DOI] [PubMed] [Google Scholar]
- Vischioni B, Van Der Valk P, Span SW, Kruyt FA, Rodriguez JA, Giaccone G. Nuclear localization of survivin is a positive prognostic factor for survival in advanced non-small cell luna cancer. Ann Oncol. 2004;15(11):1654–1660. doi: 10.1093/annonc/mdh436. [DOI] [PubMed] [Google Scholar]
- Vyas S, Asmerom Y, DeLeon DD. Resveratrol regulates IGF-II in breast cancer cells. Endocrinology. 2005;146(10):4224–4233. doi: 10.1210/en.2004-1344. [DOI] [PubMed] [Google Scholar]
- Werner H, Re GG, Drummond IA, Sukhatme VP, Rauscher FJ, Sens DA, Garvin AJ, LeRoith D, Roberts CT. Increased expression of IGF-IR gene in Wilms tumor is correlated with modulation of IGF-IR activity by the WT1 tumor gene product. Proc Natl Acad Sci USA. 1993;90(12):5828–5832. doi: 10.1073/pnas.90.12.5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CQ, Zhan X, Hu X, Kondepudi A, Perdue JF. The expression and characterization of human recombinant proIGF-II and a mutant that is defective in the O-glycosylation of its E-domain. Endocrinology. 1996;137(7):2766–2773. doi: 10.1210/endo.137.7.8770896. [DOI] [PubMed] [Google Scholar]
- Yee D, Lee AV. Crosstalk between the insulin-like growth factors and estrogens in breast cancer. J Mammary Gland Biol Neoplasia. 2000;5:107–115. doi: 10.1023/a:1009575518338. [DOI] [PubMed] [Google Scholar]
- Zhang L, Kashanchi F, Zhan Q, Brady JN, Fornace AJ, Seth P, Helman LJ. Regulation of IGF-II P3 promoter by p53: A potential mechanism for tumorigenesis. Cancer Res. 1996;56:1367–1373. [PubMed] [Google Scholar]