Abstract
Objective
To evaluate the pharmacokinetics and HIV viral load (VL) response following initiation during the third trimester of pregnancy of zidovudine (ZDV) plus standard dose lopinavir boosted with ritonavir (LPV/r), twice daily, until delivery for the prevention of mother-to-child transmission of HIV (PMTCT).
Design
Prospective study nested within a multicenter, three arm, randomised, phase III PMTCT trial in Thailand (PHPT-5, ClinicalTrials.gov Identifier: NCT00409591).
Methods
Women randomized to receive 300 mg ZDV and 400/100 mg LPV/r twice daily from 28 weeks’ gestation, or as soon as possible thereafter, until delivery had intensive steady-state 12-hour blood sampling performed. LPV/r pharmacokinetic parameters were calculated using non-compartmental analysis. Rules were defined a priori for a LPV/r dose escalation based on the proportion of women with an LPV AUC < 52 mcg.hr/mL (10th percentile for LPV AUC in non-pregnant adults). HIV-1 RNA response was assessed during the third trimester.
Results
Thirty-eight women were evaluable; at entry median (range) gestational age was 29 weeks (28–36), weight 59.5 kg (45.0–91.6), CD4 cell count 442 cells/mm3 (260–1327) and HIV-1 RNA viral load 7,818 copies/mL (<40–402,015). Geometric mean (90% confidence interval) LPV AUC, Cmax and Cmin were 64.6 mcg.hr/mL (59.7–69.8), 8.1 mcg/mL, (7.5–8.7) and 2.7 mcg/ml (2.4–3.0), respectively. 31 of 38 (81%) women had an LPV AUC above the AUC target. All women had a HIV-1 viral load less than 400 copies/mL at the time of delivery.
Conclusion
A short course of ZDV plus standard dose LPV/r initiated during the 3rd trimester of pregnancy achieved adequate LPV exposure and virologic response.
Keywords: lopinavir, pregnancy, pharmacokinetics, viral load, prevention of mother-to-child transmission of HIV
Introduction
The World Health Organization's (WHO) latest recommendations for treating HIV-infected pregnant women and preventing HIV transmission to infants promotes triple antiretroviral (ARV) treatment for all women who need therapy for their own health i.e. CD4 cell count < 350 cells/mm3 [1]. For women who are not eligible for therapy two options are proposed, zidovudine (ZDV) monotherapy from as early as 14 weeks gestation plus intrapartum single-dose nevirapine (SD-NVP), or maternal triple ARV prophylaxis. Due to its potency and safety profile lopinavir boosted with ritonavir (LPV/r) is one of the key antiretroviral components of these prophylactic strategies.
Studies in US pregnant women have shown a significant reduction in LPV plasma exposure with standard dosing (400/100 mg, twice daily) during the 3rd trimester [2] and to a lesser degree during the 2nd trimester [3]. This reduction in LPV exposure during pregnancy has been attributed to the physiological changes associated with pregnancy. Subsequently, higher LPV dosing during the 3rd trimester with 533/100 mg (4 soft-gel capsules) [3], or 600/100 mg (3 meltrex 200/50 mg tablets) [4], twice daily, have been found to achieve similar LPV exposure to that in non-pregnant adults. However, studies in European women using standard LPV/r dosing during the third trimester reported that the majority of women maintained efficacious plasma trough concentrations [5, 6]. To date, no data are available on LPV/r drug exposure during the third trimester of pregnancy in other populations.
Within the context of a phase III, randomized study in Thailand (PHPT-5 trial - ClinicalTrials.gov Identifier NCT00409591), a regimen of ZDV plus LPV/r initiated during the 3rd trimester is one of three different maternal and infant treatment strategies currently under investigation for the prevention of mother-to child transmission of HIV-1 in non immunocompromised women. The choice of the ZDV plus LPV/r combination, without lamivudine (3TC), a drug active against both HIV and the hepatitis B virus (HBV), was made to avoid the risk of hepatic toxicities after drug discontinuation in a population with a high HBV carriage rate (about 8% of pregnant women are Hepatitis BsAg carriers in Thailand). Moreover, the results of the MONARK study have shown the equivalence of LPV/r monotherapy with LPV/r in combination with ZDV and 3TC in suppressing viral replication within the first months of therapy among antiretroviral naïve patients [7].
Due to the risk of lower lopinavir exposure with standard dosing during the 3rd trimester of pregnancy, we investigated the pharmacokinetics and virologic response in the women randomized to receive ZDV plus standard dose LPV/r at the start of the PHPT-5 trial.
Materials and Methods
Study Population
The study reported herein was performed at the beginning of the ongoing PHPT-5 clinical trial, a multicenter, phase III, three arm, randomized, study investigating the efficacy of three different maternal and infant treatment strategies for the prevention of mother-to child transmission of HIV-1 in non immunocompromised women in Thailand. In one of the treatment arms, women are randomized to receive 300 mg zidovudine (ZDV), twice daily and 400/100 mg LPV/r (Aluvia® 100/25 mg tablets were used as the adult formulation is not registered in Thailand) twice daily from 28 weeks’ gestation until delivery. All HIV-infected pregnant women provided written informed consent and were enrolled if they were over 18 years of age, antiretroviral treatment naïve based on the women's clinical history except prior exposure to ZDV or SD-NVP prophylaxis for PMTCT, between 28 and 36 weeks gestational age, CD4 cell count ≥250 cells/mm3 and had the following laboratory values within 14 days of enrollment: hemoglobin > 8.5 g/dL; absolute neutrophil count >750 cells/mm3; platelets >50,000 cells/mm3; SGPT ≤5 times upper limit of normal; and serum creatinine ≤1.5 times upper limit of normal. Exclusion criteria included patients who meet the criteria of Classes III/IV of the WHO classification of HIV-associated clinical disease, evidence of pre-existing fetal anomalies incompatible with life, active tuberculosis, and contra-indication to the use of LPV/r during pregnancy or at delivery. Among the women enrolled, concomitant drugs included only: folic acid, ferrous sulphate, multivitamins, paracetamol, ranitidine and amoxicillin, none of which have reported significant drug interactions with lopinavir/ritonavir.
This study was approved by the Ethics Committees at the Harvard School of Public Health, USA, Ministry of Public Health, Thailand, Faculty of Associated Medical Sciences, Chiang Mai University, Thailand, and the local hospital ethics committees.
Pharmacokinetic Design and Stopping Rules
Women randomized to receive ZDV plus LPV/r were scheduled to have intensive 12 hour blood sampling after at least 2 weeks of treatment. Drug level analysis was performed 'real-time' and women were continually scheduled for LPV/r pharmacokinetic assessment until 25 women had evaluable PK results available. At the PK visit, a pre-dose blood sample was drawn prior to the scheduled LPV/r dose, after which LPV/r was administered with a standard low fat meal (rice soup with pork), and blood samples were collected at 1, 2, 4, 6, 8, and 12 hours after dosing. Antiretroviral drug adherence was assessed by pill counts at each study visit.
Assessment of LPV/r pharmacokinetic data was scheduled a priori to occur after the first 6, 12 and 25 women had evaluable PK data available. The target LPV area under the concentration-time curve (AUC) was ≥52 mcg.hr/mL, the 10th percentile for LPV AUC in non-pregnant adults [8, 9]. If 3 or more of the first 6 women, or 4 or more of the first 12 women, or 6 or more of the first 25 women, failed to meet the AUC target, one would have 90% confidence that the true rate of pharmacokinetic failure exceeds 10% (i.e., the lower limit of the 90% confidence interval will exceed 10%). If any of the stopping criteria were met, the LPV/r dose would be increased to 600/100 mg twice daily in all women enrolled and pharmacokinetic sampling would be repeated on an additional 25 women at that dose. The analyses were presented to the PHPT-5 Data and Safety Monitoring Board (DSBM) assisted by a panel of independent pharmacokinetic specialists.
All adverse events were graded using the Division of AIDS Table for Grading the Severity of Adult and Paediatric Adverse Events (Version 1.0, dated December 2004).
Antiretroviral Drug Level Measurement and Analysis
All blood samples were centrifuged and the plasma frozen at −20°C until analysis. Lopinavir and ritonavir plasma drug concentrations were measured using a validated High Performance Liquid Chromatography (HPLC) assay at the Faculty of Associated Medical Sciences, Chiang Mai University. The average accuracy was 103–112% and precision (inter- and intra-assay) was <4% of the coefficient of variation (CV). The lower limit of assay quantification (LLOQ) was 0.078 mcg/mL for lopinavir and 0.048 mcg/mL for ritonavir [10]. This laboratory participates in the AIDS Clinical Trial Group (ACTG), USA, Pharmacology Quality Control (Precision Testing) program [11]. Data were analyzed with WinNonLin (Version 5.2, Pharsight, USA) using non-compartment methods.
Measurement of plasma HIV-1 RNA viral load
Plasma HIV-1 RNA levels were assessed using the Abbott m2000 RealTime™ HIV-1 assay (Abbott Laboratories. Abbott Park, Illinois, U.S.A) according to the manufacturer instructions (lower limit of detection, 40 copies per millilitre).
Results
Patient Characteristics
The first 27 women initiating ZDV plus LPV/r in the PHPT-5 trial had intensive blood sampling during the third trimester of pregnancy to assess LPV/r pharmacokinetics. At study entry, median (range) age was 28 years (19–43), gestational age 30 (28–36) weeks, weight 59.9 (45.0–91.6) kg, body mass index (BMI) 26 kg/m2 (20–35), CD4 cell count 431 cells/mm3 (250–875) and HIV-1 RNA viral load 9,312 copies/mL (<40 to 402,015).
Lopinavir and ritonavir pharmacokinetics
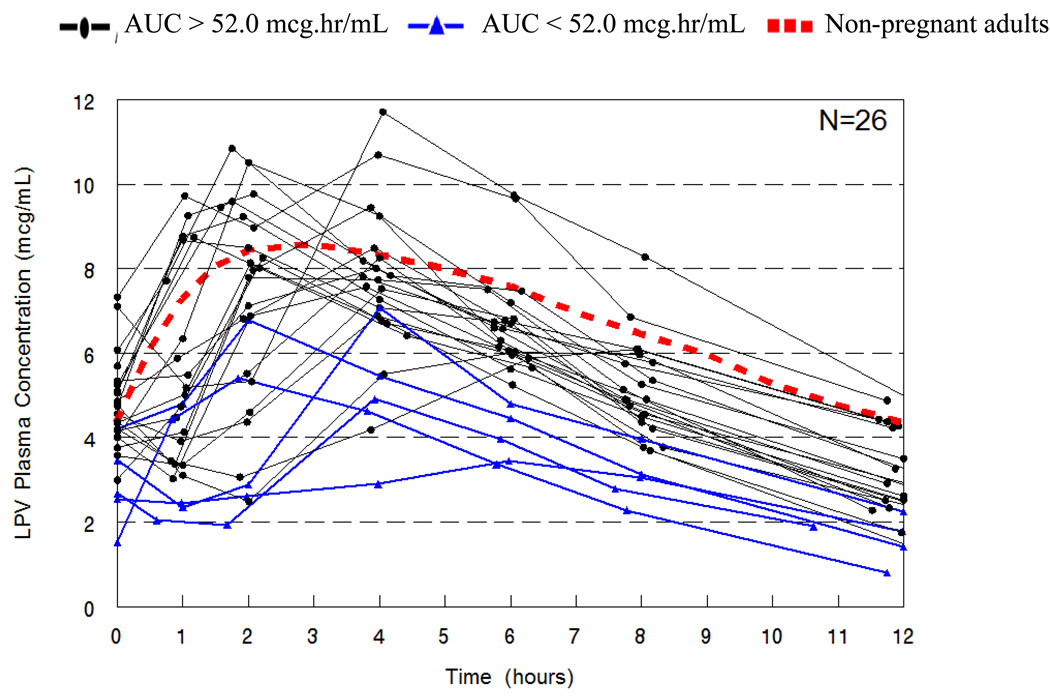
One woman was excluded from the PK analysis due to poor drug adherence. At the time of PK blood sampling, the median gestational age was 33 weeks (30–38) and the duration of LPV/r treatment was 2 weeks (2–4). Individual lopinavir concentration time curves are shown in Figure 1. At the planned interim analyses, 2 of the first 6 women and 3 of the first 12 women had an AUC below the target value of 52 mcg.hr/mL, neither reaching the pre-defined stopping criteria and enrolment was continued. LPV/r pharmacokinetic parameters are presented in Table 1. Overall, 5 of 26 (19%; 90% confidence interval (CI), 8%–36%) women had an LPV AUC below the target during the third trimester of pregnancy. The weight and BMI of women with a LPV AUC exposure above or below 52 mcg.hr/mL were not significantly different. One woman (4%) had a LPV Cmin below 1.0 mcg/mL [0.81 mcg/mL], the recommended efficacy threshold for protease inhibitor (PI) naïve patients.
Figure 1.
Individual Lopinavir Concentration vs. Time curves for HIV-infected Thai pregnant women using 400/100 mg, twice daily during the third trimester. The dashed line represents the typical 50th percentile concentrations in non-pregnant historical patients.
Table 1.
Patient characteristics at the time of PK sampling and lopinavir and ritonavir pharmacokinetic parameters during the third trimester of pregnancy in Thai women.
| Characteristics at PK Visit# | Original Analysis (n=26) | Final Analysis (n=38) |
|---|---|---|
| Weight (kg) | 61 (45–88) | 61 (45–88) |
| BMI (kg/m2) | 26 (21–36) | 25 (21–36) |
| Gestational age (Weeks) | 33 (30–38) | 33 (30–38) |
| Duration of LPV/r use (Weeks) | 2 (2–4) | 2 (2–7) |
| Lopinavir (GM 90% CI)* | ||
| AUC0–12 (µg.hr/mL) | 61.7 (56.2–67.7) | 64.6 (59.7–69.8) |
| Cmax (mcg/mL) | 7.8 (7.1–8.6) | 8.1 (7.5–8.7) |
| Tmax (median, hours) | 3.8 (1.0–8.0) | 2.2 (1.0–8.0) |
| Cpredose (mcg/mL) | 4.2 (3.8–4.7) | 4.4 (4.0–4.8) |
| C12hour (mcg/mL) | 2.6 (2.3–3.0) | 2.7 (2.4–3.1) |
| T1/2(median, hours) | 5.4 (2.9–9.6) | 5.5 (2.9–9.6) |
| CL/F (L/hr) | 6.4 (5.9–7.1) | 6.2 (5.7–6.7) |
| LPV AUC < 52.0 mcg.hr/mL | 5/26 (19%, 90% CI: 8%, 36%) |
7/38 (18%, 90% CI: 9%, 32%) |
| LPV AUC > 83.0 mcg.hr/mL | 2/26 (8%, 90% CI: 1%, 22%) |
6/38 (16%, 90% CI: 7%, 29%) |
| Ritonavir | ||
| AUC0–12 (mcg.hr/mL) | 3.1 (2.7–3.5) | 3.2 (2.8–3.5) |
| Cmax (mcg/mL) | 0.53 (0.46–0.62) | 0.54 (0.48–0.61) |
| Tmax (median, hours) | 3.9 (1.0–6.3) | 3.9 (1.0–6.3) |
| Cpredose (mcg/mL) | 0.17 (0.14–0.20) | 0.17 (0.15–0.20) |
| C12hour (mcg/mL) | 0.07 (0.06–0.09) | 0.08 (0.07–0.09) |
| T1/2(median, hours) | 3.0 (1.9–6.4) | 3.1 (1.9–6.4) |
| CL/F (L/hr) | 32. 3 (28.4–36.9) | 31.5 (28.3–35.0) |
Values: Median (range) unless otherwise stated.
Geometric Mean, 90% Confidence Interval.
AUC: Area under the curve. CL/F: apparent oral clearance
Amendment to the study: additional enrolment
The LPV/r PK results were reviewed by the members of the PHPT-5 Data and Safety Monitoring Board (DSMB) and pharmacokinetics specialists from the United States and Europe. They concluded at this time that the LPV/r dose should not be modified in the parent study but, because the criteria for increasing the dosing was close to being met, they recommended enrolling 12 additional women to decrease the possible effect of sampling fluctuations. The new stopping criterion calculated to trigger a LPV/r dose escalation to 600/100 mg twice daily was 10 or more of 38 women having an AUC <52 mcg.hr/mL. Twelve additional women randomized to receive ZDV plus LPV/r had PK blood sampling performed during the third trimester. The final baseline characteristics at study entry were: age 27 years (19–43), gestational age 29 (28–36) weeks, weight 59.6 (45.0–91.6) kg, body mass index 25 kg/m2 (20–35), CD4 cell count 441 cells/mm3 (250–1327) and HIV-1 RNA viral load 8,326 copies/mL (<40 to 402,015). Thirty-eight women were evaluable and their lopinavir and ritonavir pharmacokinetic parameters are presented in Table 1. In the final analysis, 7 of 38 (18%; 90% CI 9%, 32%) women had an LPV AUC below the AUC target and 37 of 38 (97%) women had a LPV Cmin above 1.0 mcg/mL. The weight and BMI of women with a LPV AUC exposure above or below 52 mcg.hr/mL were not significantly different.
Virologic response to ZDV plus LPV/r
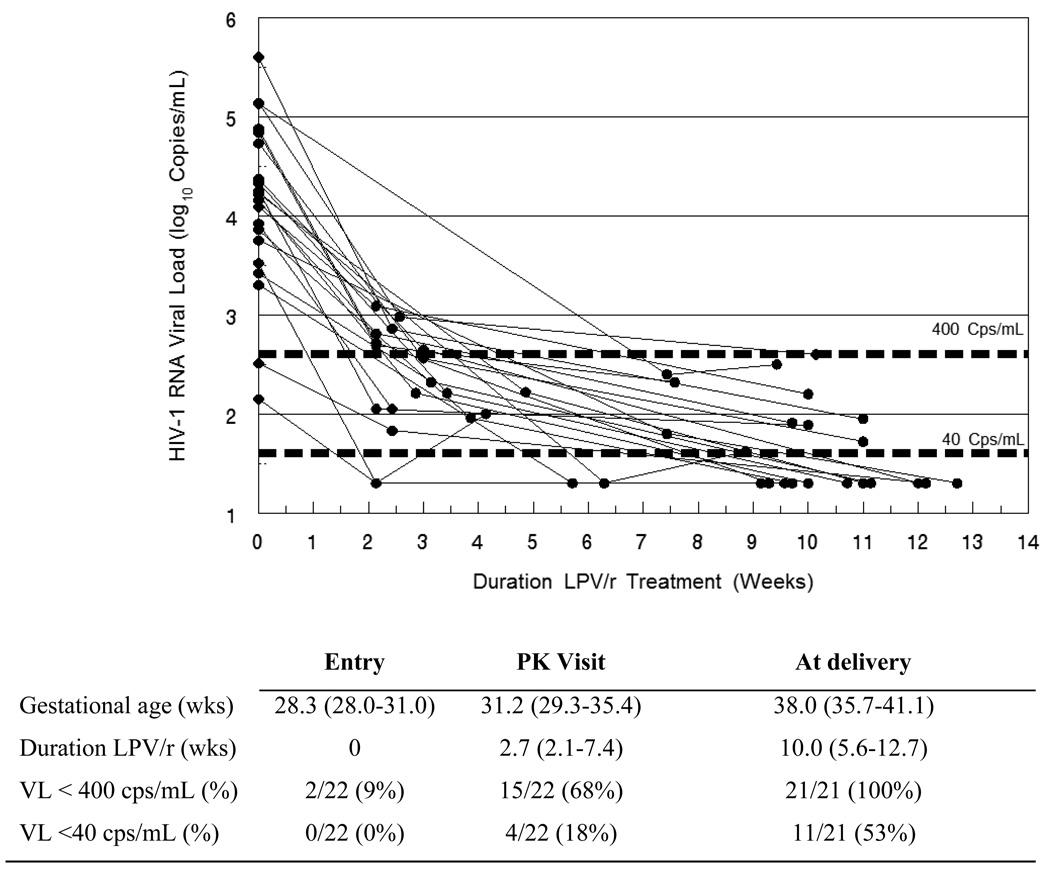
HIV-1 RNA viral loads were measured at study entry, at the PK sampling visit and at delivery. At study entry, 22 of 38 women were ARV naïve when initiating ZDV/LPV/r, while 16 women had initiated zidovudine prophylaxis before enrolling into PHPT-5. For these ARV naïve women, the median (range) duration of ZDV/LPV/r treatment at the PK sampling visit and delivery were 2.7 (2.1–7.4) and 10.0 (5.6–12.7) weeks, respectively. The dynamics of the HIV-1 RNA viral load following initiation of ZDV/LPV/r in ARV naïve women during the third trimester until delivery are shown in Figure 2. One woman stopped LPV/r between the PK visit and delivery [refer to Safety section]. All 21 ARV naïve women had a viral load <400 copies/mL at delivery, and 11/21 (53%) achieved <40 copies/mL. Among the 16 women who initiated ZDV prophylaxis prior to enrolment in PHPT-5, the median duration was 10 days (2–43) before LPV/r was added; all had a viral load <400 copies/mL and 12 (75%) had a viral load <40 copies/mL.
Figure 2.
Individual HIV-1 RNA viral load responses for 22 HIV-infected antiretroviral naïve Thai women after initiating a short course of zidovudine plus standard dose lopinavir/ritonavir during the third trimester of pregnancy until delivery.
Safety
Study medications were well tolerated. One grade 4 event was deemed probably treatment related: a woman self-reported convulsions at 30 weeks gestational age with grade 3 hyperbilirubinemia and grade 2 SGPT (ALT) elevations. LPV/r was discontinued and the woman’s hyperbilirubinemia decreased to grade 1 after 2 months. She delivered uneventfully a live baby. Two stillbirths occurred, one unexplained and one involving an abortion after physical injury, both considered unrelated to antiretroviral treatment. One women developed a grade 3 and another a grade 2 diarrhoea; both resolved within a couple of days. Five women experienced grade 3 hypercholesterolemia and/or hypertriglyceridemia which resolved after delivery in all but one case. Grade 1 events reported included nausea, vomiting, hyperlipidemia, headaches, and anaemia.
Discussion
Standard LPV/r dosing in Thai women during the third trimester provided a LPV exposure similar to that in non-pregnant adults, suggesting that a dose increase is not necessary in this population. Ritonavir pharmacokinetic parameters were similar to those in HIV-infected US pregnant women. All women administered ZDV plus LPV/r during the third trimester had a viral load below 400 copies/mL and 62% below 40 copies/mL at delivery.
Other studies have also suggested that standard LPV dosing during pregnancy was sufficient for antiretroviral treatment naïve patients. In response to the data reported in US women, several studies assessed LPV trough levels following standard 400/100 mg (3 soft gel capsules, twice daily) dosing during the third trimester. Lyons et al, reported that at median gestational age of 33 weeks (range 25–37), 1 of 21 women (5%) had an inadequate LPV trough level; 3 of 26 (14%) women had a plasma HIV RNA viral load above 50 copies/mL after a median of 10 weeks on therapy [5]. In a similarly designed study, 4 of 26 (15%) women had a subtherapeutic LPV trough level at a median gestational age of 32 weeks. However, inadequate LPV levels were not always associated with a HIV-1 RNA >50 copies/mL at the time of drug measurement; and vice versa [6]. Other studies reported similar proportions of women with inadequate LPV trough levels during the third trimester with standard LPV/r dosing, with the majority also achieving viral suppression before delivery [12, 13].
The comparison of the various published studies is made difficult by the fact that different criteria have been used to assess the adequacy of the LPV/r dosing in pregnant women, i.e. achieving drug exposures (AUCs) equivalent to those in non-pregnant adults, or maintaining trough concentrations above those reported to be associated with virological suppression. The results of the original LPV/r dose escalation study, which identified the 400/100 mg dose for further development, were used to define the AUC target for pregnant women [8, 9]. It should be noted that, because of the inherent small sample size of such studies, it is not possible to accurately study the relationship between AUC and virological efficacy, thus the adequacy of dosing after the metabolic modifications during pregnancy had to be thought in terms of the ability to reproduce a distribution of AUCs not dissimilar from that observed in non pregnant adults. The 10th percentile of the distribution of LPV AUCs observed in this adult population (52 mcg.hr/mL) was selected as the value below which LPV exposure would be considered low; and the decision rule for dose increase was designed to ensure 90% confidence that the true percentage of pregnant women with LPV exposures below this target would not exceed 10%. The alternative approach is using plasma LPV trough levels to guide virologic efficacy. A LPV trough concentration above 1.0 mcg/mL, approximately 15 times the IC50, has been reported to correlate with a HIV RNA viral load <400 copies/mL [14]; however, others have concluded that LPV trough levels do not predict virologic response in naïve patients [15]. Clearly, rationale for adopting either target exists. It is reassuring in the presented study that both the LPV exposures and trough concentrations results support the use of standard dosing in this population. More specifically, 31 of 38 (81%) Thai women had an LPV exposure above the AUC target, compared to 3 of 17 (18%) US women [2], and 97% had an LPV trough concentration above 1.0 mcg/mL. Also, all 7 women who had an LPV AUC below the 10th percentile for non-pregnant adults achieved a satisfactory HIV-RNA viral suppression at the time of delivery. Thus, for antiretroviral naïve women with no contraindicated concomitant drugs, there seems to be no indication that therapeutic drug monitoring would be necessary in this setting.
Our results confirm that although lopinavir drug exposure is reduced during the third trimester of pregnancy, by 22% in Thai women, this reduction is approximately half that observed in American women. The impact of pregnancy on lopinavir exposure may depend on the characteristics of the population. For example, the median body weight during the third trimester was considerably higher in US women than in Thai women, 90 kg versus 61 kg. Indeed, an inverse relationship between body weight and lopinavir exposure has been reported in several studies [16–18]. Lopinavir is ≥98% bound to plasma proteins, alpha-1-acid glycoprotein (AAG) and albumin, and during the third trimester decreased protein binding increases the LPV free fraction although this does not compensate for the total reduction in LPV exposure in US women [19]. Plasma proteins concentrations were not determined in the presented study but it is possible that differences in plasma proteins binding between populations could contribute towards the higher lopinavir exposure observed in Thai pregnant women. Host genetic polymorphisms could also play a role. LPV is primarily metabolized by the cytochrome P450 enzyme 3A4 (CYP3A4) and is a substrate for the drug efflux transporter P-glycoprotein, coded by the ABCB1 gene. Functional variants of the ABCB1 were not associated with lopinavir plasma concentrations [20]; however, recent evidence suggests that polymorphisms within the CYP3A and SLCO1B1 (a member of the organic anion transporting polypeptides (OATP) family) genes contribute towards variability in LPV pharmacokinetics [21, 22]. Differences between LPV/r formulations should be taken into consideration when comparing studies. The initial assessment of standard LPV/r dosing in US pregnant women used the original soft-gelatin capsule of LPV/r while the new LPV/r tablet formulation was administered in the present study. The LPV/r tablet formulation is bioequivalent to the soft gelatin capsule after administration with a moderate-fat meal but bioavailability of LPV is approximately 18% higher with tablets compared to capsules [23]. This higher bioavailability may also have contributed towards the somewhat higher LPV exposure observed during the third trimester of pregnancy in Thai women with standard dosing. Finally, sampling fluctuations and attributes such as diet, smoking or herbal intake could explain the differences between studies in lopinavir PK during pregnancy observed.
Since discontinuation of 3TC after three months of treatment in a population with a high rate of hepatitis B virus (HBV) co-infection (~8% of pregnant women are Hepatitis BsAg carriers in Thailand) can trigger HBV rebound and hepatic flare [24, 25], the regimen tested in the PHPT-5 trial did not include 3TC. Nevertheless, the HIV-1 RNA virological response after ZDV/LPV/r initiation during the third trimester was rapid. After a median duration of LPV/r treatment of 10 weeks, all women had a viral load <400 copies/mL at delivery, with 62% achieving less than 40 copies/mL. This virologic response is consistent with that reported in the MONARK trial where, using either ZDV/3TC/LPV/r or LPV/r monotherapy, approximately 60%, 80% and 90% of patients achieved a viral load below 400 copies/mL at 4, 8 and 12 weeks, respectively, and, 20%, 50% and 60% less than 50 copies/mL. The efficacy of the ZDV/LPV/r regimen for the prevention of mother-to-child transmission of HIV is still under investigation in the parent trial. However, the risk of transmission with a viral load less than 400 copies/mL has been shown to be extremely low [26].
The reduction of LPV drug exposure associated with pregnancy was less pronounced in Thai women than in US women. Standard LPV dosing appeared sufficient and the ZDV plus LPV/r regimen initiated during the third trimester of pregnancy achieved adequate virological response at delivery. These results are likely applicable for women in the many clinical settings around the world who have similarly low body weights during the third trimester of pregnancy however concomitant drug use, diet and available drug formulations should also be taken into consideration.
Acknowledgments
We would like to thank all the women who participated in the PHPT-5 trial and the study staff conducting the protocol at the sites. PHPT Data and Safety Monitoring Board (DSBM): Prof. Scott Hammer, Prof. René Ecochard, Prof. Suwachai Intaraprasert, Assoc. Prof. Rudiwilai Samakoses, Dr. Wiput Phoolcharoen. Pharmacology Consultants: Edmund Capparelli, Alice Stek, Mark Mirochnick, Jean-Marc Treluyer. This study was supported by the National Institute of Child Health and Human Development (NICHD), National Institutes of Health, USA. Grants number: R01 HD056953. Pharmaceutical support for the PHPT-5 trial is provided from: GlaxoSmithKline and Boehringer Ingelheim. Lopinavir and ritonavir for the antiretroviral drug assay were obtained through the NIH AIDS Research and Reference Program, Division of AIDS, NIAID, NIH.
T.R.C designed the PK study, developed the PK case report forms, oversaw the lopinavir/ritonavir drug level measurement, performed the data analysis and wrote the first draft of the manuscript. G.J assisted with the PK study design and statistical analysis, cowrote the PHPT-5 study and contributed to the writing of the manuscript; B.R, S.V, R.K, P.S, P.Y, S.C assisted with the study design, enrolled and monitored patients and edited the manuscript; N.N assisted with the PK study design, oversaw the virological testing, cowrote the PHPT-5 study and edited the manuscript; N.V and S.P assisted with the study design and implementation and edited the manuscript; M.L assisted with the PK study design and implementation, cowrote the PHPT-5 study and contributed to the analysis and writing of the manuscript.
Members of the PHPT-5 Team (PK study)
Phayao Provincial Hospital: Jittapol Hemvuttiphan, Ruethai Wongchai, Borwornluck Changlor, Ampai Maneekaew, Kunlaya Jansook, Saowakhon Bunchaisun. Chiang Rai Prachanukroh Hospital: Jullapong Achalapong, Subenya Jinasa, Purivis Chart, Kannikar Saisawat, Pollawat Thongsuk, Supaporn Utsaha. Mae Chan Hospital: Sudanee Buranabanjasatean, Natjaree Thuenyeanyong, Phithak Kaeha, Thanutra Taiyaithieng, Benjaporn Juntapoon. Prapokklao Hospital: Prapap Yuthavisuthi, Renoo Wongsrisai, Ubon Chanasit, Nuttupassasorn Tungtongcha, Pisut Greetanukroh. Banglamung Hospital: Kamol Boonrod, Suchada Thongsuwan, Prateep Kanjanavikai, Watcharin Kaewsaweat, Sarocha Sawatmarn. Chonburi Hospital: Nantasak Chotivanich, Tiwacha Thimakam, Nusara Krapunpongsakul, Chanida Asarath, Raewadee Wanno, Prakit Yothipitak, Suluck Soontaros, Kessarin Chaisiri. Rayong Hospital: Weerapong Suwankornsakul, Phatcharin Thuraset, Arthit Cheawchan, Sukonta Phasuk. Nakornping Hospital: Aram Limtrakul, Janjira Thonglo, Benjawan Thomyota, Autcharaporn Nakrit, Pusdee Lymthavorn. Nopparat Rajathanee Hospital: Boonsong Rawangban, Patcharaporn Krueduangkam, Thamon Wijitwong, Kantinan Leepaiboon, Pranom Poolpat. Bhumibol Adulyadej Hospital: Sinart Prommas, Marina Thitathan, Santi Winaitham, Boonruen Pengmark. Pranangklao Hospital: Surachai Pipatnakulchai, Jiradsadaporn Khanmali, Kesorn Jitmaleerat, Kingtong Wongsirikul. Hat Yai Hospital: Tapnarong Jarupanich, Namthip Kruenual, Usa Sukhaphan, Raruay Jitsakulchaidej. Nong Khai Hospital: Noossara Puarattana.aroonkorn, Waropart Pongchaisit, Sinnapa Pothinukka, Nunthiya Phomcheam, Supattra Kaengklang. Samutsakhon Hospital: Supang Varadisai, Jantra Chalasin, Yaowalak Sookbumrung, Wilai Raiva. Nakhonpathom Hospital: Rucha Kongpanichkul, Chutima Seema, Parawan Bunditwong, Panita Kapol. Samutprakarn Hospital: Prapan Sabsanong, Boonyavee Ratchanee, Kedsara Bunluesak, Arun Yaisiri, Noupporn Jenpoomjai. Lampang Hospital: Prateung Lianpongsabuddhi, Sirirat Thammajitsagul, Tiemjan Keowkarnkah, Sasipun Supong, Sunee Hanyutthapong, Wanpen Leelaporn. Vachira Phuket Hospital: Somnuk Chirayus, Sompong Wannun, Sudtida Eawsakul, Iriyaporn Kongthap, Nuttharee Pinkaew. Pathumthani Hospital: Boonrak Wiriyachoke, Chisakan Thanadechworasate, Punjamaporn Satjeanphong, Ratchadaporn Katekaraj. Panasnikom Hospital: Manoch Chakorngowit, Somsong Niamlamul, Samapond Saithong, Parinee Suebchart. Ministry of Public Health: Siripon Kanshana, Mahidol University: Suporn Koetsawang. PHPT-IRD174 Clinical Trial Unit, Chiang Mai: Sophie Le Coeur, Ken McIntosh, Pra-ornsuda Sukrakanchana, Suwalai Chalermpantmetagul, Kanchana Than-in-at, Yardpiron Taworn, Pimpinun Punyati, Angkana Thongkum, Paporn Mongkolwat, Ampika Kaewbundit, Panida Pongpunyayuen, Luc Decker, Aksorn Lueanyod, Suriyan Tanasri, Sanupong Chailert, Kanjana Yoddee, Rungruangrong Seubmongkolchai, Dujrudee Chinwong, Pongpreeda Saenchitta, Chalermpong Sanjoom.
References
- 1.WHO. World Health Organization; Use of antiretroviral drugs for treating pregnant women and preventing HIV Infection in infants. 2009 [PubMed]
- 2.Stek AM, Mirochnick M, Capparelli E, Best BM, Hu C, Burchett SK, et al. Reduced lopinavir exposure during pregnancy. Aids. 2006;20:1931–1939. doi: 10.1097/01.aids.0000247114.43714.90. [DOI] [PubMed] [Google Scholar]
- 3.Mirochnick M, Best BM, Stek AM, Capparelli E, Hu C, Burchett SK, et al. Lopinavir exposure with an increased dose during pregnancy. J Acquir Immune Defic Syndr. 2008;49:485–491. doi: 10.1097/QAI.0b013e318186edd0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Best BM, Stek AM, Hu C, Burchett SK, Rossi SS, Smith E, et al. High-Dose Lopinavir and Standard Dose Emtricitabine Pharmacokinetics During Pregnancy and Postpartum. 15th Conference on Retroviruses and Opportunistic Infections, February 3 – 6, 2008, Boston, MA, USA; Poster #629; Boston, USA. 2008. [Google Scholar]
- 5.Lyons F, Lechelt M, De Ruiter A. Steady-state lopinavir levels in third trimester of pregnancy. Aids. 2007;21:1053–1054. doi: 10.1097/QAD.0b013e3281053a1e. [DOI] [PubMed] [Google Scholar]
- 6.Manavi K, McDonald A, Al-Sharqui A. Plasma lopinavir trough levels in a group of pregnant women on lopinavir, ritonavir, zidovudine, and lamivudine. Aids. 2007;21:643–645. doi: 10.1097/QAD.0b013e328031f42e. [DOI] [PubMed] [Google Scholar]
- 7.Delfraissy JF, Flandre P, Delaugerre C, Ghosn J, Horban A, Girard PM, et al. Lopinavir/ritonavir monotherapy or plus zidovudine and lamivudine in antiretroviral-naive HIV-infected patients. Aids. 2008;22:385–393. doi: 10.1097/QAD.0b013e3282f3f16d. [DOI] [PubMed] [Google Scholar]
- 8.Kaletra Package Insert (Product Ladeling) Abbott Park, IlIinois, USA: Abbott Laboratories; 2005. [Google Scholar]
- 9.Murphy RL, Brun S, Hicks C, Eron JJ, Gulick R, King M, et al. ABT-378/ritonavir plus stavudine and lamivudine for the treatment of antiretroviral-naive adults with HIV-1 infection: 48-week results. Aids. 2001;15:F1–F9. doi: 10.1097/00002030-200101050-00002. [DOI] [PubMed] [Google Scholar]
- 10.Droste JA, Verweij-Van Wissen CP, Burger DM. Simultaneous determination of the HIV drugs indinavir, amprenavir, saquinavir, ritonavir, lopinavir, nelfinavir, the nelfinavir hydroxymetabolite M8, and nevirapine in human plasma by reversed-phase high-performance liquid chromatography. Ther Drug Monit. 2003;25:393–399. doi: 10.1097/00007691-200306000-00023. [DOI] [PubMed] [Google Scholar]
- 11.Holland DT, DiFrancesco R, Connor JD, Morse GD. Quality assurance program for pharmacokinetic assay of antiretrovirals: ACTG proficiency testing for pediatric and adult pharmacology support laboratories, 2003 to 2004: a requirement for therapeutic drug monitoring. Ther Drug Monit. 2006;28:367–374. doi: 10.1097/01.ftd.0000211817.58052.b8. [DOI] [PubMed] [Google Scholar]
- 12.Khuong-Josses MA, Boussairi A, Palette C, Mechali D. Lopinavir Drug Monitoring in 36 Pregnant Women. 14th Conference on Retroviruses and Opportunisitic Infections, Los Angeles, February 25–28, #743.2007. [Google Scholar]
- 13.Peytavin G, Francois-Pierre S, Cassard B, Truchis de P, Winter C, Visseaux B, et al. Reduced Lopinavir Exposure during Pregnancy: A Case Control Study. 14th Conference on Retroviruses and Opportunisitic Infections, Los Angeles, February 25–28, #579.2007. [Google Scholar]
- 14.Ananworanich J, Kosalaraksa P, Hill A, Siangphoe U, Bergshoeff A, Pancharoen C, et al. Pharmacokinetics and 24-week efficacy/safety of dual boosted saquinavir/lopinavir/ritonavir in nucleoside-pretreated children. Pediatr Infect Dis J. 2005;24:874–879. doi: 10.1097/01.inf.0000180578.38584.da. [DOI] [PubMed] [Google Scholar]
- 15.Chiu YL, King MS, Li J, Klein CE, Hanna GJ. Trough Lopinavir Concentrations Do not Predict Virologic Response to Lopinavir/ritonavi-based Three-Drug Regimens in Antiretroviral-Naive Patients. 8th International Workshop on Clinical Pharmacology of HIV Therapy, April 16–18, Budapest, Hungary, Poster #38.2007. [Google Scholar]
- 16.van der Leur MR, Burger DM, la Porte CJ, Koopmans PP. A retrospective TDM database analysis of interpatient variability in the pharmacokinetics of lopinavir in HIV-infected adults. Ther Drug Monit. 2006;28:650–653. doi: 10.1097/01.ftd.0000245681.12092.d6. [DOI] [PubMed] [Google Scholar]
- 17.Gibbons S, Back D, Khoo S. Variability in Lopinavir Concentrations in the Clinical Setting and Factors Affecting Concentrations. 8th International Workshop on Clinical Pharmacology of HIV Infection, Budapest, April 2007, Abstract #37.2007. [Google Scholar]
- 18.Bouillon-Pichault M, Jullien V, Piketty C, Viard JP, Morini JP, Chhun S, et al. A population analysis of weight-related differences in lopinavir pharmacokinetics and possible consequences for protease inhibitor-naive and -experienced patients. Antivir Ther. 2009;14:923–929. doi: 10.3851/IMP1414. [DOI] [PubMed] [Google Scholar]
- 19.Aweeka FT, Stek A, Best BM, Hu C, Holland D, Hermes A, et al. Lopinavir protein binding in HIV-1-infected pregnant women. HIV Med. 2009 doi: 10.1111/j.1468-1293.2009.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma Q, Brazeau D, Zingman BS, Reichman RC, Fischl MA, Gripshover BM, et al. Multidrug resistance 1 polymorphisms and trough concentrations of atazanavir and lopinavir in patients with HIV. Pharmacogenomics. 2007;8:227–235. doi: 10.2217/14622416.8.3.227. [DOI] [PubMed] [Google Scholar]
- 21.Hartkoorn RC, Kwan WS, Shallcross V, Chaikan A, Liptrott N, Egan D, et al. HIV protease inhibitors are substrates for OATP1A2, OATP1B1 and OATP1B3 and lopinavir plasma concentrations are influenced by SLCO1B1 polymorphisms. Pharmacogenet Genomics. 2010;20:112–120. doi: 10.1097/FPC.0b013e328335b02d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lubomirov R, di Iulio J, Fayet A, Colombo S, Martinez R, Marzolini C, et al. ADME pharmacogenetics: investigation of the pharmacokinetics of the antiretroviral agent lopinavir coformulated with ritonavir. Pharmacogenet Genomics. 2010;20:217–230. doi: 10.1097/FPC.0b013e328336eee4. [DOI] [PubMed] [Google Scholar]
- 23.Klein CE, Chiu YL, Awni W, Zhu T, Heuser RS, Doan T, et al. The tablet formulation of lopinavir/ritonavir provides similar bioavailability to the soft-gelatin capsule formulation with less pharmacokinetic variability and diminished food effect. J Acquir Immune Defic Syndr. 2007;44:401–410. doi: 10.1097/QAI.0b013e31803133c5. [DOI] [PubMed] [Google Scholar]
- 24.Bessesen M, Ives D, Condreay L, Lawrence S, Sherman KE. Chronic active hepatitis B exacerbations in human immunodeficiency virus-infected patients following development of resistance to or withdrawal of lamivudine. Clin Infect Dis. 1999;28:1032–1035. doi: 10.1086/514750. [DOI] [PubMed] [Google Scholar]
- 25.Lim SG, Wai CT, Rajnakova A, Kajiji T, Guan R. Fatal hepatitis B reactivation following discontinuation of nucleoside analogues for chronic hepatitis B. Gut. 2002;51:597–599. doi: 10.1136/gut.51.4.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warszawski J, Tubiana R, Le Chenadec J, Blanche S, Teglas JP, Dollfus C, et al. Mother-to-child HIV transmission despite antiretroviral therapy in the ANRS French Perinatal Cohort. Aids. 2008;22:289–299. doi: 10.1097/QAD.0b013e3282f3d63c. [DOI] [PubMed] [Google Scholar]