Abstract
Changes in the emission fluorescence intensity of pheophorbide-a (PHEO) in the presence of carboquone (CARBOQ) were used to obtain the association constant, the number of CARBOQ molecules interacting with PHEO, and the fluorescence quantum yield of the complex. Excitation spectra of mixtures of PHEO and CARBOQ in ethanol (EtOH) show an unresolved doublet in the red-most excitation band of PHEO, indicating the formation of a loose ground-state complex. The 1:1 CARBOQ–PHEO complex shows a higher fluorescence quantum yield in EtOH (0.41 ± 0.02) than in buffer solution (0.089 ± 0.002), which is also higher than that of the PHEO monomer (0.28). Quenching of the PHEO fluorescence by DNA nucleosides and double-stranded oligonucleotides was also observed and the bimolecular quenching rate constants were determined. The quenching rate constant increase as the oxidation potential of the DNA nucleoside increases. Larger quenching constants were obtained in the presence of CARBOQ suggesting that CARBOQ enhances DNA photo-oxidation, presumably by inhibiting the back–electron-transfer reaction from the photoreduced PHEO to the oxidized base. Thus, the enhanced DNA-base photosensitized oxidation by PHEO in the presence of CARBOQ may be related to the large extent by which this quinone covalently binds to DNA, as previously reported.
INTRODUCTION
Photodynamic therapy (PDT) is a cancer treatment that uses a combination of red light, a photosensitizing agent and molecular oxygen to produce a therapeutic effect (1). Porphyrins, phthalocyanines, chlorins and other dyes are currently used in photodynamic treatment of tumors due to their large molar absorption coefficients in the 500–800 nm wavelength range (2). In the presence of air, these chromophores photosensitize the production of singlet oxygen and superoxide. Singlet oxygen production, the so-called Type II pathway, is argued to be the most important step in the process of killing tumor cells. However, photoreduction or photo-oxidation of substrates, the so-called Type I pathway, has also been proposed as phototoxic events in PDT, especially in oxygen deficient environments (i.e. hypoxic environments) (3).
Solid tumors are often deficient in oxygen (4). Therefore, direct killing of these hypoxic cells by singlet oxygen is very limited (5). However, it is expected that red-light absorbing dyes could also photoreduce molecules having nearly equal or more positive redox potentials than oxygen in anoxic/hypoxic cells because these dyes are able to photoreduce oxygen. If this reducible substrate is a DNA alkylating quinone, DNA alkylation will be expected after quinone photosensitized reduction, thus inducing cell death.
For instance, diaziridinylbenzoquinones act as alkylating agents through their aziridine groups (6,7). These agents have shown significant activity against many types of tumors and have been in clinical use for several years (7). These alkylating agents contain a quinone moiety that can be reduced, as well as an aziridinyl group that can form a covalent bond with a variety of cellular components including DNA. All aziridinyl compounds can be activated to alkylate DNA by protonation of the aziridine groups followed by nucleophilic attack. Indeed, it was demonstrated that simple aziridinylbenzoquinones in an aqueous solution can cross-link to DNA before activation by a pH-dependent reduction (8).
Quinones that undergo bioreduction can have an added advantage. Although the aziridine moiety has a pK around 3–4, this value increases to around 5–6 when the quinone is reduced and, hence, the aziridine ring could react more readily with the DNA bases (9). The two-electron reduced species, the hydroquinone (QH2) (10) and the semiquinone (Q•−) of aziridinyl quinones (11), have been proposed as the DNA alkylating species.
Recently, we demonstrated that photolysis of anaerobic solutions of DNA containing either aluminum phthalocyanine tetrasulfonate, chlorin e6, pheophorbide-a (PHEO) or a novel tetracationic phthalocyanine derivative in the presence of several quinones produces quinone-DNA covalent adducts (12). Furthermore, the PHEO/carboquone (CARBOQ) couple produced the highest percentages of cross-links between DNA and the photoreduced quinone of all the quinone-dye couples tested. The results suggest that it is possible for these dyes to induce cell death in hypoxic regions of tumors if the aziridinylquinones react with the excited states of the dyes under hypoxia.
There is evidence that PHEO can oxidize guanine and adenine, but not cytosine or thymine monomers, by photoinduced electron transfer from 1PHEO* to the ground state of the DNA purine bases (13,14). Observations in this work sustain the hypothesis that CARBOQ can favorably compete with the oxidized DNA bases for the excess electron in the photoreduced PHEO in buffered solutions, thus decreasing the probability of back-electron transfer from the PHEO radical anion to the oxidized DNA base. This, in turn, results in a net increase efficiency of PHEO in oxidizing the DNA purine monomers and duplexes under anaerobic conditions by a photoinduced electron-transfer process. This knowledge is relevant for the understanding of the mechanism by which CARBOQ-DNA photoadduct formation is initiated (12).
In this work, we determined the photophysical properties of PHEO and CARBOQ mixtures in ethanol (EtOH) and buffered solutions by measuring their association constants, the number of CARBOQ molecules interacting with PHEO, and the fluorescence quantum yield of the complex. Furthermore, results for the quenching of the 1PHEO* by DNA nucleosides and DNA duplexes d(GC)10, d(AT)10 and d(IC)10 in PBS/7.4 in the absence and presence of CARBOQ are reported.
MATERIALS AND METHODS
Chemicals
PHEO was obtained from Frontier Scientific and used as received. The Drugs Synthesis and Chemistry Branch of NIH (USA) kindly provided CARBOQ. Guanosine (Guo), adenosine (Ado) and inosine (Ino) nucleosides were obtained from Lancaster Industries (CA, USA), Calbiochem (CA, USA) and Fluka, respectively, and used as received. The 20-mer d(GC)10, d(AT)10 and d(IC)10 self-complementary DNA oligonucleotide duplexes were obtained as lyophilized samples from Midland (TX, USA) and were HPLC-purified. All others chemicals were of the highest purity commercially available and were used without further purification.
Sample preparation
All solutions were freshly prepared. Oxygen was excluded from samples by keeping a positive pressure of Ar during the experiments. Deoxygenation was stopped only during the time interval when the absorption and emission spectra were recorded. Deionized water was used to prepare all PBS/7.4 solutions. The PHEO concentration was determined from its absorption spectrum using the molar absorption coefficient (15). Stock solutions of PHEO were prepared in EtOH and diluted with PBS/7.4 as needed to obtain a PHEO concentration of 1.8 ± 0.4 × 10−5M (using the monomer absorption band at 667 nm) (15) and 8.0 ± 0.5 × 10−6 M (using the monomer band at 667 nm)/1.8 ± 0.4 × 10−5 M (using the dimer band at 685 nm) (15). These concentrations were used to avoid formation of higher aggregates. Solutions of PBS/7.4 containing PHEO had an initial water/EtOH ratio of 0.9, but due to continuous deoxygenation with Ar, this ratio at the time of recording the spectra was probably higher. The CARBOQ concentration (1.4 × 10−4 M) was determined by UV-Vis spectroscopy with the information of the extinction coefficient value 1.12 × 104 M−1 cm−1 at 329 nm. The concentrations of the oligonucleotides were estimated using the molar absorption coefficients reported for poly (dAdT)·poly(dAdT) at 262 nm (6 600 M−1 cm−1) (16), for poly(dGdC)·poly(dGdC) at 254 nm (8,400 M−1 cm−1) (17) and for poly(dIdC)·poly(dIdC) at 261 nm (6,900 M−1 cm−1) (18).
Absorption and emission measurements
Absorption spectra were recorded using a HP 8453 UV-Vis photodiode array spectrophotometer. Fluorescence and excitation spectra were obtained with a Spex Fluorolog Tau 3.11 spectrofluorimeter (Spex Industries, NJ). The monochromator slits were set to 2.5 nm and corrections were made for differences in the instrument sensitivity as a function of wavelength. The emission spectrum of PHEO thin optical solutions was recorded by exciting at the second vibrational band of the first excited state (640 nm). This excitation wavelength was selected to avoid inner filter effects at the concentrations of PHEO used. Also, at this excitation wavelength, there is no need of using an optical filter to block the excitation beam, while still being able to record the emission spectrum of PHEO from 645 to 750 nm. This turns out to be important due to the considerably small fluorescence quantum yield of the PHEO (<10−4) in aqueous solutions (15). Excitation spectra were recorded at the maximum of the PHEO emission band and at the redshifted shoulder for each solution (vide infra). For the quenching experiments in the presence of the quinone, in both EtOH and PBS/7.4 solutions, equimolar concentrations of 2.0 ± 0.2 × 10−5 M were used. Quenching experiments were corrected for solvent dilution and the quenchers were added in volume increments of 0.1 mL using a high-precision micropipette (Eppendorf).
Electrochemical measurements
The reduction potentials were determined in nitrogen purged acetonitrile solutions containing 1 mM of the compound and 0.1 M tetra-N-butylammonium perchlorate using differential pulse voltammetry (DPV). A BAS CV 50 W voltammetric analyzer with a glassy carbon-working electrode was used in these determinations. An Ag/AgCl(sat) electrode was used as the reference electrode (E′ = +0.22 V versus normal hydrogen electrode [NHE]) and a platinum wire as the counter electrode. Differential pulse voltammograms were obtained in the potential range of −2.00–0.00 V, using 50 mV pulse amplitude and a 20 mV s−1 scan rate. The reduction potential values were obtained from the DPV peak potential maxima. These are almost similar to the half-wave redox potentials, E1/2, in normal polarographic measurements (19).
RESULTS AND DISCUSSION
Photophysical properties of PHEO and CARBOQ mixtures in EtOH
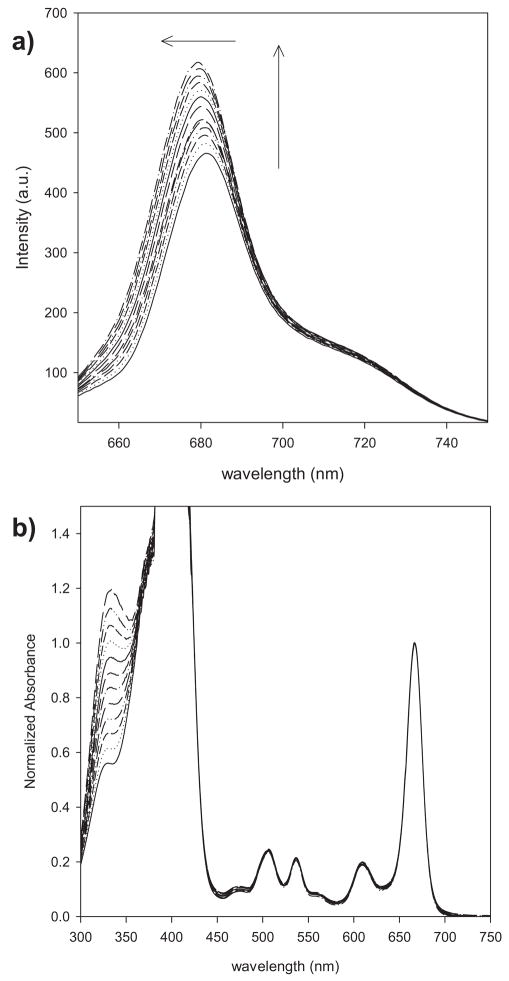
The photophysics of PHEO–CARBOQ (Fig. 1) mixtures was first studied in EtOH because in this solvent PHEO exists as a monomer (15). The emission spectrum of PHEO upon 640 nm excitation exhibited an increase in intensity and a blueshift in the emission band at 681 nm with increasing concentration of CARBOQ (Fig. 2a). The emission of CARBOQ is more than two orders of magnitude smaller than that of PHEO and thus negligible (data not shown). Hence, these results suggest that a complex is formed between PHEO and CARBOQ. The formation of this complex is expected to lead to solvent reorientation around the new exciplex dipole. If the exciplex has greater charge-transfer character, i.e. it is more polar than the singlet excited state of PHEO, then exciplex emission will be expected to occur at higher energies explaining the observed blueshift. Similar behavior has been observed for anthracene–tributhylamine exciplex in cyclohexane and THF (20) and anthracene in n-hexane (21).
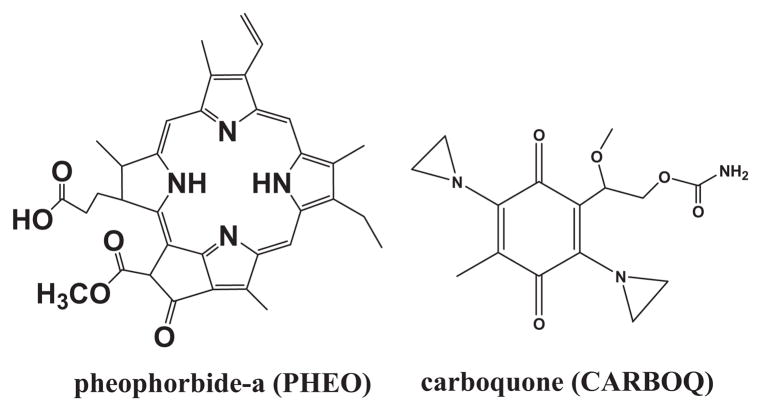
Figure 1.
Structures of pheophorbide-a (PHEO) and carboquone (CARBOQ).
Figure 2.
Emission spectra (a) and absorption spectra (b) of pheophorbide-a (PHEO) with the increasing concentrations of carboquone (CARBOQ) in ethanol. Both datasets are corrected for dilution. PHEO concentration is 1.8 × 10−5 M. CARBOQ concentration is in the range of 0.0–3.1 × 10−5 M. Arrows in (a) represent the increase in emission intensity and blue shift observed when the CARBOQ concentration was increased.
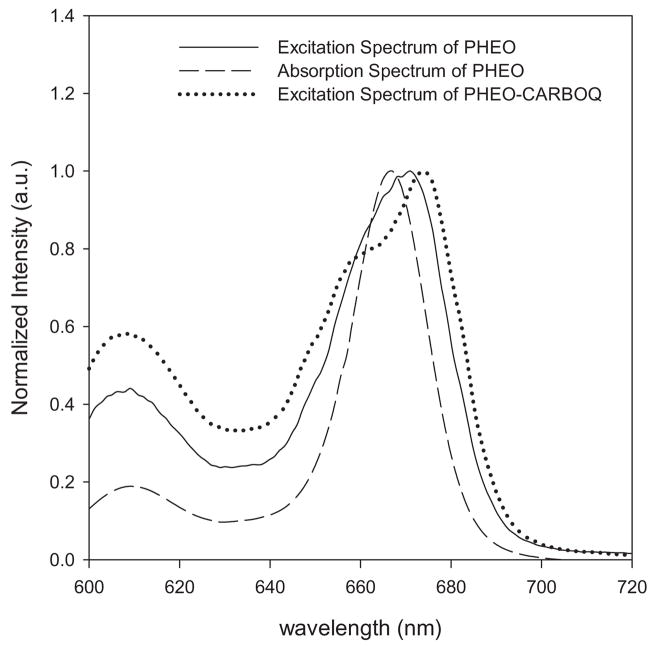
The absorption spectrum of PHEO, on the other hand, does not show band shifts or formation of new absorption bands upon addition of CARBOQ (Fig. 2b) suggesting that if a ground-state complex is formed, it is a loose complex. The increase in intensity of the absorption band at 335 nm is due to the addition of CARBOQ, which absorbs in the same spectral region. Evidence for the formation of a ground-state complex was obtained from the excitation spectra of the mixture (Fig. 3). The excitation spectra exhibit an unresolved doublet in the red-most excitation band, not observed in PHEO. This observation supports the formation of a loose ground-state complex, which forms a charge-transfer complex (exciplex) upon excitation (22). The formation of a loose complex between PHEO and CARBOQ in the ground state can explain why a new absorption band is not observed. Nonetheless, PHEO interacts more strongly with CARBOQ in the excited state, stabilizing the species and resulting in the formation of an unresolved doublet. Our results agree with previous studies on a porphyrin-quinone system (23) and its photoinduced electron-transfer behavior.
Figure 3.
Comparison of the excitation (black line) and absorption (dashed line) spectra of pheophorbide-a (PHEO) with the excitation spectrum of PHEO–carboquone (CARBOQ) mixture (dotted line) in ethanol. PHEO and CARBOQ concentrations are 1.8 × 10−5 M and 2.3 × 10−5 M, respectively.
The association constant of the ground-state complex and the number of CARBOQ molecules that interact with PHEO (n) were determined assuming the following equilibrium (24):
| (1) |
where PHEO–CARBOQn is the complex formed with an association constant Ka given by:
| (2) |
From the conservation of mass, [PHEO]0 = [PHEO–CARBOQn] + [PHEO], where [PHEO]0 is the initial concentration, [PHEO] is the concentration of unbound PHEO and [PHEO–CARBOQn] is the concentration of the ground-state complex. Then, if the ratio of the unbound PHEO to the initial PHEO concentration is associated to the corresponding fluorescence intensities, [PHEO]/[PHEO]0 = F/F0, the following equation should hold (24):
| (3) |
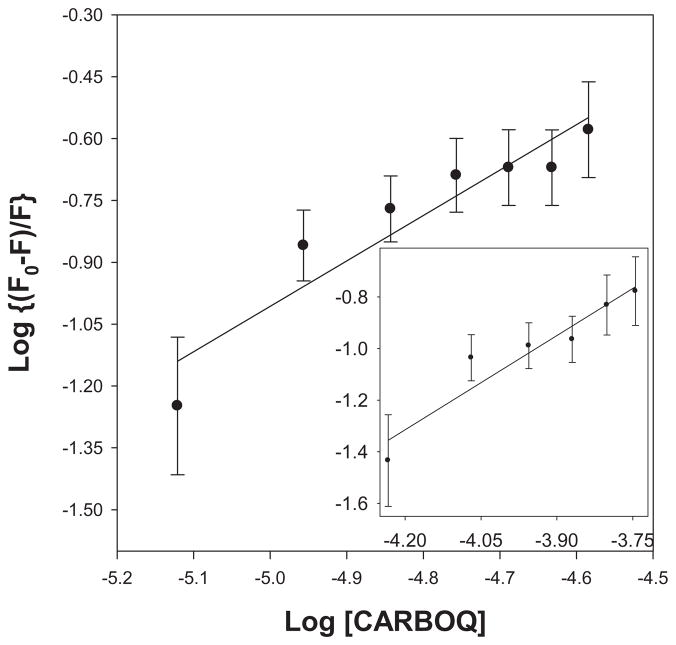
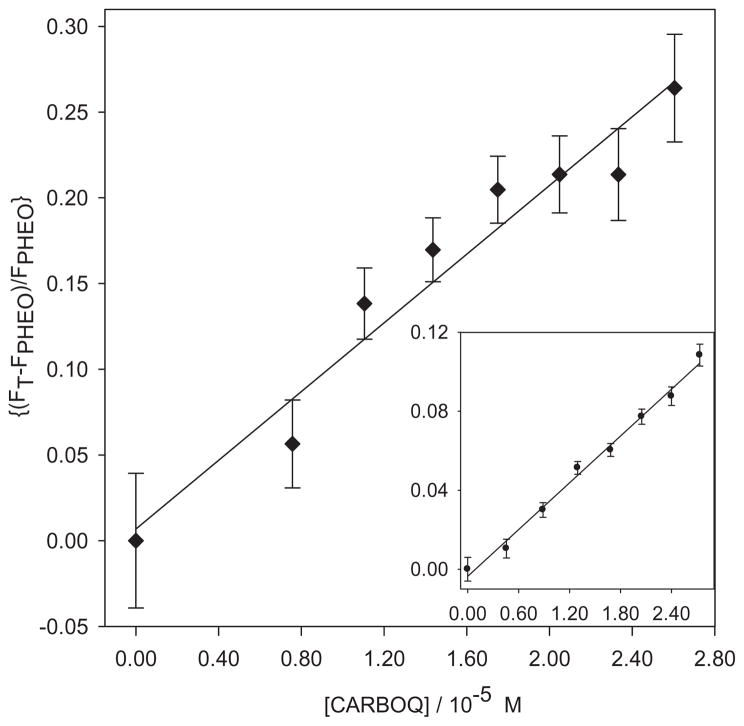
A plot of log[(F0 − F)/F] versus log[CARBOQ] should yield a straight line from which the association constant and the number of CARBOQ molecules participating in the equilibrium can be obtained (Fig. 4). An association constant of 2.5 ± 0.2 × 103 M−1 and a 1:1 PHEO–CARBOQ complex (n = 1.1 ± 0.2) was determined from a linear regression fit of the data for the PHEO and CARBOQ in EtOH. The value of this association constant corresponds to a weak interaction, similar to the interaction of chlorophyllin and ct-DNA (in the range of 10−3 M−1) (25).
Figure 4.
Determination of the association constant of pheophorbide-a (PHEO) and carboquone (CARBOQ) (Ka) and the number of CARBOQ molecules that interact with PHEO (n) in the complex in PBS/7.4 (insert: ethanol). PHEO concentration is 1.8 × 10−5 M.
The fluorescence quantum yield of the PHEO–CARBOQ complex in EtOH was estimated using Eqs. (2) and (3) and the integrated fluorescence spectra of PHEO and CARBOQ mixtures. The total fluorescence (FT) of the PHEO and CARBOQ mixture is equal to the sum of the corresponding fluorescence emissions of the individual fluorophores participating in the equilibrium:
| (4) |
and the fluorescence of a molecule (M) is related to its concentration by:
| (5) |
where ϕM is the fluorescence quantum yield of the molecule, εM is the molar absorption coefficient at the excitation wavelength, [M] is the ground-state concentration and c is a constant related to the instrument’s efficiency. Thus, subtracting the initial fluorescence of PHEO (FPHEO) to the total fluorescence, and dividing by the initial fluorescence of the mixture of PHEO, the following equation is obtained:
| (6) |
Assuming that ϕCARBOQεCARBOQ[CARBOQ] ⋘ ϕPHEOεPHEO[PHEO], equation 6 is transformed into:
| (7) |
Introducing Ka from equation 2 (using n = 1, as determined experimentally above) and assuming that the absorption coefficient of the complex is identical to that of PHEO (no changes in the absorption spectra of PHEO were observed upon CARBOQ addition; see Fig. 2b), then:
| (8) |
Thus, a plot of (FT−FPHEO)/FPHEO versus [CARBOQ] should give a straight line with intercept equal to zero (Fig. 5). From the slope of this plot, the fluorescence quantum yield of the PHEO–CARBOQ complex was estimated using the Ka value calculated from Eq. (3) and the quantum yield of PHEO 0.28 ± 0.02 (15). From this analysis, a fluorescence quantum yield of 0.41 ± 0.02 was calculated for the PHEO–CARBOQ complex in EtOH. Furthermore, this analysis predicts that higher emission intensity should be observed as the concentration of the complex increases with an increase in concentration of CARBOQ as shown in Fig. 1a. This result is in contrast to another similar porphyrin–quinone system studied by Mataga and coworkers, where the nonfluorescent character of the electron-transfer state in nonpolar solvent (benzene) was observed (23). However, in our case the PHEO–CARBOQ charge-transfer complex (exciplex) is fluorescent. It should be noted that the electronic structure of the complex and the relative location ordering of the energy levels of the relevant states (26) will determine if the complex is fluorescent or not. We have determined the half-wave reduction potentials (E1/2) of PHEO and CARBOQ as −0.944 V and −0.748 V, respectively, using Ag/AgCl as reference standard. Using the Rehm-Weller equation (27), a value of −7.6 kJ mol−1 was estimated for the free energy change of electron transfer (ΔGet) from the excited singlet state of PHEO to the ground state of CARBOQ. These experimental results support our argument that the reduction of CARBOQ by PHEO is expected to be more thermodynamically favorable from the excited singlet state of PHEO than from the ground state.
Figure 5.
Determination of the fluorescence quantum yield of the PHEO–CARBOQ exciplex in PBS/7.4 (insert: ethanol). PHEO concentration is 1.8 × 10−5 M. PHEO = pheophorbide-a; CARBOQ = carboquone.
Photophysical properties of PHEO and CARBOQ mixtures in PBS/7.4
PHEO is an amphiphilic molecule (28) and tends to aggregate in aqueous environments. It has been shown that at concentrations greater than 3 × 10−5 M, higher aggregates than the dimer are formed (15). Considering this fact, the PHEO concentration in PBS/7.4 used in our experiments was kept smaller than 3 × 10−5 M, where a monomer–dimer equilibrium would be favored (15).
The normalized absorption spectra of PHEO in PBS/7.4 (10% EtOH) showed only an increase in absorbance at 325 nm upon addition of CARBOQ, which corresponds to the absorption maximum of CARBOQ in PBS/7.4 (data not shown). Hence, no evidence of a ground-state complex formation between PHEO and CARBOQ was observed. However, previous works with a porphyrin–quinone system indicate that a loose ground-state complex is likely to be formed in polar solvents (23). Furthermore, addition of CARBOQ to PHEO in buffered solutions results in a reduction of the emission intensity of PHEO after correcting for dilution, contrary to what was observed above in EtOH solutions. Taking into consideration that only the PHEO monomer (not the dimer) fluoresces, these results suggest that CARBOQ quenches 1PHEO* in buffered solutions. It should be noted that the interaction of CARBOQ with PHEO dimer, if any, cannot be measured by fluorescence since PHEO dimer does not emit.
The formation of a ground-state complex needs to be invoked to explain the observed fluorescence quenching of PHEO by CARBOQ at the CARBOQ concentrations used. This is because the singlet excited state lifetime of PHEO is short (4.7 ns). Hence, the association constant (Ka) and n number of CARBOQ molecules interacting with PHEO molecules can be experimentally estimated using Eq. (3). An association constant of 3.2 ± 0.1 × 104 M−1 and a value of n equal to 1.2 ± 0.1 were obtained corresponding to a 1:1 PHEO–CARBOQ complex interaction. Interestingly, the association constant between PHEO and CARBOQ is higher in buffer solutions than in EtOH, probably because PHEO and CARBOQ are amphiphilic and hydrophobic molecules respectively, and are expected to associate more strongly in aqueous solutions than in EtOH.
The fluorescence quantum yield of the PHEO–CARBOQ exciplex in PBS/7.4 was determined from a plot of (FT−FPHEO)/FPHEO versus [CARBOQ] (Fig. 5) and using Eq. (8). Assuming that the quantum yield of PHEO (0.28 in EtOH) (15) is solvent independent (similar singlet excited-state lifetimes have been reported for PHEO monomer in EtOH and aqueous, buffered solutions) (29), a fluorescence quantum yield of 8.9 ± 0.2 × 10−2 was estimated for the exciplex. The radiative probability of charge-transfer complexes is small in buffered solutions (30), explaining the decrease in emission fluorescence. Furthermore, the PHEO–CARBOQ emission behavior is similar to the ethyletioporphyrin–toluquinone (EEP–TQ) intermolecular exciplex in acetone. The EEP forms a loose ground-state complex with TQ, which undergoes ultrafast deactivation via solvated electron-transfer state immediately after excitation, and the fluorescence of EEP is quenched by collisional interaction with TQ (26).
Quenching constants of 1PHEO* by DNA nucleosides in PBS/7.4
To corroborate the proposed photoinduced electron transfer from PHEO to DNA nucleosides (13), changes in the fluorescence intensity of PHEO in the presence of nucleosides were studied. Guo, Ado, Thymidine (Thd) and Ino were used as models of the DNA monomers. A decrease in the fluorescence emission intensity of PHEO was observed with the addition of Ado, Guo and Ino, but not with Thd. No evidence of ground-state complex between PHEO and the nucleosides was obtained from the changes in the absorption spectra upon addition of the nucleosides (data not shown). Because only PHEO monomer fluoresces, the fluorescence quenching by the nucleosides only provides evidence of their interaction with the singlet excited state of the PHEO monomer. However, this does not rule out a possible interaction of the nucleosides with the PHEO dimer.
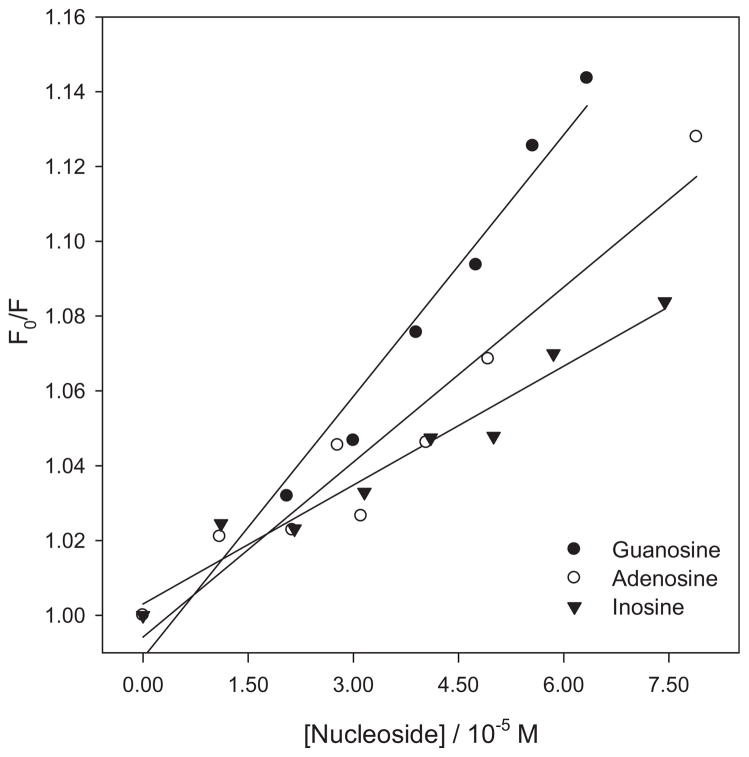
The corresponding quenching constants were determined using a Stern–Volmer plot. Figure 6 depicts a linear Stern–Volmer plot for the quenching of PHEO by the Ado, Guo and Ino nucleosides. Quenching rate constants were calculated using the reported lifetime of PHEO as 4.7 ns in PBS/7.4 (29), (Table 1). It should be noted that the value of these quenching rate constants suggests a static quenching process. However, neither the absorption or excitation spectra of the PHEO nucleoside mixture provided evidence of a ground or excited-state complex. The formation of a loose complex in the ground state between PHEO and the nucleoside could explain the high values of the quenching rate constants. This type of apparent static quenching is usually interpreted in terms of a sphere of action model, within which the probability of quenching is unity (31).
Figure 6.
Stern–Volmer plots for the quenching of the 1PHEO* fluorescence by nucleosides. Fluorescence was obtained from the area of the integrated spectrum. PHEO concentration is 1.8 × 10−5 M. PHEO = pheophorbide-a.
Table 1.
Quenching constants for the singlet excited state of PHEO by nucleosides in the presence and absence of CARBOQ.
| Guanosine |
Adenosine |
Inosine |
||||
|---|---|---|---|---|---|---|
| KS-V 103 M−1 | kq 1011M−1S−1 | KS-V 103 M−1 | kq 1011 M−1 S−1 | KS-V 103 M−1 | kq 1011 M−1 S −1 | |
| PHEO | 2.3 ± 0.2 | 4.9 ± 0.2 | 1.6 ± 0.3 | 3.4 ± 0.2 | 1.1 ± 0.2 | 2.3 ± 0.2 |
| PHEO–CARBOQ mixture | 4.1 ± 0.2 | 8.7 ± 0.3 | 2.2 ± 0.3 | 4.7 ± 0.3 | 1.6 ± 0.2 | 3.4 ± 0.3 |
| Oxidation potential (V)* | 1.29 | 1.42 | 1.5 | |||
Based on the estimated oxidation potentials of Guo (E0ox = 1.29 V versus NHE) (32), Ado (E0ox = 1.42 V versus NHE) (32) and Ino (E0ox = 1.5 V versus NHE) (33), and from the fact that the reduction potential and excited singlet state energy of PHEO are −0.56 eV versus NHE and 1.86 eV, respectively (14), the charge-transfer reaction between the 1PHEO* and Guo, Ado and Ino should be exergonic. Thus, the quenching of the 1PHEO* can be explained in terms of a photoinduced electron-transfer reaction from the nucleoside to PHEO within the sphere of action. The ordering of quenching rate constants can be rationalized from the trend followed by the electrochemical oxidation potentials of nucleosides. Moreover, no quenching of the 1PHEO* was observed when Thd (E0ox = 1.7 V versus NHE) (32) was used (data not shown).
Quenching constants of the PHEO–CARBOQ exciplex by DNA nucleosides in PBS/7.4
We have previously reported that quinones, in particular CARBOQ, can be reduced by the radical anion of PHEO produced from the photo-oxidation of ct-DNA in the presence of PHEO (12). Herein, we show that in the presence of CARBOQ, the excited singlet state of PHEO is quenched by nucleosides.
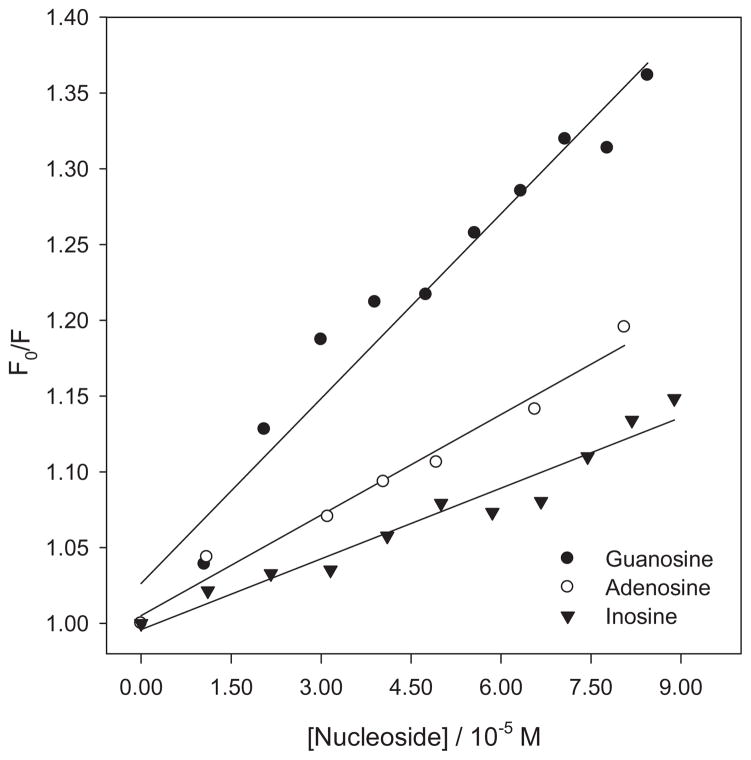
A 1:1 mixture of PHEO and CARBOQ was prepared in a buffer solution and subsequently a 4.0 × 10−4 M nucleoside solution was added in increments of 0.1 mL. Using a Ka of 3.2 ± 0.1 × 104 M−1 and the CARBOQ concentration in the solution, an initial mole fraction of 0.39 was calculated for the PHEO–CARBOQ complex. The absorption and excitation spectra of the PHEO–CARBOQ mixtures upon the addition of the nucleosides did not show evidence of a complex formation with any of the nucleosides investigated. However, a reduction in the emission intensity of the PHEO–CARBOQ mixture was observed with addition of the nucleosides. This quenching is probably due to a photoinduced electron transfer from the nucleoside to the PHEO–CARBOQ exciplex, as observed for the PHEO and nucleosides solutions. Linear Stern–Volmer plots for the quenching of the PHEO–CARBOQ exciplex by nucleosides were obtained (Fig. 7). Quenching constants for all nucleosides, shown in Table 1, were higher when the PHEO–CARBOQ mixtures were used than in the absence of the quinone. Thus, the quenching of 1PHEO* by the nucleoside increases in the presence of CARBOQ, even though only an initial fraction of 0.39 of all PHEO molecules are complexed to CARBOQ. This suggests that CARBOQ can efficiently compete with the radical cation of the nucleoside for the electron, thus resulting in an increase in the net photo-oxidation yield of the nucleosides. This can be rationalized if CARBOQ inhibits the back–electron-transfer reaction in the photo-oxidation of the nucleoside by PHEO. A similar situation was previously observed for the photosensitized oxidation of hypoxanthine by aluminum phthalocyanine tetrasulfonate (34). Quinone enhancement of hypoxanthine oxidation was proposed to be due to inhibition of back-electron transfer by semiquinone disproportionation. Thus, once the semiquinone (Q•−) is produced, it disproportionates to form the parent quinone (Q) and H2Q (Eq. 9).
Figure 7.
Stern–Volmer plot for the quenching of the PHEO–CARBOQ exciplex fluorescence by nucleosides. Fluorescence was obtained from the area of the integrated spectrum. Pheophorbide-a (PHEO) and carboquone (CARBOQ) concentrations are 1.8 × 10−5 M and 2.3 × 10−5 M, respectively.
| (9) |
The relative order of the quenching efficiencies follows a trend similar to that observed in the absence of CARBOQ, with an increase in quenching with a decrease in the oxidation potential of the nucleosides. As in the case of the titration experiment of PHEO with Thd, photo-oxidation of Thd by PHEO was not observed, as judged by the lack of fluorescence quenching when Thd was added to solutions containing PHEO and CARBOQ (data not shown). These results suggest that the formation of the PHEO–CARBOQ complex does not affect the intrinsic redox properties of 1PHEO* and those of the DNA nucleosides investigated in this work.
Quenching constants of PHEO and PHEO–CARBOQ exciplex fluorescence by double-stranded oligonucleotides in PBS/7.4
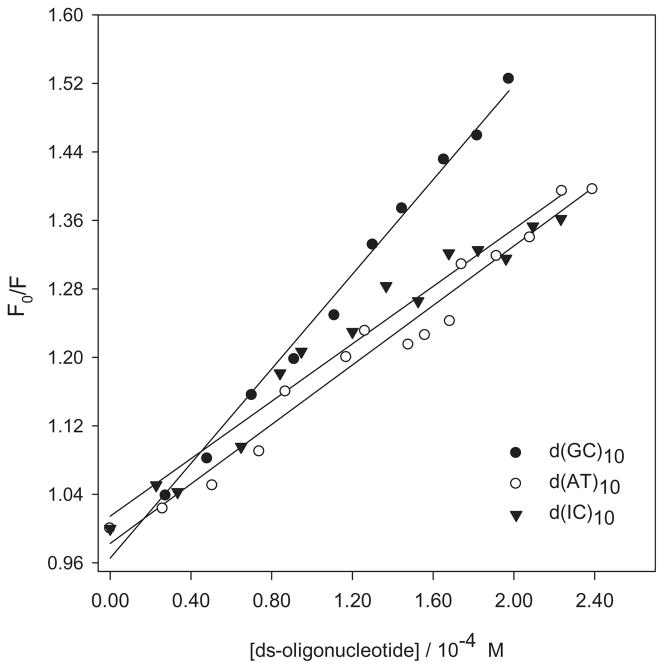
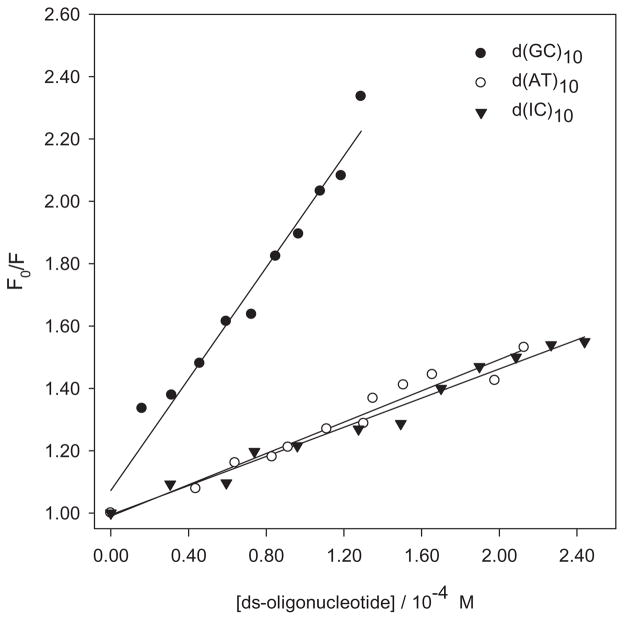
As in the case of nucleosides, no evidence of a ground-state complex between PHEO or the PHEO–CARBOQ complex with d(GC)10, d(AT)10 and d(IC)10 double-stranded oligonucleotides was observed. Nonetheless, the intensity in the emission spectra of PHEO decreased when any of the three double-strand oligonucleotides were added to the solution (after correction for dilution). Quenching rate constants, shown in Table 2, were calculated from Stern–Volmer plots for the quenching of the singlet excited state of PHEO in the presence or absence of CARBOQ by the oligonucleotides (Fig. 8, Table 2). Interestingly, the values of these quenching constants indicate that a static-controlled process is also at play. This result can be explained in terms of the sphere of action model previously mentioned (31). This suggests, once again, that a loose complex is formed in the ground state between PHEO and the double-strand oligonucleotides with no need of a diffusional encounter for the quenching to occur. Quenching efficiencies followed the order of oxidation potentials (32,33) of the nucleosides. Thus, our observations are consistent with the hypothesis that double-stranded DNA can be photo-oxidized by PHEO in buffered solutions.
Table 2.
Quenching constants for the singlet excited state of PHEO by double-stranded oligonucleotides in the presence and absence of CARBOQ.
| d(GC)10 |
d(AT)10 |
d(IC)10 |
||||
|---|---|---|---|---|---|---|
| KS-V 103 M−1 | kq 1011 M−1 S −1 | KS-V 103 M−1 | kq 1011 M−1 S −1 | KS-V 103 M−1 | kq 1011 M−1 S −1 | |
| PHEO | 2.8 ± 0.3 | 6.0 ± 0.3 | 1.7 ± 0.3 | 3.6 ± 0.3 | 1.7 ± 0.2 | 3.6 ± 0.3 |
| PHEO–CARBOQ mixture | 8.9 ± 0.2 | 19 ± 1 | 2.4 ± 0.3 | 5.1 ± 0.3 | 2.4 ± 0.2 | 5.1 ± 0.3 |
The lifetime of PHEO 4.7 ns (15) in PBS/7.4 was used. PHEO = pheophorbide-a; CARBOQ = carboquone.
Figure 8.
Stern–Volmer plot for the quenching of the 1PHEO* fluorescence by double-stranded oligonucleotides. Fluorescence was obtained from the area of the integrated spectrum. Pheophorbide-a (PHEO) concentration is 1.8 × 10−5
Quenching rate constants for all oligonucleotides were higher for the PHEO–CARBOQ mixture than for the PHEO alone (Fig. 9, Table 2). Thus, in the presence of CARBOQ the quenching of the excited singlet state of PHEO by oligonucleotides increased. This observation suggests that CARBOQ can efficiently compete with the radical cation of the oligonucleotides bases for the back-electron transfer from the reduced 1PHEO*, resulting in an increase in the net photo-oxidation yield of the double-stranded oligonucleotides.
Figure 9.
Stern–Volmer plot for the quenching of the PHEO–CARBOQ exciplex fluorescence by double-stranded oligonucleotides. Fluorescence was obtained from the area of the integrated spectrum. Pheophorbide-a (PHEO) and carboquone (CARBOQ) concentrations are 1.8 × 10−5 M and 2.3 × 10−5 M, respectively.
CONCLUSION
Our results indicate that the quenching of the PHEO excited singlet state by nucleic acid monomers and duplexes is enhanced by the presence of CARBOQ in the buffer solutions. Photo-oxidation of Guo, Ado and Ino by 1PHEO* is proposed. A correlation is found between the oxidation potential of the nucleosides and the quenching efficiency of 1PHEO*. The observation of an increase in quenching of the nucleoside/PHEO complexes in the presence of CARBOQ suggests that this quinone enhances nucleic acid base oxidation by reducing the probability of back-electron transfer from the reduced PHEO to the oxidized base. For all the nucleosides tested, Guo, the major target for oxidative DNA damage, presented the highest quenching rate constant. This is presumably due to the fact that Guo has the lowest oxidation potential of all the DNA bases and hence the electron transfer is more favored. Analogous correlations between the projected oxidation potential of the double-stranded oligonucleotides and the quenching efficiency of 1PHEO* were obtained. Here again the presence of CARBOQ enhances the photo-oxidation process probably by decreasing the back–electron-transfer process between the radical cation of the DNA purine base and the radical anion of PHEO. Therefore, CARBOQ can effectively compete with the radical cation of Ado, Guo and Ino nucleosides and double-stranded oligonucleotides for trapping the excess electron in PHEO.
The results of this work, in particular the formation of a PHEO–CARBOQ exciplex, explain satisfactorily the enhancement of the yield of DNA cross-links in irradiated CARBOQ/PHEO couple solutions (12). These results further support the hypothesis that diaziridinylquinones, such as CARBOQ, could be used as bioreductive drugs undergoing PDT Type I mechanisms, especially in hypoxic tissues such as solid tumors.
Acknowledgments
The authors express appreciation for grants P20 RP-016470, S06-GM008216, 1 R15 CA 101761-01 from NIH for financial support and to the Drug Synthesis and Chemistry Branch of NCI/NIH for supplying carboquone. Y. Díaz-Espinosa appreciates her support from the National Institute of General Medical Sciences (grant: R25GM061151). The content of this article is the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health.
References
- 1.Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. Photodynamic therapy. J Natl Cancer Inst. 1998;90:889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang Z. A review of progress in clinical photodynamic therapy. Technol Cancer Res Treat. 2005;4:283–293. doi: 10.1177/153303460500400308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ochsner M. Photophysical and photobiological processes in the photodynamic therapy of tumours. J Photochem Photobiol B, Biol. 1997;39:1–18. doi: 10.1016/s1011-1344(96)07428-3. [DOI] [PubMed] [Google Scholar]
- 4.Brown JM. Exploiting the hypoxic cancer cell: mechanisms and therapeutic strategies. Mol Med Today. 2000;6:157–162. doi: 10.1016/s1357-4310(00)01677-4. [DOI] [PubMed] [Google Scholar]
- 5.Busch TM, Hahn SM, Evans SM, Koch CJ. Depletion of tumor oxygenation during photodynamic therapy: detection by the hypoxia marker EF3 [2-(2-nitroimidazol-1[H]-yl)-N-(3,3,3-trifluoropropyl)acetamide] Cancer Res. 2000;60:2636–2642. [PubMed] [Google Scholar]
- 6.Hargreaves RH, Hartley JA, Butler J. Mechanisms of action of quinone-containing alkylating agents: DNA alkylation by aziridinylquinones. Front Biosci. 2000;5:E172–E180. doi: 10.2741/hargreav. [DOI] [PubMed] [Google Scholar]
- 7.Colombo P, Gunnarsson K, Iatropoulos M, Brughera M. Toxicological testing of cytotoxic drugs (review) Int J Oncol. 2001;19:1021–1028. doi: 10.3892/ijo.19.5.1021. [DOI] [PubMed] [Google Scholar]
- 8.Hargreaves RH, Mayalarp SP, Butler J, McAdam SR, O’Hare CC, Hartley JA. Cross-linking and sequence specific alkylation of DNA by aziridinyl quinones. 2. Structure requirements for sequence selectivity. J Med Chem. 1997;40:357–361. doi: 10.1021/jm960492j. [DOI] [PubMed] [Google Scholar]
- 9.Hoey BM, Butler J, Swallow AJ. Reductive activation of mitomycin C. Biochemistry. 1988;27:2608–2614. doi: 10.1021/bi00407a051. [DOI] [PubMed] [Google Scholar]
- 10.Fisher GR, Donis J, Gutierrez PL. Reductive metabolism of diaziquone (AZQ) in the S9 fraction of MCF-7 cells. II. Enhancement of the alkylating activity of AZQ by NAD(P)H: quinone-acceptor oxidoreductase (DT-diaphorase) Biochem Pharmacol. 1992;44:1625–1635. doi: 10.1016/0006-2952(92)90481-w. [DOI] [PubMed] [Google Scholar]
- 11.Lusthof KJ, De Mol NJ, Janssen LH, Egberink RJ, Verboom W, Reinhoudt DN. Covalent binding studies on the 14C-labeled antitumor compound 2,5-bis(1-aziridinyl)-1,4-benzoquinone. Involvement of semiquinone radical in binding to DNA, and binding to proteins and bacterial macromolecules in situ. Chem Biol Interact. 1990;76:193–209. doi: 10.1016/0009-2797(90)90088-5. [DOI] [PubMed] [Google Scholar]
- 12.Alegria AE, Cruz-Martinez N, Ghosh SK, Garcia C, Arce R. Photosensitized reduction and DNA covalent binding of aziridinylquinones. J Photochem Photobiol A, Chem. 2007;185:206–213. [Google Scholar]
- 13.Kobayashi M, Matsuda M, Kise H, Hisatome M. Electron transfer from nucleic acid base to porphyrin in the singlet excited state. Photomed Photobiol. 1995;17:117–118. [Google Scholar]
- 14.Tanielian C, Kobayashi M, Wolff C. Mechanism of photodynamic activity of pheophorbides. J Biomed Opt. 2001;6:252–256. doi: 10.1117/1.1352750. [DOI] [PubMed] [Google Scholar]
- 15.Eichwurzel I, Stiel H, Roder B. Photophysical studies of the pheophorbide a dimer. J Photochem Photobiol B, Biol. 2000;54:194–200. doi: 10.1016/s1011-1344(00)00016-6. [DOI] [PubMed] [Google Scholar]
- 16.Inman RB, Baldwin RL. Helix-random coil transitions in synthetic DNAs of alternating sequence. J Mol Biol. 1962;5:172–184. doi: 10.1016/s0022-2836(62)80082-5. [DOI] [PubMed] [Google Scholar]
- 17.Wells RD, Larson JE, Grant RC, Shortle BE, Cantor CR. Physicochemical studies on polydeoxyribonucleotides containing defined repeating nucleotide sequences. J Mol Biol. 1970;54:465–497. doi: 10.1016/0022-2836(70)90121-x. [DOI] [PubMed] [Google Scholar]
- 18.Grant RC, Harwood SJ, Wells RD. The synthesis and characterization of poly d(I-C) poly d(I-C) J Am Chem Soc. 1968;90:4474–4476. doi: 10.1021/ja01018a060. [DOI] [PubMed] [Google Scholar]
- 19.Cignitti M. Experimental Electrochemistry for Chemists: D.T. Sawyer and J.L. Roberts, Jr. (John Wiley and Sons, New York, London, Sydney, Toronto, 1974), IX+435 pp., £10.15. Bioelectrochem Bioenerg. 1976;3:636–637. [Google Scholar]
- 20.Ghoneim N. Structure of exciplexes: Solvent and temperature dependences of charge transfer character. Spectrochim Acta Part A Mol Biomol Spectrosc. 2001;57:483–489. doi: 10.1016/s1386-1425(00)00395-4. [DOI] [PubMed] [Google Scholar]
- 21.Vlahovici A, Ofenberg H. Temperature and solvent influence on the fluorescence spectrum of anthracene and anthracene-diethylaniline exciplex. J Lumin. 1982;27:413–424. [Google Scholar]
- 22.Birks JB. Excimers and exciplexes. Nature. 1967;214:1187–1190. [Google Scholar]
- 23.Mataga N, Karen A, Okada T, Nishitani S, Sakata Y, Misumi S. Picosecond laser photolysis studies of photoinduced electron transfer in porphyrin-quinone systems in solution. Detection of the short-lived exciplex state and its solvation-induced ultrafast deactivation. J Phys Chem. 1984;88:4650–4655. [Google Scholar]
- 24.Feng X, Lin Z, Yang L, Wang C, Bai C. Investigation of the interaction between acridine orange and bovine serum albumin. Talanta. 1998;47:1223–1229. doi: 10.1016/s0039-9140(98)00198-2. [DOI] [PubMed] [Google Scholar]
- 25.Neault JF, Tajmir-Riahi HA. DNA–chlorophyllin interaction. J Phys Chem B. 1998;102:1610–1614. [Google Scholar]
- 26.Mataga N. Photochemical charge transfer phenomena— Picosecond laser photolysis studies. Pure Appl Chem. 1984;56:1255–1268. [Google Scholar]
- 27.Rehm D, Weller A. Kinetics of fluorescence quenching by electron and H-atom transfer. Isr J Chem. 1970;8:259–271. [Google Scholar]
- 28.Nyman ES, Hynninen PH. Research advances in the use of tetrapyrrolic photosensitizers for photodynamic therapy. J Photochem Photobiol B, Biol. 2004;73:1–28. doi: 10.1016/j.jphotobiol.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Roeder B, Wabnitz H. Time-resolved fluorescence spectroscopy of hematoporphyrin, mesoporphyrin, pheophorbide a and chlorin e6 in ethanol and aqueous solution. J Photochem Photobiol B, Biol. 1987;1:103–113. doi: 10.1016/1011-1344(87)80010-6. [DOI] [PubMed] [Google Scholar]
- 30.Crespo-Hernández CE, Cohen B, Kohler B. Molecular spectroscopy: Complexity of excited-state dynamics in DNA (Reply) Nature. 2006;441:E8. doi: 10.1038/nature04903. [DOI] [PubMed] [Google Scholar]
- 31.Lakowicz JR. Principles of Fluorescence Spectroscopy. Springer; New York, NY: 2004. [Google Scholar]
- 32.Crespo-Hernández CE, Close DM, Gorb L, Leszczynski J. Determination of redox potentials for the Watson–Crick base pairs, DNA nucleosides, and relevant nucleoside analogues. J Phys Chem B. 2007;111:5386–5395. doi: 10.1021/jp0684224. [DOI] [PubMed] [Google Scholar]
- 33.Kelley SO, Barton JK. Electron transfer between bases in double helical DNA. Science. 1999;283:375–381. doi: 10.1126/science.283.5400.375. [DOI] [PubMed] [Google Scholar]
- 34.Alegria AE, Inostroza Y, Kumar A. Photosensitized oxidation of hypoxanthine and xanthine by aluminum phthalocyanine tetrasulfonate. Role of the alkylating quinone 2,5-dichloro-diaziridinyl-1,4-benzoquinone. Photochem Photobiol. 2008;84:1583–1588. doi: 10.1111/j.1751-1097.2008.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]