DNA sequence abnormalities have identified the CDKN1B gene as a novel contributor, and potential susceptibility gene, in the development of typical sporadic parathyroid adenomas.
Abstract
Context:
Typical nonfamilial (sporadic) parathyroid adenomas are common endocrine tumors for which no predisposing germline DNA variants and only a few clonally altered genes that drive parathyroid tumorigenesis have been identified. CDKN1B, encoding cyclin-dependent kinase inhibitor p27kip1, has recently been implicated in a multiple endocrine tumor phenotype in rats and, rarely, in a human familial MEN1 (multiple endocrine neoplasia type 1)-like disorder.
Objective:
We sought to determine whether mutation of CDKN1B might contribute to the development of common sporadic parathyroid adenomas.
Patients and Design:
We sequenced the CDKN1B gene in 86 parathyroid adenomas from patients with typical, sporadic presentations of primary hyperparathyroidism. Identified alterations were categorized as somatic or germline, and their functional consequences were examined.
Results:
CDKN1B sequence abnormalities were identified in four parathyroid adenomas. Acquired biallelic alteration of CDKN1B, resulting from somatic mutation plus loss of heterozygosity, was detected in one tumor. Germline origin was documented in two cases despite nonfamilial presentations. None of the observed alterations were found in 240 CDKN1B alleles from normal individuals, nor among more than 2,000 previously reported alleles. Most identified variants reduced p27kip1 protein levels or altered in vitro stability.
Conclusions:
In typical, sporadic parathyroid adenomas, CDKN1B mutation can be somatic and clonal, indicative of a directly conferred selective advantage in parathyroid tumorigenesis. Additionally, the identification of germline CDKN1B variants in patients with sporadic presentations provides evidence for CDKN1B as a susceptibility gene in the development of typical parathyroid adenomas.
The molecular underpinnings of common sporadic (nonfamilial) parathyroid adenomas remain incompletely understood, despite established contributions from somatic alterations of two genes, Cyclin D1 and multiple endocrine neoplasia type 1 (MEN1) (1). Clues to the pathogenesis of common sporadic tumors are sometimes obtained from the genetics of familial syndromes involving the same tumor types. For example, MEN1, the gene in which germline mutation is the major cause of MEN1, is also somatically mutated in a subset of nonfamilial parathyroid adenomas. In contrast, while germline mutation in RET, CASR, and HRPT2 cause other familial hyperparathyroid syndromes (MEN2A, familial hypocalciuric hypercalcemia, and hyperparathyroidism-jaw tumor syndrome, respectively), somatic mutation of these genes does not appear to contribute importantly to sporadic parathyroid adenomas (1). Furthermore, no specific heritable DNA variants have been confirmed to predispose to typical adenomas in the general population, and how this disorder relates to the controversy in human genetics on the importance of common vs. rare variants in predisposition to complex diseases is unknown (2, 3).
Recently, germline mutation in CDKN1B, encoding the p27kip1 (or p27) cyclin-dependent kinase inhibitor, was implicated in a rare endocrine neoplasia disorder related to MEN1 (MEN4, MIM ID: 610755) (4–6), after the finding that p27 mutation can cause a multiple endocrine tumor phenotype in rats (4). p27 binds to and inhibits cyclin–cyclin-dependent kinase complexes in the nucleus to aid in regulation of cell cycle progression at G1 (7). Abundance and localization of p27 is highly controlled, primarily by posttranscriptional mechanisms, and decreased p27 levels and cytoplasmic mislocalization have been correlated with tumorigenesis (8). Decreased expression of p27 has been noted in parathyroid adenomas (9). We hypothesized that CDKN1B mutation might contribute to the pathogenesis of typical, sporadic parathyroid adenomas.
Materials and Methods
Patients and samples
Tissue samples were obtained from 86 patients (64 females, 22 males) with typical presentations of sporadic primary hyperparathyroidism, surgically and pathologically confirmed to have parathyroid adenomas with no atypical/malignant features nor evidence of multigland disease. No patient had a history of head or neck irradiation, familial hyperparathyroidism, MEN, or previous parathyroidectomy; average age of patients at the time of surgery was 59 yr (range 28–83 yr). Blood from the same patients was obtained as a source of matched germline DNA. Blood samples were obtained from 120 individuals with no history of parathyroid disease, as population controls. A fresh piece of placenta was flash frozen to yield human placental control RNA. Samples were obtained with informed consent in accordance with Institutional Review Board-approved protocols. Genomic DNA was extracted as previously described (10). RNA was extracted from selected samples using Trizol.
CDKN1B sequencing
The entire coding region and intron-exon boundaries of CDKN1B were PCR-amplified from tumor genomic DNA. All primer sequences are listed in Supplemental Table 1 (published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org/). PCR products were purified and sequenced in both forward and reverse directions with the same primers used for PCR. Sequencing was performed by Genewiz, Inc. Resulting sequence data were analyzed and compared with the published reference sequence (RefSeq: NM_004064); variations from the reference sequence were confirmed with an independent PCR/sequencing reaction, the patient's germline DNA was examined to determine whether an observed variation was somatic or germline, and the presence of each observed variant was assessed in 240 CDKN1B alleles from normal, healthy controls. For analysis of CDKN1B transcripts, cDNA was reverse transcribed and PCR amplified using a one-step RT-PCR and p27-gene specific primers. PCR fragments were sequenced as above.
Immunohistochemistry and functional analyses
Immunohistochemistry was performed using a monoclonal anti-p27 antibody (Supplemental Materials).
Point mutations were introduced into a plasmid containing wild-type (wt) human CDKN1B cDNA. Mutant proteins were expressed in GH3 pituitary cells and their stability assessed (Supplemental Materials).
Results
The entire coding region of the CDKN1B gene was examined by direct sequencing of genomic DNA from 86 parathyroid adenomas. Nonsynonymous, intragenic CDKN1B point mutations were identified in three tumors, and one additional adenoma contained a frameshift mutation coupled with loss of heterozygosity (LOH) at CDKN1B and the surrounding genomic region. A known coding-region single nucleotide polymorphism c.326T>G (V109G, Reference SNP ID: rs2066827), was observed in 36 samples. Results are summarized in Table 1, and sequence chromatograms are shown in Fig. 1A and Supplemental Fig. 2. Each sequence variant identified in this study was sought but was not found in any of the 240 CDKN1B alleles sequenced from control individuals. Furthermore, the world literature contains documentation of more than 2,000 sequenced CDKN1B alleles, and the variants identified here have never been found (4–6, 11–21). Thus, the CDKN1B alterations discovered in this study are highly unlikely to represent benign polymorphic variants distributed commonly in the general population.
Table 1.
CDKN1B mutation summary
| Tumor/patient number | Patient age/sex | Nucleotide(s) affecteda | Heterozygous change? | Expected protein change | Present in germline? | LOH present in tumor? |
|---|---|---|---|---|---|---|
| 1 | 68 M | c.25G>A | Yes | Gly9Arg | Yes | Nod |
| 2 | 67 F | c.397C>A | Yes | Pro133Thr | NDb | Noe |
| 3 | 53 F | c.397C>A | Yes | Pro133Thr | Yes | Nof |
| 4 | 48 F | c.582del25 | No | Multiple abnormalitiesc | No | Yesg |
Nucleotide numbering begins with the A of the start codon as +1.
Normal control DNA from this patient was degraded, so presence of this mutation in the germline could not be assessed.
Frameshift after residue 193, with addition of 57 abnormal extra C-terminal amino acids, also affected by splicing abnormalities as per Fig. 1, B and C.
Supplemental Table 2 and Supplemental Fig. 2A.
LOH determination based on Comparative Genomic Hybridization and single-nucleotide polymorphism array data (Supplemental Refs. 1 and 2).
Supplemental Fig. 2B and Supplemental Ref. 2.
Fig. 1.
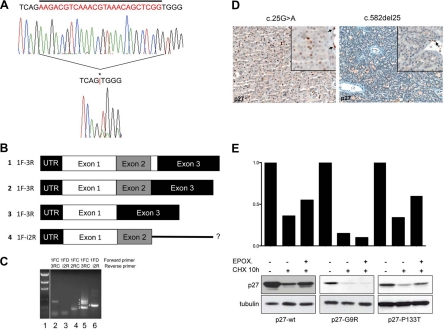
In vivo and in vitro analyses of CDKN1B mutants. A, Twenty-five base pair somatic deletion mutation identified in patient 4 (c.582del25). The top electropherogram shows the germline sequence, while the bottom electropherogram shows the tumor sequence. Sequence deleted in the tumor is indicated by a bar above the germline sequence, and the position of the mutation in the tumor sequence is indicated by an asterisk (*) and a red bar between the nucleotides flanking the deletion. The presence of clear DNA sequence downstream of the deletion, unaccompanied by overlying sequence in the normal (or other) reading frame, indicates the singular presence of the mutant allele, consistent with LOH. LOH of CDKN1B and the surrounding region in this case was also observed in comparative genomic hybridization and single nucleotide polymorphism array analyses (Supplemental Refs. 1 and 2). B, Schematic of the CDKN1B transcripts obtained by RT-PCR. Numbers 1 to 4 indicate the bands in the gel in C. Sequences of bands 1 and 2 (corresponding to lane 5 in C) were consistent with normal splicing (exon 1 to exon 2 to exon 3), the larger band 1 (350 bp) representing full-length exon 2 (line 1), likely from rare admixed normal nonparathyroid cells, and the smaller band 2 (325 bp) being exon 2 with a 25-bp deletion (line 2). Sequence analysis of band 3 (212 bp) revealed a novel isoform of p27 mRNA resulting from splicing of exon 1 directly to exon 3, skipping exon 2 (line 3). A third novel isoform of p27 was detected, corresponding to band 4 (292 bp) in C. Sequence analysis of this band was consistent with splicing of exon 1 to exon 2 with continuation into intron 2 rather than splicing to exon 3 (line 4). C, RT-PCR for p27 in normal control from placenta (lanes 2 and 3) and tumor 4 (lanes 4, 5, and 6). Forward and reverse primers used for reaction are listed above each lane. The presence of p27 mRNA was confirmed in control (lane 2) by using a forward primer in exon 1 and a reverse primer in exon 3 and in tumor (lane 4) by using a forward primer in exon 1 and a reverse primer in exon 2. Using a forward primer in exon 1 and a reverse primer in exon 3, three distinct bands were detected in the tumor sample (lane 5), while only one was present in the control (lane 2). An additional novel isoform of p27 was detected in tumor 4 using a primer in exon 1 and a primer in intron 2 (lane 6), which was absent in the control (lane 3); because the primer combination also spanned intron 1, this band was distinguishable from genomic DNA based on size. Samples included in this study were grossly dissected before RNA extraction; as such, the presence of wt p27 message in this sample was likely attributable to contamination by normal stroma, confirmed by positive p27 staining of adjacent endothelial cells. Despite the presence of mRNA, no normal p27 protein was detected in tumor cells by IHC (D, right). D, Tumor tissues from two individuals were available for immunohistochemical analysis. Left, p27 immunohistochemical staining of the parathyroid adenoma of patient 1 (bearing the c.25G>A mutation). Less than 20% of tumor cells (×200) demonstrate expression of p27. Right, p27 staining of the parathyroid adenoma of patient 4 (bearing the c.582del25 mutation) shows no p27 immunoreactivity in the tumor cells (×200), which can be better appreciated in the inset (×400). Normal endothelial cells strongly positive for p27 serve as internal control (arrows). E, Turnover of p27-wt and of p27-G9R and p27-P133T proteins was measured in cells after CHX treatment with and without the proteasome inhibitor epoxomycin. Immunoblotting was done with the indicated antibodies. p27-wt and p27-P133T have a half-life of approximately 10 h in GH3 cells; epoxomycin partially blocks p27-wt and p27-P133T degradation but not p27-G9R degradation (lower section). Thus, the instability of p27-G9R appears to be attributable in large part to proteasome-independent mechanisms. The histograms above show the band intensities normalized against α-tubulin loading controls.
One tumor contained a heterozygous single nucleotide change, also present in the individual's germline DNA, c.25G>A at base 25 in the coding region (Supplemental Fig. 2A), that would result in a Gly9Arg (G9R) substitution in the translated p27 protein product (Table 1, tumor 1). Glycine in position 9 of p27 is highly conserved across species, and its substitution to arginine might be predicted to affect phosphorylation on the adjacent Serine 10 residue, a modification that regulates subcellular localization and stability of p27 (22, 23).
Two tumors contained a heterozygous single nucleotide substitution c.397C>A (Supplemental Fig. 2B), directing a Pro133Thr (P133T) substitution in the translated p27 protein (Table 1, tumors 2 and 3). This alteration was present in matched germline DNA from one of the two unrelated individuals (Table 1, tumor 3); matched germline DNA from the second individual was degraded, so the presence of this variant in the germline could not be assessed (Table 1, tumor 2). Interestingly, both tumors with P133T also contained somatic, biallelic inactivation of MEN1 (LOH plus somatic mutations c.489del5 and c.74del29, respectively; data not shown), a finding that might not have been expected because part of menin's tumor suppressive function is thought to be mediated through its effects on p27 expression, and loss of both menin and p27 in mice was not cooperative for tumor development (24). Instead, our observations raise the possibility that acquired loss of menin may enhance the selective advantage of parathyroid cells bearing specific p27 alterations, contributing to clonal neoplastic outgrowth in vivo, and that the two defects' functional consequences may therefore not be entirely redundant. No MEN1 alterations were detected in the other two tumors with CDKN1B mutations, so p27 mutation-induced tumorigenesis can likely also proceed with the cooperation of acquired defects in genes other than MEN1.
One tumor contained a 25-bp deletion beginning at c.582 (Fig. 1A). This mutation was somatic, absent in the patient's germline DNA (Table 1, tumor 4). Included among the 25 deleted bases are the last 4 codons of CDKN1B, the stop codon, and the first bp of intron 2 (splice donor site; see Supplemental Fig. 1B). This c.582del25 mutation results in a frameshifted and/or abnormal C-terminal extension beyond the first 193 amino acids, depending on the specific resulting patterns of abnormal splicing. To determine these splicing patterns, RT-PCR was performed on RNA extracted from the parathyroid adenoma bearing this mutation. Along with some normally spliced mRNA, probably from a small component of admixed nontumor cells, three abnormal p27 mRNA species were observed and confirmed by direct sequencing (Fig. 1C). These abnormally spliced mRNAs would, if translated, result in structurally abnormal p27 proteins; such transcripts may also be unstable and unable to yield detectable protein, as appears to be the case (see below).
Immunohistochemical staining for p27 was performed on available CDKN1B tumor tissues from two individuals (Table 1, tumors 1 and 4). Tissue was unavailable from either tumor with P133T. In tumor 1, containing the heterozygous G9R alteration, tumor cells showed extremely reduced immunoreactivity for p27 when compared with normal endothelial cells (Fig. 1D). Tumor 4, containing the c.582del25 somatic mutation plus LOH, showed no staining for p27 (Fig. 1D). Compared with a series of 55 typical parathyroid adenomas, p27 expression of tumor 1 fell into the lowest quartile, and the absent staining of tumor 4 was seen in only one of the reference tumors (Supplemental Table 3).
To further investigate potential functional consequences of identified variants, plasmids were constructed to express mutant proteins. p27 stability was measured in cells treated with cyclohexamide (CHX) to block new protein synthesis. Although unavailability of parathyroid in vitro systems diminishes the likelihood of detecting tissue-specific effects of missense variants, p27-G9R was quite unstable and barely detectable after CHX treatment (Fig. 1E), while P133T did not demonstrably affect p27 stability. The instability of p27-G9R may explain the low level of p27 staining detected by IHC in tumor 1 (Fig. 1D).
Discussion
Recently, germline mutation of CDKN1B, encoding p27, was identified as the cause of a MEN phenotype in rats (MENX) and was implicated in the rare MEN1-like syndrome (MEN4) in humans, both of which include hyperparathyroidism as a component (4–6). We therefore explored the possible role of CDKN1B mutation in common sporadic parathyroid adenomas and identified sequence variants in 4 of 86 (4.6%) adenomas. The observed CDKN1B variants are likely to contribute pathogenically because 1) crucially, they occur in a gene that is already a genetically and experimentally established driver of parathyroid neoplasia in rodents (4) and is associated with a rare familial endocrine neoplasia disorder in humans (4–6); 2) they have not been observed as common or rare benign sequence variants, in more than 2,000 alleles from populations without parathyroid disease; 3) somatic mutation c.582del25 is clonal and accompanied by LOH, strongly suggesting that p27 disruption confers a selective advantage in this context; and 4) disruption in in vivo expression was found for all testable variants (G9R and c.582del25) and in vitro stability was impaired by G9R; functional consequences and/or pathogenetic mechanisms for P133T remain to be established.
The presence of germline CDKN1B sequence abnormalities in patients with sporadic parathyroid adenomas, presenting clinically in the sixth and seventh decades of life with negative family histories of parathyroid or other endocrine disease, provides novel evidence for the concept that rare predisposing alleles contribute to this disorder. Our results further predict that future studies of sporadic hyperparathyroidism will reveal additional examples, in CDKN1B and/or still other genes. In contrast to the high-frequency/small-effect genetic variants often associated with multifactorial disorders in genome-wide scans, rare germline variants are exceedingly difficult to detect in control populations (presented examples have apparent allele frequencies <0.05%) and are likely to have significant biologic effects in causing disease (2, 3). Importantly, even when carried by a few percent of patients with common sporadic disease, such variants may collectively make a larger contribution to hyperparathyroidism than do previously recognized, presumably higher-penetrance mutations (of CDKN1B as well as other genes, such as MEN1, CASR, and HRPT2) found in kindreds that are easily (but only rarely) identified in clinical practice to have familial hyperparathyroidism. To examine the potential role of CDKN1B genotyping in individualized risk assessment or management, additional study of penetrance and occurrence of de novo mutations in extended families of probands ascertained with sporadic adenomas will be important.
Acknowledgments
We thank Dr. J. Slingerland for the gift of the plasmid containing the full-length wt human CDKN1B cDNA encoding p27; Dr. L. Quintanilla-Fend for expert review of immunohistochemistry results; and Kristin Corrado for expert technical assistance.
This work was supported by Department of Health and Human Services/National Institute of Dental and Craniofacial Research (DHHS/NIDCR) Grants 5T32-DE07302 and 1F32-DE021307 (to J.C.G.) from the National Institutes of Health, the Deutsche Krebshilfe (#109223 to N.S.P.), and by the Murray-Heilig Fund in Molecular Medicine. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Dental and Craniofacial Research or the National Institutes of Health.
Disclosure Summary: The authors have nothing to declare.
Footnotes
- CHX
- Cyclohexamide
- LOH
- loss of heterozygosity
- MEN1
- multiple endocrine neoplasia type 1
- wt
- wild type.
References
- 1. Hendy GN, Arnold A. 2008. Molecular basis of PTH overexpression. In: Bilezikian JP, Raisz LG, Martin TJ. eds. Principles of bone biology. San Diego, CA: Academic Press; 1311–1326 [Google Scholar]
- 2. McClellan J, King MC. 2010. Genetic heterogeneity in human disease. Cell 141:210–217 [DOI] [PubMed] [Google Scholar]
- 3. Bodmer W, Bonilla C. 2008. Common and rare variants in multifactorial susceptibility to common diseases. Nat Genet 40:695–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pellegata NS, Quintanilla-Martinez L, Siggelkow H, Samson E, Bink K, Höfler H, Fend F, Graw J, Atkinson MJ. 2006. Germ-line mutations in p27Kip1 cause a multiple endocrine neoplasia syndrome in rats and humans. Proc Natl Acad Sci USA 103:15558–15563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Georgitsi M, Raitila A, Karhu A, van der Luijt RB, Aalfs CM, Sane T, Vierimaa O, Mäkinen MJ, Tuppurainen K, Paschke R, Gimm O, Koch CA, Gündogdu S, Lucassen A, Tischkowitz M, Izatt L, Aylwin S, Bano G, Hodgson S, De Menis E, Launonen V, Vahteristo P, Aaltonen LA. 2007. Germline CDKN1B/p27Kip1 mutation in multiple endocrine neoplasia. J Clin Endocrinol Metab 92:3321–3325 [DOI] [PubMed] [Google Scholar]
- 6. Agarwal SK, Mateo CM, Marx SJ. 2009. Rare germline mutations in cyclin-dependent kinase inhibitor genes in multiple endocrine neoplasia type 1 and related states. J Clin Endocrinol Metab 94:1826–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Viglietto G, Motti ML, Fusco A. 2002. Understanding p27(kip1) deregulation in cancer: down-regulation or mislocalization. Cell Cycle 1:394–400 [DOI] [PubMed] [Google Scholar]
- 8. Koff A. 2006. How to decrease p27Kip1 levels during tumor development. Cancer Cell 9:75–76 [DOI] [PubMed] [Google Scholar]
- 9. Buchwald PC, Akerstrom G, Westin G. 2004. Reduced p18INK4c, p21CIP1/WAF1 and p27KIP1 mRNA levels in tumours of primary and secondary hyperparathyroidism. Clin Endocrinol (Oxf) 60:389–393 [DOI] [PubMed] [Google Scholar]
- 10. Lauter KB, Arnold A. 2008. Mutational analysis of CDKN1B, a candidate tumor-suppressor gene, in refractory secondary/tertiary hyperparathyroidism. Kidney Int 73:1137–1140 [DOI] [PubMed] [Google Scholar]
- 11. Ponce-Castañeda MV, Lee MH, Latres E, Polyak K, Lacombe L, Montgomery K, Mathew S, Krauter K, Sheinfeld J, Massague J. 1995. p27Kip1: chromosomal mapping to 12p12–12p13.1 and absence of mutations in human tumors. Cancer Res 55:1211–1214 [PubMed] [Google Scholar]
- 12. Kawamata N, Morosetti R, Miller CW, Park D, Spirin KS, Nakamaki T, Takeuchi S, Hatta Y, Simpson J, Wilcyznski S, Lee Y, Bartram C, Koeffler H. 1995. Molecular analysis of the cyclin-dependent kinase inhibitor gene p27/Kip1 in human malignancies. Cancer Res 55:2266–2269 [PubMed] [Google Scholar]
- 13. Kawamata N, Seriu T, Koeffler HP, Bartram CR. 1996. Molecular analysis of the cyclin-dependent kinase inhibitor family: p16(CDKN2/MTS1/INK4A), p18(INK4C) and p27(Kip1) genes in neuroblastomas. Cancer 77:570–575 [DOI] [PubMed] [Google Scholar]
- 14. Morosétti R, Kawamata N, Gombart AF, Miller CW, Hatta Y, Hirama T, Said JW, Tomonaga M, Koeffler HP. 1995. Alterations of the p27KIP1 gene in non-Hodgkin's lymphomas and adult T-cell leukemia/lymphoma. Blood 86:1924–1930 [PubMed] [Google Scholar]
- 15. Spirin KS, Simpson JF, Takeuchi S, Kawamata N, Miller CW, Koeffler HP. 1996. p27/Kip1 mutation found in breast cancer. Cancer Res 56:2400–2404 [PubMed] [Google Scholar]
- 16. Kibel AS, Christopher M, Faith DA, Bova GS, Goodfellow PJ, Isaacs WB. 2001. Methylation and mutational analysis of p27(kip1) in prostate carcinoma. Prostate 48:248–253 [DOI] [PubMed] [Google Scholar]
- 17. Markaki EA, Stiakaki E, Zafiropoulos A, Arvanitis DA, Katzilakis N, Dimitriou H, Spandidos DA, Kalmanti M. 2006. Mutational analysis of the cell cycle inhibitor Kip1/p27 in childhood leukemia. Pediatr Blood Cancer 47:14–21 [DOI] [PubMed] [Google Scholar]
- 18. Takeuchi S, Koeffler HP, Hinton DR, Miyoshi I, Melmed S, Shimon I. 1998. Mutation and expression analysis of the cyclin-dependent kinase inhibitor gene p27/Kip1 in pituitary tumors. J Endocrinol 157:337–341 [DOI] [PubMed] [Google Scholar]
- 19. Stegmaier K, Takeuchi S, Golub TR, Bohlander SK, Bartram CR, Koeffler HP. 1996. Mutational analysis of the candidate tumor suppressor genes TEL and KIP1 in childhood acute lymphoblastic leukemia. Cancer Res 56:1413–1417 [PubMed] [Google Scholar]
- 20. Chen TC, Ng KF, Lien JM, Jeng LB, Chen MF, Hsieh LL. 2000. Mutational analysis of the p27(kip1) gene in hepatocellular carcinoma. Cancer Lett 153:169–173 [DOI] [PubMed] [Google Scholar]
- 21. Lindberg D, Akerström G, Westin G. 2007. Mutational analysis of p27 (CDKN1B) and p18 (CDKN2C) in sporadic pancreatic endocrine tumors argues against tumor-suppressor function. Neoplasia 9:533–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ishida N, Kitagawa M, Hatakeyama S, Nakayama K. 2000. Phosphorylation at serine 10, a major phosphorylation site of p27(Kip1), increases its protein stability. J Biol Chem 275:25146–25154 [DOI] [PubMed] [Google Scholar]
- 23. Rodier G, Montagnoli A, Di Marcotullio L, Coulombe P, Draetta GF, Pagano M, Meloche S. 2001. p27 cytoplasmic localization is regulated by phosphorylation on Ser10 and is not a prerequisite for its proteolysis. EMBO J 20:6672–6682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bai F, Pei XH, Nishikawa T, Smith MD, Xiong Y. 2007. p18Ink4c, but not p27Kip1, collaborates with Men1 to suppress neuroendocrine organ tumors. Mol Cell Biol 27:1495–1504 [DOI] [PMC free article] [PubMed] [Google Scholar]