Abstract
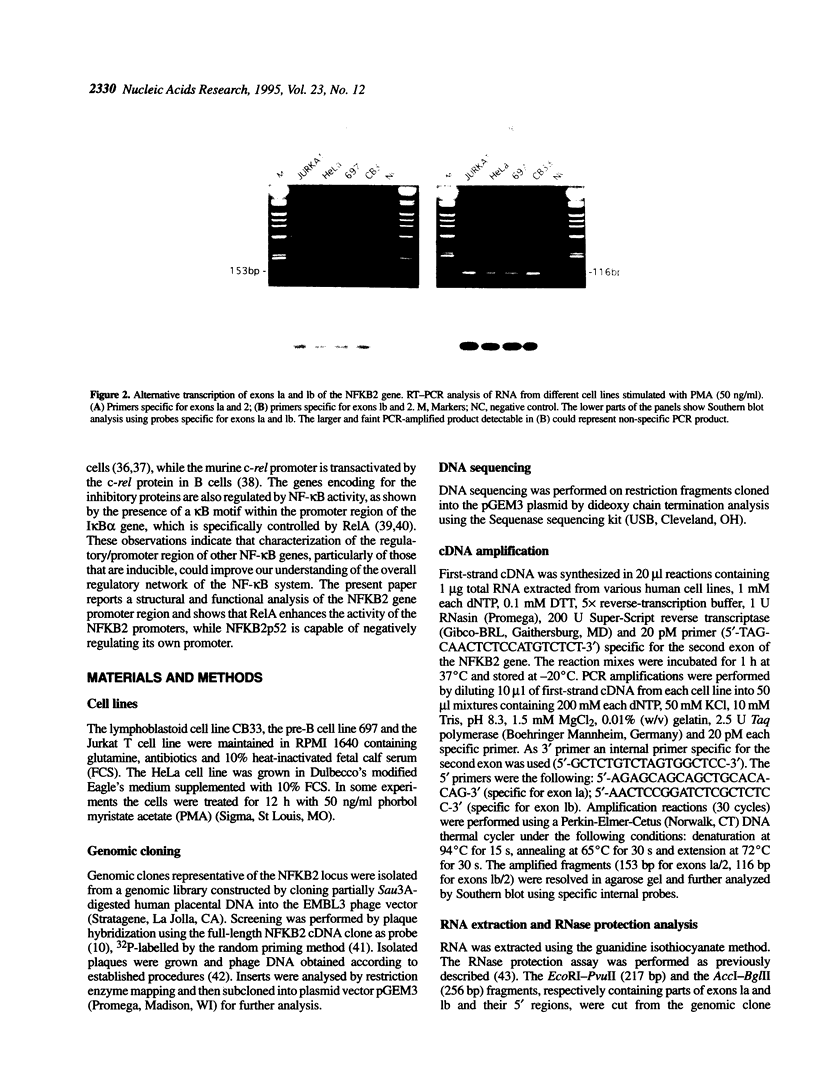
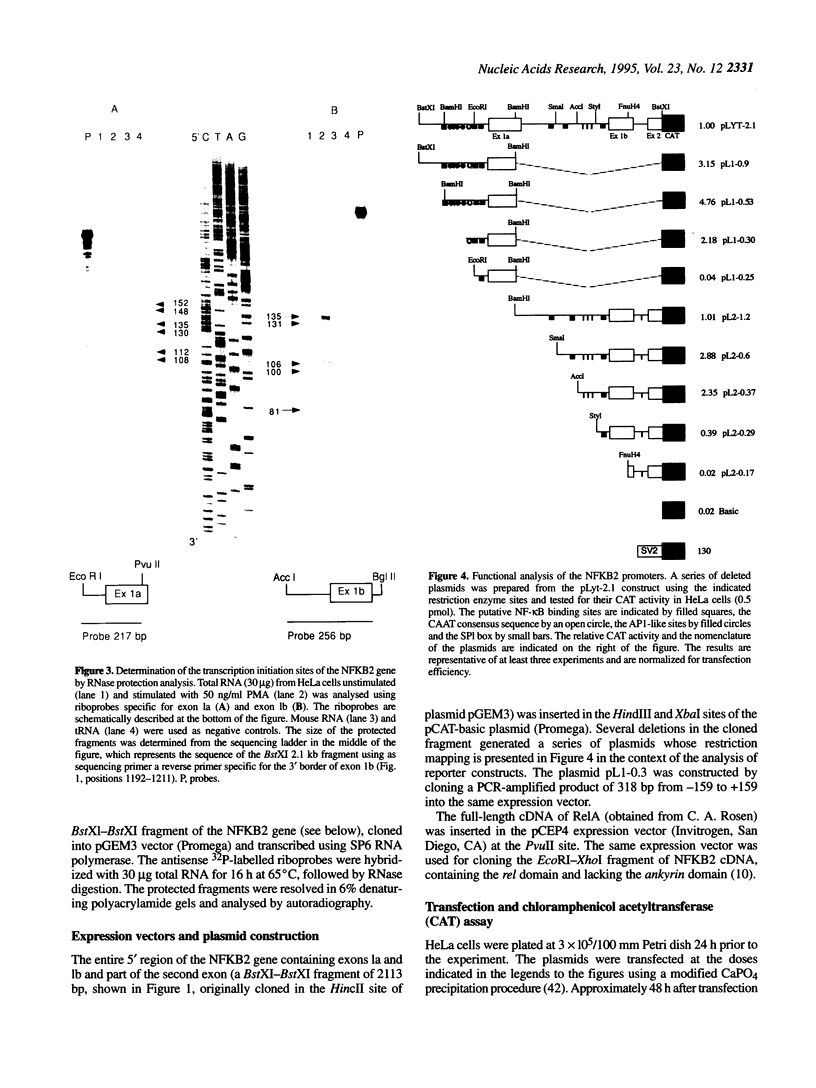
In order to clarify the transcriptional regulation of the NFKB2 gene (lyt-10, NF-kappa Bp100), we have characterized the structure and function of its promoter regions. Based on the nucleotide sequence of cDNA clones and the 5' flanking genomic region of the NFKB2 gene, RT-PCR analysis in a number of human cell lines demonstrated the presence of two alternative noncoding first exons (1a and 1b). Two distinct promoter regions, P1 and P2, were identified upstream of each exon, containing multiple sites of transcription initiation, as shown by RNase protection analysis. Sequence analysis of these regions showed a CAAT box upstream of exon 1a and high G-C content regions within both P1 and P2. Consensus binding sites for transcription factors, including SP1, AP1 and putative NF-kappa B (kappa B sites), were found upstream of each exon. In particular, six kappa B sites were identified, all but one of them capable of binding NF-kappa B complexes in vitro. Transfection in HeLa cells of plasmids containing P1 and P2 sequences linked to a chloramphenicol acetyltransferase reporter gene indicated that both P1 and P2 can act independently as promoters. Co-transfection of NF-kappa B effector plasmids (NF-kappa Bp52 and RelA) with a reporter gene linked to P1 and P2 showed that the NFKB2 promoter regions are regulated by NF-kappa B factors. RelA transactivates the NFKB2 promoter in a dose-dependent manner, whereas NF-kappa Bp52 acts as a repressor, indicating that the NFKB2 gene may be under the control of a negative feedback regulatory circuit.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anisowicz A., Messineo M., Lee S. W., Sager R. An NF-kappa B-like transcription factor mediates IL-1/TNF-alpha induction of gro in human fibroblasts. J Immunol. 1991 Jul 15;147(2):520–527. [PubMed] [Google Scholar]
- Baeuerle P. A. The inducible transcription activator NF-kappa B: regulation by distinct protein subunits. Biochim Biophys Acta. 1991 Apr 16;1072(1):63–80. doi: 10.1016/0304-419x(91)90007-8. [DOI] [PubMed] [Google Scholar]
- Blank V., Kourilsky P., Israël A. Cytoplasmic retention, DNA binding and processing of the NF-kappa B p50 precursor are controlled by a small region in its C-terminus. EMBO J. 1991 Dec;10(13):4159–4167. doi: 10.1002/j.1460-2075.1991.tb04994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bours V., Burd P. R., Brown K., Villalobos J., Park S., Ryseck R. P., Bravo R., Kelly K., Siebenlist U. A novel mitogen-inducible gene product related to p50/p105-NF-kappa B participates in transactivation through a kappa B site. Mol Cell Biol. 1992 Feb;12(2):685–695. doi: 10.1128/mcb.12.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bours V., Franzoso G., Azarenko V., Park S., Kanno T., Brown K., Siebenlist U. The oncoprotein Bcl-3 directly transactivates through kappa B motifs via association with DNA-binding p50B homodimers. Cell. 1993 Mar 12;72(5):729–739. doi: 10.1016/0092-8674(93)90401-b. [DOI] [PubMed] [Google Scholar]
- Bours V., Villalobos J., Burd P. R., Kelly K., Siebenlist U. Cloning of a mitogen-inducible gene encoding a kappa B DNA-binding protein with homology to the rel oncogene and to cell-cycle motifs. Nature. 1990 Nov 1;348(6296):76–80. doi: 10.1038/348076a0. [DOI] [PubMed] [Google Scholar]
- Brownell E., Mittereder N., Rice N. R. A human rel proto-oncogene cDNA containing an Alu fragment as a potential coding exon. Oncogene. 1989 Jul;4(7):935–942. [PubMed] [Google Scholar]
- Capobianco A. J., Gilmore T. D. Repression of the chicken c-rel promoter by vRel in chicken embryo fibroblasts is not mediated through a consensus NF-kappa B binding site. Oncogene. 1991 Dec;6(12):2203–2210. [PubMed] [Google Scholar]
- Cha Y., Deisseroth A. B. Human interferon regulatory factor 2 gene. Intron-exon organization and functional analysis of 5'-flanking region. J Biol Chem. 1994 Feb 18;269(7):5279–5287. [PubMed] [Google Scholar]
- Chang C. C., Zhang J., Lombardi L., Neri A., Dalla-Favera R. Mechanism of expression and role in transcriptional control of the proto-oncogene NFKB-2/LYT-10. Oncogene. 1994 Mar;9(3):923–933. [PubMed] [Google Scholar]
- Davis N., Ghosh S., Simmons D. L., Tempst P., Liou H. C., Baltimore D., Bose H. R., Jr Rel-associated pp40: an inhibitor of the rel family of transcription factors. Science. 1991 Sep 13;253(5025):1268–1271. doi: 10.1126/science.1891714. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fracchiolla N. S., Lombardi L., Salina M., Migliazza A., Baldini L., Berti E., Cro L., Polli E., Maiolo A. T., Neri A. Structural alterations of the NF-kappa B transcription factor lyt-10 in lymphoid malignancies. Oncogene. 1993 Oct;8(10):2839–2845. [PubMed] [Google Scholar]
- Ghosh S., Gifford A. M., Riviere L. R., Tempst P., Nolan G. P., Baltimore D. Cloning of the p50 DNA binding subunit of NF-kappa B: homology to rel and dorsal. Cell. 1990 Sep 7;62(5):1019–1029. doi: 10.1016/0092-8674(90)90276-k. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilli M., Chiu J. J., Lenardo M. J. NF-kappa B and Rel: participants in a multiform transcriptional regulatory system. Int Rev Cytol. 1993;143:1–62. doi: 10.1016/s0074-7696(08)61873-2. [DOI] [PubMed] [Google Scholar]
- Grumont R. J., Gerondakis S. Structure of a mammalian c-rel protein deduced from the nucleotide sequence of murine cDNA clones. Oncogene Res. 1989;4(1):1–8. [PubMed] [Google Scholar]
- Grumont R. J., Richardson I. B., Gaff C., Gerondakis S. rel/NF-kappa B nuclear complexes that bind kB sites in the murine c-rel promoter are required for constitutive c-rel transcription in B-cells. Cell Growth Differ. 1993 Sep;4(9):731–743. [PubMed] [Google Scholar]
- Hannink M., Temin H. M. Structure and autoregulation of the c-rel promoter. Oncogene. 1990 Dec;5(12):1843–1850. [PubMed] [Google Scholar]
- Hannink M., Temin H. M. Transactivation of gene expression by nuclear and cytoplasmic rel proteins. Mol Cell Biol. 1989 Oct;9(10):4323–4336. doi: 10.1128/mcb.9.10.4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen S. K., Nerlov C., Zabel U., Verde P., Johnsen M., Baeuerle P. A., Blasi F. A novel complex between the p65 subunit of NF-kappa B and c-Rel binds to a DNA element involved in the phorbol ester induction of the human urokinase gene. EMBO J. 1992 Jan;11(1):205–213. doi: 10.1002/j.1460-2075.1992.tb05043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskill S., Beg A. A., Tompkins S. M., Morris J. S., Yurochko A. D., Sampson-Johannes A., Mondal K., Ralph P., Baldwin A. S., Jr Characterization of an immediate-early gene induced in adherent monocytes that encodes I kappa B-like activity. Cell. 1991 Jun 28;65(7):1281–1289. doi: 10.1016/0092-8674(91)90022-q. [DOI] [PubMed] [Google Scholar]
- Hatada E. N., Nieters A., Wulczyn F. G., Naumann M., Meyer R., Nucifora G., McKeithan T. W., Scheidereit C. The ankyrin repeat domains of the NF-kappa B precursor p105 and the protooncogene bcl-3 act as specific inhibitors of NF-kappa B DNA binding. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2489–2493. doi: 10.1073/pnas.89.6.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkel T., Zabel U., van Zee K., Müller J. M., Fanning E., Baeuerle P. A. Intramolecular masking of the nuclear location signal and dimerization domain in the precursor for the p50 NF-kappa B subunit. Cell. 1992 Mar 20;68(6):1121–1133. doi: 10.1016/0092-8674(92)90083-o. [DOI] [PubMed] [Google Scholar]
- Inoue J., Kerr L. D., Kakizuka A., Verma I. M. I kappa B gamma, a 70 kd protein identical to the C-terminal half of p110 NF-kappa B: a new member of the I kappa B family. Cell. 1992 Mar 20;68(6):1109–1120. doi: 10.1016/0092-8674(92)90082-n. [DOI] [PubMed] [Google Scholar]
- Kanno M., Fromental C., Staub A., Ruffenach F., Davidson I., Chambon P. The SV40 TC-II(kappa B) and the related H-2Kb enhansons exhibit different cell type specific and inducible proto-enhancer activities, but the SV40 core sequence and the AP-2 binding site have no enhanson properties. EMBO J. 1989 Dec 20;8(13):4205–4214. doi: 10.1002/j.1460-2075.1989.tb08606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieran M., Blank V., Logeat F., Vandekerckhove J., Lottspeich F., Le Bail O., Urban M. B., Kourilsky P., Baeuerle P. A., Israël A. The DNA binding subunit of NF-kappa B is identical to factor KBF1 and homologous to the rel oncogene product. Cell. 1990 Sep 7;62(5):1007–1018. doi: 10.1016/0092-8674(90)90275-j. [DOI] [PubMed] [Google Scholar]
- Le Bail O., Schmidt-Ullrich R., Israël A. Promoter analysis of the gene encoding the I kappa B-alpha/MAD3 inhibitor of NF-kappa B: positive regulation by members of the rel/NF-kappa B family. EMBO J. 1993 Dec 15;12(13):5043–5049. doi: 10.1002/j.1460-2075.1993.tb06197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilienbaum A., Duc Dodon M., Alexandre C., Gazzolo L., Paulin D. Effect of human T-cell leukemia virus type I tax protein on activation of the human vimentin gene. J Virol. 1990 Jan;64(1):256–263. doi: 10.1128/jvi.64.1.256-263.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link D. C., Gutkind S. J., Robbins K. C., Ley T. J. Characterization of the 5' untranslated region of the human c-fgr gene and identification of the major myelomonocytic c-fgr promoter. Oncogene. 1992 May;7(5):877–884. [PubMed] [Google Scholar]
- Liou H. C., Nolan G. P., Ghosh S., Fujita T., Baltimore D. The NF-kappa B p50 precursor, p105, contains an internal I kappa B-like inhibitor that preferentially inhibits p50. EMBO J. 1992 Aug;11(8):3003–3009. doi: 10.1002/j.1460-2075.1992.tb05370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
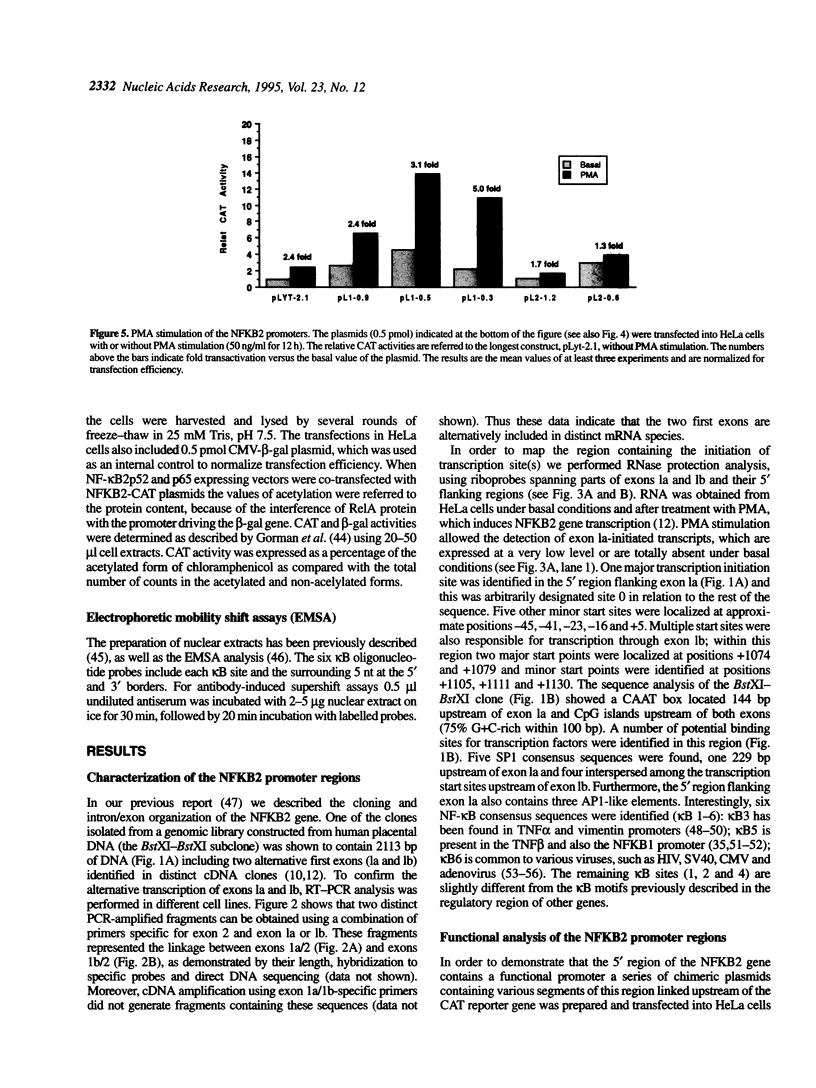
- Liptay S., Schmid R. M., Nabel E. G., Nabel G. J. Transcriptional regulation of NF-kappa B2: evidence for kappa B-mediated positive and negative autoregulation. Mol Cell Biol. 1994 Dec;14(12):7695–7703. doi: 10.1128/mcb.14.12.7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi L., Newcomb E. W., Dalla-Favera R. Pathogenesis of Burkitt lymphoma: expression of an activated c-myc oncogene causes the tumorigenic conversion of EBV-infected human B lymphoblasts. Cell. 1987 Apr 24;49(2):161–170. doi: 10.1016/0092-8674(87)90556-3. [DOI] [PubMed] [Google Scholar]
- Lux S. E., John K. M., Bennett V. Analysis of cDNA for human erythrocyte ankyrin indicates a repeated structure with homology to tissue-differentiation and cell-cycle control proteins. Nature. 1990 Mar 1;344(6261):36–42. doi: 10.1038/344036a0. [DOI] [PubMed] [Google Scholar]
- Mercurio F., DiDonato J. A., Rosette C., Karin M. p105 and p98 precursor proteins play an active role in NF-kappa B-mediated signal transduction. Genes Dev. 1993 Apr;7(4):705–718. doi: 10.1101/gad.7.4.705. [DOI] [PubMed] [Google Scholar]
- Mercurio F., Didonato J., Rosette C., Karin M. Molecular cloning and characterization of a novel Rel/NF-kappa B family member displaying structural and functional homology to NF-kappa B p50/p105. DNA Cell Biol. 1992 Sep;11(7):523–537. doi: 10.1089/dna.1992.11.523. [DOI] [PubMed] [Google Scholar]
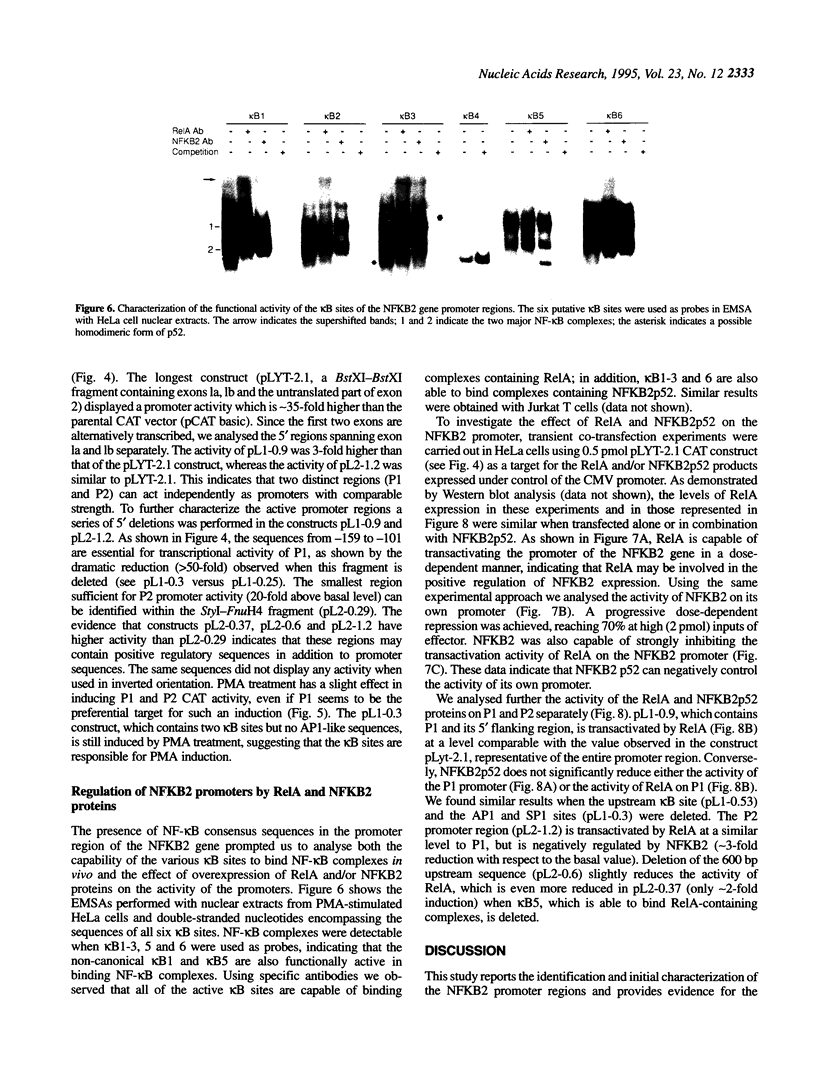
- Migliazza A., Lombardi L., Rocchi M., Trecca D., Chang C. C., Antonacci R., Fracchiolla N. S., Ciana P., Maiolo A. T., Neri A. Heterogeneous chromosomal aberrations generate 3' truncations of the NFKB2/lyt-10 gene in lymphoid malignancies. Blood. 1994 Dec 1;84(11):3850–3860. [PubMed] [Google Scholar]
- Muegge K., Durum S. K. Cytokines and transcription factors. Cytokine. 1990 Jan;2(1):1–8. doi: 10.1016/1043-4666(90)90036-s. [DOI] [PubMed] [Google Scholar]
- Nabel G., Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987 Apr 16;326(6114):711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- Neri A., Chang C. C., Lombardi L., Salina M., Corradini P., Maiolo A. T., Chaganti R. S., Dalla-Favera R. B cell lymphoma-associated chromosomal translocation involves candidate oncogene lyt-10, homologous to NF-kappa B p50. Cell. 1991 Dec 20;67(6):1075–1087. doi: 10.1016/0092-8674(91)90285-7. [DOI] [PubMed] [Google Scholar]
- Nolan G. P., Ghosh S., Liou H. C., Tempst P., Baltimore D. DNA binding and I kappa B inhibition of the cloned p65 subunit of NF-kappa B, a rel-related polypeptide. Cell. 1991 Mar 8;64(5):961–969. doi: 10.1016/0092-8674(91)90320-x. [DOI] [PubMed] [Google Scholar]
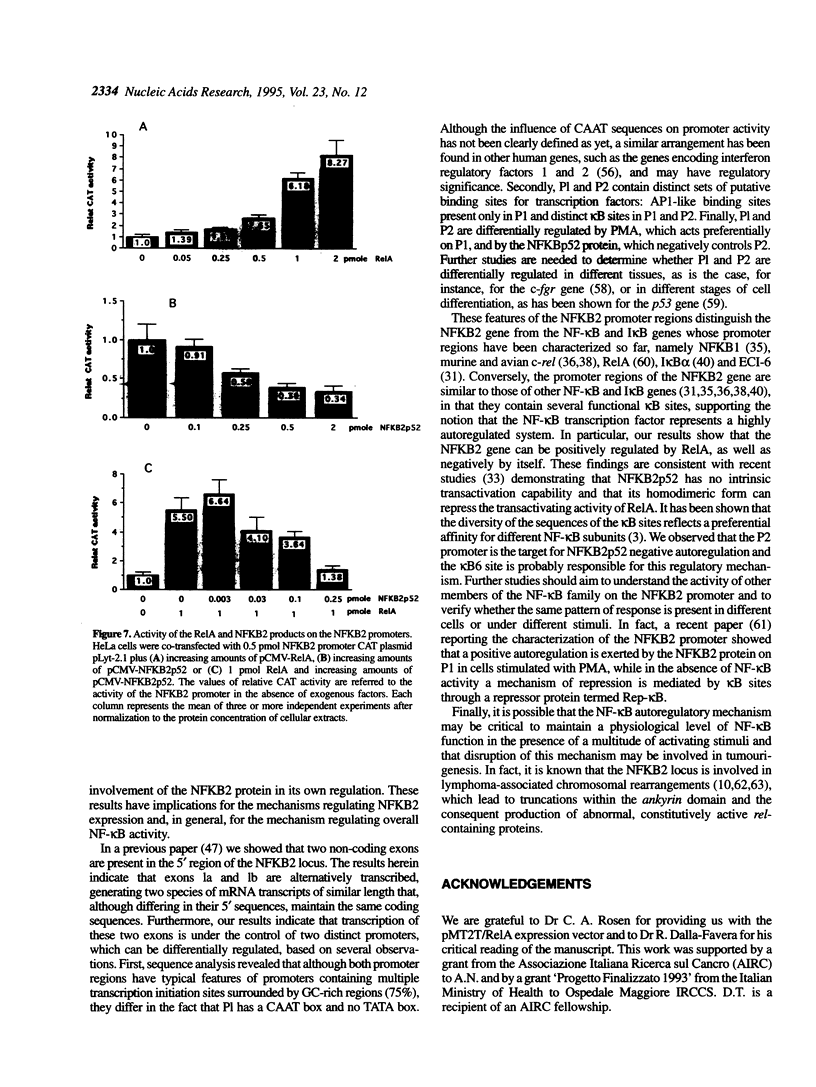
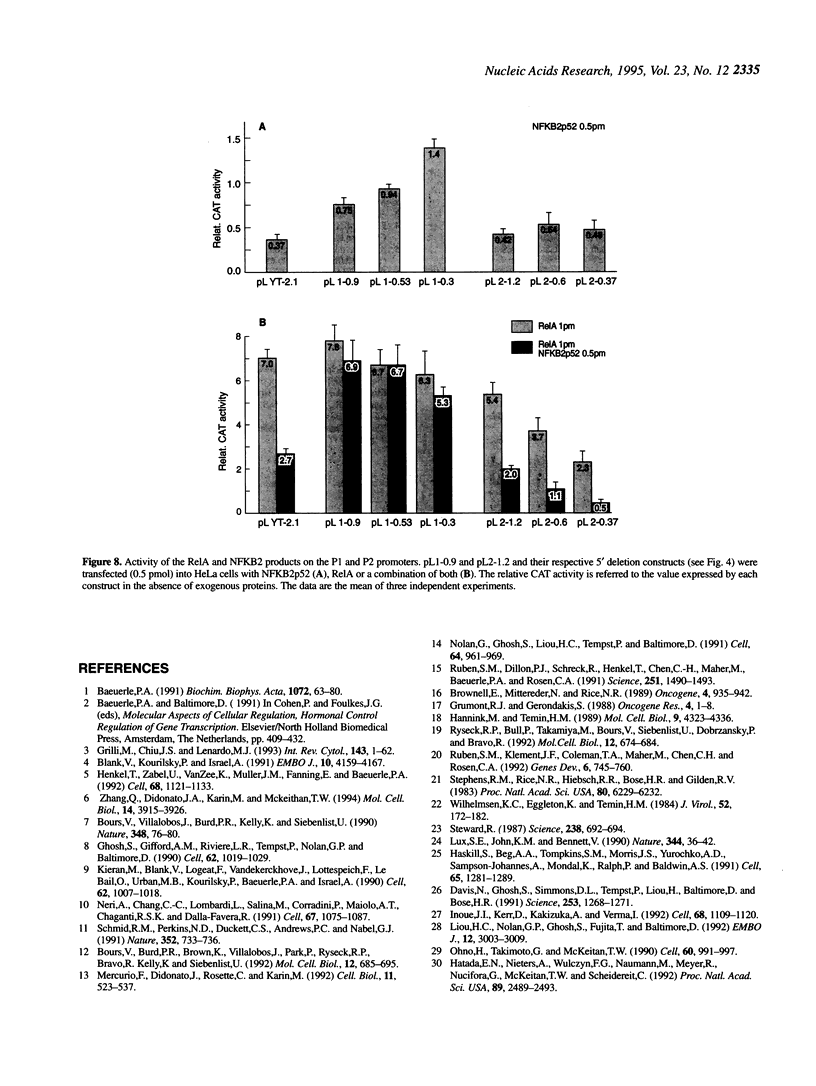
- Ohno H., Takimoto G., McKeithan T. W. The candidate proto-oncogene bcl-3 is related to genes implicated in cell lineage determination and cell cycle control. Cell. 1990 Mar 23;60(6):991–997. doi: 10.1016/0092-8674(90)90347-h. [DOI] [PubMed] [Google Scholar]
- Paul N. L., Lenardo M. J., Novak K. D., Sarr T., Tang W. L., Ruddle N. H. Lymphotoxin activation by human T-cell leukemia virus type I-infected cell lines: role for NF-kappa B. J Virol. 1990 Nov;64(11):5412–5419. doi: 10.1128/jvi.64.11.5412-5419.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman D., Rotter V. Two promoters that map to 5'-sequences of the human p53 gene are differentially regulated during terminal differentiation of human myeloid leukemic cells. Oncogene. 1989 Aug;4(8):945–953. [PubMed] [Google Scholar]
- Ruben S. M., Dillon P. J., Schreck R., Henkel T., Chen C. H., Maher M., Baeuerle P. A., Rosen C. A. Isolation of a rel-related human cDNA that potentially encodes the 65-kD subunit of NF-kappa B. Science. 1991 Mar 22;251(5000):1490–1493. doi: 10.1126/science.2006423. [DOI] [PubMed] [Google Scholar]
- Ruben S. M., Klement J. F., Coleman T. A., Maher M., Chen C. H., Rosen C. A. I-Rel: a novel rel-related protein that inhibits NF-kappa B transcriptional activity. Genes Dev. 1992 May;6(5):745–760. doi: 10.1101/gad.6.5.745. [DOI] [PubMed] [Google Scholar]
- Ryseck R. P., Bull P., Takamiya M., Bours V., Siebenlist U., Dobrzanski P., Bravo R. RelB, a new Rel family transcription activator that can interact with p50-NF-kappa B. Mol Cell Biol. 1992 Feb;12(2):674–684. doi: 10.1128/mcb.12.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambucetti L. C., Cherrington J. M., Wilkinson G. W., Mocarski E. S. NF-kappa B activation of the cytomegalovirus enhancer is mediated by a viral transactivator and by T cell stimulation. EMBO J. 1989 Dec 20;8(13):4251–4258. doi: 10.1002/j.1460-2075.1989.tb08610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid R. M., Perkins N. D., Duckett C. S., Andrews P. C., Nabel G. J. Cloning of an NF-kappa B subunit which stimulates HIV transcription in synergy with p65. Nature. 1991 Aug 22;352(6337):733–736. doi: 10.1038/352733a0. [DOI] [PubMed] [Google Scholar]
- Shakhov A. N., Collart M. A., Vassalli P., Nedospasov S. A., Jongeneel C. V. Kappa B-type enhancers are involved in lipopolysaccharide-mediated transcriptional activation of the tumor necrosis factor alpha gene in primary macrophages. J Exp Med. 1990 Jan 1;171(1):35–47. doi: 10.1084/jem.171.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. M., Rice N. R., Hiebsch R. R., Bose H. R., Jr, Gilden R. V. Nucleotide sequence of v-rel: the oncogene of reticuloendotheliosis virus. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6229–6233. doi: 10.1073/pnas.80.20.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward R. Dorsal, an embryonic polarity gene in Drosophila, is homologous to the vertebrate proto-oncogene, c-rel. Science. 1987 Oct 30;238(4827):692–694. doi: 10.1126/science.3118464. [DOI] [PubMed] [Google Scholar]
- Sun S. C., Ganchi P. A., Ballard D. W., Greene W. C. NF-kappa B controls expression of inhibitor I kappa B alpha: evidence for an inducible autoregulatory pathway. Science. 1993 Mar 26;259(5103):1912–1915. doi: 10.1126/science.8096091. [DOI] [PubMed] [Google Scholar]
- Ten R. M., Paya C. V., Israël N., Le Bail O., Mattei M. G., Virelizier J. L., Kourilsky P., Israël A. The characterization of the promoter of the gene encoding the p50 subunit of NF-kappa B indicates that it participates in its own regulation. EMBO J. 1992 Jan;11(1):195–203. doi: 10.1002/j.1460-2075.1992.tb05042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueberla K., Lu Y., Chung E., Haseltine W. A. The NF-kappa B p65 promoter. J Acquir Immune Defic Syndr. 1993 Mar;6(3):227–230. [PubMed] [Google Scholar]
- Wilhelmsen K. C., Eggleton K., Temin H. M. Nucleic acid sequences of the oncogene v-rel in reticuloendotheliosis virus strain T and its cellular homolog, the proto-oncogene c-rel. J Virol. 1984 Oct;52(1):172–182. doi: 10.1128/jvi.52.1.172-182.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. L., Garcia J., Harrich D., Pearson L., Wu F., Gaynor R. Lymphoid specific gene expression of the adenovirus early region 3 promoter is mediated by NF-kappa B binding motifs. EMBO J. 1990 Dec;9(13):4435–4442. doi: 10.1002/j.1460-2075.1990.tb07894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Chang C. C., Lombardi L., Dalla-Favera R. Rearranged NFKB2 gene in the HUT78 T-lymphoma cell line codes for a constitutively nuclear factor lacking transcriptional repressor functions. Oncogene. 1994 Jul;9(7):1931–1937. [PubMed] [Google Scholar]
- Zhang Q., Didonato J. A., Karin M., McKeithan T. W. BCL3 encodes a nuclear protein which can alter the subcellular location of NF-kappa B proteins. Mol Cell Biol. 1994 Jun;14(6):3915–3926. doi: 10.1128/mcb.14.6.3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Martin R., Vanhove B., Cheng Q., Hofer E., Csizmadia V., Winkler H., Bach F. H. Cytokine-inducible expression in endothelial cells of an I kappa B alpha-like gene is regulated by NF kappa B. EMBO J. 1993 Jul;12(7):2773–2779. doi: 10.1002/j.1460-2075.1993.tb05938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]