Higher serum testosterone is associated with higher bone mineral density, greater lean body mass, and greater total fat mass in women aged 65 and older.
Abstract
Context:
The physiological importance of endogenous testosterone (T) in older women is poorly understood.
Objective:
The aim of the study was to determine the association of higher total and free T levels with bone mineral density (BMD), lean body mass, and fat mass in elderly women.
Design:
Total and free T were measured using sensitive assays in 232 community-dwelling women aged 67–94 yr who were enrolled in the Cardiovascular Health Study and had dual-energy x-ray absorptiometry scans. Cross-sectional analyses were performed to examine associations between total and free T and BMD and body composition.
Results:
In adjusted models, total T was directly associated with BMD at the lumbar spine (P = 0.04) and hip (P = 0.001), but not body composition outcomes, in all women, and after excluding estrogen users and adjusting for estradiol (P = 0.04 and 0.01, respectively). Free T was positively related to hip BMD, lean body mass, and body fat (all P < 0.05), with more than 10% differences in each outcome between women at the highest and lowest ends of the free T range, with attenuation after excluding estrogen users and adjusting for estradiol.
Conclusions:
In the setting of the low estradiol levels found in older women, circulating T levels were associated with bone density. Women with higher free T levels had greater lean body mass, consistent with the anabolic effect of T, and, in contrast to men, greater fat mass. Mechanistic studies are required to determine whether a causal relationship exists between T, bone, and body composition in this population and the degree to which any T effects are estrogen-independent.
Little is known about the role of endogenous testosterone (T) in women over the age of 65. In older men, associations between endogenous T and bone mineral density (BMD) and body composition have been described (1–4). Furthermore, T therapy has been shown to increase vertebral BMD and skeletal muscle mass and decrease fat mass in randomized trials of older men (5–7). Androgen receptors (ARs) are expressed on osteoblasts and osteocytes; T affects osteoblastic bone formation and osteoclastic bone resorption directly through AR signaling, indirectly through its aromatization to estrogen, and through multiple other potential mechanisms (8). T may also promote myogenic differentiation of multipotent mesenchymal progenitor cells (9).
Small studies have shown that T therapy in postmenopausal women increases BMD and lean body mass, while decreasing fat mass (10–13). Furthermore, higher levels of endogenous T in postmenopausal women are associated with a decreased risk of hip fracture (14, 15). Limited data suggest a positive association of T with muscle mass in older women (16), although its association with fat mass may be opposite to that seen in men, with recent reports of associations between higher T and greater adiposity and insulin resistance in older women (17, 18). A syndrome of female androgen insufficiency has been proposed to result in persistent fatigue and sexual dysfunction with potential reductions in BMD, muscle strength, and cognition (19), although experts agree that more data are needed regarding BMD and muscle outcomes (20–22).
This cross-sectional study of 232 U.S. community-dwelling older women was conducted to determine whether T levels in women aged 65 and older are associated with BMD and body composition. We hypothesized that higher T levels would be associated with higher BMD, greater lean body mass, and more fat mass, and we predicted that associations would be stronger for free T than for total T.
Subjects and Methods
Study population
The Cardiovascular Health Study (CHS) is a population-based, longitudinal study of 5888 adults aged 65 and older (23). Enrollment of an original cohort of 5201 adults occurred between 1989 and 1990, and in 1992–1993 an additional 687 predominantly African-American participants were enrolled. Eligible individuals were identified from an age- and gender-stratified random sample of the Medicare eligibility rosters in four U.S. communities: Washington County, Maryland; Pittsburgh, Pennsylvania; Sacramento County, California; and Forsyth County, North Carolina. To be eligible, individuals had to be noninstitutionalized, expecting to remain in the area for the following 3 yr, not under active cancer treatment, not wheelchair-bound in the home, not requiring a proxy respondent at entry, and capable of providing consent. Household members of the sampled individuals were recruited, if eligible. The institutional review boards of all four sites and the coordinating center at the University of Washington in Seattle approved the study, and all participants gave informed consent.
A random sample of 368 women in both cohorts seen at the 1992–1993 study visit was later selected for measurement of total and free T, as described previously (17, 24). Total and free T measurements were repeated in women who returned to the 1994–1995 visit and had available serum (n = 311). This visit included a medical history, physical examination, assessment of health status, and phlebotomy. Our analyses are based on data from the 232 women who had dual-energy x-ray absorptiometry (DXA) scans at this visit (performed at Pittsburgh and Sacramento sites only) and were not taking bisphosphonates or corticosteroids. Total T, free T, and health characteristics did not differ between the 232 women with and the 79 women without DXA scans.
Study measures
Serum total T concentrations were measured by RIA using iodinated T as a tracer (25). This assay was developed and validated for the low range of T prevalent in HIV-infected (25–27) and postmenopausal women (28). The sensitivity, defined as hormone concentration corresponding to 90% bound in the presence and absence of analyte point, was 0.22 ng/dl (0.008 nmol/liter). The intraassay coefficient of variation (CV) was 8.2%. Interassay CVs were measured in the low, medium, and high female pool and were 13.2% in the medium pool. In other pools, interassay CVs ranged from 13 to 15%. This assay was validated against liquid chromatography-tandem mass spectrometry (LC-MS/MS) (27). These measurements demonstrated a correlation of 0.997 between the RIA and LC-MS/MS measurements. Free T concentrations were measured by a sensitive equilibrium dialysis assay (25), optimized to precisely and accurately measure low concentrations. The sensitivity of this assay is 0.3 pg/ml (1.0 pmol/liter). Ten replicates of low, medium, and high female pools were used to generate intra- and interassay CVs. The respective intraassay CVs for the low, medium, and high female pools were 5.6% for the low pool, 4.6% for the medium pool, and 2.6% for the high pool (25). The interassay CVs were in the 12–15% range. Cross-reactivity of the major steroids (dehydroepiandrosterone, dehydroepiandrosterone sulfate, dihydrotestosterone, and androstenedione) was less than 1%. Estradiol levels were measured by Esoterix, Inc. (Calabasas Hills, CA) by LC-MS/MS in non-estrogen-using women who had available serum for analysis (n = 184). The sensitivity was 1 pg/ml (3.7 pmol/liter), with an intraassay CV of 2.3% and interassay CV of 4.4% in the low range.
Baseline characteristics included age, race, weight, education, smoking status, alcohol use, activity level (blocks walked last week), and estrogen use. Medication use was determined from examination of medication bottles at the study visit. Body mass index (BMI) (kilograms/meter2), computed from objective measures, was categorized as less than 18.5, 18.5–24.9, 25–29.9, or 30 or greater.
Hip and whole body DXA scans were obtained using Hologic QDR-2000 densitometers (Hologic, Inc., Waltham, MA) with a protocol similar to that in the Study of Osteoporotic Fractures and the Fracture Intervention Trial (29, 30). Scans were read blindly at the University of California, San Francisco Reading Center using Hologic software, version 7.10. The total hip BMD was chosen as the most representative and reproducible of the hip measurements with a CV less than 0.75% (31). Lumbar spine bone density was estimated from whole body scans.
Statistical analysis
Women were categorized as being normal, having low bone mass, or osteoporotic using the World Health Organization osteoporosis classification (32) based on the total BMD of the hip; total and free T levels were compared among the three categories using a trend test for all women and repeated excluding estrogen users. Total and free T were plotted against each BMD and body composition outcome (total hip BMD, lumbar spine BMD, lean body mass, and absolute and percentage total body fat) to detect evidence for nonlinear associations. Paired t tests and one-way ANOVAs were performed to determine factors associated with each BMD and body composition outcome. Factors found to be statistically significant at a P < 0.10 level, based on two-sided tests, were included in multivariable linear regression models predicting BMD and body composition outcomes. Total and free T, BMD measures, and total lean body mass were log-transformed to achieve normality. BMD models were adjusted for age, race, weight, and estrogen use. Lean body mass models were adjusted for age, race, and activity level, whereas both total body fat and percentage body fat models were adjusted for age, race, alcohol use, and activity level. All analyses were repeated in the 189 women who were not taking estrogen and, repeated adjusting for estradiol in the 184 women in whom estradiol levels were performed.
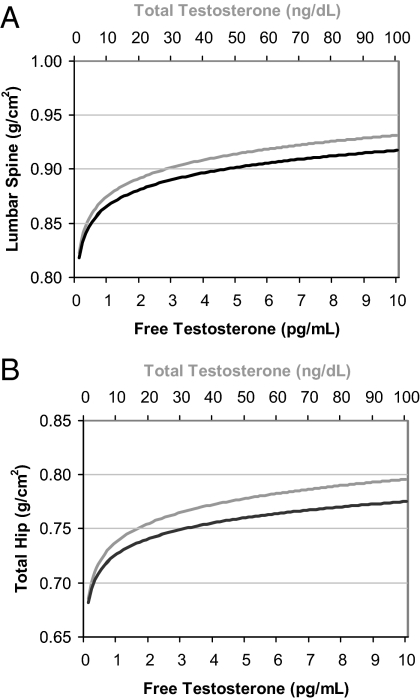
To provide estimates of the clinical significance of findings from the multivariable models, estimates of BMD and body composition outcomes were displayed graphically across a range of total and free T concentrations for a hypothetical woman whose remaining model covariates were at the predominant value for categorical variables or mean values for continuous variables. T levels were back-transformed for interpretation on the original T scales. For example, predicted hip bone densities for a 75-yr-old, 68-kg, non-estrogen-using Caucasian woman across a range of total and free T levels are displayed (see Fig. 2).
Fig. 2.
Predicted lumbar spine (A) and total hip (B) bone density for a 75-yr-old, 68-kg, non-estrogen-using Caucasian woman. Multiply by 0.0347 to convert total T concentrations from nanograms/deciliter to nanomoles/liter. Multiply by 3.47 to convert free T concentrations from picograms/milliliter to picomoles/liter.
Results
Baseline characteristics
The 232 U.S. community-dwelling women ranged in age from 67 to 94 yr, with a mean age of 75.6 yr (Table 1). Seventy-nine percent were Caucasian. The mean BMI in this cohort was 27.2 kg/m2. A small number of women (18%) reported estrogen use at the time of the study. On average, the total T level in this cohort was 12 ng/dl (0.42 nmol/liter), and the free T level was 1.6 pg/ml (5.6 pmol/liter).
Table 1.
Baseline characteristics of the study sample (n = 232)
| Measurement | |
|---|---|
| Age (yr) | 75.6 (4.6) |
| Race (% Caucasian) | 79 |
| Estrogen use (%) | 18 |
| Alcohol use (%) | |
| None | 48 |
| Any | 38 |
| Daily | 14 |
| BMI (kg/m2) | 27.2 (5) |
| Median blocks walked in last week | 12 |
| Total T (ng/dl) | 12 (14) |
| Free T (pg/ml) | 1.6 (1.6) |
Data represent mean (sd) unless otherwise stated. Multiply by 0.0347 to convert total T concentrations from ng/dl to nmol/liter. Multiply by 3.47 to convert free T concentrations from pg/ml to pmol/liter.
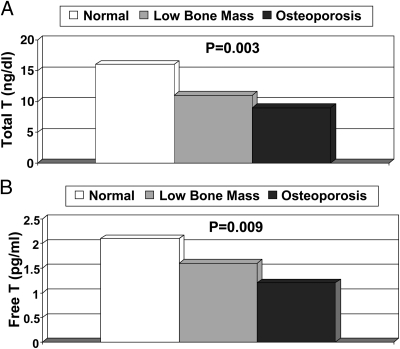
Mean total and free T levels by osteoporosis classification of the hip
Overall, there was a significant stepwise increase in hip bone mass with higher levels of total and free T (P = 0.003 and 0.009, respectively; Fig. 1). The lowest mean total T [8.6 ng/dl (0.30 nmol/liter)] and free T [1.2 pg/ml (4.2 pmol/liter)] levels were seen in women with osteoporosis of the hip; intermediate levels [11.0 ng/dl (0.38 nmol/liter) and 1.6 pg/ml (5.6 pmol/liter)] were seen in those with low bone mass; and the highest levels [16.3 ng/dl (0.57 nmol/liter) and 2.1 pg/ml (7.29 nmol/liter)] were seen in those with normal BMD. After excluding the 43 women taking estrogen, similar results were seen for osteoporosis, low bone mass, and normal BMD for total T (7.1, 11.2, and 18.5 ng/dl; P < 0.001) and free T (1.2, 1.7, and 2.3 pg/ml; P = 0.02).
Fig. 1.
Mean total T (A) and free T (B) levels by osteoporosis classification of total hip BMD. Osteoporosis was defined as a T-score ≤ 2.5 sd, low bone mass as a T-score of −1 to −2.5 sd, and normal BMD as T-score ≥ −1 sd, per World Health Organization classification (32). Multiply by 0.0347 to convert total T concentrations from nanograms/deciliter to nanomoles/liter. Multiply by 3.47 to convert free T concentrations from picograms/milliliter to picomoles/liter.
Bone density and body composition outcomes
In Table 2, we present crude and adjusted estimates of log-transformed total and free T levels with BMD and body composition outcomes. In adjusted models, total T was directly associated with BMD at the lumbar spine (P = 0.04) and hip (P = 0.001), but not with any of the body composition outcomes. The BMD associations were unchanged after excluding the estrogen users (P = 0.02 at the lumbar spine, and P = 0.004 at the hip) and additionally adjusting for estradiol levels in this subgroup (P = 0.04 and 0.01, respectively). Free T was not significantly associated with lumbar spine BMD (P = 0.12), but was positively associated with total hip BMD (P = 0.03), total lean body mass (P = 0.01), total body fat (P = 0.007), and percentage fat (P = 0.02). After exclusion of the estrogen users, free T remained associated with total body fat (P = 0.04), but not with total hip BMD, total lean body mass, and percentage body fat (P = 0.05–0.12). Additional adjustment for estradiol levels attenuated the magnitude of associations between free T and outcomes, all of which were not statistically significant. No threshold relationships were seen between total or free T and any of the outcomes.
Table 2.
Crude and adjusted models of total and free T and bone density and body composition outcomes
| Outcome | Crude β coefficient | P value | Adjusted β coefficient | P value |
|---|---|---|---|---|
| Log lumbar spine BMD | ||||
| Log total T | ||||
| All women | 0.033 | 0.02 | 0.027 | 0.04 |
| Excluding estrogen users | 0.040 | 0.01 | 0.032 | 0.02 |
| Excluding estrogen users + E2 adjusted | 0.030 | 0.04 | ||
| Log free T | ||||
| All women | 0.044 | 0.01 | 0.025 | 0.12 |
| Excluding estrogen users | 0.045 | 0.02 | 0.020 | 0.25 |
| Excluding estrogen users + E2 adjusted | 0.016 | 0.36 | ||
| Log total hip BMD | ||||
| Log total T | ||||
| All women | 0.038 | 0.002 | 0.032 | 0.001 |
| Excluding estrogen users | 0.042 | 0.002 | 0.032 | 0.004 |
| Excluding estrogen users + E2 adjusted | 0.029 | 0.01 | ||
| Log free T | ||||
| All women | 0.046 | 0.002 | 0.028 | 0.03 |
| Excluding estrogen users | 0.044 | 0.007 | 0.021 | 0.12 |
| Excluding estrogen users + E2 adjusted | 0.020 | 0.14 | ||
| Log lean body mass | ||||
| Log total T | ||||
| All women | 0.005 | 0.56 | −0.001 | 0.92 |
| Excluding estrogen users | 0.005 | 0.61 | −0.006 | 0.51 |
| Excluding estrogen users + E2 adjusted | −0.012 | 0.22 | ||
| Log free T | ||||
| All women | 0.031 | 0.005 | 0.024 | 0.01 |
| Excluding estrogen users | 0.031 | 0.009 | 0.020 | 0.06 |
| Excluding estrogen users + E2 adjusted | 0.016 | 0.16 | ||
| Total body fat (kg) | ||||
| Log total T | ||||
| All women | 0.61 | 0.39 | 0.23 | 0.73 |
| Excluding estrogen users | 0.74 | 0.36 | 0.12 | 0.87 |
| Excluding estrogen users + E2 adjusted | −0.75 | 0.31 | ||
| Log free T | ||||
| All women | 2.68 | 0.002 | 2.11 | 0.007 |
| Excluding estrogen users | 2.91 | 0.002 | 1.88 | 0.04 |
| Excluding estrogen users + E2 adjusted | 1.38 | 0.11 | ||
| Percentage body fat | ||||
| Log total T | ||||
| All women | 0.31 | 0.54 | 0.05 | 0.91 |
| Excluding estrogen users | 0.38 | 0.51 | 0.08 | 0.88 |
| Excluding estrogen users + E2 adjusted | −0.38 | 0.50 | ||
| Log free T | ||||
| All women | 1.71 | 0.005 | 1.39 | 0.02 |
| Excluding estrogen users | 1.80 | 0.008 | 1.27 | 0.05 |
| Excluding estrogen users + E2 adjusted | 1.03 | 0.10 |
β-Coefficients represent the change in outcome for a one log unit increase in T level and are not back-transformed. BMD models with all women were adjusted for age, race, weight, and estrogen use. BMD models without estrogen users were adjusted for age, race, and weight. Lean body mass models were adjusted for age, race, and activity level. Body fat models were adjusted for age, race, activity level, and alcohol use. Statistically significant values are indicated in bold. E2, Estradiol.
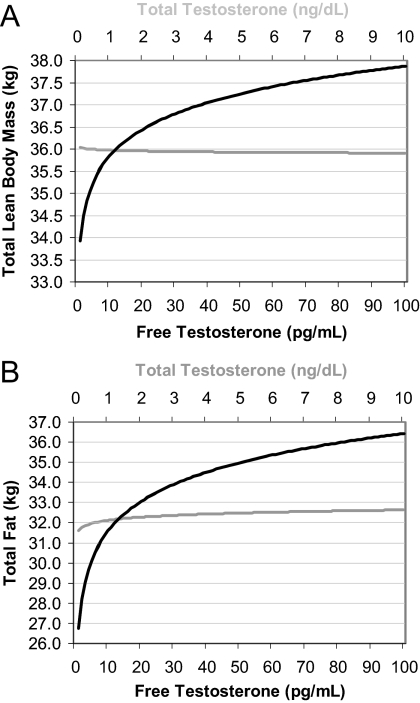
Because the estimated β coefficients are unable to be interpreted clinically, Fig. 2 displays the predicted lumbar spine and hip BMDs for a hypothetical woman whose model covariates were at the predominant value for categorical variables or mean values for continuous variables. This figure shows that predicted lumbar spine and hip BMDs increased nonlinearly and to the same degree with increasing level of total and free T, with a 0.1 g/cm2 difference between women at the highest and lowest ends of the total or free T range. A similar effect was seen in the relationship between free T and predicted lean body mass (3.9-kg difference) and fat mass (9.7-kg difference) for a 68-kg woman, but not for total T, with no difference in body composition across the spectrum of total T values (Fig. 3).
Fig. 3.
Predicted lean body mass (A) and fat mass (B) for a 75-yr-old, nondrinking, average activity, Caucasian woman. Multiply by 0.0347 to convert total T concentrations from nanograms/deciliter to nanomoles/liter. Multiply by 3.47 to convert free T concentrations from picograms/milliliter to picomoles/liter.
Discussion
Our findings indicate that older women with higher circulating concentrations of T have significantly greater BMD, independent of weight and other confounding factors, suggesting that circulating androgens may play a role in maintenance of bone density in the setting of the low estradiol levels typical for this age group of women. Women with higher free T levels also had greater lean body mass, consistent with an anabolic effect of T, even at the low levels seen in women of this age group. However, in contrast to what is seen in men, women with higher free T levels had higher absolute and total percentage body fat than their counterparts with lower free T levels.
Our findings of associations between T and BMD in older women are consistent with several other studies showing a positive relationship with BMD (1, 2, 16, 33, 34) and decreased incidence of fracture (14, 15), although not with three studies reporting an absence of association between T and fracture risk in women (35–37). Our findings are also concordant with four small controlled T administration trials, three in postmenopausal women and the other in women with hypopituitarism, that demonstrated gains in BMD with T use (10–12, 38). Our observational study extends the association between T and BMD to elderly women, as the only published analysis conducted exclusively in women aged 65 and older. Furthermore, our study employed a more sensitive T assay than previous studies have, and it is the only one with measurements of free T by dialysis.
T can potentially affect bone density through multiple mechanisms, including through its direct effects on the ARs in trabecular and cortical bone (8). Aromatization of T to estradiol by adipose tissue or locally at bone, with subsequent stimulation of estrogen receptors in bone, plays an important role in the action of T on the bone (39–41). In women of the advanced age of our study participants, T is the major source of circulating estradiol. T may also regulate local production of cytokines and growth factors in bone, including IL-6, IL-1β, TGF-β, and IGFs (8). The anabolic effects of T on muscle mass and strength could also indirectly affect bone mass. Additional studies are needed to elucidate the independence of the roles of physical activity and sex steroids on bone (42).
Additionally, in our investigation, we found that older women with higher free T levels had significantly greater lean body mass, which is consistent with the anabolic effects of T. Two other cross-sectional studies of postmenopausal women have shown correlations between free T and lean body mass (16, 43), although these analyses were not adjusted for relevant covariates. Interestingly, in contrast to what has been shown in men, our study found that higher free T levels were associated with greater fat mass. This is consistent with other data showing that postmenopausal women with higher free T levels have greater BMI and are more insulin resistant (17, 18, 24), and it raises the question of whether the effects of T on body composition are gender dimorphic. We prefer an alternative explanation, that the increase in androgen production with increasing adiposity originally described in polycystic ovary syndrome occurs across a woman's life span. Women with greater adiposity are more likely to have hyperinsulinemia due to insulin resistance, which could then lead to insulin-stimulated T production by the postmenopausal ovary. We have recently published a mechanistic study in postmenopausal women that supports this explanation (44). Data from this study suggest that T is a marker of adiposity and insulin resistance in older women and does not play a causal role in fat synthesis.
Because estrogen supplementation may have effects on bone and body composition that are incompletely accounted for by adjustment, we repeated all of our analyses after excluding estrogen users. Interestingly, this had no effect on the findings of a relationship between total T and BMD, but it did result in a decrease in both the magnitude and statistical significance of the relationships between free T and outcomes. The diminishment of associations, by 9–25%, is likely due to a true difference between estrogen users and non-users, although there was also a decrease in statistical power to detect associations due to a reduced sample size. Additional adjustment for estradiol, which has been shown to have important effects on analyses of T and outcomes (45), led to further decreases in the magnitude of the associations. However, an important issue should be considered in the models with both free T and estradiol. Estradiol concentrations are a marker of T concentrations, particularly in women with high adiposity, because the major source of estradiol in the postmenopausal woman is aromatization of T. In addition, the free T assay, due to the low concentration that the assay is attempting to measure, has more assay variability than a tandem mass spectrometry assay of estradiol. When modeled together, the more precise assay, in this case estradiol, may predominate due to assay characteristics, not underlying biology. Additional studies are required to determine the specific roles of estrogens vs. androgens in bone density and body composition in elderly women.
The strengths of our study include its population-based sample of older women, available measured covariates, and the use of highly sensitive assays of T and estradiol. However, our analyses are cross-sectional in nature, which limits inferences about causality and directionality of association. In addition, DXA measurement of lean body mass includes body water and internal organs in addition to muscle mass and is not the “gold standard” for assessing this tissue compartment.
In conclusion, our study demonstrated that higher endogenous free T levels are associated with higher BMD, greater lean body mass, and greater total fat mass in women aged 65 and older. The differences in BMD and muscle mass across the spectrum of free T levels were not only statistically significant, but also clinically significant, at greater than 10%. There are now a multitude of medications to prevent or treat osteoporosis, but no pharmacological therapies for age-related sarcopenia or frailty. The hypothesis that T therapy might potentially improve bone density and sarcopenia across a range of T levels that are physiological for women, either through T supplementation or through administration of selective AR modulators, should be explored. Further studies are needed to refine the appropriate target populations and to examine the risks and benefits of exogenous T in older women.
Acknowledgments
This work was supported by National Institute on Aging Grant K23 AG19161; an American Federation for Aging Research/Pfizer Research grant; contracts N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, and N01-HC-45133, and Grant U01 HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke; and the Intramural Research Program of the National Institute on Aging. A full list of principal Cardiovascular Health Study investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AR
- Androgen receptor
- BMD
- bone mineral density
- BMI
- body mass index
- CV
- coefficient of variation
- DXA
- dual-energy x-ray absorptiometry
- LC-MS/MS
- liquid chromatography-tandem mass spectrometry
- T
- testosterone.
References
- 1. Khosla S, Melton LJ, 3rd, Atkinson EJ, O'Fallon WM, Klee GG, Riggs BL. 1998. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J Clin Endocrinol Metab 83:2266–2274 [DOI] [PubMed] [Google Scholar]
- 2. Greendale GA, Edelstein S, Barrett-Connor E. 1997. Endogenous sex steroids and bone mineral density in older women and men: the Rancho Bernardo Study. J Bone Miner Res 12:1833–1843 [DOI] [PubMed] [Google Scholar]
- 3. van den Beld AW, de Jong FH, Grobbee DE, Pols HA, Lamberts SW. 2000. Measures of bioavailable serum testosterone and estradiol and their relationships with muscle strength, bone density, and body composition in elderly men. J Clin Endocrinol Metab 85:3276–3282 [DOI] [PubMed] [Google Scholar]
- 4. LeBlanc ES, Nielson CM, Marshall LM, Lapidus JA, Barrett-Connor E, Ensrud KE, Hoffman AR, Laughlin G, Ohlsson C, Orwoll ES. 2009. The effects of serum testosterone, estradiol, and sex hormone binding globulin levels on fracture risk in older men. J Clin Endocrinol Metab 94:3337–3346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Snyder PJ, Peachey H, Hannoush P, Berlin JA, Loh L, Lenrow DA, Holmes JH, Dlewati A, Santanna J, Rosen CJ, Strom BL. 1999. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab 84:2647–2653 [DOI] [PubMed] [Google Scholar]
- 6. Blackman MR, Sorkin JD, Münzer T, Bellantoni MF, Busby-Whitehead J, Stevens TE, Jayme J, O'Connor KG, Christmas C, Tobin JD, Stewart KJ, Cottrell E, St Clair C, Pabst KM, Harman SM. 2002. Growth hormone and sex steroid administration in healthy aged women and men: a randomized controlled trial. JAMA 288:2282–2292 [DOI] [PubMed] [Google Scholar]
- 7. Sattler FR, Castaneda-Sceppa C, Binder EF, Schroeder ET, Wang Y, Bhasin S, Kawakubo M, Stewart Y, Yarasheski KE, Ulloor J, Colletti P, Roubenoff R, Azen SP. 2009. Testosterone and growth hormone improve body composition and muscle performance in older men. J Clin Endocrinol Metab 94:1991–2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clarke BL, Khosla S. 2009. Androgens and bone. Steroids 74:296–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sinha-Hikim I, Taylor WE, Gonzalez-Cadavid NF, Zheng W, Bhasin S. 2004. Androgen receptor in human skeletal muscle and cultured muscle satellite cells: up-regulation by androgen treatment. J Clin Endocrinol Metab 89:5245–5255 [DOI] [PubMed] [Google Scholar]
- 10. Davis SR, McCloud P, Strauss BJ, Burger H. 1995. Testosterone enhances estradiol's effects on postmenopausal bone density and sexuality. Maturitas 21:227–236 [DOI] [PubMed] [Google Scholar]
- 11. Barrett-Connor E, Young R, Notelovitz M, Sullivan J, Wiita B, Yang HM, Nolan J. 1999. A two-year, double-blind comparison of estrogen-androgen and conjugated estrogens in surgically menopausal women. Effects on bone mineral density, symptoms and lipid profiles. J Reprod Med 44:1012–1020 [PubMed] [Google Scholar]
- 12. Miller BE, De Souza MJ, Slade K, Luciano AA. 2000. Sublingual administration of micronized estradiol and progesterone, with and without micronized testosterone: effect on biochemical markers of bone metabolism and bone mineral density. Menopause 7:318–326 [DOI] [PubMed] [Google Scholar]
- 13. Dobs AS, Nguyen T, Pace C, Roberts CP. 2002. Differential effects of oral estrogen versus oral estrogen-androgen replacement therapy on body composition in postmenopausal women. J Clin Endocrinol Metab 87:1509–1516 [DOI] [PubMed] [Google Scholar]
- 14. Cummings SR, Browner WS, Bauer D, Stone K, Ensrud K, Jamal S, Ettinger B. 1998. Endogenous hormones and the risk of hip and vertebral fractures among older women. Study of Osteoporotic Fractures Research Group. N Engl J Med 339:733–738 [DOI] [PubMed] [Google Scholar]
- 15. Lee JS, LaCroix AZ, Wu L, Cauley JA, Jackson RD, Kooperberg C, Leboff MS, Robbins J, Lewis CE, Bauer DC, Cummings SR. 2008. Associations of serum sex hormone-binding globulin and sex hormone concentrations with hip fracture risk in postmenopausal women. J Clin Endocrinol Metab 93:1796–1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van Geel TA, Geusens PP, Winkens B, Sels JP, Dinant GJ. 2009. Measures of bioavailable serum testosterone and estradiol and their relationships with muscle mass, muscle strength and bone mineral density in postmenopausal women: a cross-sectional study. Eur J Endocrinol 160:681–687 [DOI] [PubMed] [Google Scholar]
- 17. Patel SM, Ratcliffe SJ, Reilly MP, Weinstein R, Bhasin S, Blackman MR, Cauley JA, Sutton-Tyrrell K, Robbins J, Fried LP, Cappola AR. 2009. Higher serum testosterone concentration in older women is associated with insulin resistance, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab 94:4776–4784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kalyani RR, Franco M, Dobs AS, Ouyang P, Vaidya D, Bertoni A, Gapstur SM, Golden SH. 2009. The association of endogenous sex hormones, adiposity, and insulin resistance with incident diabetes in postmenopausal women. J Clin Endocrinol Metab 94:4127–4135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bachmann G, Bancroft J, Braunstein G, Burger H, Davis S, Dennerstein L, Goldstein I, Guay A, Leiblum S, Lobo R, Notelovitz M, Rosen R, Sarrel P, Sherwin B, Simon J, Simpson E, Shifren J, Spark R, Traish A. 2002. Female androgen insufficiency: the Princeton consensus statement on definition, classification, and assessment. Fertil Steril 77:660–665 [DOI] [PubMed] [Google Scholar]
- 20. 2005. The role of testosterone therapy in postmenopausal women: position statement of The North American Menopause Society. Menopause 12:497–511 [DOI] [PubMed] [Google Scholar]
- 21. Wierman ME, Basson R, Davis SR, Khosla S, Miller KK, Rosner W, Santoro N. 2006. Androgen therapy in women: an Endocrine Society Clinical Practice guideline. J Clin Endocrinol Metab 91:3697–3710 [DOI] [PubMed] [Google Scholar]
- 22. Braunstein GD. 2007. The Endocrine Society Clinical Practice Guideline and The North American Menopause Society position statement on androgen therapy in women: another one of Yogi's forks. J Clin Endocrinol Metab 92:4091–4093 [DOI] [PubMed] [Google Scholar]
- 23. Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A. 1991. The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1:263–276 [DOI] [PubMed] [Google Scholar]
- 24. Cappola AR, Ratcliffe SJ, Bhasin S, Blackman MR, Cauley J, Robbins J, Zmuda JM, Harris T, Fried LP. 2007. Determinants of serum total and free testosterone levels in women over the age of 65 years. J Clin Endocrinol Metab 92:509–516 [DOI] [PubMed] [Google Scholar]
- 25. Sinha-Hikim I, Arver S, Beall G, Shen R, Guerrero M, Sattler F, Shikuma C, Nelson JC, Landgren BM, Mazer NA, Bhasin S. 1998. The use of a sensitive equilibrium dialysis method for the measurement of free testosterone levels in healthy, cycling women and in human immunodeficiency virus-infected women. J Clin Endocrinol Metab 83:1312–1318 [DOI] [PubMed] [Google Scholar]
- 26. Javanbakht M, Singh AB, Mazer NA, Beall G, Sinha-Hikim I, Shen R, Bhasin S. 2000. Pharmacokinetics of a novel testosterone matrix transdermal system in healthy, premenopausal women and women infected with the human immunodeficiency virus. J Clin Endocrinol Metab 85:2395–2401 [DOI] [PubMed] [Google Scholar]
- 27. Choi HH, Gray PB, Storer TW, Calof OM, Woodhouse L, Singh AB, Padero C, Mac RP, Sinha-Hikim I, Shen R, Dzekov J, Dzekov C, Kushnir MM, Rockwood AL, Meikle AW, Lee ML, Hays RD, Bhasin S. 2005. Effects of testosterone replacement in human immunodeficiency virus-infected women with weight loss. J Clin Endocrinol Metab 90:1531–1541 [DOI] [PubMed] [Google Scholar]
- 28. Singh AB, Lee ML, Sinha-Hikim I, Kushnir M, Meikle W, Rockwood A, Afework S, Bhasin S. 2006. Pharmacokinetics of a testosterone gel in healthy postmenopausal women. J Clin Endocrinol Metab 91:136–144 [DOI] [PubMed] [Google Scholar]
- 29. Cummings SR, Black DM, Nevitt MC, Browner WS, Cauley JA, Genant HK, Mascioli SR, Scott JC, Seeley DG, Steiger P. 1990. Appendicular bone density and age predict hip fracture in women. The Study of Osteoporotic Fractures Research Group. JAMA 263:665–668 [PubMed] [Google Scholar]
- 30. Black DM, Reiss TF, Nevitt MC, Cauley J, Karpf D, Cummings SR. 1993. Design of the Fracture Intervention Trial. Osteoporos Int 3(Suppl 3):S29–S39 [DOI] [PubMed] [Google Scholar]
- 31. Robbins J, Hirsch C, Whitmer R, Cauley J, Harris T. 2001. The association of bone mineral density and depression in an older population. J Am Geriatr Soc 49:732–736 [DOI] [PubMed] [Google Scholar]
- 32. 2007. The World Health Organization assessment of osteoporosis at the primary health care level. Summary report of a WHO Scientific Group. Geneva: World Health Organization [Google Scholar]
- 33. Tok EC, Ertunc D, Oz U, Camdeviren H, Ozdemir G, Dilek S. 2004. The effect of circulating androgens on bone mineral density in postmenopausal women. Maturitas 48:235–242 [DOI] [PubMed] [Google Scholar]
- 34. Lambrinoudaki I, Christodoulakos G, Aravantinos L, Antoniou A, Rizos D, Chondros C, Kountouris A, Chrysofakis G, Creatsas G. 2006. Endogenous sex steroids and bone mineral density in healthy Greek postmenopausal women. J Bone Miner Metab 24:65–71 [DOI] [PubMed] [Google Scholar]
- 35. Garnero P, Sornay-Rendu E, Claustrat B, Delmas PD. 2000. Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: the OFELY study. J Bone Miner Res 15:1526–1536 [DOI] [PubMed] [Google Scholar]
- 36. Goderie-Plomp HW, van der Klift M, de Ronde W, Hofman A, de Jong FH, Pols HA. 2004. Endogenous sex hormones, sex hormone-binding globulin, and the risk of incident vertebral fractures in elderly men and women: the Rotterdam Study. J Clin Endocrinol Metab 89:3261–3269 [DOI] [PubMed] [Google Scholar]
- 37. Bjørnerem A, Ahmed LA, Joakimsen RM, Berntsen GK, Fønnebø V, Jørgensen L, Øian P, Seeman E, Straume B. 2007. A prospective study of sex steroids, sex hormone-binding globulin, and non-vertebral fractures in women and men: the Tromso Study. Eur J Endocrinol 157:119–125 [DOI] [PubMed] [Google Scholar]
- 38. Miller KK, Biller BM, Beauregard C, Lipman JG, Jones J, Schoenfeld D, Sherman JC, Swearingen B, Loeffler J, Klibanski A. 2006. Effects of testosterone replacement in androgen-deficient women with hypopituitarism: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab 91:1683–1690 [DOI] [PubMed] [Google Scholar]
- 39. Burnett-Bowie SA, McKay EA, Lee H, Leder BZ. 2009. Effects of aromatase inhibition on bone mineral density and bone turnover in older men with low testosterone levels. J Clin Endocrinol Metab 94:4785–4792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Somner J, McLellan S, Cheung J, Mak YT, Frost ML, Knapp KM, Wierzbicki AS, Wheeler M, Fogelman I, Ralston SH, Hampson GN. 2004. Polymorphisms in the P450 c17 (17-hydroxylase/17,20-Lyase) and P450 c19 (aromatase) genes: association with serum sex steroid concentrations and bone mineral density in postmenopausal women. J Clin Endocrinol Metab 89:344–351 [DOI] [PubMed] [Google Scholar]
- 41. Sjögren K, Lagerquist M, Moverare-Skrtic S, Andersson N, Windahl SH, Swanson C, Mohan S, Poutanen M, Ohlsson C. 2009. Elevated aromatase expression in osteoblasts leads to increased bone mass without systemic adverse effects. J Bone Miner Res 24:1263–1270 [DOI] [PubMed] [Google Scholar]
- 42. McTiernan A, Wu L, Chen C, Chlebowski R, Mossavar-Rahmani Y, Modugno F, Perri MG, Stanczyk FZ, Van Horn L, Wang CY. 2006. Relation of BMI and physical activity to sex hormones in postmenopausal women. Obesity (Silver Spring) 14:1662–1677 [DOI] [PubMed] [Google Scholar]
- 43. Gower BA, Nyman L. 2000. Associations among oral estrogen use, free testosterone concentration, and lean body mass among postmenopausal women. J Clin Endocrinol Metab 85:4476–4480 [DOI] [PubMed] [Google Scholar]
- 44. Patel SM, Iqbal N, Kaul S, Ratcliffe SJ, Rickels MR, Reilly MP, Scattergood T, Basu A, Fuller C, Cappola AR. 2010. Effects of metformin and leuprolide acetate on insulin resistance and testosterone levels in nondiabetic postmenopausal women: a randomized, placebo-controlled trial. Fertil Steril 94:2161–2166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Braunstein GD, Johnson BD, Stanczyk FZ, Bittner V, Berga SL, Shaw L, Hodgson TK, Paul-Labrador M, Azziz R, Merz CN. 2008. Relations between endogenous androgens and estrogens in postmenopausal women with suspected ischemic heart disease. J Clin Endocrinol Metab 93:4268–4275 [DOI] [PMC free article] [PubMed] [Google Scholar]