Serum and intratesticular androstenedione and intratesticular DHEA suppress with gonadotropin suppression, and are similarly stimulated by hCG, but serum DHEA remains unchanged.
Abstract
Introduction:
Concentrations of intratesticular (IT) testosterone (T) are known to be 100–200 times those of serum T; however, the IT concentrations of T's precursors, their testicular to serum gradients, gonadotropin dependence, and response to stimulation with human chorionic gonadotropin (hCG) have not been studied in detail. We hypothesized that serum and IT androstenedione (ADD) and IT dehydroepiandrosterone (DHEA) would be significantly suppressed by the administration of a GnRH antagonist and increased when stimulated by hCG, without a similar suppression of serum DHEA.
Methods:
We suppressed gonadotropins in 23 normal men with the GnRH antagonist acyline and randomly assigned them to one of four doses of hCG, 0, 15, 60, or 125 IU sc every other day for 10 d. Blood and IT fluid for the measurement of serum and IT hormones were obtained at baseline and after 10 d of treatment.
Results:
Baseline IT ADD [median (25th, 75th percentile)] was 629 (308, 860) nmol/liter, and IT DHEA was 564 (411, 879) nmol/liter, which were 175 and 27 times higher than their respective serum concentrations. IT ADD and IT DHEA were suppressed by 98 and 82%, respectively, by acyline and significantly increased with hCG administration. Likewise, serum ADD was suppressed by 50%, but serum DHEA was unchanged.
Discussion:
ADD and DHEA are highly concentrated within the human testes compared with serum. Serum and IT ADD and IT DHEA are markedly suppressed with GnRH administration and stimulated by hCG, but serum DHEA is not, suggesting that most circulating DHEA is not of testicular origin.
Intratesticular (IT) testosterone (T) is required for spermatogenesis. In men with normal spermatogenesis, IT T concentrations are known to be 100–200 times greater than those in the serum (1–6). However, these high concentrations of IT T are not essential for spermatogenesis because spermatogenesis has been observed with much lower concentrations of IT T in both rats (7) and men (8). In the context of low IT T, such as with experimental male hormonal contraception regimens, it is possible that other IT androgens, such as androstenedione (ADD) and dehydroepiandrosterone (DHEA), may play a role in supporting spermatogenesis. Previous work has suggested that DHEA may support spermatogenesis in rats (9, 10), either from an adrenal or testicular source, in which case it may function as a paracrine stimulatory signal. Nonetheless, the IT concentrations of these androgenic precursors of T biosynthesis in man have not been studied in detail.
Previous studies examining IT ADD and IT DHEA relied on testicular tissue obtained either at the time of orchidectomy from prostate cancer patients or from testicular biopsies of infertile patients (11–15). Only two studies enrolled normal controls to further characterize the IT hormonal milieu (16, 17), but the testicular biopsies in these studies involved the use of general anesthesia, which can affect steroidogenesis by suppressing LH secretion from the pituitary (18). Additionally, because of the rarity of testicular biopsy in normal men, these studies involved small numbers of subjects, which may have adversely affected the precision of their estimates for IT ADD and IT DHEA.
To overcome this limitation, Jarow and colleagues (2) developed a minimally invasive fine-needle aspiration technique that allows for sampling of IT fluid in normal men without the requirement for general anesthesia. This technique makes it possible to assess the IT hormone concentrations in normal, healthy fertile men without underlying medical problems such as prostate cancer or infertility. Several studies have since used fine-needle aspiration to study IT hormones, but these studies have focused on IT T and IT dihydrotestosterone (DHT) (2, 4, 6), and the IT concentration of T precursors such as IT ADD and IT DHEA has not been studied using this technique.
Knowledge regarding the concentrations of IT ADD and IT DHEA and their regulation by LH may provide insights into the role of these hormones in spermatogenesis, particularly in the setting of low IT T, as may be observed in some men with infertility and/or during treatment with experimental forms of male hormonal contraception. In addition, knowledge of IT ADD and IT DHEA may enhance our understanding of T biosynthesis in vivo and could aid in the development of novel inhibitors of T biosynthesis. Such inhibitors could be useful in the treatment of androgen-sensitive disease or improve the efficacy of male hormonal contraceptives.
Therefore, to improve our understanding of testicular steroidogenesis in normal men, we measured IT T, IT ADD, and IT DHEA by fine-needle aspiration in a large group of healthy, fertile men. Measurements were performed before and after suppression of gonadotropins with the GnRH antagonist acyline and restimulation with low doses of human chorionic gonadotropin (hCG). We hypothesized that serum and IT ADD and DHEA would suppress with administration of a GnRH antagonist and increase when stimulated by low doses of hCG, without a similar suppression of serum DHEA. In addition, we hypothesized that IT ADD and IT DHEA would be much lower than IT T at baseline, reflecting their rapid conversion to T in the testes.
Subjects and Methods
Subjects
The study design has been reported previously (19). Briefly, healthy men, 18–50 yr old, with normal serum gonadotropins, serum T concentrations, and normal seminal fluid analyses were enrolled. After enrollment, subjects were assigned to one of the treatment groups by a random number sequence and also randomized to the order of the unilateral testicular fine-needle aspirations (right vs. left testis on d 1 vs. d 10). All subjects had a baseline testicular fine-needle aspiration on d 1, which was performed using a scrotal block with 1% lidocaine buffered 1:10 with sodium bicarbonate injected into the spermatic cord. Next, a blood sample was obtained for assessment of serum hormones, and a unilateral testicular aspiration was performed as previously described (2, 6, 19, 20). After the testicular aspiration on d 1, all subjects received a sc injection of the GnRH antagonist acyline (NeoMPS, San Diego, CA) at a dose of 300 μg/kg into the abdominal skin. Subjects then received the first dose of hCG (Pregnyl; Organon, Roseland, NJ) based on treatment group randomization: group 1 received placebo hCG (normal saline) sc every other day for five doses, group 2 received 15 IU hCG sc every other day for five doses, group 3 received 60 IU hCG sc every other day for five doses, and group 4 received 125 IU hCG sc every other day for five doses. On d 10, subjects underwent a testicular fine-needle aspiration of the other testis, following the same protocol outlined above for d 1. On d 40, subjects had a follow-up visit to ensure their testicular examination, serum and semen parameters had all returned to normal. The University of Washington Institutional Review Board approved the study, and all subjects provided written, informed consent before study procedures. The study was registered in advance on www.clinicaltrials.gov as NCT 00839319.
Measurements
Testicular fluid samples were immediately placed on ice and centrifuged at 300 × g to remove any aspirated cells; the supernatant fluid was decanted and stored at −70 C. Serum was stored at −20 C. Testicular fluid and serum samples were assayed simultaneously for T, ADD, and DHEA by liquid chromatography-tandem mass spectrometry on a Waters Aquity UPLC coupled with a Micromass Premiere-XE tandem quadrupole mass spectrometer (Waters Corp., Milford, MA) using a modification of our previously described method (6, 19, 21). The midrange pooled intra- and interassay coefficients of variation were 4.9 and 7.4% for T, 3.5 and 20.6% for ADD, and 7.6 and 15.4% for DHEA. The assay sensitivity was less than 0.1 pmol/liter for T, less than 0.03 nmol/liter for ADD, and less than 0.07 nmol/liter for DHEA.
Serum LH and FSH concentrations were quantified by immunofluorometric assay (8). The sensitivity of the LH assay was 0.019 IU/liter, and the intra- and interassay coefficients of variation for a midrange pooled value of 1.2 IU/liter was 3.2 and 12.5%, respectively. The sensitivity of the FSH assay was 0.016 IU/liter, and the intra- and interassay coefficients of variation were 2.9 and 6.1% for a midrange pooled value of 0.96 IU/liter. Serum hCG was measured by immunofluorometric assay (Delfia, Wallac, Inc., Turku, Finland). The hCG assay used in this study is specific for the intact heterodimer and was calibrated against the against the 4th International Standard for Chorionic Gonadotropin (75/589) (22). The intra- and interassay coefficients of variation for hCG were 3.4 and 3.7%, respectively, and the lower limit of detection was less than 1 IU/liter. All samples for all subjects were batched and measured in a single assay.
Statistical analysis
Due to nonnormality, the data are expressed as medians and 25th and 75th percentiles. Six subjects (one each in groups 1 and 2 and two each in groups 3 and 4) had serum LH values above 1.2 IU/liter on d 7. These subjects were excluded from further analysis because their IT hormones were affected by normal serum concentrations of LH. Therefore, analysis of baseline and end-of-treatment hormone concentrations was performed on the 23 subjects who suppressed serum LH below the lower limit of the normal range. Due to nonnormality, comparisons of hormone concentrations between groups were performed in a nonparametric fashion using Kruskal-Wallis ANOVA with a Wilcoxon rank-sum post hoc test. Correlations between serum hormone levels and IT hormones, and between IT hormones, were performed using the Spearman technique. No corrections were made for multiple comparisons. All statistical analyses were performed using STATA version 10.0 (StataCorp, College Station, TX). For all comparisons, an α of <0.05 was considered significant.
Results
Subjects
A description of the number of subjects screened and enrolled for this study, along with data for IT T and IT DHT in a larger group, has been previously published (19). There were no serious adverse events during the study. Median testicular aspirate volume was 10 μl both at baseline and after 10 d of treatment.
Baseline hormone concentrations
Baseline serum and IT hormone concentrations and demographics are reported in Table 1. IT ADD was roughly 25% of IT T, and IT DHEA was 23% of IT T. At baseline, median IT T was 169 times higher than serum T, IT ADD was 175 times higher than serum ADD, and median IT DHEA was 27 times higher than serum DHEA. There were no statistically significant differences between any of the treatment groups for any of the measurements at baseline.
Table 1.
Median baseline characteristics and serum and IT hormones of 23 participants by treatment group (25th, 75th interquartile range)
| Group 1, 0 IU hCG (n = 6) | Group 2, 15 IU hCG (n = 7) | Group 3, 60 IU hCG (n = 5) | Group 4, 125 IU hCG (n = 5) | All subjects (n = 23) | |
|---|---|---|---|---|---|
| Age (yr) | 21 (20, 26) | 25 (20,29) | 22 (20, 24) | 22 (21, 26) | 22 (20,26) |
| Body mass index (kg/m2) | 24.8 (23.6, 26.3) | 24.1 (23.2, 26.7) | 24.9 (21.2, 26.3) | 25.8 (22.9, 26) | 24.9 (23.2, 26.3) |
| Serum hormones | |||||
| LH (IU/liter) | 3.5 (3.1, 4.8) | 3 (2.6, 4.9) | 3.4 (3.4, 4.9) | 2.9 (2.3, 3.7) | 3.4 (2.6, 4.9) |
| FSH (IU/liter) | 2.7 (1.2, 3.4) | 2.4 (2, 2.8) | 2.6 (2.2, 3.2) | 2.2 (1.9, 2.5) | 2.4 (1.9, 3.1) |
| T (nmol/liter) | 13 (11.3, 16.9) | 15 (11.4, 20.9) | 14.2 (12.7, 14.9) | 16.8 (14.4, 18.6) | 14.5 (11.4, 17.3) |
| ADD (nmol/liter) | 4.2 (3.6, 5) | 3.8 (3.3, 6.8) | 3.5 (3.3, 3.7) | 2.6 (2.5, 3.4) | 3.6 (3.3, 4.5) |
| DHEA (nmol/liter) | 31.9 (16.1, 43.2) | 25.5 (11.8, 33.1) | 20.9 (19, 24.2) | 16.1 (13.9, 18.2) | 20.8 (16, 32.8) |
| SHBG (nmol/liter) | 24 (16, 30) | 35 (21, 43) | 28 (26, 39) | 30 (21, 37) | 21 (15, 33) |
| IT hormones | |||||
| T (nmol/liter) | 3467 (2508, 3839) | 2425 (1700, 3380) | 1821 (1753, 2412) | 3502 (2305, 3959) | 2449 (1820, 3669) |
| ADD (nmol/liter) | 530 (265, 645) | 811 (246, 956) | 629 (336, 766) | 395 (352, 808) | 629 (308, 860) |
| DHEA (nmol/liter) | 650 (454, 879) | 526 (411, 911) | 458 (393, 565) | 676 (518, 693) | 564 (411, 879) |
IT hormone concentrations were strongly correlated with one another at baseline. In particular, IT T correlated strongly with both IT ADD (r = 0.48; P = 0.02) and IT DHEA (r = 0.76; P < 0.01), and IT ADD correlated with IT DHEA (r = 0.79; P < 0.001). Neither IT ADD nor IT DHEA correlated significantly with serum T at baseline. In addition, neither IT ADD nor IT DHEA correlated with serum ADD or serum DHEA at baseline. Lastly, none of the IT hormones correlated with serum LH, FSH, DHT, estradiol, 17-hydroxyprogesterone, SHBG, age, or body mass index at baseline.
Posttreatment hormone concentrations
Posttreatment serum and IT hormone data are presented in Table 2. Treatment of normal men with acyline alone resulted in a 98% drop in IT ADD from 530 (265, 645) nmol/liter to 8 (2, 9) nmol/liter and an 82% drop in IT DHEA from 650 (454, 879) nmol/liter to 102 (66, 160) nmol/liter. IT T fell by 97% after treatment with acyline from 3467 (2508, 3839) to 77 (40, 122) nmol/liter.
Table 2.
Posttreatment median serum and IT hormones of 23 participants by treatment group (25th, 75th interquartile range)
| Group 1, 0 IU hCG (n = 6) | Group 2, 15 IU hCG (n = 7) | Group 3, 60 IU hCG (n = 5) | Group 4, 125 IU hCG (n = 5) | |
|---|---|---|---|---|
| LH (IU/liter) | 0.13 (0.07, 0.17) | 0.26 (0.14, 0.65) | 0.16 (0.16, 0.49) | 0.33 (0.28, 0.39) |
| FSH (IU/liter) | 0.37 (0.17, 0.44) | 0.27 (0.26, 0.6) | 0.27 (0.24, 0.29) | 0.28 (0.25, 0.31) |
| T (nmol/liter) | 0.46 (0.39, 0.67) | 1.1 (0.39, 1.2) | 3.1 (1.1, 3.8) | 8.2 (6.3, 12) |
| ADD (nmol/liter) | 2.3 (1.6, 2.9) | 1.6 (1.5, 4.7) | 2.1 (2.0, 2.5) | 2.6 (2.5, 3.4) |
| DHEA (nmol/liter) | 20.8 (15.2, 27.8) | 13.0 (10.5, 23.4) | 13.8 (13.6, 23.3) | 11.1 (9.9, 13.9) |
| IT T (nmol/liter) | 77 (40, 122) | 104 (66, 244) | 174 (139, 319) | 923 (895, 1017) |
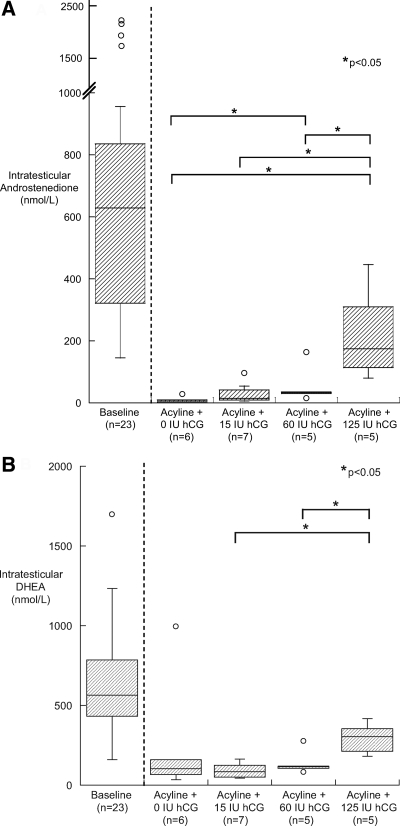
After 10 d of treatment with hCG, IT ADD and IT DHEA increased in proportion to hCG dose (Fig. 1, A and B). This effect was significant in the 125-IU hCG group compared with the lower-dose groups, but the 125-IU hCG dose did not fully restore IT ADD and IT DHEA to baseline levels.
Fig. 1.
Box plot of IT ADD (A) and IT DHEA (B) in gonadotropin-suppressed subjects on d 10 by treatment group (n = 6 for the 0-IU hCG group, n = 7 for the 15-IU hCG group, and n = 5 for the 60-IU hCG group and 125-IU hCG group).
Treatment of normal men with acyline resulted in a significant reduction in serum ADD of 45% (P = 0.03). Serum ADD remained significantly reduced compared with baseline after 10 d of treatment with the two lower doses of hCG, but the 125-IU dose of hCG returned serum ADD to baseline values. Serum DHEA was reduced in a nonsignificant fashion with both acyline alone and in response to hCG treatment.
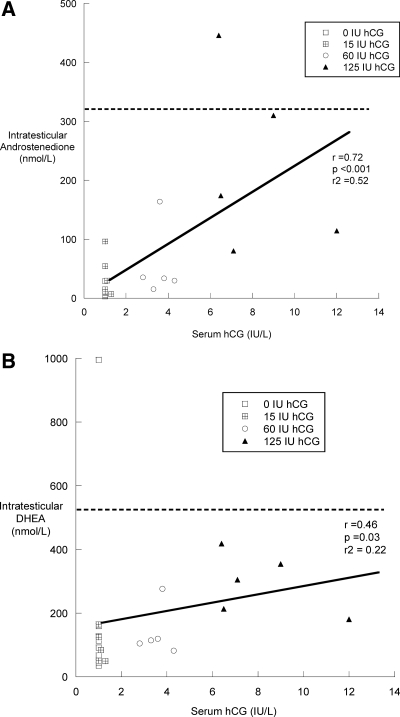
IT and serum hormone concentrations after 10 d of treatment with hCG were highly correlated with one another. IT T correlated with IT ADD (r = 0.95; P < 0.01) and IT DHEA (r = 0.84; P < 0.01). In contrast to the situation at baseline, treatment serum T was highly correlated with IT ADD (r = 0.88; P < 0.01) and IT DHEA (r = 0.62; P < 0.01), but not with serum ADD or serum DHEA. Lastly, both IT ADD and IT DHEA correlated significantly with serum hCG after 10 d of hCG treatment (Fig. 2, A and B).
Fig. 2.
Correlations between posttreatment serum hCG and IT ADD (A) and IT DHEA (B) for all subjects receiving hCG (n = 23). The dotted line represents the limit of the baseline range.
Discussion
In this report, we have used testicular aspiration to determine the IT concentration of the two main T precursors IT ADD and IT DHEA in the human testes of a large group of normal healthy men. In addition, we have used gonadotropin suppression with a GnRH antagonist to examine the concentrations of serum and IT ADD and DHEA in a gonadotropin deprived state and after re-stimulation with various low doses of hCG. This is the first study to measure IT ADD and IT DHEA in a large number of healthy, fertile men using testicular aspiration. Our results describe the range of IT ADD and IT DHEA concentrations in normal men and demonstrate that the IT concentrations of these hormones are significantly higher than their serum concentrations, yet are only about 25% the concentration of IT T.
Our findings are in contrast to a previous study that examined IT T and IT ADD in men with varicoceles and found nearly identical concentrations of these hormones in both testicular biopsy samples and spermatic vein blood (15). Our results are more similar to those observed previously by Rajfer et al. (12) in a smaller sample. In our study, IT ADD was only 23% of IT T, implying that ADD is rapidly converted to T in the IT environment. This relative preservation of IT T over IT ADD appears to be particularly true in the setting of gonadotropin suppression where the IT ADD is reduced to 10% of the concentration of IT T. These results suggest that it is unlikely that IT ADD or IT DHEA play a major role in supporting spermatogenesis in a low IT T setting because these compounds are rapidly converted to IT T. In contrast to IT ADD, IT DHEA, although present in a similar concentration to IT ADD at baseline, is less affected by gonadotropin suppression, suggesting that conversion of IT ADD to IT T by 17β-hydroxysteroid dehydrogenase is more robust than the conversion of IT DHEA to IT ADD by 3β-hydroxysteroid dehydrogenase. Although IT DHEA was reduced dramatically with gonadotropin suppression, serum DHEA was not significantly lower compared with baseline. These data are consistent with the conclusion that a significant fraction of circulating DHEA is adrenal in origin (23). Lastly, in contrast to serum DHEA, serum ADD decreased by more than 50% after treatment with acyline, implying that the testis is the major source of serum ADD.
The concentrations of IT hormones in our study were higher than those measured by De la Torre (17) and colleagues who analyzed testicular biopsies in men under general anesthesia, consistent with the notion that general anesthesia may transiently suppress steroidogenesis (18). Therefore, to avoid this potential confounder, future studies of IT steroid concentration in normal men should focus on the use of the testicular aspiration under local anesthesia.
Our study had several weaknesses. One was that 20% of subjects whose gonadotropins did not suppress completely with acyline were subsequently removed from the analysis. A previous study using acyline (24) showed uniform suppression of gonadotropins in normal young men. It remains unclear why acyline did not uniformly suppress gonadotropin levels in this study. There were no obvious differences in subject characteristics between the two studies that would explain this failure. A second caveat to our work is the difficulty in controlling for the timing of LH pulses in the baseline measurement of the IT hormones. Such pulses have been shown to affect serum concentrations of sex steroids (25). The ultradian pulsatility of LH is the likely explanation for the absence of a correlation between serum LH and steroid hormones at baseline. In contrast, correlations between steroid hormones and hCG are more evident during stimulation likely due to the stable serum concentrations of hCG during treatment (26). Due to the limited volume of fluid aspirated, we were unable to measure estradiol or other key IT T precursors including progesterone, pregnenolone, 17-hydroxyprogesterone, and 17-hydroxypregnenolone. The measurement of the IT concentration of these steroids will be the subject of future research. In addition, although testicular fine-needle aspiration has been associated with testicular hemorrhage in a small number of infertile men (27), testicular fine-needle aspiration for the measurement of IT steroids appears to be safe in normal men, without evidence of testicular hemorrhage or any adverse impact on endocrine function as assessed by serum hormone measurements.
In conclusion, this study expands the information available regarding IT androgen concentrations and shows that IT ADD and IT DHEA are both markedly suppressed with gonadotropin suppression and respond similarly to LH-like stimulation with hCG. This information will be useful in future studies examining the concentration of other T precursors alone and after the administration of enzyme-specific inhibitors of steroidogenesis such as ketoconazole or abiraterone acetate. Increased knowledge of in vivo T biosynthesis may have utility in treatment of androgen-dependent disease such as prostate cancer and aid in the development of male hormonal contraceptives.
Acknowledgments
We thank Ms. Iris Nielsen, Ms. Marilyn Busher, Ms. Dorothy McGuiness, and Ms. Connie Pete for their assistance with this study as well as our study volunteers without whom this research would not be possible.
The National Institute of Child Health and Human Development supported this work through cooperative agreements U54 HD-12629 and U54 HD-42454 as part of the specialized Cooperative Centers Program in Reproductive Research and the Cooperative Contraceptive Research Centers Program. M.Y.R. is supported, in part, by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Grant K12 HD053984. S.T.P. is supported by the National Institute of Aging, a Division of the National Institutes of Health, Grant K23 AG027238. A.M.M. is supported by the Department of Veterans Affairs.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ADD
- Androstenedione
- DHEA
- dehydroepiandrosterone
- DHT
- dihydrotestosterone
- hCG
- human chorionic gonadotropin
- IT
- intratesticular
- T
- testosterone.
References
- 1. Morse HC, Horike N, Rowley MJ, Heller CG. 1973. Testosterone concentrations in testes of normal men: effects of testosterone propionate administration. J Clin Endocrinol Metab 37:882–886 [DOI] [PubMed] [Google Scholar]
- 2. Jarow JP, Chen H, Rosner TW, Trentacoste S, Zirkin BR. 2001. Assessment of the androgen environment within the human testis: minimally invasive method to obtain intratesticular fluid. J Androl 22:640–645 [PubMed] [Google Scholar]
- 3. McLachlan RI, O'Donnell L, Stanton PG, Balourdos G, Frydenberg M, de Kretser DM, Robertson DM. 2002. Effects of testosterone plus medroxyprogesterone acetate on semen quality, reproductive hormones, and germ cell populations in normal young men. J Clin Endocrinol Metab 87:546–556 [DOI] [PubMed] [Google Scholar]
- 4. Zhao M, Baker SD, Yan X, Zhao Y, Wright WW, Zirkin BR, Jarow JP. 2004. Simultaneous determination of steroid composition of human testicular fluid using liquid chromatography tandem mass spectrometry. Steroids 69:721–726 [DOI] [PubMed] [Google Scholar]
- 5. Matthiesson KL, Stanton PG, O'Donnell L, Meachem SJ, Amory JK, Berger R, Bremner WJ, McLachlan RI. 2005. Effects of testosterone and levonorgestrel combined with a 5α-reductase inhibitor or gonadotropin-releasing hormone antagonist on spermatogenesis and intratesticular steroid levels in normal men. J Clin Endocrinol Metab 90:5647–5655 [DOI] [PubMed] [Google Scholar]
- 6. Roth MY, Lin K, Amory JK, Matsumoto AM, Anawalt BD, Snyder CN, Kalhorn TF, Bremner WJ, Page ST. 2010. Serum LH correlates highly with intratesticular steroid levels in normal men. J Androl 31:138–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zirkin BR, Santulli R, Awoniyi CA, Ewing LL. 1989. Maintenance of advanced spermatogenic cells in the adult rat testis: quantitative relationship to testosterone concentration within the testis. Endocrinology 124:3043–3049 [DOI] [PubMed] [Google Scholar]
- 8. Page ST, Kalhorn TF, Bremner WJ, Anawalt BD, Matsumoto AM, Amory JK. 2007. Intratesticular androgens and spermatogenesis during severe gonadotropin suppression induced by male hormonal contraceptive treatment. J Androl 28:734–741 [DOI] [PubMed] [Google Scholar]
- 9. Saxena N, Paul PK. 1987. Influence of adrenocortical hormones on the onset of spermatogenesis in rats. Indian J Exp Biol 25:296–301 [PubMed] [Google Scholar]
- 10. Saxena N, Paul PK. 1988. Role of adrenal in maintenance of spermatogenesis in rats. Indian J Exp Biol 26:932–936 [PubMed] [Google Scholar]
- 11. Takahashi J, Higashi Y, LaNasa JA, Yoshida K, Winters SJ, Oshima H, Troen P. 1983. Studies of the human testis. XVIII. Simultaneous measurement of nine intratesticular steroids: evidence for reduced mitochondrial function in testis of elderly men. J Clin Endocrinol Metab 56:1178–1187 [DOI] [PubMed] [Google Scholar]
- 12. Rajfer J, Sikka SC, Rivera F, Handelsman DJ. 1986. Mechanism of inhibition of human testicular steroidogenesis by oral ketoconazole. J Clin Endocrinol Metab 63:1193–1198 [DOI] [PubMed] [Google Scholar]
- 13. Rajfer J, Sikka SC, Swerdloff RS. 1987. Lack of a direct effect of gonadotropin hormone-releasing hormone agonist on human testicular steroidogenesis. J Clin Endocrinol Metab 64:62–67 [DOI] [PubMed] [Google Scholar]
- 14. Weusten JJ, Smals AG, Hofman JA, Kloppenborg PW, Benraad TJ. 1987. Early time sequence in pregnenolone metabolism to testosterone in homogenates of human and rat testis. Endocrinology 120:1909–1913 [DOI] [PubMed] [Google Scholar]
- 15. Winters SJ, Takahashi J, Troen P. 1999. Secretion of testosterone and its delta4 precursor steroids into spermatic vein blood in men with varicocele-associated infertility. J Clin Endocrinol Metab 84:997–1001 [DOI] [PubMed] [Google Scholar]
- 16. Marie E, Galeraud-Denis I, Carreau S. 2001. Increased testicular steroid concentrations in patients with idiopathic infertility and normal FSH levels. Arch Androl 47:177–184 [DOI] [PubMed] [Google Scholar]
- 17. de la Torre B, Norén S, Hedman M, Ritzén M, Diczfalusy E. 1982. Intratesticular and plasma steroid profiles in fertile and infertile men. Int J Androl 5:367–378 [DOI] [PubMed] [Google Scholar]
- 18. Glass AR, Smith CE, Kidd GS, Vigersky RA. 1978. Response of the hypothalamic-pituitary-testicular axis to surgery. Fertil Steril 30:560–564 [PubMed] [Google Scholar]
- 19. Roth MY, Page ST, Lin K, Anawalt BD, Matsumoto AM, Snyder CN, Marck BT, Bremner WJ, Amory JK. 2010. Dose-dependent increase in intratesticular testosterone by very low-dose human chorionic gonadotropin in normal men with experimental gonadotropin deficiency. J Clin Endocrinol Metab 95:3806–3813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Coviello AD, Matsumoto AM, Bremner WJ, Herbst KL, Amory JK, Anawalt BD, Sutton PR, Wright WW, Brown TR, Yan X, Zirkin BR, Jarow JP. 2005. Low-dose human chorionic gonadotropin maintains intratesticular testosterone in normal men with testosterone-induced gonadotropin suppression. J Clin Endocrinol Metab 90:2595–2602 [DOI] [PubMed] [Google Scholar]
- 21. Kalhorn TF, Page ST, Howald WN, Mostaghel EA, Nelson PS. 2007. Analysis of testosterone and dihydrotestosterone from biological fluids as the oxime derivatives using high-performance liquid chro-matography/tandem mass spectrometry. Rapid Commun Mass Spectrom 21:3200–3206 [DOI] [PubMed] [Google Scholar]
- 22. Sturgeon CM, Berger P, Bidart JM, Birken S, Burns C, Norman RJ, Stenman UH. 2009. Differences in recognition of the 1st WHO international reference reagents for hCG-related isoforms by diagnostic immunoassays for human chorionic gonadotropin. Clin Chem 55:1484–1491 [DOI] [PubMed] [Google Scholar]
- 23. Zappulla F, Ventura D, Capelli M, Cassio A, Balsamo A, Frejaville E, Bolelli G, Cacciari E. 1981. Gonadal and adrenal secretion of dehydroepiandrosterone sulfate in prepubertal and pubertal subjects. J Endocrinol Invest 4:197–202 [DOI] [PubMed] [Google Scholar]
- 24. Herbst KL, Coviello AD, Page S, Amory JK, Anawalt BD, Bremner WJ. 2004. A single dose of the potent gonadotropin-releasing hormone antagonist acyline suppresses gonadotropins and testosterone for 2 weeks in healthy young men. J Clin Endocrinol Metab 89:5959–5965 [DOI] [PubMed] [Google Scholar]
- 25. Matsumoto AM, Bremner WJ. 1984. Modulation of pulsatile gonadotropin secretion by testosterone in man. J Clin Endocrinol Metab 58:609–614 [DOI] [PubMed] [Google Scholar]
- 26. Trinchard-Lugan I, Khan A, Porchet HC, Munafo A. 2002. Pharmacokinetics and pharmacodynamics of recombinant human chorionic gonadotropin in healthy male and female volunteers. Reprod Biomed Online 4:106–115 [DOI] [PubMed] [Google Scholar]
- 27. Friedler S, Raziel A, Strassburger D, Soffer Y, Komarovsky D, Ron-El R. 1997. Testicular sperm retrieval by percutaneous fine needle sperm aspiration compared with testicular sperm extraction by open biopsy in men with non-obstructive azoospermia. Hum Reprod 12:1488–1493 [DOI] [PubMed] [Google Scholar]