SP-A from endometrium/decidua selectively inhibits PGF2α, and the observed decrease in decidua SP-A may be critical to “decidual activation” and onset of labor at term.
Abstract
Context:
Labor is characterized by “decidual activation” with production of inflammatory mediators. Recent data suggest that surfactant protein-A (SP-A) may be critical to the onset of labor in mice. Whether this is also true in humans is unclear.
Objectives:
The aim was to investigate: 1) the expression of SP-A at the maternal-fetal interface; 2) the effect of SP-A on the production of inflammatory mediators by human decidua; and 3) the association between single nucleotide polymorphisms in maternal SP-A genes and spontaneous preterm birth.
Research Design and Methods:
In situ expression of SP-A was investigated by immunohistochemistry and quantitative RT-PCR. Term decidual stromal cells were isolated, purified, and treated with/without SP-A (1–100 μg/ml), IL-1β, and/or thrombin. Levels of inflammatory mediators [IL-6, IL-8, TNFα, matrix metalloproteinase-3, monocyte chemotactic protein-1, IL-1β, PGE2, prostaglandin F2α (PGF2α)] and angiogenic factors (soluble fms-like tyrosine kinase-1, vascular endothelial growth factor) were measured in conditioned supernatant by ELISA and corrected for protein content. The effect of SP-A on eicosanoid gene expression was measured by quantitative RT-PCR.
Results:
SP-A localized to endometrium/decidua. High-dose SP-A (100 μg/ml) inhibited PGF2α by term decidual stromal cells without affecting the production of other inflammatory mediators, and this effect occurred at a posttranscriptional level. Decidual SP-A expression decreased significantly with labor. Single nucleotide polymorphisms in the SP-A genes do not appear to be associated with preterm birth.
Conclusions:
SP-A is produced by human endometrium/decidua, where it significantly and selectively inhibits PGF2α production. Its expression decreases with labor. These novel observations suggest that decidual SP-A likely plays a critical role in regulating prostaglandin production within the uterus, culminating at term in decidual activation and the onset of labor.
Pulmonary surfactant is a developmentally regulated lipoprotein complex produced by type II pneumocytes, which is required to prevent alveolar collapse during expiration. Lipids, which make up 90% of surfactant, are critical for normal pulmonary function. The role of the surfactant-associated proteins, which make up the remaining 10%, is less well understood. Four surfactant-associated proteins have been identified: surfactant protein-A (SP-A), SP-B, SP-C, and SP-D. SP-B and SP-C are relatively lipophilic and play a critical role in stabilizing the surfactant complex within the lungs and facilitating normal pulmonary function after birth. In contrast, SP-A and SP-D are relatively hydrophilic C-type collagenous lectins (collectins) that, in addition to their role as regulators of surfactant structure and homeostasis, also appear to be important regulators of the innate immune system. For example, SP-A binds to and opsonizes a variety of bacteria and viruses, thereby enhancing their phagocytosis by innate immune cells such as alveolar macrophages (1–3). SP-A is the predominant protein in the fetal lung and amniotic fluid and can be measured in amniotic fluid from 20 wk of pregnancy (4, 5).
There is substantial evidence to suggest that the fetus is in control of the timing of labor, but the mechanism(s) by which this is achieved is not well understood. Recent data suggest that pulmonary SP-A may provide the trigger for labor in mice (6). In this murine model, Condon et al. (6) demonstrated that: 1) levels of SP-A in amniotic fluid increase with increasing gestational age; 2) intraamniotic administration of SP-A on embryonic day 15 of gestation resulted in preterm labor and birth; and 3) intraamniotic administration of a neutralizing antibody against SP-A caused prolonged gestation. The authors hypothesize that SP-A produced by the maturing lungs of the fetal pups is secreted into the amniotic cavity where it activates resident macrophages. These activated fetal macrophages then infiltrate the maternal tissues of the uterus and induce a potent proinflammatory response characterized by an increase in proinflammatory mediators [including IL-1β and up-regulation of nuclear factor-κB leading to parturition (6)].
Whether SP-A has a role to play in labor in humans is not known, although concentrations of SP-A in amniotic fluid increase progressively throughout gestation, reaching a peak at term and then decreasing during spontaneous labor at term (4, 5). Although there is no evidence in humans of immune cell infiltration into the maternal tissues of the uterus at term, human labor—like that of the mouse (6)—is characterized by “decidual activation” with production of prostaglandins [predominantly prostaglandin F2α (PGF2α)] and other inflammatory mediators (7). This study investigates for the first time whether SP-A is produced by human endometrium/decidua and, if so, whether it is capable of regulating the production of inflammatory mediators in this tissue.
The human SP-A locus has been localized to chromosome 10q22-23 and consists of two functional genes, SP-A1 and SP-A2, which are highly conserved between species (8–10). Although a number of single nucleotide polymorphisms (SNPs) have been identified in human SP-A genes, the association between these genetic variants and preterm birth (PTB) has not previously been examined. This study investigates for the first time the association between SNPs in human SP-A genes and spontaneous PTB. To this end, we chose to focus specifically on the Pro62Pro (A/G) SNP in the SP-A1 gene (rs1136451) because: 1) it is well characterized; 2) it exists in strong linkage disequilibrium with the other functional SNPs in this gene (11); and 3) carriage of this genetic variant has been associated with an increased risk of respiratory diseases, including neonatal respiratory distress syndrome, bronchopulmonary dysplasia, and adult respiratory distress syndrome (12, 13), and otitis media (14).
Materials and Methods
Tissues
For immunohistochemistry, endometrial tissues were collected from healthy nonpregnant women (n = 3), and placenta/fetal membranes were collected at cesarean delivery from women before (n = 3) and after (n = 5) labor. For functional experiments, placenta/fetal membranes (n = 14) were obtained from uncomplicated term singleton deliveries at elective cesarean before the onset of labor. Decidua was scraped from fetal membranes and either used immediately to prepare fresh decidual stromal cells (DSCs) or snap-frozen in liquid nitrogen. Intrauterine infection was excluded on the basis of clinical criteria (absence of fever, maternal/fetal tachycardia, uterine tenderness), laboratory investigations (white cell count), and the absence of histological chorioamnionitis. All tissues were collected in accordance with requirements of the Institutional Review Boards at Yale University (New Haven, CT) and Pennsylvania State University (Hershey, PA).
Immunohistochemical studies
Serial sections (4 μm) of paraffin-embedded tissues were labeled as described (15) (see Supplemental Data, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). Primary antibodies included mouse monoclonal antihuman cytokeratin 7 (M7018, 1:200; Dako, Glostrup, Denmark), mouse monoclonal antihuman vimentin (M0725, 1:200; Dako), and rabbit polyclonal anti-SP-A (H-148, SC-13977, 1:100; Santa Cruz Biotechnology, Santa Cruz, CA). Negative controls were prepared by substituting preimmune serum for the corresponding preimmune antibody. Quantitative analysis of each tissue section was performed using AxioVision and corresponding digital image processing software (Carl Zeiss Microimaging, Inc., Thornwood, NY). A computer-generated H-score (in relative light units) was assigned for a representative tissue section and corrected for background light intensity as described (15, 16). Results are reported as mean ± sem from a minimum of five separate readings from three separate tissue sections.
Isolation and culture of term DSCs
Term DSCs were prepared and cultured as described (15, 17). Thereafter, DSCs were stimulated for 24 or 48 h with or without SP-A (1–100 μg/ml), and conditioned supernatant stored at −80 C until further analysis. SP-A was purified from bronchoalveolar lavage fluid collected from patients with human alveolar proteinosis as described (18–20) (see Supplemental Data). In select experiments, DSCs were stimulated with or without SP-A (1–100 μg/ml) for 6 h, and RNA was extracted for quantitative RT-PCR (RT-qPCR). To ensure positive controls for all cytokines, DSCs were also stimulated with or without IL-1β (1–10 ng/ml; R&D Systems, Minneapolis, MN) or thrombin (2.5 U/ml; Sigma-Aldrich, St. Louis, MO). In select experiments, cell viability at the end of the treatment period was measured using the MTT Cell Proliferation assay (ACTT Bioproducts, Manassas, VA).
Enzyme-linked immunosorbent assay (ELISA)
Levels of IL-6, IL-8, TNFα, matrix metalloproteinase-3 (MMP-3), monocyte chemotactic protein-1 (MCP-1), IL-1β, soluble fms-like tyrosine kinase-1 (sFlt-1), vascular endothelial growth factor (VEGF), IL-1β, PGE2, and PGF2α were measured in conditioned supernatant by specific ELISA according to instructions provided by the manufacturer (Cayman Chemical Company, Ann Arbor, MI, for PGE2 and PGF2α; R&D Systems, for the rest) (see Supplemental Data). In all cases, total protein content was measured using the Bradford protein assay (Bio-Rad Laboratories, Hercules, CA) (see Supplemental Data), and levels of cytokines were normalized to protein content.
Real-time RT-qPCR
Total RNA was extracted, and RT-qPCR was performed using oligonucleotide primers specific for human cytosolic phospholipase A2, cyclooxygenase (COX)-1, COX-2, and microsomal prostaglandin S synthase (mPGS) (see Supplemental Data and Supplemental Table 1). In all cases, mRNA levels were corrected for expression of the housekeeping gene, β-actin.
Statistical analysis for ELISA and RT-qPCR experiments (see Supplemental Data)
Study population for SP-A SNP analysis
Consecutive patients with unexplained spontaneous PTB (cases) and uncomplicated term deliveries (controls) were identified from the March of Dimes Perinatal Emphasis Research Initiative project (MOD no. 20-FY03-30; Principal Investigator, Charles J. Lockwood, M.D.) at New York University (New York, NY) and Yale University (New Haven, CT) from January 1989 through June 2005. Cases were matched (1:2) with controls. The study was approved by the Institutional Review Boards of both institutions. Written consent was obtained from all subjects before peripheral blood collection. Blood was centrifuged, and buffy coats were stored at −80 C before analysis. All specimens were linked with demographic, medical, and obstetrical data abstracted from the maternal and neonatal medical records. The diagnosis of spontaneous PTB required evidence of regular uterine contractions and progressive cervical dilatation as well as the absence of any identifiable causes for PTB (such as intrauterine infection) or an indicated PTB (such as placenta previa or prior classical cesarean).
DNA extraction and SNP analysis
Maternal genomic DNA (n = 435) was extracted from stored buffy coats using standard guanidine HCl or phenol-chloroform extraction protocols. Using the high-throughput automated MALDI-TOF-MS (matrix-assisted laser desorption/ionization time-of-flight mass spectrometry) platform with individualized assay designs created by automated spectrodesign software (Sequenom Inc., San Diego, CA), DNA was genotyped for Pro62Pro (A/G) polymorphism in SP-A1 gene (see Supplemental Data). χ2 statistics were used to evaluate the relationship between PTB and SP-A haplotype. Logistic regression models were then used to model the relationships between epidemiological factors (race, prior preterm delivery, infection, stress), genotype, and spontaneous PTB. Under a log-additive genetic model, our sample size conferred 80% power to detect an odds ratio of 2.5, assuming a two-tailed statistical significance level of ≤0.05 (Quanto version 1.0; http://hydra.usc.edu/gxe) (21).
Results
Localization of SP-A in endometrium/decidua
SP-A was localized to the maternal endometrium/decidua, with strong staining in both the endometrial glands and stromal cells (Fig. 1).
Fig. 1.
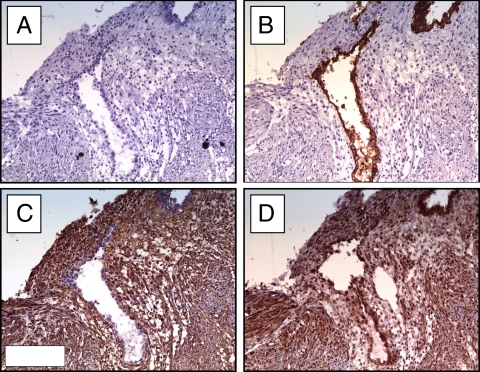
Immunohistochemical localization of SP-A in the maternal endometrium. Representative serial sections of endometrial tissues collected from a healthy nonpregnant woman in the early proliferative phase (d 6–7) of the menstrual cycle were stained for cytokeratin 7 (B; to identify endometrial glands), vimentin (C; to identify endometrial stromal cells), and SP-A (D) as described in Materials and Methods. As expected, cytokeratin stained endometrial glandular epithelial cells, but not stromal cells (B). In contrast, vimentin stained stromal but not glandular cells (C). Strong staining for SP-A is evident in both glandular and stromal cells (D). Negative controls using second antibody only showed the absence of nonspecific binding (A). Magnification, ×20.
Effect of SP-A on prostaglandin production by term DSCs
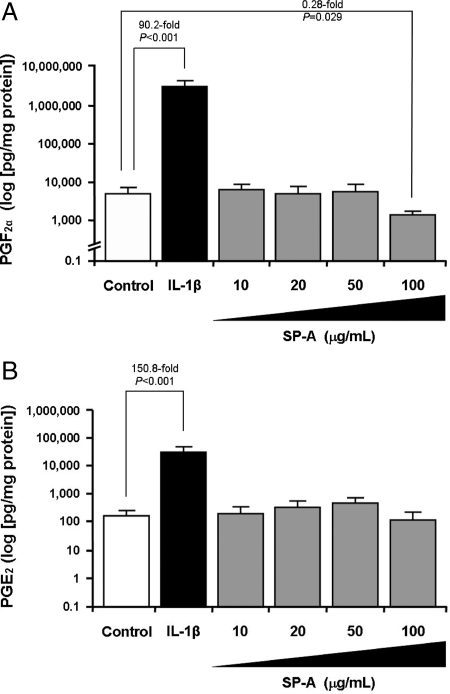
High-dose SP-A (100 μg/ml) significantly inhibited PGF2α production by term DSCs (4406 ± 1555 vs. 1079 ± 118 pg/mg protein per 24 h; P = 0.029) (Fig. 2A), which was independent of the hormonal milieu (Supplemental Fig. 1) or the presence of IL-1β or thrombin (Supplemental Fig. 2). This effect was not seen with low-dose SP-A (1–50 μg/ml) (Fig. 2A). SP-A did not affect PGE2 production (Fig. 2B). These observations were similar at both 24 and 48 h (data not shown). IL-1β (positive control) significantly up-regulated PGF2α and PGE2 release (Fig. 2), and SP-A did not abrogate the IL-1β-mediated increase in PGF2α production (Supplemental Fig. 2). This SP-A-mediated inhibition of PGF2α by term DSCs was not due to cell toxicity because: 1) it was selective to PGF2α (Figs. 2B and 3); 2) the cells showed no apparent morphological changes after SP-A treatment; and 3) there was no decrease in cell viability as measured by MTT Cell Proliferation assay (ACTT Bioproducts) after SP-A treatment (data not shown).
Fig. 2.
Effects of SP-A on prostaglandin production by term DSCs. Term DSCs were isolated, purified, treated with 17β-estradiol (E2; 10−8 m), medroxyprogesterone acetate (MPA; 10−7 m), both, or vehicle for 7 d, and then stimulated with SP-A (1–100 μg/ml) for 24 or 48 h as described in Materials and Methods. Positive controls included IL-1β (1 ng/ml). Levels of PGF2α (A) and PGE2 (B) were measured in conditioned supernatant by specific ELISA and corrected for protein content. Results are shown for E2 + MPA pretreatment and 24-h incubation only. Data are presented as mean ± sem from a minimum of four separate experiments performed in triplicate. Statistical differences are shown.
Fig. 3.
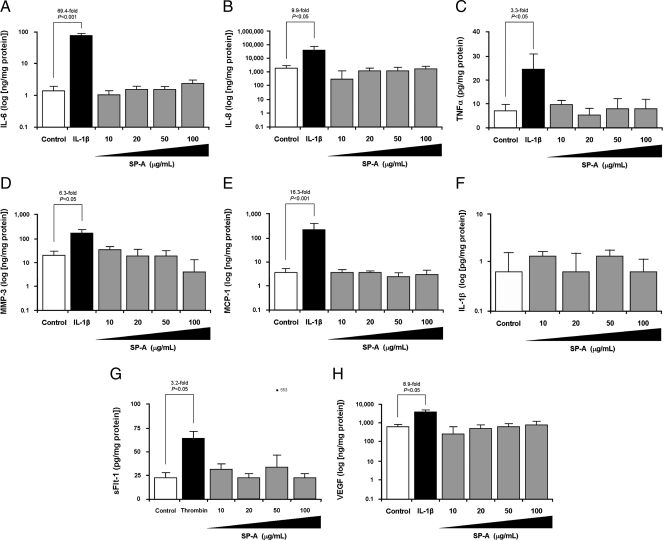
Effects of SP-A on the production of select cytokines and angiogenic factors by term DSCs. Term DSCs were isolated, purified, treated with 17β-estradiol (E2; 10−8 m), medroxyprogesterone acetate (MPA; 10−7 m), both, or vehicle for 7 d, and then stimulated with SP-A (1–100 μg/ml) for 24 or 48 h as described in Materials and Methods. Positive controls included IL-1β (1 ng/ml) or thrombin (2.5 U/ml). Levels of select cytokines and angiogenic factors including IL-6 (A), IL-8 (B), TNFα (C), MMP-3 (D), MCP-1 (E), IL-1β (F), sFlt-1 (G), and VEGF (H) were measured in conditioned supernatant by specific ELISA and corrected for protein content. Results are shown for E2 + MPA pretreatment and 24-h incubation only. Data are presented as mean ± sem from a minimum of four separate experiments performed in triplicate. Statistical differences are shown.
Effect of SP-A on production of cytokines and angiogenic factors by term DSCs
Levels of IL-6, IL-8, TNFα, MMP-3, MCP-1, IL-1β, sFlt-1, and VEGF were unaffected by SP-A at either high- or low-dose (Fig. 3, A–H). IL-1β (positive control) up-regulated the production of IL-6, IL-8, TNFα, MMP-3, MCP-1 (Fig. 3, A–E), and VEGF (Fig. 3H) by term DSCs (P < 0.05, for all). Consistent with prior publications using first-trimester DSCs (17, 22), IL-1β did not stimulate sFlt-1 production by term DSCs (data not shown). For this reason, thrombin was used as a positive control and, in keeping with prior reports (17, 22), thrombin (2.5 U/ml) significantly increased sFlt-1 production by term DSCs (Fig. 3G; P < 0.05).
Effect of SP-A on expression of eicosanoid genes by term DSCs
Transcripts for cytosolic phospholipase A2, COX-1, COX-2, and microsomal prostaglandin S synthase were identified in term DSCs, and their expression was not altered by stimulation with SP-A (Supplemental Fig. 3).
Effect of labor on SP-A expression in term decidua
SP-A expression was examined in term placentae by immunohistochemistry before and after labor. SP-A was highly expressed in decidual (vimentin-positive) cells from tissues collected before labor (Fig. 4, A–D) and was significantly reduced in tissues collected after labor (Fig. 4, E–H) (P = 0.008; Fig. 4I). The presence of SP-A in DSCs was confirmed by immunocytochemistry and RT-qPCR (data not shown). SP-A was essentially absent from trophoblast (cytokeratin 7-positive) cells in tissues collected before and after labor as evidenced by immunohistochemistry (Fig. 4) and RT-qPCR (data not shown).
Fig. 4.
Immunohistochemical studies of SP-A expression in placental tissues before and after labor. Serial sections of placental tissues collected from women before (A–D) and after (E–H) the onset of labor were stained for cytokeratin 7 (B and F; to identify trophoblast cells), vimentin (C and G; to identify decidual cells), and SP-A (D and H) as described in the Materials and Methods section. Representative serial images are shown. Before labor, vimentin-positive decidual cells (C) showed strong staining for SP-A (D). Staining was most intense within the nucleus, but was also evident in the cytoplasm (D, inset). After labor, however, the vimentin-positive decidual cells (G) showed little staining for SP-A (H). Quantitative H-score analysis confirmed the decrease in SP-A staining intensity with the onset of labor (I). Negative controls (A and E) used second antibody only and showed the absence of nonspecific binding. Magnification, ×10 (insets, ×40).
Association between maternal SP-A polymorphisms and spontaneous PTB
A total of 145 women with spontaneous PTB (cases) and 290 women with uncomplicated term deliveries (controls) were identified. After excluding subjects with insufficient DNA (n = 5), technical failures (n = 6), or incomplete clinical data (n = 23), 133 cases (91.7% of initial subjects) and 268 controls (92.4%) were included in the final analysis (Supplemental Fig. 4). There were no statistically significant differences in maternal age at delivery, gravidity, parity, maternal weight, and body mass index between cases and controls (Supplemental Table 2). As expected, the median gestational age at delivery [35.2 wk (interquartile range, 32.9, 36.4), vs. 39.7 wk (39.0, 40.7)], birth weight, and Apgar scores were significantly lower among cases vs. controls, whereas neonatal intensive care unit admission rates were significantly higher. Compared with controls, cases were more likely to have a history of a prior PTB (P = 0.03), be a multiple pregnancy (P = 0.02), and be complicated by first-trimester bleeding (P < 0.001), bacterial vaginosis (P = 0.007), preterm premature rupture of membranes (P < 0.001), preeclampsia/eclampsia (P < 0.001), and/or pregestational diabetes (P < 0.001). Moreover, as markers of impending PTB, such pregnancies were also more likely to have been diagnosed with preterm labor (P < 0.001) and to have received tocolytic therapy (P = 0.003) and/or antenatal corticosteroids (P < 0.001) (Supplemental Table 2).
Genotyping for Pro62Pro (A/G) polymorphism in human SP-A1 gene was successful in 98.6% (424 of 430) of samples. Hardy-Weinberg genotype distributions were as expected in both cases (P = 0.99) and controls (P = 0.24). The genotype distribution frequency in the overall population was similar to that previously reported (14, 23), with no difference in genotype frequencies between cases and controls (P = 0.59) or in A vs. G allele frequencies between cases and controls (odds ratio, 1.0; 95% confidence interval, 0.7, 1.5; P = 0.92) (Table 1). Multivariate analysis showed no association between Pro62Pro (A/G) genotype and PTB when controlled for maternal age, ethnicity, gravidity, parity, history of prior PTB, route of delivery, or neonatal outcome (P = 0.619). The racial distribution of the population was 82.1% Hispanic, 9.0% Asian, 3.8% Black, and 5.1% White (Supplemental Table 2). The numbers of patients of individual races were insufficient for the presentation of data stratified by race; however, no significant within-race differences were observed (P > 0.88 for all races).
Table 1.
Maternal SP-A genotype distribution between spontaneous PTB cases and controls
| Genotype | Total study population (n = 401) | Spontaneous PTB |
P value | |
|---|---|---|---|---|
| Yes (cases) (n = 133) | No (controls) (n = 268) | |||
| Pro62Pro (A/G) | ||||
| AA | 257 (64.1%) | 85 (63.9%) | 172 (64.2%) | 0.59 |
| AG | 134 (33.4%) | 45 (33.8%) | 89 (33.2%) | |
| GG | 10 (2.5%) | 3 (2.3%) | 7 (2.6%) | |
Discussion
There is substantial evidence to suggest that the fetus is in control of the timing of labor, but the mechanism(s) by which this is achieved is not well understood. SP-A has been implicated in the onset of labor in mice (6) and has been shown to bind to toll-like receptors and induce the production of proinflammatory cytokines, including IL-1β, IL-6, and TNF-α (24). Given these reports and the central role that decidual activation is known to play in the onset of labor (7), we chose to investigate whether SP-A is produced by human endometrium/decidua and, if so, whether it is capable of regulating the production of select inflammatory mediators and angiogenic factors in this tissue.
It has long been argued that SP-A expression in humans is limited to the respiratory system. However, recent studies have shown that it is also produced in a number of tissues within the reproductive tract, including the vaginal mucosa (25), fetal membranes (26–29), and chorionic and placental trophoblast cells (27, 30). The traditional teaching that SP-A in amniotic fluid comes from the fetal lungs is thus being challenged. Indeed, amniotic fluid SP-A levels were measured in a case of tracheal atresia in which there was no communication between the fetal lungs and amniotic cavity and were noted to be normal (26). In this study, we demonstrate for the first time that SP-A is produced by the maternal endometrium/decidua (Fig. 1) and that its expression decreases with labor (Fig. 4). This is consistent with prior reports that amniotic fluid SP-A levels decrease with labor (5). Taken together, these data suggest that the maternal decidua, and not the fetal lungs, may be the primary source of SP-A in amniotic fluid.
The majority of publications on SP-A have focused on its role in pulmonary function, but recent research suggests that it may have a far more important role to play as a regulator of innate immunity. Indeed, SP-A knockout mice demonstrate no reproductive phenotype and no abnormalities in lung function, but a marked decrease in the ability to clear bacterial antigens within the lungs (31, 32). SP-A appears to act by binding to specific cell surface receptors on microorganisms and immune cells and has been shown to modulate the functions of phagocytic cells both in vitro and in vivo (33). The role of SP-A on immune function at the maternal-fetal interface has not been extensively investigated. Here, we demonstrate that SP-A significantly and selectively inhibited PGF2α production by term DSCs without affecting the production of other inflammatory mediators (Figs. 2 and 3). This effect was seen with high (100 μg/ml), but not low, concentrations of SP-A (1, 10, 20, or 50 μg/ml) (Fig. 2). Concentrations of SP-A in amniotic fluid at term range from 2–25 μg/ml (5, 34). Although the “physiological” concentration of SP-A in the maternal decidua in vivo is not known, data presented in this manuscript demonstrating that SP-A is made in this tissue and that the decidua may be the primary source of this protein in amniotic fluid (26) suggest that SP-A concentrations within the maternal decidua may well be significantly higher than that in amniotic fluid.
Endogenous levels of prostaglandins in the endometrium/decidua are lower in early pregnancy than in the endometrium at any stage of the menstrual cycle (35). These findings, along with the observation that prostaglandin precursors are present in far higher concentrations than primary (biologically active) prostaglandins in amniotic fluid and most uterine tissues, and that administration of exogenous prostaglandins, iv, intraamniotically, or vaginally in all species examined and at any stage of gestation, have the ability to induce abortion, support the hypothesis that pregnancy is maintained by a mechanism that tonically suppresses uterine prostaglandin synthesis throughout gestation. The factor(s) responsible for this suppression remains elusive, but the current study suggests that SP-A may be a promising candidate. The molecular mechanism(s) by which SP-A suppresses PGF2α production in term DSCs is not known, but this effect appears to be regulated at a posttranscriptional level because SP-A did not significantly alter the expression of key eicosanoid enzymes in term DSCs in vitro (Supplemental Fig. 3). Alternatively, as demonstrated in lung epithelial cells, SP-A may interfere with phospholipase A2 activity (and thereby the release of arachidonic acid and prostaglandin synthesis) through a direct protein-protein interaction with peroxiredoxin 6, a member of the peroxiredoxin superfamily of selenium-independent peroxidases (36).
Familial clustering, racial disparities, and the high incidence of recurrent PTB all suggest a critical role for maternal genetic factors in the timing of labor. Despite their importance, the maternal genetic factors associated with spontaneous PTB are not well understood. Here, we investigate the association between SNPs in the human SP-A genes and spontaneous PTB. The human SP-A locus has been localized to chromosome 10q22-23 and consists of two functional genes, SP-A1 and SP-A2 (9, 10). Their promoter sequences have been well characterized, and transcription is regulated by several steroid and polypeptide hormones including glucocorticoids and cAMP (37, 38). Each gene encodes a distinct 248 amino acid protein (SP-A1 and SP-A2, respectively) with a molecular mass of 32–36 kDa. Within the alveolus, two SP-A1 and one SP-A2 protein combine to form a trimer, and six trimers come together to create a bouquet-like octadecameric complex (molecular mass, 650 kDa) known as SP-A. Both the SP-A1 and SP-A2 genes are highly polymorphic. To date, more than 30 allelic variants have been characterized for the SP-A genes. By convention, these are designated by 6An for SP-A1 gene haplotypes and 1An for SP-A2 gene haplotypes. Of these, four SP-A1 gene alleles (6A, 6A2, 6A3, 6A4) and six SP-A2 gene alleles (1A, 1A0, 1A1, 1A2, 1A3, 1A5) have been observed at a high frequency in the general population and appear to be functionally important (11–14, 39, 40). For example, carriage of several of these variants have been shown to affect the ability of SP-A to stimulate TNFα production in immortalized cell lines in vitro (39). Similarly, sequence variability within the 3′- and 5′-untranslated regions have been shown to differentially regulate expression of the SP-A genes both under basal conditions and in response to dexamethasone (40). Most importantly, carriage of these genetic variants has been shown to translate into an increased risk for a number of clinical disorders, including respiratory diseases [respiratory distress syndrome, bronchopulmonary dysplasia, adult respiratory distress syndrome, chronic obstructive pulmonary disease, and susceptibility to tuberculosis (11–13)] and otitis media (14). To investigate the association between these genetic variations and spontaneous PTB, we chose initially to measure only the well-characterized synonymous Pro62Pro (A/G) SNP in the SP-A1 gene (rs1136451), which is located in the 5′ end of the second translated exon of this relatively small (5 kb) gene on the long arm of chromosome 10, with the objective of refining haplotype analysis if this particular tag SNP showed even a weak association. This is because all of the genetic variants in both the SP-A1 and SP-A2 genes have been shown to exist in strong linkage disequilibrium (14, 41), making it unnecessary to measure them all separately. Of note, the Pro62Pro (A/G) SNP in the SP-A1 gene is silent, meaning that it is not in the coding sequence and, as such, does not result in an amnio acid substitution. Using established methodology and a well-characterized population, we have found no support for the hypothesis that SNPs in the maternal SP-A genes are associated with spontaneous PTB.
In summary, we demonstrate that SP-A is produced by human endometrium/decidua, where it acts to significantly and selectively inhibit PGF2α production without affecting the production of other select inflammatory mediators and angiogenic factors (including IL-6, IL-8, TNFα, MMP-3, MCP-1, IL-1β, PGE2, sFlt-1, and VEGF). This effect appears to be regulated at a posttranscriptional level. Expression of SP-A within the decidua decreases with labor. Taken together, these novel observations suggest that decidual SP-A may play a critical role in regulating prostaglandin production within the uterus, culminating at term in decidual activation and the onset of labor. Further studies are needed to examine the relative contribution of decidual SP-A to levels measured in the amniotic fluid throughout gestation and to better define the role of decidual SP-A in the onset of labor, both at term and preterm.
Acknowledgments
This work was supported by the National Institutes of Health/National Institute of Child Health and Human Development (5-K12-HD000849-23, to E.R.N.) and the March of Dimes (21-FY05-1250, to E.R.N.).
Disclosure Summary: The authors have no conflicts of interest to declare.
Footnotes
- COX
- Cyclooxygenase
- DSC
- decidual stromal cell
- MCP-1
- monocyte chemotactic protein-1
- MMP-3
- matrix metalloproteinase-3
- PGF2α
- prostaglandin F2α
- PTB
- preterm birth
- RT-qPCR
- quantitative RT-PCR
- sFlt-1
- soluble fms-like tyrosine kinase-1
- SNP
- single nucleotide polymorphism
- SP-A
- surfactant protein-A
- VEGF
- vascular endothelial growth factor.
References
- 1. Wright JR. 2005. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol 5:58–68 [DOI] [PubMed] [Google Scholar]
- 2. Dobbs LG, Wright JR, Hawgood S, Gonzalez R, Venstrom K, Nellenbogen J. 1987. Pulmonary surfactant and its components inhibit secretion of phosphatidylcholine from cultured rat alveolar type II cells. Proc Natl Acad Sci USA 84:1010–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McCormack FX, Whitsett JA. 2002. The pulmonary collectins, SPA and SP-D, orchestrate innate immunity in the lung. J Clin Invest 109:707–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pryhuber GS, Hull WM, Fink I, McMahan MJ, Whitsett JA. 1991. Ontogeny of surfactant proteins A and B in human amniotic fluid as indices of fetal lung maturity. Pediatr Res 30:597–605 [DOI] [PubMed] [Google Scholar]
- 5. Chaiworapongsa T, Hong JS, Hull WM, Kim CJ, Gomez R, Mazor M, Romero R, Whitsett JA. 2008. The concentration of surfactant protein-A in amniotic fluid decreases in spontaneous human parturition at term. J Matern Fetal Neonatal Med 21:652–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Condon JC, Jeyasuria P, Faust JM, Mendelson CR. 2004. Surfactant protein secreted by the maturing mouse fetal lung acts as a hormone that signals the initiation of parturition. Proc Natl Acad Sci USA 101:4978–4983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Casey ML, MacDonald PC. 1988. Biomolecular processes in the initiation of parturition: decidual activation. Clin Obstet Gynecol 31:533–552 [DOI] [PubMed] [Google Scholar]
- 8. White RT, Damm D, Miller J, Spratt K, Schilling J, Hawgood S, Benson B, Cordell B. 1985. Isolation and characterization of the human pulmonary surfactant apoprotein gene. Nature 317:361–363 [DOI] [PubMed] [Google Scholar]
- 9. Hoover RR, Floros J. 1998. Organization of the human SP-A and SP-D loci at 10q22–q23. Physical and radiation hybrid mapping reveal gene order and orientation. Am J Respir Cell Mol Biol 18:353–362 [DOI] [PubMed] [Google Scholar]
- 10. Floros J, Hoover RR. 1998. Genetics of the hydrophilic surfactant proteins A and D. Biochim Biophys Acta 1408:312–322 [DOI] [PubMed] [Google Scholar]
- 11. Hallman M, Haataja R. 2006. Surfactant protein polymorphisms and neonatal lung disease. Semin Perinatol 30:350–361 [DOI] [PubMed] [Google Scholar]
- 12. Heinrich S, Hartl D, Griese M. 2006. Surfactant protein A—from genes to human lung diseases. Curr Med Chem 13:3239–3252 [DOI] [PubMed] [Google Scholar]
- 13. Haataja R, Rämet M, Marttila R, Hallman M. 2000. Surfactant proteins A and B as interactive genetic determinants of neonatal respiratory distress syndrome. Hum Mol Genet 9:2751–2760 [DOI] [PubMed] [Google Scholar]
- 14. Pettigrew MM, Gent JF, Zhu Y, Triche EW, Belanger KD, Holford TR, Bracken MB, Leaderer BP. 2006. Association of surfactant protein A polymorphisms with otitis media in infants at risk for asthma. BMC Med Genet 7:68–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Snegovskikh VV, Schatz F, Arcuri F, Toti P, Kayisli UA, Murk W, Guoyang Luo, Lockwood CJ, Norwitz ER. 2009. Intra-amniotic infection upregulates decidual cell vascular endothelial growth factor (VEGF) and neuropilin-1 and -2 expression: implications for infection-related preterm birth. Reprod Sci 16:767–780 [DOI] [PubMed] [Google Scholar]
- 16. Budwit-Novotny DA, McCarty KS, Cox EB, Soper JT, Mutch DG, Creasman WT, Flowers JL, McCarty KS., Jr 1986. Immunohistochemical analyses of estrogen receptor in endometrial adenocarcinoma using a monoclonal antibody. Cancer Res 46:5419–5425 [PubMed] [Google Scholar]
- 17. Norwitz ER, Snegovskikh V, Schatz F, Foyouzi N, Rahman M, Buchwalder L, Lee HJ, Funai EF, Buhimschi CS, Buhimschi IA, Lockwood CJ. 2007. Progestin inhibits and thrombin stimulates the plasminogen activator/inhibitor system in term decidual stromal cells: implications for parturition. Am J Obstet Gynecol 196:382.e1–382.e8 [DOI] [PubMed] [Google Scholar]
- 18. McIntosh JC, Mervin-Blake S, Conner E, Wright JR. 1996. Surfactant protein A protects growing cells and reduces TNF-α activity from LPS-stimulated macrophages. Am J Physiol 271:L310–L319 [DOI] [PubMed] [Google Scholar]
- 19. Lin PM, Wright JR. 2006. Surfactant protein A binds to IgG and enhances phagocytosis of IgG-opsonized erythrocytes. Am J Physiol Lung Cell Mol Physiol 291:L1199–L1206 [DOI] [PubMed] [Google Scholar]
- 20. Kunzmann S, Wright JR, Steinhilber W, Kramer BW, Blaser K, Speer CP, Schmidt-Weber C. 2006. TGF-β1 in SP-A preparations influence immune suppressive properties of SP-A on human CD4+ T lymphocytes. Am J Physiol Lung Cell Mol Physiol 291:L747–L756 [DOI] [PubMed] [Google Scholar]
- 21. Gauderman WJ. 2002. Sample size requirements for association studies of gene-gene interaction. Am J Epidemiol 155:478–484 [DOI] [PubMed] [Google Scholar]
- 22. Lockwood CJ, Toti P, Arcuri F, Norwitz E, Funai EF, Huang ST, Buchwalder LF, Krikun G, Schatz F. 2007. Thrombin regulates soluble fms-like tyrosine kinase-1 (sFlt-1) expression in first trimester decidua: implications for preeclampsia. Am J Pathol 170:1398– 1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rämet M, Haataja R, Marttila R, Hämäläinen AM, Knip M, Hallman M. 2000. Human surfactant protein-A gene locus for genetic studies in the Finnish population. Dis Markers 16:119–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guillot L, Balloy V, McCormack FX, Golenbock DT, Chignard M, Si-Tahar M. 2002. Cutting edge: the immunostimulatory activity of the lung surfactant protein-A involves Toll-like receptor 4. J Immunol 168:5989–5992 [DOI] [PubMed] [Google Scholar]
- 25. MacNeill C, Umstead TM, Phelps DS, Lin Z, Floros J, Shearer DA, Weisz J. 2004. Surfactant protein A, an innate immune factor, is expressed in the vaginal mucosa and is present in vaginal lavage fluid. Immunology 111:91–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Han YM, Romero R, Kim YM, Kim JS, Richani K, Friel LA, Kusanovic JP, Jeanty C, Vitale S, Nien JK, Espinoza J, Kim CJ. 2007. Surfactant protein-A mRNA expression by human fetal membranes is increased in histological chorioamnionitis but not in spontaneous labour at term. J Pathol 211:489–496 [DOI] [PubMed] [Google Scholar]
- 27. Sun K, Brockman D, Campos B, Pitzer B, Myatt L. 2006. Induction of surfactant protein A expression by cortisol facilitates prostaglandin synthesis in human chorionic trophoblasts. J Clin Endocrinol Metab 91:4988–4994 [DOI] [PubMed] [Google Scholar]
- 28. Lee DC, Romero R, Kim CJ, Chaiworapongsa T, Tarca AL, Lee J, Suh YL, Mazaki-Tovi S, Vaisbuch E, Mittal P, Draghici S, Erez O, Kusanovic JP, Hassan SS, Kim JS. 2010. Surfactant protein-A as an anti-inflammatory component in the amnion: implications for human pregnancy. J Immunol 184:6479–6491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Miyamura K, Malhotra R, Hoppe HJ, Reid KB, Phizackerley PJ, Macpherson P, López Bernal A. 1994. Surfactant proteins A (SP-A) and D (SP-D): levels in human amniotic fluid and localization in the fetal membranes. Biochim Biophys Acta 1210:303–307 [DOI] [PubMed] [Google Scholar]
- 30. Sati L, Seval-Celik Y, Demir R. 2010. Lung surfactant proteins in the early human placenta. Histochem Cell Biol 133:85–93 [DOI] [PubMed] [Google Scholar]
- 31. Korfhagen TR, Bruno MD, Ross GF, Huelsman KM, Ikegami M, Jobe AH, Wert SE, Stripp BR, Morris RE, Glasser SW, Bachurski CJ, Iwamoto HS, Whitsett JA. 1996. Altered surfactant function and structure in SP-A gene targeted mice. Proc Natl Acad Sci USA 93:9594–9599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ikegami M, Korfhagen TR, Whitsett JA, Bruno MD, Wert SE, Wada K, Jobe AH. 1998. Characteristics of surfactant from SP-A-deficient mice. Am J Physiol 275:L247–L254 [DOI] [PubMed] [Google Scholar]
- 33. Crouch E, Wright JR. 2001. Surfactant proteins A and D and pulmonary host defense. Annu Rev Physiol 63:521–554 [DOI] [PubMed] [Google Scholar]
- 34. Chaiworapongsa T, Hong JS, Hull WM, Romero R, Whitsett JA. 2008. Amniotic fluid concentration of surfactant proteins in intra-amniotic infection. J Matern Fetal Neonatal Med 21:663–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Norwitz ER, Wilson T. 2000. Secretory component: a potential regulator of endometrium/decidual prostaglandin production in early human pregnancy. Am J Obstet Gynecol 183:108–117 [DOI] [PubMed] [Google Scholar]
- 36. Wu YZ, Manevich Y, Baldwin JL, Dodia C, Yu K, Feinstein SI, Fisher AB. 2006. Interaction of surfactant protein A with peroxiredoxin 6 regulates phospholipase A2 activity. J Biol Chem 281:7515–7525 [DOI] [PubMed] [Google Scholar]
- 37. Odom MJ, Snyder JM, Boggaram V, Mendelson CR. 1988. Glucocorticoid regulation of the major surfactant associated protein (SP-A) and its messenger ribonucleic acid and of morphological development of human fetal lung in vitro. Endocrinology 123:1712–1720 [DOI] [PubMed] [Google Scholar]
- 38. Alcorn JL, Islam KN, Young PP, Mendelson CR. 2004. Glucocorticoid inhibition of SP-A gene expression in lung type II cells is mediated via the TTF-1-binding element. Am J Physiol Lung Cell Mol Physiol 286:L767–L776 [DOI] [PubMed] [Google Scholar]
- 39. Wang G, Phelps DS, Umstead TM, Floros J. 2000. Human SP-A protein variants derived from one or both genes stimulate TNF-α production in the THP-1 cell line. Am J Physiol Lung Cell Mol Physiol 278:L946–L954 [DOI] [PubMed] [Google Scholar]
- 40. Wang G, Guo X, Floros J. 2005. Differences in the translation efficiency and mRNA stability mediated by 5′-UTR splice variants of human SP-A1 and SP-A2 genes. Am J Physiol Lung Cell Mol Physiol 289:L497–L508 [DOI] [PubMed] [Google Scholar]
- 41. Floros J, DiAngelo S, Koptides M, Karinch AM, Rogan PK, Nielsen H, Spragg RG, Watterberg K, Deiter G. 1996. Human SP-A locus: allele frequencies and linkage disequilibrium between the two surfactant protein genes. Am J Respir Cell Mol Biol 15:489–498 [DOI] [PubMed] [Google Scholar]