The overproduction of androstenedione and testosterone in women with PCOS is consistent with excessive steroidogenesis in the delta-5 pathway.
Abstract
Context:
In women with polycystic ovary syndrome (PCOS), the basis for ovarian androgen overproduction involves an overall increase of steroidogenesis, notably in the delta-4 pathway. However, in vitro studies have suggested that excessive androgen production occurs predominantly through the delta-5 pathway.
Objective:
This study was performed to assess androgen dose-responses after human chorionic gonadotropin (hCG) stimulation in PCOS and normal women.
Design:
We conducted a prospective study to compare androgen production after iv hCG in PCOS and normal women.
Setting:
The study was conducted in a General Clinical Research Center in an academic medical center.
Participants:
Women with PCOS (age, 18–37 yr; n = 10) and normal ovulatory controls (age, 18–37 yr; n = 11) were recruited.
Interventions:
For dose-response studies, blood samples were obtained before and at 0.5, 24, and 48 h after iv recombinant hCG (1, 10, 25, 100, and 250 μg). A subset of subjects underwent frequent blood sampling over 24 h after iv injection of 25 μg of recombinant hCG.
Main Outcome Measure(s):
We measured basal and stimulated serum 17-hydroxyprogesterone (17-OHP), androstenedione (A), testosterone (T), dehydroepiandrosterone, estradiol, and progesterone responses after hCG administration.
Results:
In PCOS women, maximal A and T production was observed at the lowest doses of hCG, whereas responses were minimal in normal women. Incremental responses of 17-OHP, estradiol, and progesterone were greater in PCOS compared to normal women.
Conclusion:
In PCOS women, maximal A and T responses to hCG relative to those of 17-OHP are consistent with ovarian androgen overproduction via the delta-5 pathway.
In women with polycystic ovary syndrome (PCOS), a cardinal physiological abnormality is excessive ovarian androgen production. This process is driven by increased pituitary LH secretion and enhanced theca cell responsiveness to gonadotropin stimulation. The latter consideration is essential to the pathophysiology of this disorder because hyperandrogenemia is predominant among PCOS women, whereas serum levels of LH may be minimally increased or normal. In PCOS, the basis for ovarian androgen overproduction involves an increase in overall steroidogenesis, notably in the delta-4 pathway (1–4). The most notable feature has been exaggerated serum 17-hydroxyprogesterone (17-OHP) responses to gonadotropin stimulation (1–3, 5–9). Moreover, in PCOS women, heightened 17-OHP responses to human chorionic gonadotropin (hCG) stimulation were unchanged after GnRH agonist suppression of gonadotropin secretion, suggesting an intrinsic defect of theca cell steroidogenesis (8).
These clinical observations are consistent with in vitro studies that have demonstrated excess theca cell androgen production on a per-cell basis resulting from overexpression of multiple steroidogenic enzymes, including CYP17 (10, 11). However, evidence of delta-4 17,20-lyase activity was minimal, which suggested, in contrast to in vivo studies, that excessive androgen production occurred predominantly through the delta-5 steroid pathway. In light of these findings, we conducted a study to examine androgen dose-responsiveness and time-course patterns after hCG stimulation in women with PCOS and normal women to discern the nature of androgen overproduction.
Subjects and Methods
Participants
Ten women with PCOS and 11 normal women were recruited. PCOS subjects exhibited clinical and/or biochemical evidence of hyperandrogenism and were either oligomenorrheic or amenorrheic. Oligomenorrhea was defined as irregular menstrual bleeding occurring less than six times a year. Each PCOS subject had polycystic ovaries by ultrasound. PCOS and normal women had comparable mean ages (±se) of 28.5 ± 1.1 and 30.0 ± 1.3 yr, respectively. Mean body mass index (BMI) was higher in PCOS subjects (37.7 ± 2.1 vs. 29.1 ± 2.2 kg/m2, respectively; P < 0.02). Late-onset congenital adrenal hyperplasia was excluded by serum 17-OHP less than 2 ng/ml. Circulating TSH and prolactin were normal among all subjects. No subject had received hormone medication for 2 months before study. The study was approved by the Human Research Protection Program at the University of California, San Diego (UCSD), and written informed consent was obtained from each participant.
Procedures
Subjects were admitted to the General Clinical Research Center at UCSD on each day of testing. Each subject received recombinant hCG (r-hCG) as an iv bolus at doses of 1, 10, 25, 150, and 250 μg. r-hCG was administered iv. In normal subjects, r-hCG was given during the midfollicular phase of separate menstrual cycles. In PCOS women, r-hCG was administered on a random day with an interval between doses of at least 2 wk. The order of doses was randomized before selection by study personnel. Blood samples were obtained at −30, 0, 0.5, 24, and 48 h after r-hCG administration.
To determine peak androgen responses after r-hCG administration, five PCOS women and four normal controls underwent frequent blood sampling over 24 h after 25 μg of r-hCG. Blood samples were obtained at −120, −60, 0, 1, 2, 3, 4, 6, 8, 10, 12, 16, 20, and 24 h after r-hCG.
None of the PCOS subjects had experienced recent ovulation, as evidenced by absence of recent menstrual bleeding for 2 months before study and serum progesterone (P4) less than 2.0 ng/ml.
Assays
Serum LH and FSH were measured by RIA with intra- and interassay coefficients of variation (CVs) of 5.4 and 8.0%, respectively, for LH; and 3.0 and 4.6%, respectively, for FSH (Diagnostic Products Corp., Los Angeles, CA). Serum hCG was measured by chemiluminescent immunometric assay with intra- and interassay CVs of 5.4 and 9.9%, respectively (Immulite 1000; Siemens Healthcare Diagnostics, Deerfield, IL).
Serum estradiol (E2), androstenedione (A), and testosterone (T) were measured by RIA with intraassay CV less than 7%. Serum 17-OHP, P4, dehydroepiandrosterone (DHEA), and DHEA sulfate (DHEA-S) were measured by RIA with intraassay CV less than 7% (Diagnostic Systems Laboratories, Inc., Webster, TX). Serum anti-Müllerian hormone (AMH) was measured by ELISA with intra- and interassay CVs less than 4%.
Statistical analysis
Hormone measures were transformed to natural log values to minimize the impact of skewed distributions. Baseline hormone values for PCOS and normal women were compared by two-sided, two-sample t tests. In the dose-response studies, 17-OHP, A, T, DHEA, E2, and P4 responses were compared within and between groups after administration of 1, 10, 25, 100, and 250 μg of r-hCG. Linear mixed-effect models (12) were used to test the interaction of dose with diagnostic group on each assay. We modeled the mean level of an assay, Y24, at 24 h for subject i at dose dj as: Y24i(dj)=β0 + β1PCOSi + β2dj + β3PCOSidj + αi0 + εij, where PCOSi is an indicator variable for PCOS vs. normal, βs are fixed effects, and α and ε are Gaussian distributed random effects and residual error. We also adjusted for BMI at baseline and for the response at time 0, Y0i. The parameter of interest is β3, the difference in mean rate of change in Y24 per unit dose. Residual plots from models with dose on the microgram scale revealed heteroscedasticity that was stabilized after log transforming the doses. To ascertain the doses at which groups were significantly differentiated, we modeled dose as a categorical variable in a mixed-effects model of repeated measures (MMRM) (13). For all analyses, P values less than 0.05 were considered statistically significant. Statistical analyses were performed using the R statistical computing software (14).
Results
Baseline hormone concentrations in PCOS and normal women
Baseline circulating hormone levels are shown in Table 1. In women with PCOS, serum 17-OHP, A, and LH levels were significantly greater than those of normal controls. Serum T and AMH were higher in PCOS than normal controls, but did not achieve statistical significance, likely reflecting the limited number of subjects (P = 0.09 and P = 0.1, respectively). Serum FSH, E2, P4, DHEA, and DHEA-S were similar between groups.
Table 1.
Basal circulating hormone levels in PCOS and normal controls
| Controls (n = 11) | PCOS (n = 10) | |
|---|---|---|
| LH (mIU/ml) | 3.2 ± 0.6 | 6.1 ± 0.9a |
| FSH (mIU/ml) | 5.4 ± 0.4 | 5.0 ± 0.4 |
| 17-OHP (ng/ml) | 0.77 ± 0.4 | 1.11 ± 0.6a |
| A (ng/ml) | 0.97 ± 0.5 | 1.54 ± 0.5a |
| T (ng/ml) | 0.29 ± 0.2 | 0.44 ± 0.2 |
| DHEA (ng/ml) | 3.5. ±1.9 | 5.2 ± 4.1 |
| DHEA-S (ng/ml) | 2007 ± 1026 | 2227 ± 1133 |
| E2 (pg/ml) | 46.8 ± 18.6 | 49.6 ± 12.0 |
| P4 (ng/ml) | 1.0 ± 0.1 | 1.0 ± 0.1 |
| AMH (ng/ml) | 2.9 ± 0.7 | 5.3. ±1.2 |
Data are expressed as mean ± se. To convert to SI units, multiply by the following conversion factors: 17-OHP, 3.03; A, 3.49; T, 3.47; DHEA, 3.47; DHEA-S, 0.0027; E2, 3.67; P4, 3.18; and AMH, 7.14.
P < 0.05 PCOS vs. normal controls.
Serum hCG levels in response to r-hCG administration
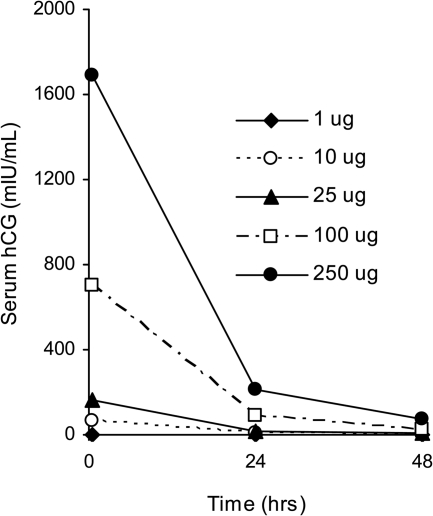
Circulating levels of hCG after each iv dose of r-hCG are shown in Fig. 1. Baseline serum hCG levels were undetectable before stimulation. Highest concentrations were observed soon after injection at 30 min. In both groups, increases of serum hCG were similar and dose-related; therefore, the data were combined. Maximum values of hCG were 2 ± 1, 62 ± 8, 164 ± 13, 705 ± 50, and 1687 ± 120 mIU/ml at doses of 1, 10, 25, 100, and 250 μg, respectively. The decline in hCG followed a double exponential curve with an initial fast component reflecting clearance from the circulation and a later slow component resulting from tissue redistribution.
Fig. 1.
Mean serum hCG concentrations after iv administration of r-hCG in a combined group of five PCOS and five normal women. The baseline hCG levels in all subjects were undetectable. The early maximal increase at each dose represents the mean serum hCG value 30 min after injection.
In general, r-hCG given iv was well tolerated in all subjects. In normal women receiving 250 μg, minimal vaginal bleeding in two subjects and breast tenderness in one subject were noted during the first week of administration. There were no reported side effects in the PCOS group.
Androgen dose-responses to r-hCG administration
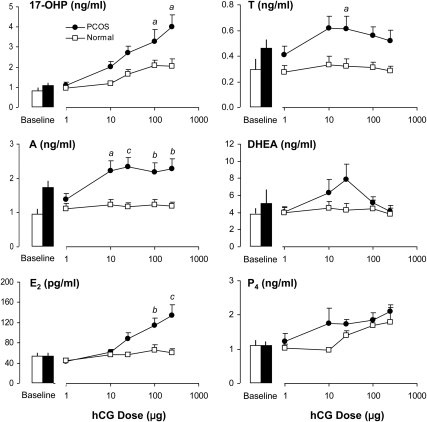
In PCOS and normal women, serum 17-OHP responses 24 h after 1, 10, 25, 100, and 250 μg of r-hCG are shown in Fig. 2. According to analysis by the linear mixed-effect model, women with PCOS exhibited significant (P < 0.001) and progressively increasing 17-OHP dose-responsiveness to r-hCG administration that was greatest at 250 μg (Table 2). In normal controls, similar patterns of responsiveness (P < 0.001) were observed, although the maximal mean response occurred at 100 μg. PCOS women had a significantly greater rate of 17-OHP response per dose of r-hCG than normal controls (P = 0.005). In addition, with respect to increasing r-hCG dose, the incremental fold-change from basal values in the PCOS group was higher than that noted for normal women as shown in Table 2. The MMRM revealed the individual doses of hCG at which stimulated 17-OHP responses were significantly greater in PCOS compared with those of normal women, 100 and 250 μg (P < 0.05).
Fig. 2.
Mean (±se) baseline and 24-h serum 17-OHP, A, T, DHEA, E2, and P4 responses to iv administration of 1, 10, 25, 100, and 250 ìg of r-hCG in PCOS and normal women. Using linear mixed-effect models analyses, significant increases of 17-OHP, E2, and P4 were observed in both groups. By comparison, incremental A responses were significant only in PCOS women. Between groups, 17-OHP and A responses were significantly greater in PCOS women. Serum T responses were also higher compared to normal women, but the difference did not achieve statistical significance. As determined by MMRM, significant differences between groups in response to a fixed dose of hCG are indicated by a (P < 0.05), b (P < 0.01), and c (P < 0.001).
Table 2.
Serum 17-OHP responses to increasing doses of r-hCG in PCOS and normal women
| Group | 17-OHP responses to r-hCG |
||||
|---|---|---|---|---|---|
| 1 μg | 10 μg | 25 μg | 100 μg | 250 μg | |
| Normal | |||||
| Maximum concentration (ng/ml) | 1.0 ± 0.5 | 1.2 ± 0.5 | 1.7 ± 0.5 | 2.1 ± 0.5 | 2.1 ± 0.5 |
| Fold change | 1.5 ± 0.2 | 1.4 ± 0.1 | 2.0 ± 0.3 | 2.6 ± 0.2 | 2.8 ± 0.4 |
| PCOS | |||||
| Maximum concentration (ng/ml) | 1.1 ± 0.1 | 2.0 ± 0.3 | 2.7 ± 0.3 | 3.3 ± 0.6a | 4.0 ± 0.6a |
| Fold change | 1.1 ± 0.1 | 1.6 ± 0.1 | 2.6 ± 0.2 | 3.9 ± 0.3a | 4.4 ± 0.2a |
Data are expressed as mean ± se.
P < 0.05, PCOS vs. normal.
Increasing doses of r-hCG also provoked significant increases of A (P < 0.001) and T (P = 0.04) in PCOS women (Fig. 2). Surprisingly, maximal production of A was apparent at the 10-μg dose and remained so despite increments of hCG up to a dose of 250 μg. Maximal serum T responses were maintained up to a dose of 25 μg, after which a nonsignificant decline was observed. These findings are in marked distinction to the pattern of dose-responsiveness by 17-OHP that was maximal at 250 μg. Production of 17-OHP at 10 μg relative to baseline values was 33% of maximal response observed at 250 μg. By comparison, the mean A response at 10 μg was 92% of that at 250 μg, whereas the T response at 10 μg was 100% of that observed at the 25-μg dose. Surprisingly, normal women failed to demonstrate incremental responses of A and T to progressive doses of r-hCG. As a result, serum A dose-responses to r-hCG were significantly greater in PCOS than in normal women (P = 0.006). Also, at each dose of hCG, serum A responses in PCOS women were significantly greater than normal controls (Fig. 2). Mean serum T responses were also greater in PCOS women, although the difference was not statistically significant (P = 0.13), most likely due to variability among subjects. However, assessment of individual dose-effect revealed that stimulated T values at 25 μg were significantly higher than corresponding responses in normal women (P < 0.05).
Serum DHEA responses to r-hCG in PCOS women revealed a somewhat biphasic pattern characterized by initial increases up to a dose of 25 μg, followed by a lack of response at higher doses (Fig. 2). This variable dose-response was not statistically significant. In the normal group of women, DHEA dose-responses were not observed, and there were no differences between groups.
E2 and P4 dose-responses to r-hCG administration
As shown in Fig. 2, r-hCG injection induced a rise of serum E2 in PCOS women at a dose of 25 μg, followed by increasing responses to 100 and 250 μg (P = < 0.001). In normal women, incremental E2 dose-responses were also significant (P = 0.03), although r-hCG-stimulated production was considerably less than that of the PCOS group. Serum P4 levels increased significantly after r-hCG in both PCOS women (P < 0.001) and normal controls (P < 0.001). Between-group comparisons of E2 and P4 responses revealed that PCOS women had higher serum levels of E2 than those in normal women (P = 0.002), whereas P4 responses were similar in both groups.
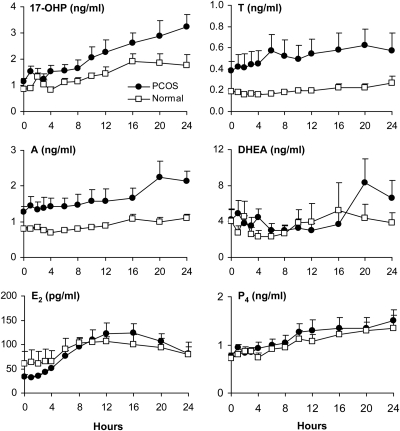
Time-course of steroid hormone responses to r-hCG
The 24-h time-course patterns of serum androgens, E2, and P4 were assessed in five PCOS subjects and four normal women (Fig. 3). The 25-μg dose was selected as a relative midmaximal stimulatory dose based on 17-OHP responses. In PCOS women, 17-OHP, A, and T gradually increased after r-hCG, reaching peak levels at 24 h. In normal women, a similar response pattern of lower magnitude was observed for 17-OHP. By comparison, serum A and T were minimally responsive over 24 h, which underscored negligible dose-responsiveness in these women. Despite considerable overlap between groups, circulating DHEA levels in PCOS women gradually peaked at 20–24 h, compared with minimal change in normal women. Serum E2 levels in both groups achieved highest values at 12–16 h, after which there was a gradual decline. Serum P4 showed modest and similar rises over 24 h in both groups.
Fig. 3.
Mean (±se) serum levels of 17-OHP, A, T, DHEA, E2, and P4 over 24 h after iv administration of 25 ìg r-hCG in five PCOS women and four normal controls.
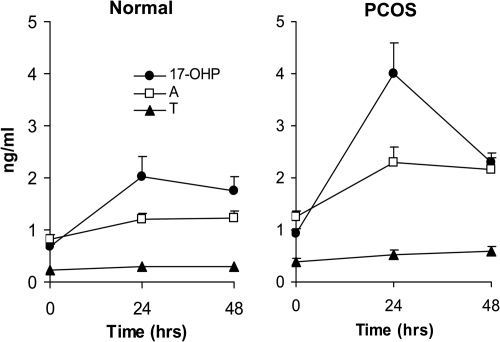
Extended blood sampling after r-hCG revealed that at 48 h, circulating 17-OHP in PCOS women had declined substantially, whereas serum A and T remained unchanged (Fig. 4). In normal women, 17-OHP levels at 48 h were unaltered compared with 24 h values, whereas serum A and T remained minimally responsive (Fig. 5).
Fig. 4.
Mean (±se) serum levels of 17-OHP, A, and T at baseline, 24, and 48 h after iv administration of 250 ìg r-hCG in PCOS and normal women.
Fig. 5.
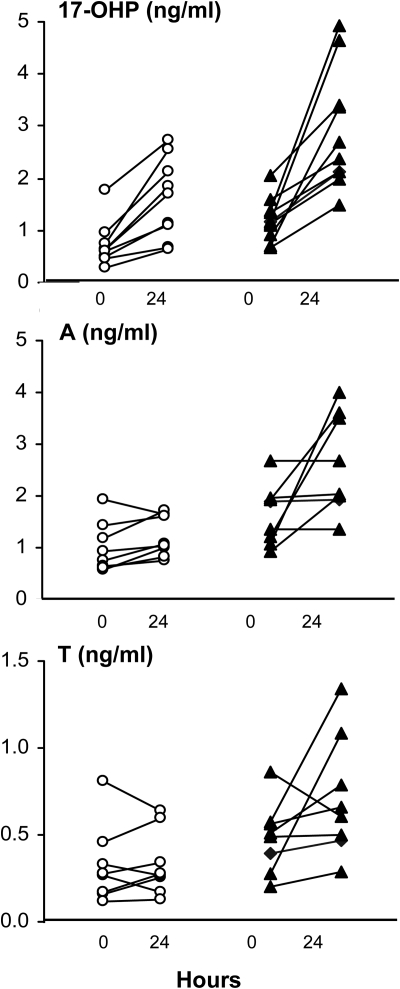
Serum 17-OHP, A, and T responses in individual women with PCOS (black triangles) and normal controls (open circles) before and 24 h after iv administration of 25 μg r-hCG. Variable androgen responsiveness in PCOS women was similar at all doses of r-hCG.
Effect of BMI on stimulated steroid responses to hCG
Women with PCOS were significantly heavier by weight than normal controls, as indicated by BMI values. However, consideration of BMI in the statistical analysis by linear mixed-effect models failed to demonstrate an influence on steroid responses to hCG.
Discussion
The results of this study have demonstrated that in PCOS women androgen dose-responses to iv hCG administration are characterized by maximal A and T production at low stimulatory doses, whereas in normal women A and T responses were essentially nonexistent at all doses of hCG. By comparison, increments of serum 17-OHP were dose-dependent, with peak production occurring at the highest doses in both PCOS and normal women. Increases of DHEA after hCG were not observed within or between groups. Serum E2 dose-responses in PCOS women were clearly greater than those seen in normal women, whereas P4 responses were significantly increased in both groups.
Enhanced A and T responsiveness to iv hCG in the PCOS group was underscored by minimal androgen responses observed in normal women. This may have reflected a relatively low level of theca cell responsiveness in normal women. Alternatively, the interval of blood sampling may have been of insufficient length to detect a rise in these androgens because significant increases in both A and T were only observed in normal women at 96 h after hCG given im (15, 16). This delayed rise was likely the result of elevated serum hCG levels that tended to persist for several days after im administration (17).
A distinctive finding of this study was that maximal A and T production occurred at a rather low dose of hCG (10 μg), whereas further increases were not observed despite nearly 25-fold higher increases in dose. The dramatic dose-effectiveness of 10 μg indicated that in PCOS theca cells A and T production are exquisitely responsive to hCG stimulation, particularly when compared with the minimal increases noted in normal women. Interestingly, the fold changes from basal values for A and T at 10 μg were comparable to peak responses reported previously in PCOS women stimulated with higher doses of hCG (15). The marked androgen response to low-dose hCG may have been due to the mode of delivery. Based on the pharmacokinetics of a given dose of hCG administered im or sc, iv injection results in much higher peak circulating levels that occur immediately (17).
In PCOS women, increased 17-OHP responses to gonadotropin stimulation have been previously well documented (3, 7–9, 15, 18, 19). In our study, comparison of dose-response patterns indicated that the mean fold change per dose was greater in PCOS women than that of normal individuals, which is at some variance with 17-OHP responses observed in vitro. In stimulated PCOS and normal theca cell cultures, mean fold increases of androgens above basal levels were similar, although there were significant increases in both basal and stimulated androgen production per PCOS theca cell compared with normal cells (10, 11). The difference between in vivo and in vitro responses may have reflected the presence of endogenous factors that amplify theca cell androgen production in PCOS women, such as insulin. Nevertheless, the capacity to generate more 17-OHP per dose of hCG does not preclude the notion that women with PCOS exhibit increased androgen production on a per-cell basis.
Compared with peak responses of A and T in PCOS women receiving low-dose hCG, maximal stimulation of 17-OHP was observed at the highest doses of hCG. Previous studies using single-dose gonadotropin administration in women with functional ovarian hyperandrogenism have suggested an overall up-regulation of ovarian steroidogenesis with emphasis on CYP17-driven 17-OHP and A production (1, 3, 4). In these women, GnRH-stimulated rises of A were relatively less than those of 17-OHP, which suggested inefficient 17,20-lyase activity (3). In vitro studies have shown that PCOS and normal theca cells treated with LH demonstrated no difference in A/17-OHP and A/DHEA ratios, although the A/P4 ratio was significantly higher in PCOS theca cells, which suggested higher conversion of P4 to A. Subsequently, a rigorous study using propagated PCOS theca cells demonstrated that stimulated P4, 17-OHP, and T production was greater than that of normal cells (11). In addition, radiolabeled pregnenolone was more rapidly metabolized to 17-OHP and DHEA and eventually to A, compared with normal cells (11). This increased steroid output was accompanied by greater enzymatic activities of CYP11A, 3β-hydroxysteroid dehydrogenase (3β-HSD), and 17β-HSD. In particular, 3β-HSD enzyme activity appeared to be more efficient because 60% of tritiated DHEA was converted to A in PCOS theca cells over 12 h, whereas only a 30% conversion occurred in normal cells over 48 h. Notably, although it was shown that 17-hydroxylation was increased in PCOS theca cells compared with normal cells, evidence of delta-4 17,20-lyase activity was lacking in both. It was concluded that androgen biosynthesis occurred predominantly through the delta-5 pathway in PCOS as well as normal theca cells. The lack or inefficiency of delta-4 17,20-lyase activity may explain, at least in part, the discordant dose-responses of 17-OHP compared with those exhibited by A and T in our PCOS women. The accumulation of A and T with minimal 17-OHP production at a low dose of hCG is compatible with hyperactivity of the delta-5 steroid pathway combined with increased 3β-HSD enzyme activity, a stable property of the PCOS theca cell (11). At higher doses of hCG, incremental changes in 17-OHP responsiveness likely arose from combined overexpression of both 17-hydroxylase and 3β-HSD enzymes.
A mechanism to account for the inactivity of delta-4 17,20-lyase has not been elucidated. Studies of rat theca tissue have demonstrated that LH may exert dose-dependent effects on 17,20-lyase activity. In vitro, low amounts of LH stimulate CYP17, whereas high amounts inhibit the activities of this enzyme, in particular, 17,20-lyase (20, 21). Alternatively, E2 has been shown to suppress 17,20-lyase activity in human testes, and this effect may be operative in the ovary after hCG administration (22–24). Koivunen et al. (15) showed that in PCOS women, significant serum 17-OHP responses after hCG were accompanied by corresponding temporal increases in circulating E2 and suggested a possible role for E2 in the regulation of 17,20-lyase activity. Serum E2 responses to hCG in our subjects peaked at 12–16 h, which may have exerted an inhibitory effect on 17,20-lyase activity and potentially contributed to enhanced 17-OHP dose-responses as suggested by Koivunen et al. (15). However, in men as well as women, tamoxifen apparently failed to alter 17-OHP, A, or T responses to hCG (25). A tenable consideration is that a defect of delta-4 17,20-lyase activity may reflect an inherent property of CYP17 gene expression in the human theca cell. When human CYP17 was transfected into monkey kidney COS-1 cells or modified yeast cells, ample production of 17-OHP from P4 was observed, whereas subsequent conversion to A was undetectable (26, 27). In contrast, 17-hydroxyolase and 17,20-lyase activities in the delta-5 pathway were clearly evident. Enzyme kinetic studies have shown that the catalytic efficiency (Vmax/Km) for the 17,20-lyase reaction was approximately 100-fold greater for delta-5 17-hydroxypregnenolone compared with that of delta-4 17-OHP (28). These findings indicate that human 17,20-lyase activity in the delta-4 pathway is minimal relative to that of delta-5 enzyme activity. As a result, it follows that androgen production must proceed through DHEA and not via 17-OHP.
Although we observed in PCOS women progressive increases in serum DHEA levels after hCG up to a dose of 25 μg, these changes were not significant or different from those of normal women. Our results are consistent with previous studies that have failed to demonstrate increases of DHEA or DHEA-S after high doses (5,000–10,000 IU) of hCG in both PCOS and normal women (8, 29).
Incremental E2 dose-responses were observed in PCOS women, whereas only small increases were apparent in normal women. The source of E2 production may have been of theca cell origin because aromatase expression has been demonstrated previously (30). Alternatively, the rather large amounts of A and T produced by hCG may have provided excess substrate for aromatization by granulosa cells responsive to ambient FSH secretion.
Our findings should be viewed with some caution because stimulated responses do not necessarily reflect steroid production rates. Rather, the results serve as an indirect assessment of steroidogenic activity in normal and PCOS women. Elevated basal T levels in PCOS women were not statistically different from controls, which we attributed to the small number of subjects in this group. The chromatographic RIA method used to determine androgen concentrations has been well validated and established by our lab. Another limitation is the increased BMI in the PCOS group compared with normal women, which may have contributed to increased basal and GnRH-stimulated 17-OHP levels (31). However, consideration of BMI in our statistical analysis using liner mixed-effect models did not alter the outcome of steroid responses to hCG. Increased 17-OHP responses in PCOS women also have been linked to worsening insulin resistance, which may have influenced PCOS theca cell responsiveness to iv hCG (9, 32–35). An effect of hyperinsulinemia is unclear because our subjects were nonfasting.
In summary, the results of our dose-response studies in PCOS women have revealed an exquisite responsiveness of A and T to hCG stimulation and support the concept that excessive androgen production is derived predominantly through the delta-5 steroidogenic pathway. In addition, serum A and T responses relative to those of 17-OHP are consistent with dysregulation of CYP17 and inefficiency of 17,20-lyase activity in women with this disorder. The greater fold increases in hCG-stimulated androgen values in PCOS women are compatible with increased production on a per-cell basis and also suggest that other endogenous factors may contribute to overall hyperandrogenemia.
Acknowledgments
We thank Walter L. Miller, M.D., for his generous review of the manuscript. We also thank Jeff Wong for his expert technical assistance.
Clinical Trial Registration no.: NCT00747617.
This research was supported by the Eunice Kennedy Shriver NICHD/NIH through cooperative agreement (U54 HD12303-28) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research and in part by NIH grant MO1 RR00827.
Disclosure Summary: The authors have nothing to declare.
Footnotes
- A
- Androstenedione
- AMH
- anti-Müllerian hormone
- BMI
- body mass index
- CV
- coefficient of variation
- DHEA
- dehydroepiandrosterone
- DHEA-S
- DHEA sulfate
- E2
- estradiol
- hCG
- human chorionic gonadotropin
- 3β-HSD
- 3β-hydroxysteroid dehydrogenase
- MMRM
- mixed-effects model of repeated measures
- 17-OHP
- 17-hydroxyprogesterone
- P4
- progesterone
- PCOS
- polycystic ovary syndrome
- r-hCG
- recombinant hCG
- T
- testosterone.
References
- 1. Barnes RB, Rosenfield RL, Burstein S, Ehrmann DA. 1989. Pituitary-ovarian responses to nafarelin testing in the polycystic ovary syndrome. N Engl J Med 320:559–565 [DOI] [PubMed] [Google Scholar]
- 2. Rosenfield RL, Barnes RB, Cara JF, Lucky AW. 1990. Dysregulation of cytochrome P450c 17 α as the cause of polycystic ovarian syndrome. Fertil Steril 53:785–791 [PubMed] [Google Scholar]
- 3. Rosenfield RL, Barnes RB, Ehrmann DA. 1994. Studies of the nature of 17-hydroxyprogesterone hyperresponsiveness to gonadotropin-releasing hormone agonist challenge in functional ovarian hyperandrogenism. J Clin Endocrinol Metab 79:1686–1692 [DOI] [PubMed] [Google Scholar]
- 4. Ehrmann DA, Barnes RB, Rosenfield RL. 1995. Polycystic ovary syndrome as a form of functional ovarian hyperandrogenism due to dysregulation of androgen secretion. Endocr Rev 16:322–353 [DOI] [PubMed] [Google Scholar]
- 5. Sahin Y, Kele°timur F. 1993. 17-Hydroxyprogesterone response to buserelin testing in the polycystic ovary syndrome. Clin Endocrinol (Oxf) 39:151–155 [DOI] [PubMed] [Google Scholar]
- 6. McCartney CR, Bellows AB, Gingrich MB, Hu Y, Evans WS, Marshall JC, Veldhuis JD. 2004. Exaggerated 17-hydroxyprogesterone response to intravenous infusions of recombinant human LH in women with polycystic ovary syndrome. Am J Physiol Endocrinol Metab 286:E902–E908 [DOI] [PubMed] [Google Scholar]
- 7. Ibañez L, Hall JE, Potau N, Carrascosa A, Prat N, Taylor AE. 1996. Ovarian 17-hydroxyprogesterone hyperresponsiveness to gonadotropin-releasing hormone (GnRH) agonist challenge in women with polycystic ovary syndrome is not mediated by luteinizing hormone hypersecretion: evidence from GnRH agonist and human chorionic gonadotropin stimulation testing. J Clin Endocrinol Metab 81:4103–4107 [DOI] [PubMed] [Google Scholar]
- 8. Gilling-Smith C, Story H, Rogers V, Franks S. 1997. Evidence for a primary abnormality of thecal cell steroidogenesis in the polycystic ovary syndrome. Clin Endocrinol (Oxf) 47:93–99 [DOI] [PubMed] [Google Scholar]
- 9. Pasquali R, Patton L, Pocognoli P, Cognigni GE, Gambineri A. 2007. 17-Hydroxyprogesterone responses to gonadotropin-releasing hormone disclose distinct phenotypes of functional ovarian hyperandrogenism and polycystic ovary syndrome. J Clin Endocrinol Metab 92:4208–4217 [DOI] [PubMed] [Google Scholar]
- 10. Gilling-Smith C, Willis DS, Beard RW, Franks S. 1994. Hypersecretion of androstenedione by isolated thecal cells from polycystic ovaries. J Clin Endocrinol Metab 79:1158–1165 [DOI] [PubMed] [Google Scholar]
- 11. Nelson VL, Legro RS, Strauss JF, 3rd, McAllister JM. 1999. Augmented androgen production is a stable steroidogenic phenotype of propagated theca cells from polycystic ovaries. Mol Endocrinol 13:946–957 [DOI] [PubMed] [Google Scholar]
- 12. Laird NM, Ware JH. 1982. Random-effects models for longitudinal data. Biometrics 38:963–974 [PubMed] [Google Scholar]
- 13. Mallinckrodt CH, Clark WS, David SR. 2001. Accounting for dropout bias using mixed-effects models. J Biopharm Stat 11:9–21 [DOI] [PubMed] [Google Scholar]
- 14. R Development Core Team 2009. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 15. Koivunen RM, Morin-Papunen LC, Ruokonen A, Tapanainen JS, Martikainen HK. 2001. Ovarian steroidogenic response to human chorionic gonadotropin in obese women with polycystic ovary syndrome: effect of metformin. Hum Reprod 16:2546–2551 [DOI] [PubMed] [Google Scholar]
- 16. Piltonen T, Koivunen R, Ruokonen A, Tapanainen JS. 2003. Ovarian age-related responsiveness to human chorionic gonadotropin. J Clin Endocrinol Metab 88:3327–3332 [DOI] [PubMed] [Google Scholar]
- 17. Trinchard-Lugan I, Khan A, Porchet HC, Munafo A. 2002. Pharmacokinetics and pharmacodynamics of recombinant human chorionic gonadotropin in healthy male and female volunteers. Reprod Biomed Online 4:106–115 [DOI] [PubMed] [Google Scholar]
- 18. Levrant SG, Barnes RB, Rosenfield RL. 1997. A pilot study of the human chorionic gonadotropin test for ovarian hyperandrogenism. Hum Reprod 12:1416–1420 [DOI] [PubMed] [Google Scholar]
- 19. Piltonen T, Koivunen R, Perheentupa A, Morin-Papunen L, Ruokonen A, Tapanainen JS. 2004. Ovarian age-related responsiveness to human chorionic gonadotropin in women with polycystic ovary syndrome. J Clin Endocrinol Metab 89:3769–3775 [DOI] [PubMed] [Google Scholar]
- 20. Bogovich K, Richards JS. 1982. Androgen biosynthesis in developing ovarian follicles: evidence that luteinizing hormone regulates thecal 17α-hydroxylase and C17-20-lyase activities. Endocrinology 111:1201–1208 [DOI] [PubMed] [Google Scholar]
- 21. Eckstein B, Greenbaum O, Cohen S. 1985. Kinetic studies on ovarian C-17,20-lyase activity: effect of luteinizing hormone surge. Endocrinology 117:2376–2382 [DOI] [PubMed] [Google Scholar]
- 22. Forest MG. 1979. Pattern of the response of testosterone and its precursors to human chorionic gonadotropin stimulation in relation to age in infants and children. J Clin Endocrinol Metab 49:132–137 [DOI] [PubMed] [Google Scholar]
- 23. Martikainen H, Huhtaniemi I, Vihko R. 1980. Response of peripheral serum sex steroids and some of their precursors to a single injection of hCG in adult men. Clin Endocrinol (Oxf) 13:157–166 [DOI] [PubMed] [Google Scholar]
- 24. Tapanainen J, Martikainen H, Dunkel L, Perheentupa J, Vihko R. 1983. Steroidogenic response to a single injection of hCG in pre- and early pubertal cryptorchid boys. Clin Endocrinol (Oxf) 18:355–362 [DOI] [PubMed] [Google Scholar]
- 25. Levrant SG, Barnes RB, Rosenfield RL. hCG-stimulated 17,20 lyase activity is unaffected by tamoxifen in PCOS or normal subjects. Program of the 76th Annual Meeting of The Endocrine Society, Anaheim, CA, 1994, p 410 (Abstract 837) [Google Scholar]
- 26. Lin D, Harikrishna JA, Moore CC, Jones KL, Miller WL. 1991. Missense mutation serine106—proline causes 17 α-hydroxylase deficiency. J Biol Chem 266:15992–15998 [PubMed] [Google Scholar]
- 27. Lin D, Black SM, Nagahama Y, Miller WL. 1993. Steroid 17 α-hydroxylase and 17,20-lyase activities of P450c17: contributions of serine 106 and P450 reductase. Endocrinology 132:2498–2506 [DOI] [PubMed] [Google Scholar]
- 28. Auchus RJ, Lee TC, Miller WL. 1998. Cytochrome b5 augments the 17,20-lyase activity of human P450c17 without direct electron transfer. J Biol Chem 273:3158–3165 [DOI] [PubMed] [Google Scholar]
- 29. Piltonen T, Koivunen R, Morin-Papunen L, Ruokonen A, Huhtaniemi IT, Tapanainen JS. 2002. Ovarian and adrenal steroid production: regulatory role of LH/HCG. Hum Reprod 17:620–624 [DOI] [PubMed] [Google Scholar]
- 30. Bogovich K, Richards JS. 1984. Androgen synthesis during follicular development: evidence that rat granulosa cell 17-ketosteroid reductase is independent of hormonal regulation. Biol Reprod 31:122–131 [DOI] [PubMed] [Google Scholar]
- 31. Jakubowicz DJ, Nestler JE. 1997. 17α-Hydroxyprogesterone responses to leuprolide and serum androgens in obese women with and without polycystic ovary syndrome offer dietary weight loss. J Clin Endocrinol Metab 82:556–560 [DOI] [PubMed] [Google Scholar]
- 32. Barbieri RL, Makris A, Randall RW, Daniels G, Kistner RW, Ryan KJ. 1986. Insulin stimulates androgen accumulation in incubations of ovarian stroma obtained from women with hyperandrogenism. J Clin Endocrinol Metab 62:904–910 [DOI] [PubMed] [Google Scholar]
- 33. Cara JF, Rosenfield RL. 1988. Insulin-like growth factor I and insulin potentiate luteinizing hormone-induced androgen synthesis by rat ovarian thecal-interstitial cells. Endocrinology 123:733–739 [DOI] [PubMed] [Google Scholar]
- 34. Bergh C, Carlsson B, Olsson JH, Selleskog U, Hillensjö T. 1993. Regulation of androgen production in cultured human thecal cells by insulin-like growth factor I and insulin. Fertil Steril 59:323–331 [DOI] [PubMed] [Google Scholar]
- 35. Nestler JE, Powers LP, Matt DW, Steingold KA, Plymate SR, Rittmaster RS, Clore JN, Blackard WG. 1991. A direct effect of hyperinsulinemia on serum sex hormone-binding globulin levels in obese women with the polycystic ovary syndrome. J Clin Endocrinol Metab 72:83–89 [DOI] [PubMed] [Google Scholar]