Vitamin D3 might be a potential novel therapeutic agent for safe and nonsurgical treatment of uterine fibroids.
Abstract
Background:
Uterine leiomyomas (fibroids) are the most common benign estrogen-dependent tumors of premenopausal women. TGF-β3 up-regulates the synthesis of many of extracellular matrix proteins that are associated with tissue fibrosis.
Objective:
To examine the effect of 1,25-dihydroxyvitamin D3 (vitamin D3) on TGF-β3-induced fibrosis-related protein expression in immortalized human uterine leiomyoma (HuLM) cells.
Methods:
HuLM cells were treated with TGF-β3 with or without vitamin D3. Western blot analyses were employed to test the effect of vitamin D3 on TGF-β3-induced protein expression of collagen type 1, fibronectin, and plasminogen activator inhibitor-1 proteins. Western blots as well as immunofluorescence analyses were used to verify the effect of vitamin D3 on TGF-β3-induced Smad activation involved in extracellular matrix protein synthesis and deposition, which ultimately lead to tissue fibrosis.
Results:
We observed that TGF-β3 induced fibronectin and collagen type 1 protein expression in HuLM cells, and that effect was suppressed by vitamin D3. TGF-β3 also induced protein expression of plasminogen activator inhibitor-1, an important TGF-β target, in HuLM cells, which was also inhibited by vitamin D3. Additionally, TGF-β3 induced phosphorylation of Smad2 as well as nuclear translocation of Smad2 and Smad3 in HuLM cells, whereas vitamin D significantly reduced all these TGF-β3-mediated effects. Therefore, our results suggest that vitamin D3 has consistently reduced TGF-β3 effects that are involved in the process of fibrosis in human leiomyoma cells.
Conclusion:
Vitamin D3 is an antifibrotic factor that might be potentially useful as a novel therapeutic for nonsurgical treatment of benign uterine fibroids.
Uterine leiomyomas are the most common benign tumors of premenopausal women and are associated with excessive vaginal bleeding, pelvic pain, recurrent miscarriage, and preterm labor (1, 2). They are the most commonly cited reason for hysterectomy in the United States (3). The initiating factors that lead to the development of uterine leiomyomas are not well understood. However, evidence supports that ovarian steroids estrogen and progesterone are important factors for fibroid growth (4, 5). Uterine leiomyomas are three to four times more prevalent in African-American women than White women (6). Vitamin D deficiency is about 10 times more prevalent in African-Americans (40–45%) compared with Caucasians (4%) (7). The exact reasons for this higher occurrence of vitamin D deficiency are not well known (6, 7). Our recent reports support that the differential ethnic distribution of specific functional genetic variants in genes of estrogen-metabolizing enzymes such as catechol-O-methyltransferase (COMT) are associated with uterine fibroids susceptibility in African-American women (8, 9). Additionally, increased COMT RNA and protein expression was also observed in uterine leiomyoma in comparison with adjacent normal myometrium (10). More recently, we have reported that vitamin D3 effectively inhibited the proliferation of human leiomyoma cells and that effect was mediated, at least partially, via the COMT gene (11).
TGF-βs are multifunctional peptides that regulate diverse biological functions (12, 13). TGF-β1, -β2, and -β3 have been identified in a variety of normal and transformed mammalian cells and tissues (12). The mRNAs and proteins for TGF-β1, TGF-β2, and TGF-β3 and their receptors have been detected in both human myometrium and leiomyomas (14, 15). The biological functions of TGF-βs in their target tissues are mediated through three specific cell surface receptors such as receptor type I, II, and III (16, 17). The type I and type II receptors are serine/threonine kinases, whereas the type III receptor (endoglin) acts as a cell surface binding protein (17, 18). The multifunctional effects of TGF-βs are elicited through the oligomeric complex formation between the type I and type II serine-threonine kinase receptors. TGF-β initiates signals by binding to the type II receptor (TβRII) and stabilizes the heteromeric complex with the type I receptor (TβRI), and the TβRI is transphosphorylated and activated by the TβRII. Activated TβRI then propagates the signals through interaction with and phosphorylation of receptor-regulated Smads (19). The Smad proteins are divided into three distinct classes based on their structure and function in signaling by TGF-β family members. The receptor-regulated Smads (R-Smads) are phosphorylated on two serine residues at the C terminus and thus activated in a ligand-specific manner. The receptor-regulated Smads Smad2 and Smad3 mediate signaling by TGF-β and activin, whereas Smad1, Smad5, and Smad8 are involved in bone morphogenetic protein signaling. Once Smad2 and Smad3 are phosphorylated and activated by TβRI, they form heteromeric complexes with Smad4 (common Smad) and then translocate to the nucleus where they modulate the transcription of TGF-β target genes (13, 19, 20).
TGF-βs are known as profibrotic cytokines that are overexpressed in a wide range of fibrotic tissues including uterine leiomyomas (21–23). TGF-βs are the key regulators of cell growth, differentiation, inflammation, apoptosis, and tissue remodeling, and these processes are important to lead to tissue fibrosis (22). TGF-βs up-regulate the synthesis of many of the extracellular matrix (ECM) proteins that are involved in fibrosis (20). These multifunctional peptides promote fibroproliferative and tumorigenic processes in different organ systems including uterine leiomyoma (23). TGF-β3 is elevated 3- to 5-fold in leiomyomas compared with myometrium tissues as well as in primary cultures (24–26). TGF-β3 plays an essential role in ECM overproduction in uterine leiomyomas by inducing the expression of collagen type 1, fibronectin, laminin, and proteoglycans (27). These ECM-related genes such as collagen type 1 and fibronectin were overexpressed in uterine leiomyoma (28, 29). Additionally, microscopy analyses demonstrated that ECM collagen fibril spatial structures were disoriented and loosely packed compared with the parallel orientation and closely packed manner in matched normal myometrium (28, 30).
Vitamin D3 is a strong growth inhibitor that induces apoptosis in human breast cancer cells (31). A recent study from our laboratory demonstrated the dose-dependent growth-inhibitory effect of vitamin D3 on immortalized human uterine leiomyoma (HuLM) cells (11). Bläuer et al. (32) also demonstrated the dose-dependent inhibition of myometrial and leiomyoma cell proliferation by vitamin D3 treatment in vitro. Vitamin D3 also functions as a strong antifibrotic factor. Vitamin D3-mediated reduction of the expression of collagen and key profibrotic factors has been recently shown in mesenchymal multipotent cells (33). However, the effect of vitamin D3 on the reduction of TGF-β-induced tumorigenic and fibrosis-related protein expressions in uterine leiomyoma cells is yet to be determined. In this report, we aimed to investigate the effects of vitamin D3 on TGF-β3-induced fibrosis-related protein expressions in HuLM cells.
Materials and Methods
Cell lines and cultures
HuLM cells were a kind gift from Dr. Darlene Dixon (National Institute of Environmental Health Sciences, Research Triangle Park, NC) (34). These cells were cultured as we have described previously (11).
Reagents and antibodies
TGF-β3 and 1,25-dihydroxyvitamin D3 were purchased from Sigma Chemical Co. (St. Louis, MO). Nuclear and cytoplasmic extraction reagents were purchased from Pierce Biotechnology (Rockford, IL). Antibodies were purchased as follows: anti-phospho-Smad2 and anti-phospho-ERK from Cell Signaling (Danvers, MA); anti-Smad2 and anti-Smad3 from Zymed Laboratories Inc. (San Francisco, CA); anti-collagen type 1 from Fitzgerald (Concord, MA); anti-Smad4, anti-cyclin D1, anti-Cdk2, anti-Cdk4, anti-Cdk6, anti-ERK, anti-plasminogen activator inhibitor-1 (anti-PAI-1), anti-poly ADP-ribose polymerase (PARP), and anti-Rho GDP-dissociation inhibitor (Rho-GDI) from Santa Cruz Biotechnology (Santa Cruz, CA); and antifibronectin and anti-β-actin from Sigma.
Western blot analyses
HuLM cells were serum starved and then treated with TGF-β3 (5 ng/ml) with or without different concentrations of vitamin D3, as indicated. Equal amounts of cell lysates were assayed by Western blot analyses as described previously (35). The antigen-antibody complex was detected with an enhanced chemiluminescence detection system. Specific protein bands were visualized after exposure to autoradiography films and developed by using automatic x-ray developer. The intensity of each protein band was quantified and normalized against corresponding β-actin.
Immunoprecipitation analyses
Immunoprecipitation analyses were performed as we have described previously (35). Briefly, lysates were prepared from HuLM cells treated with TGF-β3 (10 ng/ml) with or without vitamin D3. An equal amount (1.5 mg) of each clear cell lysate was incubated with both anti-Smad2 and anti-Smad3 (2 μg each) antibodies for 2.5 h at 4 C, followed by incubation with 25 μl of protein G-Sepharose (Sigma) for an additional 1 h. Immune complexes were precipitated, washed, and analyzed by Western blotting with mouse anti-Smad4 antibodies. The expression of Smad proteins in the above cell lysates was also verified by Western blot analyses.
Nuclear and cytoplasmic fraction extraction
HuLM cells were serum starved and treated with TGF-β3 (5 ng/ml) with or without different concentrations of vitamin D3 (0.1 or 1 μm) for 30 min, 2 h, and 4 h. At the end of each time point, cells were washed with ice-cold PBS, scraped, and transferred into separate Eppendorf tubes. Cell pellets were collected after a brief centrifugation at 4 C. Nuclear and cytoplasmic extraction fractions were prepared from those cell pellets according to the manufacturer's instruction. A 15-μg amount of each cytoplasmic and nuclear fraction was subjected to Western blot analyses for Smads localization/expression, and to ensure the extraction efficiency, PARP (a nuclear protein) and Rho-GDI (a cytoplasmic protein) were determined as internal controls.
Immunofluorescence analyses
HuLM cells were cultured on glass cover slides, serum starved, and treated with TGF-β3 (5 ng/ml) with or without different concentrations of vitamin D3 (0.1 and 1 μm) for 4 h. Cells were fixed in 3.7% formaldehyde and permeabilized in 0.2% Triton X-100 for 10 min, and nonspecific binding was inhibited by blocking with 10% fetal bovine serum in PBS for 30 min. Slides were then incubated with 10 μg/ml rabbit polyclonal anti-Smad2 or anti-Smad3 antibodies for 1 h at room temperature followed by incubation for 1 h with carbocyanine 3 (CY3)-conjugated rabbit secondary antibody. Staining signals for Smad2 and Smad3 were visualized by fluorescent confocal microscopy, and the signals were compared between untreated and vitamin D3-treated HuLM cells.
Statistical analysis
All statistical analyses were performed using paired t test. The paired t test was used to assess the significance of differences in control and vitamin D3-treated data points. Values were considered to be statistically significant when P < 0.05 .
Results
Vitamin D3 inhibits TGF-β3-induced fibronectin protein expression in human leiomyoma cells
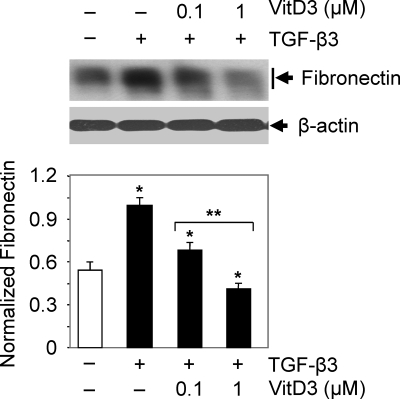
To determine whether vitamin D3 affects TGF-β3-induced fibronectin protein expression, we performed Western blot analyses using lysates from HuLM cells treated with TGF-β3 (5 ng/ml) with or without vitamin D3. TGF-β3 significantly induced (2-fold, P < 0.05) fibronectin protein expression at 24 h when compared with untreated control. However, treatment with vitamin D3 significantly reduced the TGF-β3-induced fibronectin gene expression in a concentration-dependent manner in HuLM cells (Fig. 1). Therefore, these results suggest that vitamin D3 inhibits TGF-β3-induced fibronectin gene expression in HuLM cells.
Fig. 1.
Effect of vitamin D3 on TGF-β3-induced fibronectin protein expression in human leiomyoma cells. HuLM cells were serum starved and treated with TGF-β3 (5 ng/ml) with or without increasing concentrations of vitamin D3 (VitD3) (0.1 and 1 μm) for 24 h. Equal amounts of each protein lysate were analyzed by Western blotting using antifibronectin antibody. Western blot with β-actin antibody was used as a loading control. The intensity of each protein band was quantified and normalized with corresponding β-actin. *, P < 0.05 compared with corresponding control; **, P < 0.05 when compared between vitamin D3 doses.
Vitamin D3 inhibits TGF-β3-induced collagen type 1 protein expression in human leiomyoma cells
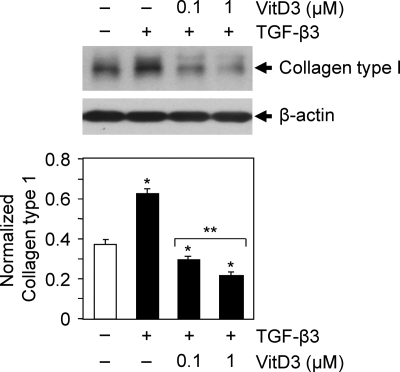
To test whether vitamin D3 affects TGF-β3-induced collagen type 1 protein expression in HuLM cells, we performed similar Western blot analyses as described above. HuLM cells were treated with TGF-β3 with or without vitamin D3 for 24 h as indicated. Cell lysates were analyzed by Western blots using anti-collagen type 1 antibody. We found that TGF-β3 induced collagen type 1 protein expression in HuLM cells when compared with untreated control. However, treatment with vitamin D3 significantly reduced the TGF-β3-induced collagen type 1 protein expression in HuLM cells in a dose-dependent manner (Fig. 2, P < 0.05). Thus, these results suggest that vitamin D3 has potential to reduce the TGF-β3-induced collagen type 1 gene expression in HuLM cells.
Fig. 2.
Effect of vitamin D3 on TGF-β3-induced collage type 1 protein expression in human leiomyoma cells. HuLM cells were serum starved and treated with TGF-β3 (5 ng/ml) with or without increasing concentrations of vitamin D3 (VitD3) (0.1 and 1 μm) for 24 h. Equal amounts of each protein lysate were analyzed by Western blotting using anti-collagen type 1 antibody. The intensity of each protein band was quantified and normalized with corresponding β-actin as indicated above. *, P < 0.05 compared with corresponding control; **, P < 0.05 when compared between vitamin D3 doses.
Vitamin D3 inhibits TGF-β3-induced PAI-1 protein expression in human leiomyoma cells
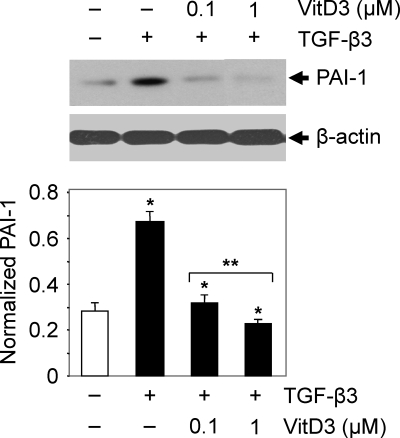
The PAI-1 gene is a target of TGF-β. To test whether PAI-1 protein expression is induced in HuLM cells by TGF-β3 and whether vitamin D3 reduces this TGF-β3-induced PAI-1 expression, we performed Western blot analyses. HuLM cells were treated with TGF-β3 in the presence or absence of vitamin D3 for 12 h. An equal amount of each lysate was analyzed for PAI-1 protein expression using anti-PAI-1 antibody. PAI-1 protein could be detected at a molecular mass of 50 kDa. We observed that TGF-β3 induced a 2-fold increase in PAI-1 expression when compared with untreated control (Fig. 3). However, treatment with vitamin D3 significantly reduced the TGF-β3-induced PAI-1 expression in HuLM cells in a dose-dependent manner (Fig. 3, P < 0.05). These results suggest the potential role of vitamin D3 in reduction of TGF-β3-induced PAI-1 gene expression in HuLM cells.
Fig. 3.
Effect of vitamin D3 on TGF-β3-induced PAI-1 protein expression in human leiomyoma cells. HuLM cells were serum starved and treated with TGF-β3 (5 ng/ml) with or without increasing concentrations of vitamin D3 (VitD3) (0.1 and 1 μm) for 12 h. Equal amounts of each protein lysate were analyzed by Western blotting using anti-PAI-1 antibody. The intensity of each protein band was quantified and normalized with corresponding β-actin. *, P < 0.05 compared with corresponding control; **, P < 0.05 when compared between vitamin D3 doses.
Vitamin D3 reduces TGF-β3-induced prooncogenic protein expression in human leiomyoma cells
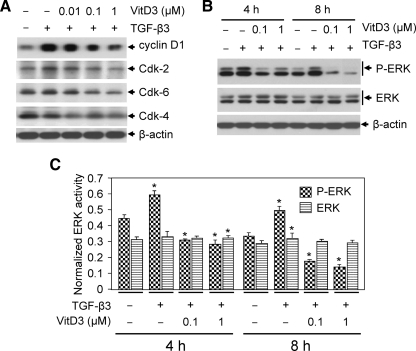
To test the effect of vitamin D3 on TGF-β3-induced cell cycle regulatory gene expression, we performed immunoblot analyses using protein lysates from HuLM cells treated with TGF-β3 with or without vitamin D3. We found that TGF-β3 induced cyclin D1 protein expression in HuLM cells, whereas vitamin D3 suppressed that expression in a dose-dependent manner (Fig. 4A). TGF-β3 also weakly induced Cdk2 and Cdk6 protein expression in HuLM cells, which was significantly (P < 0.05) inhibited by vitamin D3 (Fig. 4A). Although TGF-β3 did not induce the level of Cdk4 protein, vitamin D3 was still able to significantly (P < 0.05) reduce its basal level of expression in HuLM cells (Fig. 4A). Furthermore, to verify whether vitamin D3 has any effect on TGF-β3-mediated ERK activation, we performed Western blot analyses. TGF-β3 induced ERK phosphorylation that was suppressed by vitamin D3 treatment in HuLM cells (Fig. 4B). However, the level of total ERK was not affected by TGF-β3 or by vitamin D3 in HuLM cells (Fig. 4, B and C). These results suggest an important role of vitamin D3 on TGF-β3-induced prooncogenic cyclin D1 gene expression as well as the growth-promoting ERK activation in HuLM cells.
Fig. 4.
Effect of vitamin D3 on TGF-β3-induced prooncogenic and growth-related protein expression in human leiomyoma cells. A, HuLM cells were serum starved and treated with TGF-β3 (5 ng/ml) with or without increasing concentrations of vitamin D3 (VitD3) (0.01, 0.1, and 1 μm) for 4 h. Equal amounts of each protein lysate were analyzed by Western blotting as indicated. B, HuLM cells were treated with TGF-β3 (5 ng/ml) with or without different doses of vitamin D3 (VitD3) (0.1 and 1 μm) for 4 and 8 h. Equal amounts of each protein lysate were analyzed using anti-phospho-ERK and anti-ERK antibodies. β-Actin was used as loading control. C, The intensity of each protein band was quantified and normalized with corresponding β-actin. *, P < 0.05 compared with corresponding control.
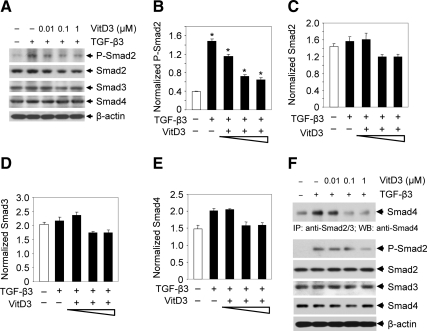
Vitamin D3 reduces TGF-β3-induced phosphorylation of Smad2 and the complex formation between Smad2, Smad3, and Smad4 in human leiomyoma cells
To verify whether TGF-β3 activates Smad signaling in HuLM cells and whether vitamin D3 can effectively reverse this effect, HuLM cells were treated with 5 ng/ml TGF-β3 with or without vitamin D3, and then cell lysates were used to perform Western blot analyses. TGF-β3 induced phosphorylation of Smad2 (∼2.5-fold, P < 0.05), whereas treatment with vitamin D3 significantly reduced the TGF-β3-induced Smad2 phosphorylation in a dose-dependent manner (P < 0.05,Fig. 5, A and B). Treatment with TGF-β3 or vitamin D3 did not significantly alter the expression levels of Smad2, Smad3, and Smad4 (Fig. 5, C–E). To further test whether reduced phosphorylation of Smad2 by vitamin D3 can inhibit the functional complex formation between Smad2, Smad3, and Smad4, we performed immunoprecipitation experiments after treating HuLM cells with TGF-β3 with or without vitamin D3. We observed that TGF-β3 induced heteromeric complex formation between Smad2, Smad3, and Smad4 and that complex formation was significantly reduced by vitamin D3 (Fig. 5F, top panel, P < 0.05). We also observed that vitamin D3 reduced phosphorylation of Smad2 in the above lysates, whereas the total levels of Smad2, Smad3, and Smad4 were not altered (Fig. 5F, bottom panel). Therefore, the above results suggest that TGF-β3 activates Smad signaling by phosphorylation of Smad2 and by complex formation between Smad2, Smad3, and Smad4 in human leiomyoma cells, whereas vitamin D3 can suppress these effects, which in turn would result in inhibition of TGF-β signaling.
Fig. 5.
Effect of vitamin D3 on TGF-β3-induced phosphorylation of Smad2 and the complex formation between Smad2, Smad3, and Smad4 in human leiomyoma cells. A, HuLM cells were serum starved and treated with TGF-β3 (5 ng/ml) with or without increasing concentrations of vitamin D3 (VitD3) (0.1 and 1 μm) for 4 h. Protein lysates were analyzed by Western blotting using antibodies against phospho-Smad2, Smad2, Smad3, and Smad4. Western blot with β-actin was used as loading control. B–E, The intensity of each protein band was quantified and normalized with corresponding β-actin. *, P < 0.05 compared with corresponding control. F, HuLM cells were preincubated for 2 h in serum-free medium and then treated with TGF-β3 (10.0 ng/ml) with or without increasing concentrations of vitamin D3 (VitD3) (0.01, 0.1, and 1 μm) for 1 h as indicated. Cell lysates were subjected to immunoprecipitation with anti-Smad2 and anti-Smad3 antibodies, and the immunoprecipitates were analyzed by immunoblotting with anti-Smad4 antibody (top panel). The above protein lysates were also used to verify the expression of phospho-Smad2, Smad2, Smad3, and Smad4 proteins by Western blot analyses.
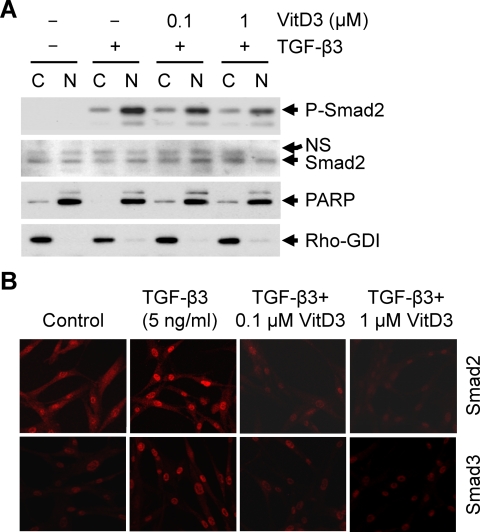
Vitamin D3 reduces TGF-β3-induced nuclear translocation of Smad2 and Smad3 in human leiomyoma cells
To determine whether vitamin D3 affects TGF-β3-induced phosphorylation/activation and nuclear localization of Smad proteins, we performed Western blot analyses using nuclear and cytosolic extracts prepared from HuLM cells that were treated with TGF-β3 with or without vitamin D3. TGF-β3 induced nuclear translocation of phospho-Smad2 within 4 h (Fig. 6A). Additionally, vitamin D3 treatment showed a dose-dependent reduction of TGF-β3-induced nuclear translocation of phospho-Smad2 (Fig. 6A). The expression of PARP was almost exclusively localized in the nuclear fraction, whereas the expression of Rho-GDI was almost exclusively localized in the cytosolic fraction (Fig. 6A, bottom panel). To further confirm the effect of vitamin D3 on TGF-β3-induced nuclear translocation of Smad2 and Smad3, immunofluorescence analyses were performed using HuLM cells treated with TGF-β3 with or without vitamin D3. We found that TGF-β3 induced nuclear translocation of Smad2 and Smad3, whereas vitamin D3 treatment greatly reduced TGF-β3-induced nuclear translocation in a dose-dependent manner (Fig. 6B). Additionally, cytoplasmic Smad2 and Smad3 stains were also reduced by vitamin D3 treatment when compared with untreated control cells. Therefore, these results suggest that vitamin D3 inhibits TGF-β3 effects by reducing the phosphorylation of Smad2 and by inhibiting the nuclear translocation of both Smad2 and Smad3 in HuLM cells.
Fig. 6.
Effect of vitamin D3 on TGF-β3-induced nuclear translocation of Smad2 and Smad3 in human leiomyoma cells. A, Cytoplasmic and nuclear fractions were prepared from HuLM cells treated with TGF-β3 (5 ng/ml) with or without increasing concentrations of vitamin D3 (VitD3) (0.1 and 1 μm) as indicated. Equal amounts of each fraction (15 μg) were subjected to Western blot analyses using anti-phospho-Smad2 and anti-Smad2 antibodies. Anti-PARP (a nuclear protein) and anti-Rho-GDI (a cytoplasmic protein) antibodies were used to ensure the extraction efficiency. B, Immunofluorescence analyses of HuLM cells after the treatment with TGF-β3 (5 ng/ml) with or without increasing concentrations of vitamin D3 (VitD3) (0.1 and 1 μm) for 4 h. Cells were fixed, permeabilized, and incubated with anti-Smad2 or anti-Smad3 antibodies. Smad proteins were stained by incubating the slides with CY3-conjugated secondary antibody and then visualized by laser confocal microscopy. We used Nikon TE2000-U C1 confocal laser scanning microscopy to collect fluorescence emission with a 505- to 550-nm filter set. All confocal pictures were captured at ×200 magnification under similar conditions. The EZ C1 confocal software was used subsequently to reconstruct serial z-plane images into a three-dimensional rendering. Images from each treatment condition were transferred to PowerPoint and shown. The intensity of red staining signals (CY3-conjugated Smad3 or Smad4) were visually compared between untreated control and TGF-β3-treated data points and also compared between TGF-β3-treated and both TGF-β3- and vitamin D3-treated data points.
Discussion
In the present study, we have demonstrated the effects of vitamin D3 on TGF-β3 functions that are involved in the process of fibrosis. Although earlier studies have demonstrated that African-American women are more susceptible to uterine leiomyomas, and they also have higher prevalence of vitamin D deficiency than White women (6, 7), the potential link between these two distinct conditions has not been explored previously.
Studies have demonstrated that the development of uterine leiomyomas is associated with excessive synthesis and deposition of ECM, which leads to tissue fibrosis. Therefore, we have tested the effect of TGF-β3 on fibrosis-related gene expressions in HuLM cells. We observed that TGF-β3 stimulates ECM protein expression of collagen type 1, fibronectin, and PAI-1 in HuLM cells. Although TGF-βs have three isoforms, TGF-β3 is the isoform most commonly involved in ECM deposition and in the process of fibrosis (26). Therefore, the present study was undertaken to determine whether treatment with vitamin D3 can reverse TGF-β3-induced protein expression in HuLM. We have used HuLM cells as a model of uterine leiomyoma, because these well-characterized cells are representative of human leiomyoma pathophysiology and characteristically express both estrogen and progesterone receptors (34). Uterine leiomyoma exhibits increased expression of TGF-β3 protein and mRNA compared with adjacent normal myometrium (36). Increasing TGF-β3 concentration leads to increased expression of various ECM components such as collagen 1A1, fibronectin, and proteoglycans in human myometrial and leiomyoma cell lines (27). Taken together, it is reasonable to assume a central role of TGF-β3 in early transformational events of normal myometrium to the leiomyomatous phenotype and that elevated TGF-β3 subsequently results in overexpression and secretion of ECM proteins leading to gradual increase in the size of growing fibroid lesions. Thus, TGF-β3 may contribute to the increasing size of leiomyomas both by increasing cell proliferation as well as potentially increasing ECM deposition. Additionally, the increase in ECM in the new fibrotic cells has been shown to further activate secretion of TGF-β3 in the ECM compartment, which in essence creates a pathological loop resulting in continued gradual increase in tumor bulk (27). TGF-β3 plays an essential role in ECM overproduction in human leiomyomas by stimulating the expression of collagen type 1, fibronectin, laminin, and proteoglycans (16, 17, 37). Consistent with those published studies, we have also observed the induction of collagen type 1, fibronectin, and PAI-1 protein expression by TGF-β3 in HuLM cells (Figs. 1–3). The Smads signaling pathway is crucial for simultaneous activation of several fibrillar collagen genes by TGF-β. TGF-β up-regulation of COL7A1 gene expression is mediated by rapid and transient binding of a Smad-containing complex to a specific region of the COL7A1 promoter (38). Additionally, ECM-related genes whose expression is modulated by TGF-β, such as PAI-1, COL1A2, and the b5 integrin have also been identified as potential Smad targets (39–41). In this study, we found that TGF-β3 significantly induced collagen type 1, fibronectin, and PAI-1 protein expression, which was significantly reduced by vitamin D3 as shown in Figs. 1–3. Therefore, our study supports the notion that with increased TGF-β3 stimulation, uterine leiomyoma cells can undergo molecular alterations that may increase the fibrotic phenotype of these tumor cells, which can be effectively suppressed by vitamin D3 and, in turn, can reduce the fibrotic phenotype of these leiomyoma cells.
PAI-1 gene is a target of TGF-β, and it is associated in the process of fibrosis (42). We observed significant induction of PAI-1 protein expression by TGF-β3 in HuLM cells (Fig. 3). Vitamin D3 treatment completely abolished the TGF-β3-induced PAI-1 protein expression in HuLM cells, supporting the potential role of vitamin D3 in the regulation of TGF-β induced fibrosis in human leiomyoma cells. Similar results were also observed with collagen and fibronectin protein expression (Figs. 1 and 2).
In addition to Smads, TGF-β also activates non-Smad pathways including ERK, p38 MAPK, c-Jun N-terminal kinase, and phosphatidylinositol 3-kinase, which are known to be involved in prooncogenic functions of TGF-β (43). We observed that TGF-β3 induced ERK phosphorylation in HuLM cells, whereas vitamin D3 suppressed the TGF-β3-induced ERK phosphorylation, suggesting that vitamin D3 can potentially exert antioncogenic role in uterine leiomyoma cells. The cyclin D1 and cyclin-dependent kinases are frequently overexpressed in carcinogenesis (44). We found that TGF-β3 induced the expression of prooncogenic cyclin D1 and Cdk2 in HuLM cells, whereas the levels of those proteins were reduced by vitamin D3 as shown in Fig. 4. These observations clearly demonstrate the antioncogenic and growth inhibitory functions of vitamin D3 in the regulation of leiomyoma cell growth traits that are mediated by TGF-β3.
TGF-β primarily exerts its biological function by activating the downstream Smad signaling pathway (19). In an attempt to determine whether vitamin D3 affects Smad signaling, we performed Western blot and immunoprecipitation analyses using human leiomyoma cells as shown in Fig. 5, A–F. Our results demonstrated that TGF-β3 induced activation of Smad signaling by phosphorylation of Smad2 and by induction of functionally complex formation between Smad2, Smad3, and Smad4 in HuLM cells, whereas vitamin D3 inhibited those effects in a dose-dependent manner. Additionally, vitamin D3 significantly reduced the phosphorylation of Smad2 in the nucleus as well as inhibited nuclear translocation of both Smad2 and Smad3, which is induced by TGF-β3 in HuLM cells (Fig. 6). Thus, our observation demonstrated an important role of vitamin D3 in regulating TGF-β3-induced activation and nuclear translocation of Smad proteins and, as a result, inhibited transcriptional activation of TGF-β3 target genes that are related to the process of fibrosis.
In conclusion, TGF-β3 induced fibronectin, collagen type 1, and PAI-1 protein expression in HuLM cells, whereas vitamin D3 reduced all the TGF-β3-induced protein expression. Vitamin D3 also inhibited prooncogenic cyclin D1 and Cdk2 protein expression, which was induced by TGF-β3 in HuLM cells. Vitamin D3 inhibited TGF-β3-induced phosphorylation of Smad2, functional complex formation between Smad2, Smad3, and Smad4, and the nuclear translocation of both Smad2 and Smad3 in HuLM cells. Therefore, taken together, our results strongly suggest that vitamin D3 can abrogate the TGF-β3-induced profibrotic gene profile as well as tumor-promoting functions in HuLM cells that might be involved in the development and progression of uterine leiomyoma and thus begin to propose vitamin D3 as a potential therapeutic agent for the safe nonsurgical treatment of uterine fibroid. Additionally, it suggests that vitamin D3 deficiency might be a potential risk factor for the development of uterine fibroids. In fact, our preliminary data support that notion (45–47) and suggest an inverse correlation between serum vitamin D3 level and fibroid tumor burden (48). Additional basic and translational research is needed to further delineate the role of vitamin D in fibroid biology.
Acknowledgments
We appreciate Dr. Chakradhari Sharan at the Center for Women's Health Research, Meharry Medical College, for his excellent help.
This work was supported by National Institutes of Health Grants NIH/NICHD 1 R01 HD046228-01 (to A.A-H.), Research Centers in Minority Institutions Grant G12 RR03032, RCMI pilot 2 G12 RR003032-26 (to S.K.H.), U01NS041071, and U54NS041071 and also by Wal-Mart (Study No. WPP2009-01 to S.K.H).
Disclosure Summary: The authors in this paper have no conflict of interest or anything else to disclose.
Footnotes
- COMT
- Catechol-O-methyltransferase
- CY3
- carbocyanine 3
- ECM
- extracellular matrix
- HuLM
- human uterine leiomyoma
- PAI-1
- plasminogen activator inhibitor-1
- PARP
- poly ADP-ribose polymerase
- Rho-GDI
- Rho GDP-dissociation inhibitor
- TβRI
- TGF-β type I receptor
- TβRII
- TGF-β type II receptor.
References
- 1. Farhi J, Ashkenazi J, Feldberg D, Dicker D, Orvieto R, Ben Rafael Z. 1995. Effect of uterine leiomyomata on the results of in-vitro fertilization treatment. Hum Reprod 10:2576–2578 [DOI] [PubMed] [Google Scholar]
- 2. Surrey ES, Lietz AK, Schoolcraft WB. 2001. Impact of intramural leiomyomata in patients with a normal endometrial cavity on in vitro fertilization-embryo transfer cycle outcome. Fertil Steril 75:405–410 [DOI] [PubMed] [Google Scholar]
- 3. Wilcox LS, Koonin LM, Pokras R, Strauss LT, Xia Z, Peterson HB. 1994. Hysterectomy in the United States, 1988–1990. Obstet Gynecol 83:549–555 [DOI] [PubMed] [Google Scholar]
- 4. Wilson EA, Yang F, Rees ED. 1980. Estradiol and progesterone binding in uterine leiomyomata and in normal uterine tissues. Obstet Gynecol 55:20–24 [PubMed] [Google Scholar]
- 5. Rein MS, Barbieri RL, Friedman AJ. 1995. Progesterone: a critical role in the pathogenesis of uterine myomas. Am J Obstet Gynecol 172:14–18 [DOI] [PubMed] [Google Scholar]
- 6. Baird DD, Dunson DB. 2003. Why is parity protective for uterine fibroids? Epidemiology 14:247–250 [DOI] [PubMed] [Google Scholar]
- 7. Nesby-O'Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, Allen C, Doughertly C, Gunter EW, Bowman BA. 2002. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr 76:187–192 [DOI] [PubMed] [Google Scholar]
- 8. Al-Hendy A, Salama SA. 2006. Ethnic distribution of estrogen receptor-alpha polymorphism is associated with a higher prevalence of uterine leiomyomas in black Americans. Fertil Steril 86:686–693 [DOI] [PubMed] [Google Scholar]
- 9. Salama SA, Nasr AB, Dubey RK, Al-Hendy A. 2006. Estrogen metabolite 2-methoxyestradiol induces apoptosis and inhibits cell proliferation and collagen production in rat and human leiomyoma cells: a potential medicinal treatment for uterine fibroids. J Soc Gynecol Investig 13:542–550 [DOI] [PubMed] [Google Scholar]
- 10. Salama SA, Ho SL, Wang HQ, Tenhunen J, Tilgmann C, Al-Hendy A. 2006. Hormonal regulation of catechol-O-methyl transferase activity in women with uterine leiomyomas. Fertil Steril 86:259–262 [DOI] [PubMed] [Google Scholar]
- 11. Sharan C, Halder SK, Thota T, Nair S, Jaleel T, Al-Hendy A. 2011. Vitamin D inhibits proliferation of human uterine leiomyoma cells via catechol-O-methyltransferase. Fertil Steril 95:247–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Massagué J. 1990. The transforming growth factor-β family. Annu Rev Cell Biol 6:597–641 [DOI] [PubMed] [Google Scholar]
- 13. Heldin CH, Miyazono K, ten Dijke P. 1997. TGF-β signalling from cell membrane to nucleus through SMAD proteins. Nature 390:465–471 [DOI] [PubMed] [Google Scholar]
- 14. Tang XM, Dou Q, Zhao Y, McLean F, Davis J, Chegini N. 1997. The expression of transforming growth factor-βs and TGF-β receptor mRNA and protein and the effect of TGF-βs on human myometrial smooth muscle cells in vitro. Mol Hum Reprod 3:233–240 [DOI] [PubMed] [Google Scholar]
- 15. Chegini N, Zhao Y, Williams RS, Flanders KC. 1994. Human uterine tissue throughout the menstrual cycle expresses transforming growth factor-β1 (TGFβ1), TGFβ2, TGFβ3, and TGFβ type II receptor messenger ribonucleic acid and protein and contains [125I]TGFβ1-binding sites. Endocrinology 135:439–449 [DOI] [PubMed] [Google Scholar]
- 16. Massagué J, Cheifetz S, Boyd FT, Andres JL. 1990. TGF-β receptors and TGF-β binding proteoglycans: recent progress in identifying their functional properties. Ann NY Acad Sci 593:59–72 [DOI] [PubMed] [Google Scholar]
- 17. Massagué J. 1992. Receptors for the TGF-β family. Cell 69:1067–1070 [DOI] [PubMed] [Google Scholar]
- 18. Massagué J. 1996. TGFβ signaling: receptors, transducers, and Mad proteins. Cell 85:947–950 [DOI] [PubMed] [Google Scholar]
- 19. Attisano L, Wrana JL. 2000. Smads as transcriptional co-modulators. Curr Opin Cell Biol 12:235–243 [DOI] [PubMed] [Google Scholar]
- 20. Massagué J, Wotton D. 2000. Transcriptional control by the TGF-β/Smad signaling system. EMBO J 19:1745–1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Verrecchia F, Mauviel A. 2002. Transforming growth factor-β signaling through the Smad pathway: role in extracellular matrix gene expression and regulation. J Invest Dermatol 118:211–215 [DOI] [PubMed] [Google Scholar]
- 22. Schnaper HW, Hayashida T, Hubchak SC, Poncelet AC. 2003. TGF-β signal transduction and mesangial cell fibrogenesis. Am J Physiol Renal Physiol 284:F243–F252 [DOI] [PubMed] [Google Scholar]
- 23. Luo X, Ding L, Xu J, Chegini N. 2005. Gene expression profiling of leiomyoma and myometrial smooth muscle cells in response to transforming growth factor-β. Endocrinology 146:1097–1118 [DOI] [PubMed] [Google Scholar]
- 24. Arici A, Sozen I. 2000. Transforming growth factor-β3 is expressed at high levels in leiomyoma where it stimulates fibronectin expression and cell proliferation. Fertil Steril 73:1006–1011 [DOI] [PubMed] [Google Scholar]
- 25. Lee BS, Nowak RA. 2001. Human leiomyoma smooth muscle cells show increased expression of transforming growth factor-β3 (TGF β3) and altered responses to the antiproliferative effects of TGF β. J Clin Endocrinol Metab 86:913–920 [DOI] [PubMed] [Google Scholar]
- 26. Malik M, Catherino WH. 2007. Novel method to characterize primary cultures of leiomyoma and myometrium with the use of confirmatory biomarker gene arrays. Fertil Steril 87:1166–1172 [DOI] [PubMed] [Google Scholar]
- 27. Norian JM, Malik M, Parker CY, Joseph D, Leppert PC, Segars JH, Catherino WH. 2009. Transforming growth factor β3 regulates the versican variants in the extracellular matrix-rich uterine leiomyomas. Reprod Sci 16:1153–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stewart EA, Friedman AJ, Peck K, Nowak RA. 1994. Relative overexpression of collagen type I and collagen type III messenger ribonucleic acids by uterine leiomyomas during the proliferative phase of the menstrual cycle. J Clin Endocrinol Metab 79:900–906 [DOI] [PubMed] [Google Scholar]
- 29. Ding L, Xu J, Luo X, Chegini N. 2004. Gonadotropin releasing hormone and transforming growth factor β activate mitogen-activated protein kinase/extracellularly regulated kinase and differentially regulate fibronectin, type I collagen, and plasminogen activator inhibitor-1 expression in leiomyoma and myometrial smooth muscle cells. J Clin Endocrinol Metab 89:5549–5557 [DOI] [PubMed] [Google Scholar]
- 30. Leppert PC, Baginski T, Prupas C, Catherino WH, Pletcher S, Segars JH. 2004. Comparative ultrastructure of collagen fibrils in uterine leiomyomas and normal myometrium. Fertil Steril 82(Suppl 3):1182–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ylikomi T, Laaksi I, Lou YR, Martikainen P, Miettinen S, Pennanen P, Purmonen S, Syvälä H, Vienonen A, Tuohimaa P. 2002. Antiproliferative action of vitamin D. Vitam Horm 64:357–406 [DOI] [PubMed] [Google Scholar]
- 32. Bläuer M, Rovio PH, Ylikomi T, Heinonen PK. 2009. Vitamin D inhibits myometrial and leiomyoma cell proliferation in vitro. Fertil Steril 91:1919–1925 [DOI] [PubMed] [Google Scholar]
- 33. Artaza JN, Norris KC. 2009. Vitamin D reduces the expression of collagen and key profibrotic factors by inducing an antifibrotic phenotype in mesenchymal multipotent cells. J Endocrinol 200:207–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Carney SA, Tahara H, Swartz CD, Risinger JI, He H, Moore AB, Haseman JK, Barrett JC, Dixon D. 2002. Immortalization of human uterine leiomyoma and myometrial cell lines after induction of telomerase activity: molecular and phenotypic characteristics. Lab Invest 82:719–728 [DOI] [PubMed] [Google Scholar]
- 35. Halder SK, Beauchamp RD, Datta PK. 2005. Smad7 induces tumorigenicity by blocking TGF-β-induced growth inhibition and apoptosis. Exp Cell Res 307:231–246 [DOI] [PubMed] [Google Scholar]
- 36. Stewart AJ, Westley BR, May FE. 1992. Modulation of the proliferative response of breast cancer cells to growth factors by oestrogen. Br J Cancer 66:640–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Barnard JA, Lyons RM, Moses HL. 1990. The cell biology of transforming growth factor β. Biochim Biophys Acta 1032:79–87 [DOI] [PubMed] [Google Scholar]
- 38. Vindevoghel L, Kon A, Lechleider RJ, Uitto J, Roberts AB, Mauviel A. 1998. Smad-dependent transcriptional activation of human type VII collagen gene (COL7A1) promoter by transforming growth factor-β. J Biol Chem 273:13053–13057 [DOI] [PubMed] [Google Scholar]
- 39. Chen SJ, Artlett CM, Jimenez SA, Varga J. 1998. Modulation of human alpha1(I) procollagen gene activity by interaction with Sp1 and Sp3 transcription factors in vitro. Gene 215:101–110 [DOI] [PubMed] [Google Scholar]
- 40. Dennler S, Itoh S, Vivien D, ten Dijke P, Huet S, Gauthier JM. 1998. Direct binding of Smad3 and Smad4 to critical TGF β-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J 17:3091–3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lai CF, Feng X, Nishimura R, Teitelbaum SL, Avioli LV, Ross FP, Cheng SL. 2000. Transforming growth factor-β up-regulates the β5 integrin subunit expression via Sp1 and Smad signaling. J Biol Chem 275:36400–36406 [DOI] [PubMed] [Google Scholar]
- 42. Samarakoon R, Higgins PJ. 2008. Integration of non-SMAD and SMAD signaling in TGF-β1-induced plasminogen activator inhibitor type-1 gene expression in vascular smooth muscle cells. Thromb Haemost 100:976–983 [PMC free article] [PubMed] [Google Scholar]
- 43. Moustakas A, Heldin CH. 2005. Non-Smad TGF-β signals. J Cell Sci 118:3573–3584 [DOI] [PubMed] [Google Scholar]
- 44. Zhang T, Nanney LB, Luongo C, Lamps L, Heppner KJ, DuBois RN, Beauchamp RD. 1997. Concurrent overexpression of cyclin D1 and cyclin-dependent kinase 4 (Cdk4) in intestinal adenomas from multiple intestinal neoplasia (Min) mice and human familial adenomatous polyposis patients. Cancer Res 57:169–175 [PubMed] [Google Scholar]
- 45. Halder SK, Al-Hendy A. 2010. 1,25-Dihydroxyvitamin D3 reduces transforming growth factor (TGF) β3-induced profibrotic gene expressions in human uterine leiomyoma cells. Reprod Sci 17(Suppl):713 [Google Scholar]
- 46. Halder SK, Sharan C, Al-Hendy A. 2010. 1,25-Dihydroxyvitamin D3 reduces growth of uterine leiomyomas in Eker rats. Reprod Sci 17(Suppl):99520697142 [Google Scholar]
- 47. Halder SK, Sharan C, Harirah H, Al-Hendy A. 2010. Lower serum levels of vitamin D3 is a risk factor for uterine fibroids in African Americans. Reprod Sci 17(Suppl):72420445008 [Google Scholar]
- 48. Abdelraheem MS, Al-Hendy A. 2010. Serum vitamin D3 level inversely correlates with total fibroid tumor burden in women with symptomatic uterine fibroid. Fertil Steril 94(Suppl):S74 [Google Scholar]