Based on culture-independent molecular methods using sequence analysis of 16S rRNA genes, most of the predominant bacterial species in the oral cavity have been identified (1,2,3,6,14,17,20,26,28,30,36,39,40,46,48). Collectively speaking, there are about 620 predominant oral bacterial species, of which about 35% have not yet been cultivated in vitro. Whereas there is considerable debate as to what defines a bacterial species (53,54), the 16S rRNA approach defines, in general, a species (or more precisely a phylotype) as 16S rRNA gene sequences of strains or cloned 16S rRNA inserts with >98.5% similarity. Consequently, strain or clone sequences with <98.5% similarity to previously defined phylotypes are considered representatives of new phylotypes (for details, see the Human Oral Microbiome Database [HOMD, http://www.homd.org/]). More recently, based on evaluations of 35,000 16S rRNA gene sequences from about 400 patients, it is estimated that there are approximately 1,200 predominant species (unpublished data, HOMD). In contrast, investigators in a recent study utilizing pyrosequencing analysis of 197,600 sequences derived from the oral cavity have suggested that the microbial diversity of the human oral microbiome is much greater with approximately 19,000 phylotypes (27). However, most of these phylotypes are those found at very low densities.
Bacterial species colonizing the surfaces of the human oral cavity are known to play an important role in oral health and disease and thus a rapid and accurate means of identification is crucial. Traditionally, identification has been based on phenotypic and biochemical criteria, including microscopy, biochemical reactivity, growth conditions, dye and immunofluorescence staining, bacterial end product analysis, cell membrane composition, and antibiotic sensitivity. However, these tests are labor-intensive and costly, providing sometimes inconsistent results that make identification rather tentative. This can be due to strain variation within a species. More recently, molecular DNA-based techniques have been used to identify bacteria directly from clinical samples circumventing the need for in vitro cultivation. Results from these studies implicate specific bacterial species or complexes of species that are associated with oral health and disease. In general, there are 3 main categories of molecular microbial analyses to consider, namely 1) PCR-based methods, including single target PCR, multiplex PCR and quantitative PCR; 2) DNA-DNA hybridization methods such as in situ hybridization, checkerboard hybridization, and 16S rRNA-based microarrays; and 3) sequencing methods including the latest, next-generation sequencing (NGS) techniques, such as pyrosequencing, real-time single-molecule DNA sequencing, and nanopore-based sequencing. The focus of this review is to describe the current status of these DNA-based, culture-independent methods for use or potential use in molecular microbial diagnosis.
PCR-based methods
Single target PCR applications
Many studies have utilized PCR-based methods to detect specific species directly from oral clinical samples. These studies focused on the detection of a few species typically associated with the putative periodontal and caries pathogens, such as Porphyromonas gingivalis, Tannerella forsythia, Treponema denticola, Streptococcus mutans, and Aggregatibacter actinomycetemcomitans (13,31,34,47,55). In previous studies of sequence analysis of 16S rRNA genes from the oral cavity, a number of bacterial species were implicated as candidates as putative pathogens for periodontitis, including the more traditional pathogenic species, such as P. gingivalis, T. denticola and T. forsythia (36). Species or phylotype-specific PCR primers were designed and were subsequently used in highly stringent, individual PCR reactions to detect the prevalence of target species in plaque samples of healthy subjects and diseased subjects (29). These investigators confirmed that several additional species, including those that have not yet been grown in vitro, were associated with oral health or periodontitis.
Multiplex PCR
This technique is an expansion of single target PCR methodology in which more than one pair of species-specific primers are used in a single PCR assay that allows for the simultaneous detection of multiple species. Such assays have been used to simultaneously detect A. actinomycetemcomitans, T. forsythia, and P. gingivalis (21,57,62). Optimization of multiplex PCR can be laborious to establish, but ultimately these assays are quite sensitive with detection limits of 10 to 100 cells per PCR reaction (57).
MicroDent® Test is a commercially available method using multiplex PCR that tests for 5 oral species and has been used to compare the microbial profiles of subgingival plaque samples in oral health and periodontitis (19,52).
Real-time PCR
Real-time PCR, also referred to as qPCR, qRT-PCR, RT-qPCR and kinetic PCR, is a method to quantify the copy numbers of DNA in clinical samples. There are two types of real time PCR, namely an intercalator-based method and a probe-based method. The intercalator-based method, also known as SYBR Green method, intercalates SYBR green which binds to newly synthesized double-stranded DNA producing a fluorescently-labeled PCR amplicon. The probe-based method, or TaqMan PCR, is more specific in that it utilizes a fluorogenic-labeled probe that binds only to its complementary sequence in the internal portion of the generated PCR amplicon. Real-time PCR has been used to detect and quantify several periodontal pathogens including A. actinomycetemcomitans, P. gingivalis, Prevotella intermedia, the tetQ gene, and total bacteria, in clinical samples (5,7,32,33).
MYPERIOPATH™ from OralDNAlabs™ is a commercially available service that utilizes TaqMan PCR which determines the microbial profiles of 13 putative periodontal pathogens from oral specimens provided by clinicians. Treatment considerations and follow up recommendations are given with the final report.
DNA-DNA hybridization methods
Fluorescence in situ hybridization (FISH)
Fluorescence in situ hybridization (FISH), or more specifically whole cell hybridization, can be used to quantify, determine the spatial configuration, and demonstrate the morphology of individual bacterial cells in complex natural communities, such as dental plaque (4). Basically, fluorescently labeled, rRNA-targeted oligonucleotides are hybridized to partially fixed, whole cells on microscope slides and are visualized using fluorescence or confocal fluorescence microscopy. Known oral bacterial species including A. actinomycetemcomitans, P. gingivalis, Actinomyces spp. and Streptococcus spp. (15,16,44,45) and phylotypes known only from 16S rRNA sequence analysis (35,58) have been detected using FISH. Furthermore, whole-cell hybridizations in solution can be combined with flow cytometry for analysis of mixed microbial populations (63).
Checkerboard hybridization
In order to make definitive bacterial associations with oral health and disease states, the microbial profiles of large numbers of clinical samples must be determined. Consequently in 1994, a method was introduced that enabled the hybridization of 45 DNA samples against 30 DNA probes (i.e., up to 1350 simultaneous hybridizations) on a single support membrane (51). There are two types of checkerboard hybridization, one utilizing whole genomic DNA probes that are hybridized to sample DNA on the membrane (51), and the other utilizing labeled 16S rRNA amplicons that are hybridized to 16S rRNA-based probes that are on the membrane (36). This latter method has been referred to as reverse-capture, 16S rRNA-based oligonucleotide checkerboard hybridization. In both methods, hybridization signals are typically detected using chemifluorescence procedures.
Although there is potential use for checkerboard hybridization as a diagnostic tool, the checkerboard hybridization methodology is used more routinely for research purposes. Most of the publications have used the whole genomic DNA probes checkerboard hybridization to study the roles of bacteria in oral health and disease. In a landmark paper, Socransky et al (49) analyzed 185 subjects representing about 13,000 plaque samples using whole genomic DNA probes to 40 bacterial cultivable species in checkerboard hybridization assays to define bacterial complexes, rather than individual species, that were involved in oral health and periodontal disease. Many publications since then have utilized whole genomic checkerboard hybridization to answer many biologic, research-related questions in oral ecology (23,24,50). Recently, checkerboard hybridization has also been used for the quantification of multiple inflammatory mediators in gingival crevicular fluid (GCF) samples (56)
The reverse-capture checkerboard hybridization has also been used to determine the role of bacteria in oral health and disease, including caries of the primary dentition (6,11), caries of the secondary dentition (3), necrotizing ulcerative periodontitis (39), and periodontal diseases associated with HIV infected individuals (2). The advantage of the reverse capture method is that species that have only been identified as 16S rRNA phylotypes, e.g., the “uncultivables”, can be monitored just as easily as known cultivable species (38).
Oligonucleotide microarray technology
As an extension of the 16S rRNA-based, reverse-capture DNA-DNA checkerboard hybridization, the Human Oral Microbe Identification Microarray, or HOMIM, was developed in order to examine the complex oral microbial diversity in a single hybridization on glass slides (38,41,42). This high sample-throughput, 16S rRNA-based technology allows the simultaneous detection about 300 key and predominant bacterial species, including species that have not yet been cultivated (for details, see http://mim.forsyth.org).
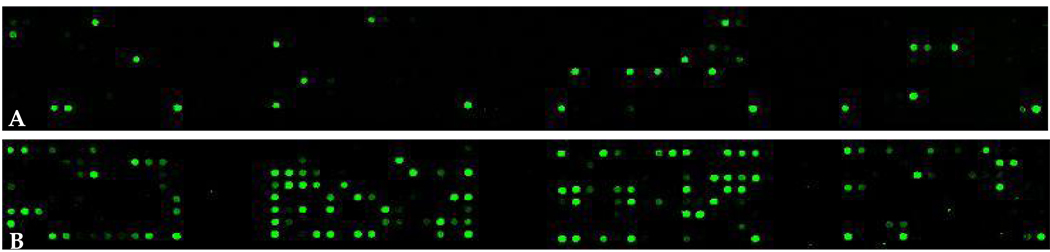
Briefly, 16S rRNA-based, reverse-capture oligonucleotide probes (typically 18 to 20 bases) are synthesized with a 5’ - (C6)-amine modified base and eight spacer thymidines. Probes are printed on aldehyde-coated glass slides. 16S rRNA genes are PCR-amplified from DNA isolated from clinical samples using 16S rRNA universal forward and reverse primers and labeled via incorporation of Cy3-dCTP in a second nested PCR (41). Labeled amplicons are hybridized to the probes on the slides. Details of the protocol have been described (41). Typical arrays are shown in Figure 1.
Figure 1.
Microbial profiles of clinical samples. HOMIMs of DNA isolated from subgingival plaque from a healthy site of a healthy subject (A) and from a 5 mm pocket of a subject with periodontitis (B) are compared using 461 oligonucleotide probes that were organized phylogenetically. Note that the diseased profile is considerably more diverse than the healthy profile.
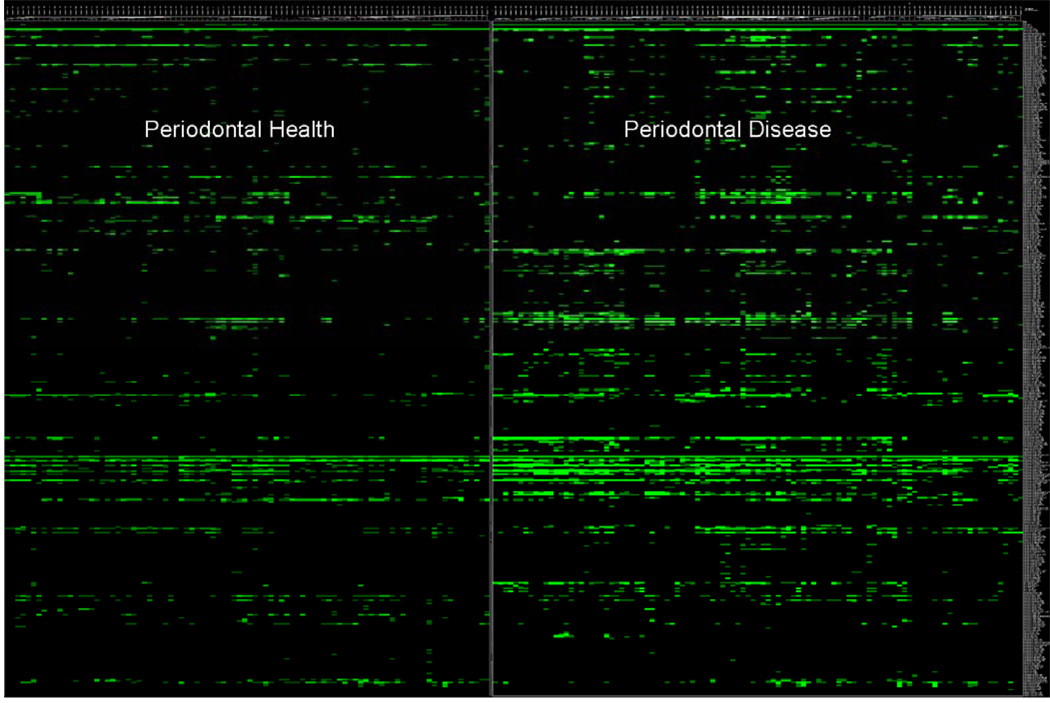
To analyze data from HOMIM arrays, individual signals are translated to a “bar code” format and are normalized by comparing individual signal intensities to the average of signals from universal probes. The bands correspond to presence or absence and band intensities correspond to 1+, 2+, 3+4+, or 5+—thus more intense bands reflect higher proportions. Figure 2 illustrates the bar code format of HOMIMs comparing the microbial profiles of approximately 300 bacterial species from subjects with periodontal health and periodontitis. HOMIMs have been used to define the microflora of root surface caries in the elderly (41) and to compare and define the normal flora in 5 sites in the oral cavity of the elderly (42). Most recently, HOMIM has been used to compare the subgingival microbiota of refractory periodontitis, successfully treated periodontitis, and periodontally healthy (10)
Figure 2.
Bacterial profiles of 461 bacterial taxa (representing about 300 species) comparing subgingival plaque from 105 healthy sites in periodontally healthy subjects (n=20) to 154 diseased sites from periodontally diseased subjects (n=47). Differences in profiles can be seen at a glance. (Courtesy of A.P. Colombo)
One reverse-capture, 16S rRNA-based microarray is currently commercially available for diagnostic use. ParoCheck® DNA chip targets 20 oral bacterial species and has been used to determine the microbial profiles in clinical samples including endodontic lesions (59) and normal microflora of gingival biopsies (18).
Recently, high density 16S rRNA-based microarrays have also been developed. The Phylochip as developed by the Affymetrix Corporation ® and Lawrence Berkeley Labs can detect up to 32,000 16S rRNA phylotypes (9). Huyghe et al. (25) reported on a similar microarray design to study complex bacterial communities that targets 9,500 16S rRNA phylotypes. Most of the studies using such microarrays have focused on analysis of environmental samples. Probes on these high-density microarrays target mainly family and genus rather than species level distinctions.
Sequencing methods
Kumar et al (30) used culture-independent, quantitative analysis of subgingival plaque samples to identify putative pathogenic and health associated species. In this study, DNA from samples was analyzed using ribosomal 16S cloning and subsequent sequencing, but the results were subjected to quantitative analysis. Most studies of this type are descriptive in nature. This technique would be far too laborious to be used as a diagnostic tool.
Next generation sequencing, or NGS, is the newest technology for high-throughput genomic analysis using a pyrosequencing platform (43). Most NGS technologies eliminate the need for cloning and sequencing by amplifying a single DNA molecule (60,61). The 3 main technologies for NGS are as follows: 1) 454 pyrosequencing in which DNA is fragmented and amplified with special adaptors in an emulsion PCR which bind to an agarose bead. This amplification produces up to 1 million copies around one bead. This methodology allows reads 400,000 DNAs that are each about 250 bases in length although newer technology allows for longer reads of up to 500 bp; 2) SOLiD which is similar to 454 pyrosequencing in that fragmented DNA is amplified on an agarose bead. However, this technique utilizes the incorporation of a ligase and universal oligonucleotides. 3) Illumina/Solexa methodology also utilizes fragmented DNA and specialized adaptors, but attaches to a slide rather than a bead. SOLid and Illumina allow for millions of reads, albeit only 35 to 50 bp.
Even newer technologies such as real-time, single-molecule DNA sequencing, which uses RNA polymerase (22), and nanopore sequencing (8,12), which measures the change in current as a single DNA molecule is driven through a tiny pore, allows reading over a thousand bases per second. Such technology greatly reduces the cost per sample.
Concluding remarks
Most of the DNA-based, culture-independent methods described in this review are used more for research purposes in order to answer specific scientific questions rather than for use in molecular microbial diagnosis. For example, 16S rRNA-based and DNA hybridization-based methods are being used to identify the role of particular bacterial species or bacterial complexes in oral infectious diseases, oral cancer, and systemic diseases associated with oral bacteria. Consequently, specific bacterial profiles may be useful to determine those people at risk for disease, e.g., a “unhealthy” profile may be an early indicator of disease. As the scientific questions are answered and the technology improves, then many of these techniques will likely be utilized as diagnostic tools.
Acknowledgements
Previously unpublished work cited in this paper was supported by NIH grant DE11443 from the National Institute of Dental and Craniofacial Research.
References
- 1.Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43:5721–5732. doi: 10.1128/JCM.43.11.5721-5732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aas JA, Barbuto SM, Alpagot T, Olsen I, Dewhirst FE, Paster BJ. Subgingival plaque microbiota in HIV+ patients. J Clin Periodontol. 2007;34:189–195. doi: 10.1111/j.1600-051X.2006.01034.x. [DOI] [PubMed] [Google Scholar]
- 3.Aas JA, Griffen AL, Dardis SR, Lee AM, Olsen I, Dewhirst FE, Leys EJ, Paster BJ. Bacteria of dental caries in primary and permanent teeth in children and young adults. J Clin Microbiol. 2008;46:1407–1417. doi: 10.1128/JCM.01410-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amann RI, Ludwig W, Schleifer K. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev. 1995;59:143–169. doi: 10.1128/mr.59.1.143-169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atieh MA. Accuracy of real-time polymerase chain reaction versus anaerobic culture in detection of Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis: a meta-analysis. J Periodontol. 2008;79:1620–1629. doi: 10.1902/jop.2008.070668. [DOI] [PubMed] [Google Scholar]
- 6.Becker MR, Paster BJ, Leys EJ, Moeschberger ML, Kenyon SG, Galvin JL, Boches SK, Dewhirst FE, Griffen AL. Molecular analysis of bacterial species associated with early childhood caries. J Clin Microbiol. 2002;40:1001–1009. doi: 10.1128/JCM.40.3.1001-1009.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boutaga K, Savelkoul PH, Winkel EG, van Winkelhoff AJ. Comparison of subgingival bacterial sampling with oral lavage for detection and quantification of periodontal pathogens by real-time polymerase chain reaction. J Periodontol. 2007;78:79–86. doi: 10.1902/jop.2007.060078. [DOI] [PubMed] [Google Scholar]
- 8.Branton D, Deamer DW, Marziali A, Bayley H, Benner SA, Butler T, Di Ventra M, Garaj S, Hibbs A, Huang X, Jovanovich SB, Krstic PS, Lindsay S, Ling XS, Mastrangelo CH, Meller A, Oliver JS, Pershin YV, Ramsey JM, Riehn R, Soni GV, Tabard-Cossa V, Wanunu M, Wiggin M, Schloss JA. The potential and challenges of nanopore sequencing. Nat Biotechnol. 2008;26:1146–1153. doi: 10.1038/nbt.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brodie EL, Desantis TZ, Joyner DC, Baek SM, Larsen JT, Andersen GL, Hazen TC, Richardson PM, Herman DJ, Tokunaga TK, Wan JM, Firestone MK. Application of a high-density oligonucleotide microarray approach to study bacterial population dynamics during uranium reduction and reoxidation. Appl Environ Microbiol. 2006;72:6288–6298. doi: 10.1128/AEM.00246-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colombo APV, Boches SK, Cotton SL, Goodson JM, Kent R, Haffajee AD, Socransky SS, Hasturk H, van Dyke TE, Paster BJ. Subgingival microbiota of refractory periodontitis determined by microarray (HOMIM) J Dent Res. 2009;88 [Google Scholar]
- 11.Corby PM, Lyons-Weiler J, Bretz WA, Hart TC, Aas JA, Boumenna T, Goss J, Corby AL, Junior HM, Weyant RJ, Paster BJ. Microbial risk indicators in early childhood caries. J Clin Microbiol. 2005;43:5753–5759. doi: 10.1128/JCM.43.11.5753-5759.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deamer DW, Akeson M. Nanopores and nucleic acids: prospects for ultrarapid sequencing. Trends Biotechnol. 2000;18:147–151. doi: 10.1016/s0167-7799(00)01426-8. [DOI] [PubMed] [Google Scholar]
- 13.de Lillo A, Booth V, Kyriacou L, Weightman AJ, Wade WG. Culture-independent identification of periodontitis-associated Porphyromonas and Tannerella populations by targeted molecular analysis. J Clin Microbiol. 2004;42:5523–5527. doi: 10.1128/JCM.42.12.5523-5527.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dewhirst FE, Tamer MA, Ericson RE, Lau CN, Levanos VA, Boches SK, Galvin JL, Paster BJ. The diversity of periodontal spirochetes by 16S rRNA analysis. Oral Microbiol Immunol. 2000;15:196–202. doi: 10.1034/j.1399-302x.2000.150308.x. [DOI] [PubMed] [Google Scholar]
- 15.Diaz PI, Chalmers NI, Rickard AH, Kong C, Milburn CL, Palmer RJ, Jr, Kolenbrander PE. Molecular characterization of subject-specific oral microflora during initial colonization of enamel. Appl Environ Microbiol. 2006;72:2837–2848. doi: 10.1128/AEM.72.4.2837-2848.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dige I, Raarup MK, Nyengaard JR, Kilian M, Nyvad B. Actinomyces naeslundii in initial dental biofilm formation. Microbiology. 2009 Apr 30; doi: 10.1099/mic.0.027706-0. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Dymock D, Weightman AJ, Scully C, Wade WG. Molecular analysis of microflora associated with dentoalveolar abscess. J Clin Microbiol. 1996;34:537–542. doi: 10.1128/jcm.34.3.537-542.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eberhard J, Menzel N, Dommisch H, Winter J, Jepsen S, Mutters R. The stage of native biofilm formation determines the gene expression of human beta-defensin-2, psoriasin, ribonuclease 7 and inflammatory mediators: a novel approach for stimulation of keratinocytes with in situ formed biofilms. Oral Microbiol Immunol. 2008;23:21–28. doi: 10.1111/j.1399-302X.2007.00385.x. [DOI] [PubMed] [Google Scholar]
- 19.Eick S, Pfister W. Comparison of microbial cultivation and a commercial PCR based method for detection of periodontopathogenic species in subgingival plaque samples. J Clin Periodontol. 2002;29:638–644. doi: 10.1034/j.1600-051x.2002.290708.x. [DOI] [PubMed] [Google Scholar]
- 20.Faveri M, Mayer MPA, Feres M, De Figueiredo LC, Dewhirst FE, Paster BJ. Microbiological diversity of generalized aggressive periodontitis by 16SrRNA clonal analysis. Oral Microbiol Immunol. 2008;23:112–118. doi: 10.1111/j.1399-302X.2007.00397.x. [DOI] [PubMed] [Google Scholar]
- 21.Garcia L, Tercero JC, Legido B, Ramos JA, Alemany J, Sanz M. Rapid detection of Actinobacillus actinomycetemcomitans, Prevotella intermedia, and Porphyromona gingivalis by multiplex PCR. J Periodont Res. 1998;33:59–64. doi: 10.1111/j.1600-0765.1998.tb02292.x. [DOI] [PubMed] [Google Scholar]
- 22.Greenleaf WJ, Block SM. Single-molecule, motion-based DNA sequencing using RNA polymerase. Science. 2006;313:801. doi: 10.1126/science.1130105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haffajee AD, Patel M, Socransky SS. Microbiological changes associated with four different periodontal therapies for the treatment of chronic periodontitis. Oral Microbiol Immunol. 2008;23:148–157. doi: 10.1111/j.1399-302X.2007.00403.x. [DOI] [PubMed] [Google Scholar]
- 24.Haffajee AD, Socransky SS, Patel MR, Song X. Microbial complexes in supragingival plaque. Oral Microbiol Immunol. 2008;23:196–205. doi: 10.1111/j.1399-302X.2007.00411.x. [DOI] [PubMed] [Google Scholar]
- 25.Huyghe A, Francois P, Charbonnier Y, Bento M, Bonetti E-J, Paster BJ, Bolivar I, Baratti-Mayer D, Pittet D, Schrenzel J. A novel microarray design strategy to study complex bacterial communities. Appl Environment Microbiol. 2008;74:1876–1885. doi: 10.1128/AEM.01722-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kazor CE, Mitchell PM, Lee AM, Stokes LN, Loesche WJ, Dewhirst FE, Paster BJ. Diversity of bacterial populations on the tongue dorsum in halitosis and in health. J Clin Microbiol. 2003;41:558–563. doi: 10.1128/JCM.41.2.558-563.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keijser BJ, Zaura E, Huse SM, van der Vossen JM, Schuren FH, Montijn RC, ten Cate JM, Crielaard W. Pyrosequencing analysis of the oral microflora of healthy adults. J Dent Res. 2008;87:1016–1020. doi: 10.1177/154405910808701104. [DOI] [PubMed] [Google Scholar]
- 28.Kroes I, Kroes PW, Relman DA. Bacterial diversity within the human subgingival crevice. PNAS. 1999;96:14547–14552. doi: 10.1073/pnas.96.25.14547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar PS, Griffen AL, Barton JA, Paster BJ, Moeschberger ML, Leys EJ. New bacterial species associated with chronic periodontitis. J Dent Res. 2003;82:338–344. doi: 10.1177/154405910308200503. [DOI] [PubMed] [Google Scholar]
- 30.Kumar PS, Griffen AL, Moeschberger ML, Leys EJ. Identification of candidate periodontal pathogens and beneficial species by quantitative 16S clonal analysis. J Clin Microbiol. 2005 Aug;43(8):3944–3955. doi: 10.1128/JCM.43.8.3944-3955.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leys EJ, Lyons SR, Moeschberger ML, Rumpf RW, Griffen AL. Association of Bacteroides forsythus and a novel Bacteroides phylotype with periodontitis. J Clin Microbiol. 2002;40:821–825. doi: 10.1128/JCM.40.3.821-825.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lyons SR, Griffen AL, Leys EJ. Quantitative real-time PCR for Porphyromonas gingivalis and total bacteria. J Clin Microbiol. 2000;38:2362–2365. doi: 10.1128/jcm.38.6.2362-2365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maeda H, Fujimoto C, Haruki Y, Maeda T, Kokeguchi S, Petelin M, Arai H, Tanimoto I, Nishimura F, Takashiba S. Quantitative real-time PCR using TaqMan and SYBR Green for Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, Prevotella intermedia, tetQ gene and total bacteria. FEMS Immunol Med Microbiol. 2003;39:81–86. doi: 10.1016/S0928-8244(03)00224-4. [DOI] [PubMed] [Google Scholar]
- 34.Okada M, Soda Y, Hayashi F, Doi T, Suzuki J, Miura K, Kozai K. Longitudinal study of dental caries incidence associated with Streptococcus mutans and Streptococcus sobrinus in pre-school children. J Med Microbiol. 2005;54:661–665. doi: 10.1099/jmm.0.46069-0. [DOI] [PubMed] [Google Scholar]
- 35.Ouverney CC, Armitage GC, Relman DA. Single-cell enumeration of an uncultivated TM7 subgroup in the human subgingival crevice. Appl Environ Microbiol. 2003;69:6294–6298. doi: 10.1128/AEM.69.10.6294-6298.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paster BJ, Bartoszyk IM, Dewhirst FE. Identification of oral streptococci using PCR-based, reverse-capture, checkerboard hybridization. Methods Cell Sci. 1998;20:223–231. [Google Scholar]
- 37.Paster BJ, Boches SK, Galvin JL, Ericson RE, Lau CN, Levanos VA, Sahasrabudhe A, Dewhirst FE. Bacterial diversity in human subgingival plaque. J Bacteriol. 2001;183:3770–3783. doi: 10.1128/JB.183.12.3770-3783.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paster BJ, Olsen I, Aas JA, Dewhirst FE. The breadth of bacterial diversity in the human periodontal pocket and other oral sites. Periodontol 2000. 2006;42:80–87. doi: 10.1111/j.1600-0757.2006.00174.x. [DOI] [PubMed] [Google Scholar]
- 39.Paster BJ, Russell MK, Alpagot T, Lee AM, Boches SK, Galvin JL, Dewhirst FE. Bacterial diversity in necrotizing ulcerative periodontitis in HIV-positive subjects. Ann Periodontol. 2002;7:8–16. doi: 10.1902/annals.2002.7.1.8. [DOI] [PubMed] [Google Scholar]
- 40.Preza D, Olsen I, Aas JA, Willumsen T, Grinde B, Paster BJ. Bacterial profiles of root caries in elderly patients. J Clin Microbiol. 2008;46:2015–2021. doi: 10.1128/JCM.02411-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Preza D, Olsen I, Willumsen T, Boches SK, Cotton SL, Grinde B, Paster BJ. Microarray analysis of the microflora of root caries in elderly. Eur J Clin Microbiol Infect Dis. 2008;46:2015–2021. doi: 10.1007/s10096-008-0662-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Preza D, Olsen I, Willumsen T, Grinde B, Paster BJ. Diversity and site-specificity of the oral microflora in the elderly. Eur J Clin Microbiol Infect Dis. 2009 doi: 10.1007/s10096-009-0743-3. Online 10.1007/s10096-009-0743-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ronaghi M, Uhlen M, Nyren P. A sequencing method based on real-time pyrophosphate. Science. 1998;281:363–365. doi: 10.1126/science.281.5375.363. [DOI] [PubMed] [Google Scholar]
- 44.Rudney JD, Chen R, Sedgewick GJ. Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, and Tannerella forsythensis are components of a polymicrobial intracellular flora within human buccal cells. J Dent Res. 2005;84:59–63. doi: 10.1177/154405910508400110. [DOI] [PubMed] [Google Scholar]
- 45.Rudney JD, Chen R, Zhang G. Streptococci dominate the diverse flora within buccal cells. J Dent Res. 2005;84:1165–1171. doi: 10.1177/154405910508401214. [DOI] [PubMed] [Google Scholar]
- 46.Sakamoto M, Umeda M, Benno Y. Molecular analysis of human oral microbiota. J Periodontal Res. 2005;40:277–285. doi: 10.1111/j.1600-0765.2005.00793.x. [DOI] [PubMed] [Google Scholar]
- 47.Sanz M, Lau L, Herrera D, Morillo JM, Silva A. Methods of detection of Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis and Tannerella forsythensis in periodontal microbiology, with special emphasis on advanced molecular techniques: a review. J Clin Periodontol. 2004;31:1034–1047. doi: 10.1111/j.1600-051X.2004.00609.x. [DOI] [PubMed] [Google Scholar]
- 48.Siqueira JF, Jr, Rôças IN. The microbiota of acute apical abscesses. J Dent Res. 2009;88:61–65. doi: 10.1177/0022034508328124. [DOI] [PubMed] [Google Scholar]
- 49.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 50.Socransky SS, Haffajee AD, Smith C, Martin L, Haffajee JA, Uzel NG, Goodson JM. Use of checkerboard DNA-DNA hybridization to study complex microbial ecosystems. Oral Microbiol Immunol. 2004;19:352–362. doi: 10.1111/j.1399-302x.2004.00168.x. [DOI] [PubMed] [Google Scholar]
- 51.Socransky SS, Smith C, Martin L, Paster BJ, Dewhirst FE, Levin AE. "Checkerboard" DNA-DNA hybridization. Biotechniques. 1994;17:788–792. [PubMed] [Google Scholar]
- 52.Squeri R, La Fauci V, Cannavò G, Lo Giudice G, Sindoni L. Identification of the microorganisms responsible for periodontopathy by multiplex RT-PCR. J Prev Med Hyg. 2006;47:142–145. [PubMed] [Google Scholar]
- 53.Stackebrandt E, Frederiksen W, Garrity GM, Grimont PA, Kämpfer P, Maiden MC, Nesme X, Rosselló-Mora R, Swings J, Trüper HG, Vauterin L, Ward AC, Whitman WB. Report of the ad hoc committee for the re-evaluation of the species definition in bacteriology. Int J Syst Evol Microbiol. 2002;52:1043–1047. doi: 10.1099/00207713-52-3-1043. [DOI] [PubMed] [Google Scholar]
- 54.Staley JT. The bacterial species dilemma and the genomic-phylogenetic species concept. Philos Trans R Soc Lond B Biol Sci. 2006;361:1899–1909. doi: 10.1098/rstb.2006.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tanner AC, Paster BJ, Lu SC, Kanasi E, Kent R, Jr, Van Dyke T, Sonis ST. Subgingival and tongue microbiota during early periodontitis. J Dent Res. 2006;85:318–323. doi: 10.1177/154405910608500407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Teles RP, Sakellari D, Konstantinidis A, Socransky SS, Haffajee AD. Application of the checkerboard immunoblotting technique to the quantification of host biomarkers in gingival crevicular fluid. J Periodontol. 2009;80:447–456. doi: 10.1902/jop.2009.080440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tran SD, Rudney JD. Improved multiplex PCR using conserved and species-specific 16S rRNA gene primers for simultaneous detection of Actinobacillus actinomycetemcomitans, Bacteroides forsythus, and Porphyromonas gingivalis. J Clin Microbiol. 1999;37:3504–3508. doi: 10.1128/jcm.37.11.3504-3508.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vartoukian SR, Palmer RM, Wade WG. Diversity and morphology of members of the phylum 'Synergistetes' in periodontal health and disease. Appl Environ Microbiol. 2009 Apr 3; doi: 10.1128/AEM.02763-08. E-publication ahead of print. PMID19346352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vianna ME, Horz HP, Gomes BP, Conrads G. Microarrays complement culture methods for identification of bacteria in endodontic infections. Oral Microbiol Immunol. 2005;20:253–258. doi: 10.1111/j.1399-302X.2005.00221.x. [DOI] [PubMed] [Google Scholar]
- 60.Voelkerding KV, Dames SA, Durtschi JD. Next-generation sequencing: from basic research to diagnostics. Clin Chem. 2009;55:641–658. doi: 10.1373/clinchem.2008.112789. [DOI] [PubMed] [Google Scholar]
- 61.von Bubnoff A. Next-generation sequencing: the race is on. Cell. 2008;132:721–723. doi: 10.1016/j.cell.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 62.Wahlfors J, Meurman JH, Vaisanen P, Alakuijala P, Korhonen A, Torkko H, Janne J. Simultaneous detection of Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis by a rapid PCR method. J Dent Res. 1995;74:1796–1801. doi: 10.1177/00220345950740111301. [DOI] [PubMed] [Google Scholar]
- 63.Wallner G, Amann R, Beisker W. Optimizing fluorescent in situ hybridization of suspended cells with rRNA-targeted oligonucleotide probes for the flow cytometric identification of microorganisms. Cytometry. 1993;14:136–143. doi: 10.1002/cyto.990140205. [DOI] [PubMed] [Google Scholar]