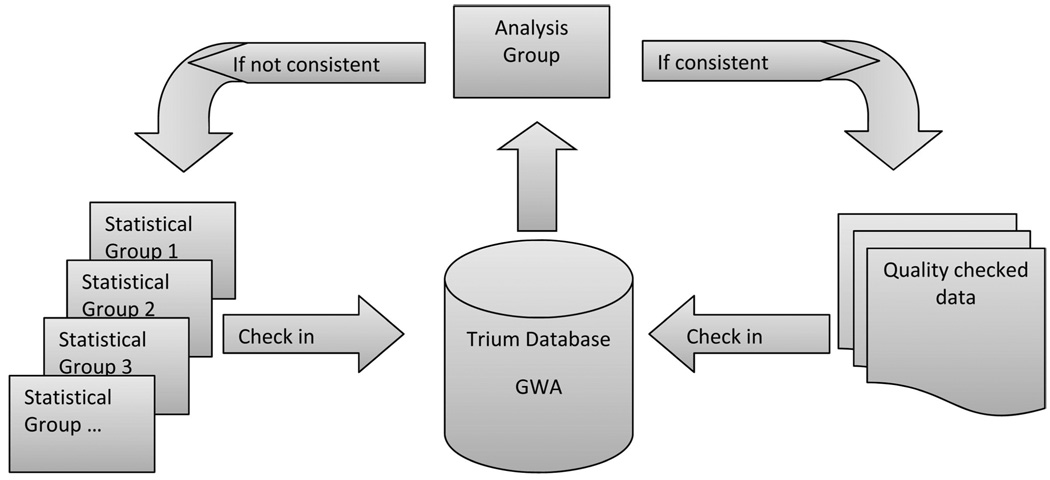
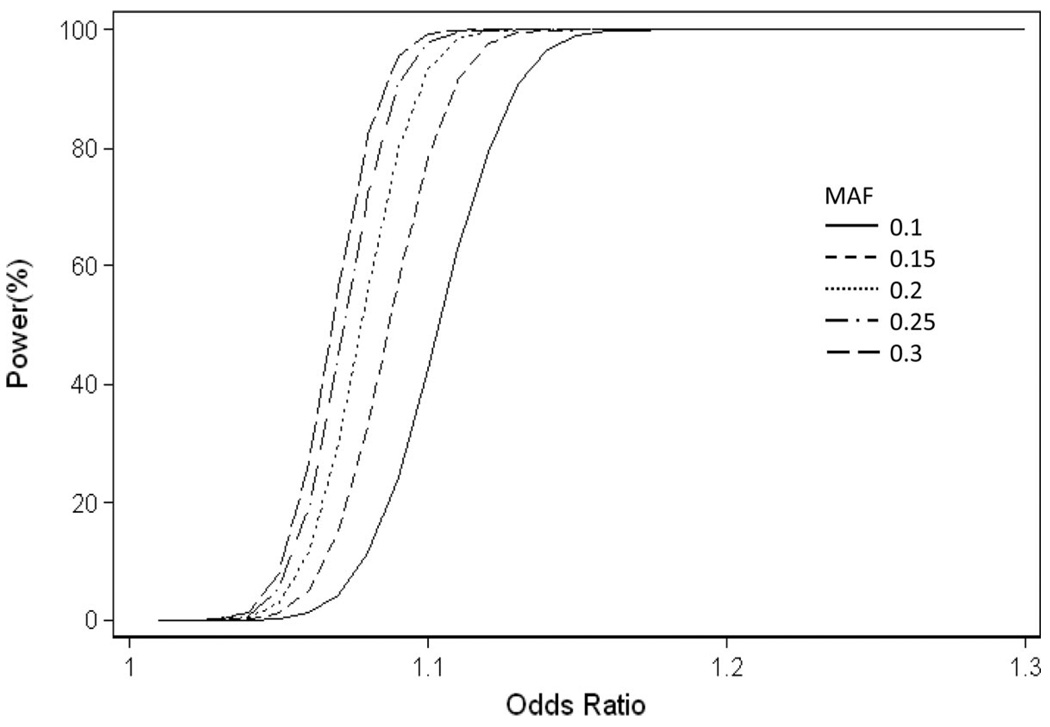
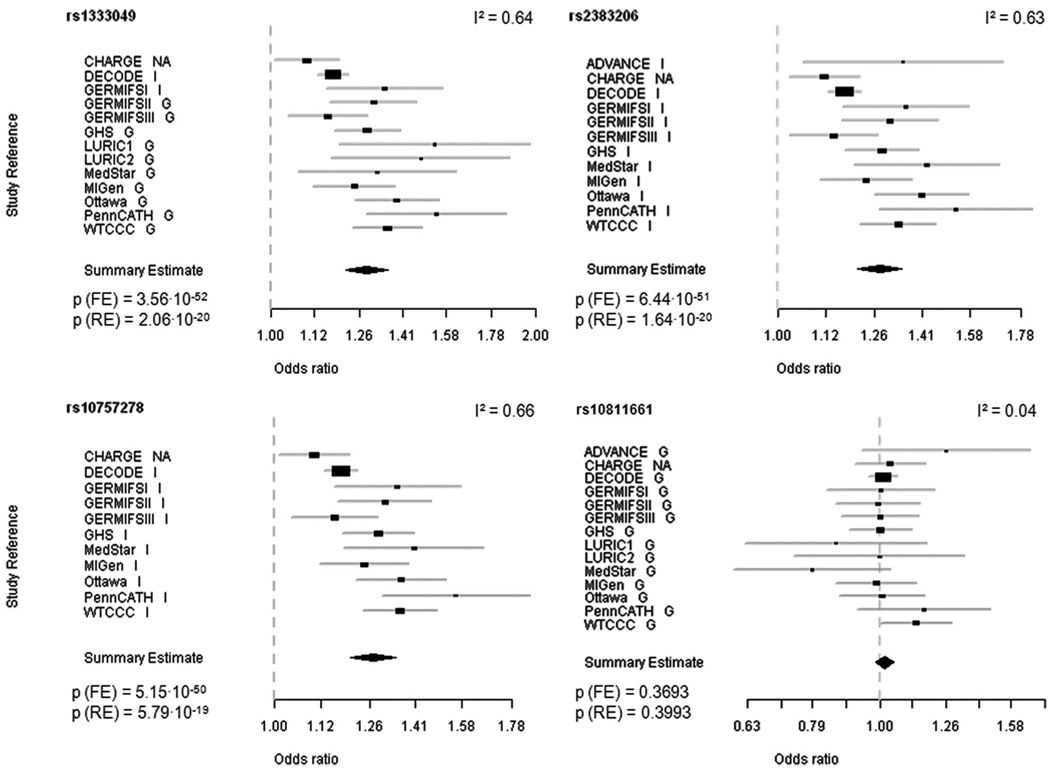
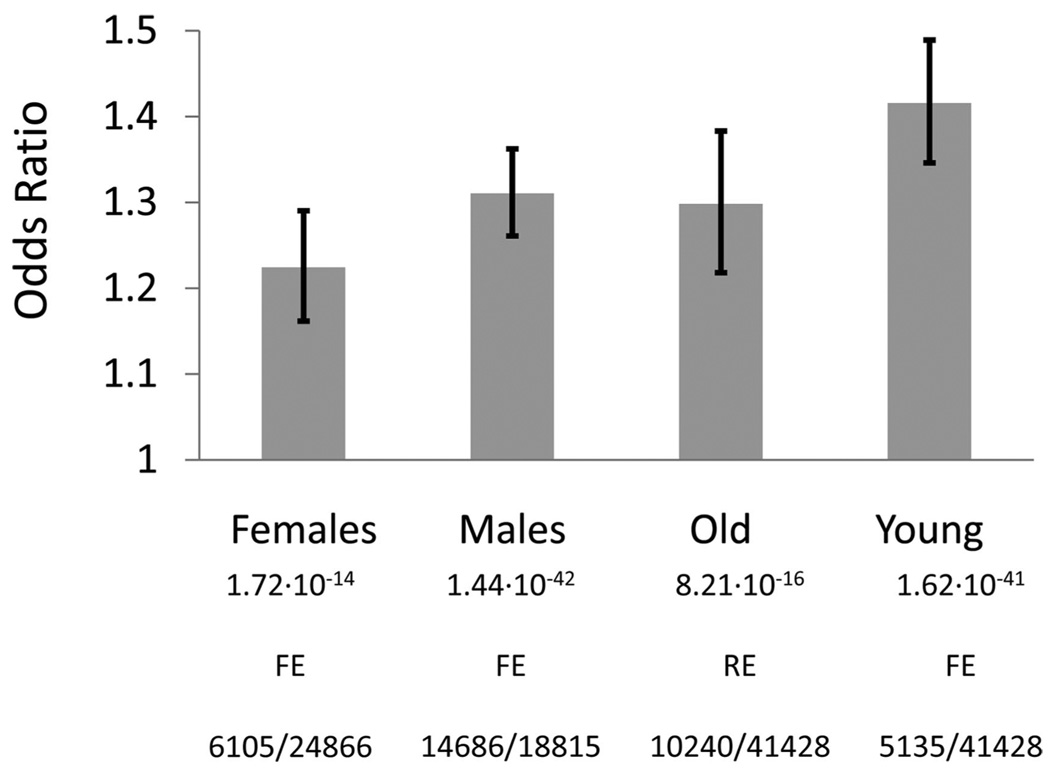
Michael Preuss
Michael Preuss
1Institut für Medizinische Biometrie und Statistik (M.P., I.R.K., A.S., A.Z.) and Medizinische Klinik II (M.P., J.E., H.S.), Universität zu Lübeck, Lübeck, Germany; Department of Health Sciences (J.R.T, C.P.N.), University of Leicester, Leicester, UK; Hudson Alpha Institute (D.A.), Huntsville, Ala; Department of Medicine (T.L.A., T.Q.), Stanford University School of Medicine, Stanford, Calif; Medizinische Klinik und Poliklinik (S.B.), Johannes-Gutenberg Universität Mainz, Universitätsmedizin, Mainz, Germany; University of Texas Health Science Center (E.B.), Human Genetics Center and Institute of Molecular Medicine, Houston, Tex; The John & Jennifer Ruddy Canadian Cardiovascular Genetics Center (L.C., R.M., R.R., A.F.R.S., G.W.), University of Ottawa, Ottawa, Ontario, Canada; Department of Biostatistics and Epidemiology (A.C.), Boston University, Boston, Mass; National Heart, Lung, and Blood Institute (A.C., C.J.O.), Framingham Heart Study, Framingham, Mass; LIGHT Research Institute (A.S.H.), Faculty of Medicine and Health, University of Leeds, Leeds, UK; The Blavatnik School of Computer Science and the Department of Molecular Microbiology and Biotechnology (E.H.), Tel-Aviv University, Tel-Aviv, Israel; International Computer Science Institute (E.H.), Berkeley, Calif; Klinik und Poliklinik für Innere Medizin II (C.H.), Universität Regensburg, Regensburg, Germany; deCODE Genetics (H.H., G.T., U.T.), 101 Reykjavik, Iceland; Science Center (R.L.), Tampere University Hospital, Tampere, Finland; Biostatistics and Epidemiology (M.L.), University of Pennsylvania, Philadelphia, Pa; Synlab Center of Laboratory Diagnostics Heidelberg (W.M.), Heidelberg, Germany; Clinical Institute of Medicine and Chemical Laboratory Diagnostics (W.M.), Medical University of Graz, Graz, Austria; Institute of Public Health, Social and Preventive Medicine (W.M.), Faculty of Medicine Mannheim, University of Heidelberg, Germany; Cardiovascular Research Center and Cardiology Division (K.M., S.K.), Massachusetts General Hospital, Boston, Mass; Center for Human Genetic Research (K.M., B.F.V., S.K.), Massachusetts General Hospital, Boston, Mass; Program in Medical and Population Genetics (K.M., B.F.V., S.K.), Broad Institute of Harvard and Massachusetts Institute of Technology, Cambridge, Mass; Cardiovascular Research Institute (M.S.B., S.E.E.), MedStar Research Institute, Washington Hospital Center, Washington, DC; Cardiology Division (C.J.O.), Massachusetts General Hospital, Boston, Mass; The Institute for Translational Medicine and Therapeutics (D.J.R., M.P.R.), School of Medicine, University of Pennsylvania, Philadelphia, Pa; The Cardiovascular Institute (D.J.R., M.P.R.), University of Pennsylvania, Philadelphia, Pa; University of Iceland (U.T.), Faculty of Medicine, 101 Reykjavik, Iceland; Department of Medicine (B.F.V.), Harvard Medical School, Boston, Mass; Department of Cardiovascular Sciences (N.J.S.), University of Leicester, Glenfield Hospital, Leicester, UK.
Dipl-Inform Med
1,
Inke R König
Inke R König
1Institut für Medizinische Biometrie und Statistik (M.P., I.R.K., A.S., A.Z.) and Medizinische Klinik II (M.P., J.E., H.S.), Universität zu Lübeck, Lübeck, Germany; Department of Health Sciences (J.R.T, C.P.N.), University of Leicester, Leicester, UK; Hudson Alpha Institute (D.A.), Huntsville, Ala; Department of Medicine (T.L.A., T.Q.), Stanford University School of Medicine, Stanford, Calif; Medizinische Klinik und Poliklinik (S.B.), Johannes-Gutenberg Universität Mainz, Universitätsmedizin, Mainz, Germany; University of Texas Health Science Center (E.B.), Human Genetics Center and Institute of Molecular Medicine, Houston, Tex; The John & Jennifer Ruddy Canadian Cardiovascular Genetics Center (L.C., R.M., R.R., A.F.R.S., G.W.), University of Ottawa, Ottawa, Ontario, Canada; Department of Biostatistics and Epidemiology (A.C.), Boston University, Boston, Mass; National Heart, Lung, and Blood Institute (A.C., C.J.O.), Framingham Heart Study, Framingham, Mass; LIGHT Research Institute (A.S.H.), Faculty of Medicine and Health, University of Leeds, Leeds, UK; The Blavatnik School of Computer Science and the Department of Molecular Microbiology and Biotechnology (E.H.), Tel-Aviv University, Tel-Aviv, Israel; International Computer Science Institute (E.H.), Berkeley, Calif; Klinik und Poliklinik für Innere Medizin II (C.H.), Universität Regensburg, Regensburg, Germany; deCODE Genetics (H.H., G.T., U.T.), 101 Reykjavik, Iceland; Science Center (R.L.), Tampere University Hospital, Tampere, Finland; Biostatistics and Epidemiology (M.L.), University of Pennsylvania, Philadelphia, Pa; Synlab Center of Laboratory Diagnostics Heidelberg (W.M.), Heidelberg, Germany; Clinical Institute of Medicine and Chemical Laboratory Diagnostics (W.M.), Medical University of Graz, Graz, Austria; Institute of Public Health, Social and Preventive Medicine (W.M.), Faculty of Medicine Mannheim, University of Heidelberg, Germany; Cardiovascular Research Center and Cardiology Division (K.M., S.K.), Massachusetts General Hospital, Boston, Mass; Center for Human Genetic Research (K.M., B.F.V., S.K.), Massachusetts General Hospital, Boston, Mass; Program in Medical and Population Genetics (K.M., B.F.V., S.K.), Broad Institute of Harvard and Massachusetts Institute of Technology, Cambridge, Mass; Cardiovascular Research Institute (M.S.B., S.E.E.), MedStar Research Institute, Washington Hospital Center, Washington, DC; Cardiology Division (C.J.O.), Massachusetts General Hospital, Boston, Mass; The Institute for Translational Medicine and Therapeutics (D.J.R., M.P.R.), School of Medicine, University of Pennsylvania, Philadelphia, Pa; The Cardiovascular Institute (D.J.R., M.P.R.), University of Pennsylvania, Philadelphia, Pa; University of Iceland (U.T.), Faculty of Medicine, 101 Reykjavik, Iceland; Department of Medicine (B.F.V.), Harvard Medical School, Boston, Mass; Department of Cardiovascular Sciences (N.J.S.), University of Leicester, Glenfield Hospital, Leicester, UK.
Prof Dr rer biol hum
1,
John R Thompson
John R Thompson, PhD
1Institut für Medizinische Biometrie und Statistik (M.P., I.R.K., A.S., A.Z.) and Medizinische Klinik II (M.P., J.E., H.S.), Universität zu Lübeck, Lübeck, Germany; Department of Health Sciences (J.R.T, C.P.N.), University of Leicester, Leicester, UK; Hudson Alpha Institute (D.A.), Huntsville, Ala; Department of Medicine (T.L.A., T.Q.), Stanford University School of Medicine, Stanford, Calif; Medizinische Klinik und Poliklinik (S.B.), Johannes-Gutenberg Universität Mainz, Universitätsmedizin, Mainz, Germany; University of Texas Health Science Center (E.B.), Human Genetics Center and Institute of Molecular Medicine, Houston, Tex; The John & Jennifer Ruddy Canadian Cardiovascular Genetics Center (L.C., R.M., R.R., A.F.R.S., G.W.), University of Ottawa, Ottawa, Ontario, Canada; Department of Biostatistics and Epidemiology (A.C.), Boston University, Boston, Mass; National Heart, Lung, and Blood Institute (A.C., C.J.O.), Framingham Heart Study, Framingham, Mass; LIGHT Research Institute (A.S.H.), Faculty of Medicine and Health, University of Leeds, Leeds, UK; The Blavatnik School of Computer Science and the Department of Molecular Microbiology and Biotechnology (E.H.), Tel-Aviv University, Tel-Aviv, Israel; International Computer Science Institute (E.H.), Berkeley, Calif; Klinik und Poliklinik für Innere Medizin II (C.H.), Universität Regensburg, Regensburg, Germany; deCODE Genetics (H.H., G.T., U.T.), 101 Reykjavik, Iceland; Science Center (R.L.), Tampere University Hospital, Tampere, Finland; Biostatistics and Epidemiology (M.L.), University of Pennsylvania, Philadelphia, Pa; Synlab Center of Laboratory Diagnostics Heidelberg (W.M.), Heidelberg, Germany; Clinical Institute of Medicine and Chemical Laboratory Diagnostics (W.M.), Medical University of Graz, Graz, Austria; Institute of Public Health, Social and Preventive Medicine (W.M.), Faculty of Medicine Mannheim, University of Heidelberg, Germany; Cardiovascular Research Center and Cardiology Division (K.M., S.K.), Massachusetts General Hospital, Boston, Mass; Center for Human Genetic Research (K.M., B.F.V., S.K.), Massachusetts General Hospital, Boston, Mass; Program in Medical and Population Genetics (K.M., B.F.V., S.K.), Broad Institute of Harvard and Massachusetts Institute of Technology, Cambridge, Mass; Cardiovascular Research Institute (M.S.B., S.E.E.), MedStar Research Institute, Washington Hospital Center, Washington, DC; Cardiology Division (C.J.O.), Massachusetts General Hospital, Boston, Mass; The Institute for Translational Medicine and Therapeutics (D.J.R., M.P.R.), School of Medicine, University of Pennsylvania, Philadelphia, Pa; The Cardiovascular Institute (D.J.R., M.P.R.), University of Pennsylvania, Philadelphia, Pa; University of Iceland (U.T.), Faculty of Medicine, 101 Reykjavik, Iceland; Department of Medicine (B.F.V.), Harvard Medical School, Boston, Mass; Department of Cardiovascular Sciences (N.J.S.), University of Leicester, Glenfield Hospital, Leicester, UK.
1,
Jeanette Erdmann
Jeanette Erdmann
1Institut für Medizinische Biometrie und Statistik (M.P., I.R.K., A.S., A.Z.) and Medizinische Klinik II (M.P., J.E., H.S.), Universität zu Lübeck, Lübeck, Germany; Department of Health Sciences (J.R.T, C.P.N.), University of Leicester, Leicester, UK; Hudson Alpha Institute (D.A.), Huntsville, Ala; Department of Medicine (T.L.A., T.Q.), Stanford University School of Medicine, Stanford, Calif; Medizinische Klinik und Poliklinik (S.B.), Johannes-Gutenberg Universität Mainz, Universitätsmedizin, Mainz, Germany; University of Texas Health Science Center (E.B.), Human Genetics Center and Institute of Molecular Medicine, Houston, Tex; The John & Jennifer Ruddy Canadian Cardiovascular Genetics Center (L.C., R.M., R.R., A.F.R.S., G.W.), University of Ottawa, Ottawa, Ontario, Canada; Department of Biostatistics and Epidemiology (A.C.), Boston University, Boston, Mass; National Heart, Lung, and Blood Institute (A.C., C.J.O.), Framingham Heart Study, Framingham, Mass; LIGHT Research Institute (A.S.H.), Faculty of Medicine and Health, University of Leeds, Leeds, UK; The Blavatnik School of Computer Science and the Department of Molecular Microbiology and Biotechnology (E.H.), Tel-Aviv University, Tel-Aviv, Israel; International Computer Science Institute (E.H.), Berkeley, Calif; Klinik und Poliklinik für Innere Medizin II (C.H.), Universität Regensburg, Regensburg, Germany; deCODE Genetics (H.H., G.T., U.T.), 101 Reykjavik, Iceland; Science Center (R.L.), Tampere University Hospital, Tampere, Finland; Biostatistics and Epidemiology (M.L.), University of Pennsylvania, Philadelphia, Pa; Synlab Center of Laboratory Diagnostics Heidelberg (W.M.), Heidelberg, Germany; Clinical Institute of Medicine and Chemical Laboratory Diagnostics (W.M.), Medical University of Graz, Graz, Austria; Institute of Public Health, Social and Preventive Medicine (W.M.), Faculty of Medicine Mannheim, University of Heidelberg, Germany; Cardiovascular Research Center and Cardiology Division (K.M., S.K.), Massachusetts General Hospital, Boston, Mass; Center for Human Genetic Research (K.M., B.F.V., S.K.), Massachusetts General Hospital, Boston, Mass; Program in Medical and Population Genetics (K.M., B.F.V., S.K.), Broad Institute of Harvard and Massachusetts Institute of Technology, Cambridge, Mass; Cardiovascular Research Institute (M.S.B., S.E.E.), MedStar Research Institute, Washington Hospital Center, Washington, DC; Cardiology Division (C.J.O.), Massachusetts General Hospital, Boston, Mass; The Institute for Translational Medicine and Therapeutics (D.J.R., M.P.R.), School of Medicine, University of Pennsylvania, Philadelphia, Pa; The Cardiovascular Institute (D.J.R., M.P.R.), University of Pennsylvania, Philadelphia, Pa; University of Iceland (U.T.), Faculty of Medicine, 101 Reykjavik, Iceland; Department of Medicine (B.F.V.), Harvard Medical School, Boston, Mass; Department of Cardiovascular Sciences (N.J.S.), University of Leicester, Glenfield Hospital, Leicester, UK.
Prof Dr rer nat
1,
Devin Absher
Devin Absher, PhD
1Institut für Medizinische Biometrie und Statistik (M.P., I.R.K., A.S., A.Z.) and Medizinische Klinik II (M.P., J.E., H.S.), Universität zu Lübeck, Lübeck, Germany; Department of Health Sciences (J.R.T, C.P.N.), University of Leicester, Leicester, UK; Hudson Alpha Institute (D.A.), Huntsville, Ala; Department of Medicine (T.L.A., T.Q.), Stanford University School of Medicine, Stanford, Calif; Medizinische Klinik und Poliklinik (S.B.), Johannes-Gutenberg Universität Mainz, Universitätsmedizin, Mainz, Germany; University of Texas Health Science Center (E.B.), Human Genetics Center and Institute of Molecular Medicine, Houston, Tex; The John & Jennifer Ruddy Canadian Cardiovascular Genetics Center (L.C., R.M., R.R., A.F.R.S., G.W.), University of Ottawa, Ottawa, Ontario, Canada; Department of Biostatistics and Epidemiology (A.C.), Boston University, Boston, Mass; National Heart, Lung, and Blood Institute (A.C., C.J.O.), Framingham Heart Study, Framingham, Mass; LIGHT Research Institute (A.S.H.), Faculty of Medicine and Health, University of Leeds, Leeds, UK; The Blavatnik School of Computer Science and the Department of Molecular Microbiology and Biotechnology (E.H.), Tel-Aviv University, Tel-Aviv, Israel; International Computer Science Institute (E.H.), Berkeley, Calif; Klinik und Poliklinik für Innere Medizin II (C.H.), Universität Regensburg, Regensburg, Germany; deCODE Genetics (H.H., G.T., U.T.), 101 Reykjavik, Iceland; Science Center (R.L.), Tampere University Hospital, Tampere, Finland; Biostatistics and Epidemiology (M.L.), University of Pennsylvania, Philadelphia, Pa; Synlab Center of Laboratory Diagnostics Heidelberg (W.M.), Heidelberg, Germany; Clinical Institute of Medicine and Chemical Laboratory Diagnostics (W.M.), Medical University of Graz, Graz, Austria; Institute of Public Health, Social and Preventive Medicine (W.M.), Faculty of Medicine Mannheim, University of Heidelberg, Germany; Cardiovascular Research Center and Cardiology Division (K.M., S.K.), Massachusetts General Hospital, Boston, Mass; Center for Human Genetic Research (K.M., B.F.V., S.K.), Massachusetts General Hospital, Boston, Mass; Program in Medical and Population Genetics (K.M., B.F.V., S.K.), Broad Institute of Harvard and Massachusetts Institute of Technology, Cambridge, Mass; Cardiovascular Research Institute (M.S.B., S.E.E.), MedStar Research Institute, Washington Hospital Center, Washington, DC; Cardiology Division (C.J.O.), Massachusetts General Hospital, Boston, Mass; The Institute for Translational Medicine and Therapeutics (D.J.R., M.P.R.), School of Medicine, University of Pennsylvania, Philadelphia, Pa; The Cardiovascular Institute (D.J.R., M.P.R.), University of Pennsylvania, Philadelphia, Pa; University of Iceland (U.T.), Faculty of Medicine, 101 Reykjavik, Iceland; Department of Medicine (B.F.V.), Harvard Medical School, Boston, Mass; Department of Cardiovascular Sciences (N.J.S.), University of Leicester, Glenfield Hospital, Leicester, UK.
1,
Themistocles L Assimes
Themistocles L Assimes, MD, PhD
1Institut für Medizinische Biometrie und Statistik (M.P., I.R.K., A.S., A.Z.) and Medizinische Klinik II (M.P., J.E., H.S.), Universität zu Lübeck, Lübeck, Germany; Department of Health Sciences (J.R.T, C.P.N.), University of Leicester, Leicester, UK; Hudson Alpha Institute (D.A.), Huntsville, Ala; Department of Medicine (T.L.A., T.Q.), Stanford University School of Medicine, Stanford, Calif; Medizinische Klinik und Poliklinik (S.B.), Johannes-Gutenberg Universität Mainz, Universitätsmedizin, Mainz, Germany; University of Texas Health Science Center (E.B.), Human Genetics Center and Institute of Molecular Medicine, Houston, Tex; The John & Jennifer Ruddy Canadian Cardiovascular Genetics Center (L.C., R.M., R.R., A.F.R.S., G.W.), University of Ottawa, Ottawa, Ontario, Canada; Department of Biostatistics and Epidemiology (A.C.), Boston University, Boston, Mass; National Heart, Lung, and Blood Institute (A.C., C.J.O.), Framingham Heart Study, Framingham, Mass; LIGHT Research Institute (A.S.H.), Faculty of Medicine and Health, University of Leeds, Leeds, UK; The Blavatnik School of Computer Science and the Department of Molecular Microbiology and Biotechnology (E.H.), Tel-Aviv University, Tel-Aviv, Israel; International Computer Science Institute (E.H.), Berkeley, Calif; Klinik und Poliklinik für Innere Medizin II (C.H.), Universität Regensburg, Regensburg, Germany; deCODE Genetics (H.H., G.T., U.T.), 101 Reykjavik, Iceland; Science Center (R.L.), Tampere University Hospital, Tampere, Finland; Biostatistics and Epidemiology (M.L.), University of Pennsylvania, Philadelphia, Pa; Synlab Center of Laboratory Diagnostics Heidelberg (W.M.), Heidelberg, Germany; Clinical Institute of Medicine and Chemical Laboratory Diagnostics (W.M.), Medical University of Graz, Graz, Austria; Institute of Public Health, Social and Preventive Medicine (W.M.), Faculty of Medicine Mannheim, University of Heidelberg, Germany; Cardiovascular Research Center and Cardiology Division (K.M., S.K.), Massachusetts General Hospital, Boston, Mass; Center for Human Genetic Research (K.M., B.F.V., S.K.), Massachusetts General Hospital, Boston, Mass; Program in Medical and Population Genetics (K.M., B.F.V., S.K.), Broad Institute of Harvard and Massachusetts Institute of Technology, Cambridge, Mass; Cardiovascular Research Institute (M.S.B., S.E.E.), MedStar Research Institute, Washington Hospital Center, Washington, DC; Cardiology Division (C.J.O.), Massachusetts General Hospital, Boston, Mass; The Institute for Translational Medicine and Therapeutics (D.J.R., M.P.R.), School of Medicine, University of Pennsylvania, Philadelphia, Pa; The Cardiovascular Institute (D.J.R., M.P.R.), University of Pennsylvania, Philadelphia, Pa; University of Iceland (U.T.), Faculty of Medicine, 101 Reykjavik, Iceland; Department of Medicine (B.F.V.), Harvard Medical School, Boston, Mass; Department of Cardiovascular Sciences (N.J.S.), University of Leicester, Glenfield Hospital, Leicester, UK.
1,
Stefan Blankenberg
Stefan Blankenberg
1Institut für Medizinische Biometrie und Statistik (M.P., I.R.K., A.S., A.Z.) and Medizinische Klinik II (M.P., J.E., H.S.), Universität zu Lübeck, Lübeck, Germany; Department of Health Sciences (J.R.T, C.P.N.), University of Leicester, Leicester, UK; Hudson Alpha Institute (D.A.), Huntsville, Ala; Department of Medicine (T.L.A., T.Q.), Stanford University School of Medicine, Stanford, Calif; Medizinische Klinik und Poliklinik (S.B.), Johannes-Gutenberg Universität Mainz, Universitätsmedizin, Mainz, Germany; University of Texas Health Science Center (E.B.), Human Genetics Center and Institute of Molecular Medicine, Houston, Tex; The John & Jennifer Ruddy Canadian Cardiovascular Genetics Center (L.C., R.M., R.R., A.F.R.S., G.W.), University of Ottawa, Ottawa, Ontario, Canada; Department of Biostatistics and Epidemiology (A.C.), Boston University, Boston, Mass; National Heart, Lung, and Blood Institute (A.C., C.J.O.), Framingham Heart Study, Framingham, Mass; LIGHT Research Institute (A.S.H.), Faculty of Medicine and Health, University of Leeds, Leeds, UK; The Blavatnik School of Computer Science and the Department of Molecular Microbiology and Biotechnology (E.H.), Tel-Aviv University, Tel-Aviv, Israel; International Computer Science Institute (E.H.), Berkeley, Calif; Klinik und Poliklinik für Innere Medizin II (C.H.), Universität Regensburg, Regensburg, Germany; deCODE Genetics (H.H., G.T., U.T.), 101 Reykjavik, Iceland; Science Center (R.L.), Tampere University Hospital, Tampere, Finland; Biostatistics and Epidemiology (M.L.), University of Pennsylvania, Philadelphia, Pa; Synlab Center of Laboratory Diagnostics Heidelberg (W.M.), Heidelberg, Germany; Clinical Institute of Medicine and Chemical Laboratory Diagnostics (W.M.), Medical University of Graz, Graz, Austria; Institute of Public Health, Social and Preventive Medicine (W.M.), Faculty of Medicine Mannheim, University of Heidelberg, Germany; Cardiovascular Research Center and Cardiology Division (K.M., S.K.), Massachusetts General Hospital, Boston, Mass; Center for Human Genetic Research (K.M., B.F.V., S.K.), Massachusetts General Hospital, Boston, Mass; Program in Medical and Population Genetics (K.M., B.F.V., S.K.), Broad Institute of Harvard and Massachusetts Institute of Technology, Cambridge, Mass; Cardiovascular Research Institute (M.S.B., S.E.E.), MedStar Research Institute, Washington Hospital Center, Washington, DC; Cardiology Division (C.J.O.), Massachusetts General Hospital, Boston, Mass; The Institute for Translational Medicine and Therapeutics (D.J.R., M.P.R.), School of Medicine, University of Pennsylvania, Philadelphia, Pa; The Cardiovascular Institute (D.J.R., M.P.R.), University of Pennsylvania, Philadelphia, Pa; University of Iceland (U.T.), Faculty of Medicine, 101 Reykjavik, Iceland; Department of Medicine (B.F.V.), Harvard Medical School, Boston, Mass; Department of Cardiovascular Sciences (N.J.S.), University of Leicester, Glenfield Hospital, Leicester, UK.
Prof Dr med
1,
Eric Boerwinkle
Eric Boerwinkle, PhD
1Institut für Medizinische Biometrie und Statistik (M.P., I.R.K., A.S., A.Z.) and Medizinische Klinik II (M.P., J.E., H.S.), Universität zu Lübeck, Lübeck, Germany; Department of Health Sciences (J.R.T, C.P.N.), University of Leicester, Leicester, UK; Hudson Alpha Institute (D.A.), Huntsville, Ala; Department of Medicine (T.L.A., T.Q.), Stanford University School of Medicine, Stanford, Calif; Medizinische Klinik und Poliklinik (S.B.), Johannes-Gutenberg Universität Mainz, Universitätsmedizin, Mainz, Germany; University of Texas Health Science Center (E.B.), Human Genetics Center and Institute of Molecular Medicine, Houston, Tex; The John & Jennifer Ruddy Canadian Cardiovascular Genetics Center (L.C., R.M., R.R., A.F.R.S., G.W.), University of Ottawa, Ottawa, Ontario, Canada; Department of Biostatistics and Epidemiology (A.C.), Boston University, Boston, Mass; National Heart, Lung, and Blood Institute (A.C., C.J.O.), Framingham Heart Study, Framingham, Mass; LIGHT Research Institute (A.S.H.), Faculty of Medicine and Health, University of Leeds, Leeds, UK; The Blavatnik School of Computer Science and the Department of Molecular Microbiology and Biotechnology (E.H.), Tel-Aviv University, Tel-Aviv, Israel; International Computer Science Institute (E.H.), Berkeley, Calif; Klinik und Poliklinik für Innere Medizin II (C.H.), Universität Regensburg, Regensburg, Germany; deCODE Genetics (H.H., G.T., U.T.), 101 Reykjavik, Iceland; Science Center (R.L.), Tampere University Hospital, Tampere, Finland; Biostatistics and Epidemiology (M.L.), University of Pennsylvania, Philadelphia, Pa; Synlab Center of Laboratory Diagnostics Heidelberg (W.M.), Heidelberg, Germany; Clinical Institute of Medicine and Chemical Laboratory Diagnostics (W.M.), Medical University of Graz, Graz, Austria; Institute of Public Health, Social and Preventive Medicine (W.M.), Faculty of Medicine Mannheim, University of Heidelberg, Germany; Cardiovascular Research Center and Cardiology Division (K.M., S.K.), Massachusetts General Hospital, Boston, Mass; Center for Human Genetic Research (K.M., B.F.V., S.K.), Massachusetts General Hospital, Boston, Mass; Program in Medical and Population Genetics (K.M., B.F.V., S.K.), Broad Institute of Harvard and Massachusetts Institute of Technology, Cambridge, Mass; Cardiovascular Research Institute (M.S.B., S.E.E.), MedStar Research Institute, Washington Hospital Center, Washington, DC; Cardiology Division (C.J.O.), Massachusetts General Hospital, Boston, Mass; The Institute for Translational Medicine and Therapeutics (D.J.R., M.P.R.), School of Medicine, University of Pennsylvania, Philadelphia, Pa; The Cardiovascular Institute (D.J.R., M.P.R.), University of Pennsylvania, Philadelphia, Pa; University of Iceland (U.T.), Faculty of Medicine, 101 Reykjavik, Iceland; Department of Medicine (B.F.V.), Harvard Medical School, Boston, Mass; Department of Cardiovascular Sciences (N.J.S.), University of Leicester, Glenfield Hospital, Leicester, UK.
1,
Li Chen
Li Chen, MSc
1Institut für Medizinische Biometrie und Statistik (M.P., I.R.K., A.S., A.Z.) and Medizinische Klinik II (M.P., J.E., H.S.), Universität zu Lübeck, Lübeck, Germany; Department of Health Sciences (J.R.T, C.P.N.), University of Leicester, Leicester, UK; Hudson Alpha Institute (D.A.), Huntsville, Ala; Department of Medicine (T.L.A., T.Q.), Stanford University School of Medicine, Stanford, Calif; Medizinische Klinik und Poliklinik (S.B.), Johannes-Gutenberg Universität Mainz, Universitätsmedizin, Mainz, Germany; University of Texas Health Science Center (E.B.), Human Genetics Center and Institute of Molecular Medicine, Houston, Tex; The John & Jennifer Ruddy Canadian Cardiovascular Genetics Center (L.C., R.M., R.R., A.F.R.S., G.W.), University of Ottawa, Ottawa, Ontario, Canada; Department of Biostatistics and Epidemiology (A.C.), Boston University, Boston, Mass; National Heart, Lung, and Blood Institute (A.C., C.J.O.), Framingham Heart Study, Framingham, Mass; LIGHT Research Institute (A.S.H.), Faculty of Medicine and Health, University of Leeds, Leeds, UK; The Blavatnik School of Computer Science and the Department of Molecular Microbiology and Biotechnology (E.H.), Tel-Aviv University, Tel-Aviv, Israel; International Computer Science Institute (E.H.), Berkeley, Calif; Klinik und Poliklinik für Innere Medizin II (C.H.), Universität Regensburg, Regensburg, Germany; deCODE Genetics (H.H., G.T., U.T.), 101 Reykjavik, Iceland; Science Center (R.L.), Tampere University Hospital, Tampere, Finland; Biostatistics and Epidemiology (M.L.), University of Pennsylvania, Philadelphia, Pa; Synlab Center of Laboratory Diagnostics Heidelberg (W.M.), Heidelberg, Germany; Clinical Institute of Medicine and Chemical Laboratory Diagnostics (W.M.), Medical University of Graz, Graz, Austria; Institute of Public Health, Social and Preventive Medicine (W.M.), Faculty of Medicine Mannheim, University of Heidelberg, Germany; Cardiovascular Research Center and Cardiology Division (K.M., S.K.), Massachusetts General Hospital, Boston, Mass; Center for Human Genetic Research (K.M., B.F.V., S.K.), Massachusetts General Hospital, Boston, Mass; Program in Medical and Population Genetics (K.M., B.F.V., S.K.), Broad Institute of Harvard and Massachusetts Institute of Technology, Cambridge, Mass; Cardiovascular Research Institute (M.S.B., S.E.E.), MedStar Research Institute, Washington Hospital Center, Washington, DC; Cardiology Division (C.J.O.), Massachusetts General Hospital, Boston, Mass; The Institute for Translational Medicine and Therapeutics (D.J.R., M.P.R.), School of Medicine, University of Pennsylvania, Philadelphia, Pa; The Cardiovascular Institute (D.J.R., M.P.R.), University of Pennsylvania, Philadelphia, Pa; University of Iceland (U.T.), Faculty of Medicine, 101 Reykjavik, Iceland; Department of Medicine (B.F.V.), Harvard Medical School, Boston, Mass; Department of Cardiovascular Sciences (N.J.S.), University of Leicester, Glenfield Hospital, Leicester, UK.
1,
L Adrienne Cupples
L Adrienne Cupples, PhD
1Institut für Medizinische Biometrie und Statistik (M.P., I.R.K., A.S., A.Z.) and Medizinische Klinik II (M.P., J.E., H.S.), Universität zu Lübeck, Lübeck, Germany; Department of Health Sciences (J.R.T, C.P.N.), University of Leicester, Leicester, UK; Hudson Alpha Institute (D.A.), Huntsville, Ala; Department of Medicine (T.L.A., T.Q.), Stanford University School of Medicine, Stanford, Calif; Medizinische Klinik und Poliklinik (S.B.), Johannes-Gutenberg Universität Mainz, Universitätsmedizin, Mainz, Germany; University of Texas Health Science Center (E.B.), Human Genetics Center and Institute of Molecular Medicine, Houston, Tex; The John & Jennifer Ruddy Canadian Cardiovascular Genetics Center (L.C., R.M., R.R., A.F.R.S., G.W.), University of Ottawa, Ottawa, Ontario, Canada; Department of Biostatistics and Epidemiology (A.C.), Boston University, Boston, Mass; National Heart, Lung, and Blood Institute (A.C., C.J.O.), Framingham Heart Study, Framingham, Mass; LIGHT Research Institute (A.S.H.), Faculty of Medicine and Health, University of Leeds, Leeds, UK; The Blavatnik School of Computer Science and the Department of Molecular Microbiology and Biotechnology (E.H.), Tel-Aviv University, Tel-Aviv, Israel; International Computer Science Institute (E.H.), Berkeley, Calif; Klinik und Poliklinik für Innere Medizin II (C.H.), Universität Regensburg, Regensburg, Germany; deCODE Genetics (H.H., G.T., U.T.), 101 Reykjavik, Iceland; Science Center (R.L.), Tampere University Hospital, Tampere, Finland; Biostatistics and Epidemiology (M.L.), University of Pennsylvania, Philadelphia, Pa; Synlab Center of Laboratory Diagnostics Heidelberg (W.M.), Heidelberg, Germany; Clinical Institute of Medicine and Chemical Laboratory Diagnostics (W.M.), Medical University of Graz, Graz, Austria; Institute of Public Health, Social and Preventive Medicine (W.M.), Faculty of Medicine Mannheim, University of Heidelberg, Germany; Cardiovascular Research Center and Cardiology Division (K.M., S.K.), Massachusetts General Hospital, Boston, Mass; Center for Human Genetic Research (K.M., B.F.V., S.K.), Massachusetts General Hospital, Boston, Mass; Program in Medical and Population Genetics (K.M., B.F.V., S.K.), Broad Institute of Harvard and Massachusetts Institute of Technology, Cambridge, Mass; Cardiovascular Research Institute (M.S.B., S.E.E.), MedStar Research Institute, Washington Hospital Center, Washington, DC; Cardiology Division (C.J.O.), Massachusetts General Hospital, Boston, Mass; The Institute for Translational Medicine and Therapeutics (D.J.R., M.P.R.), School of Medicine, University of Pennsylvania, Philadelphia, Pa; The Cardiovascular Institute (D.J.R., M.P.R.), University of Pennsylvania, Philadelphia, Pa; University of Iceland (U.T.), Faculty of Medicine, 101 Reykjavik, Iceland; Department of Medicine (B.F.V.), Harvard Medical School, Boston, Mass; Department of Cardiovascular Sciences (N.J.S.), University of Leicester, Glenfield Hospital, Leicester, UK.
1,
Alistair S Hall
Alistair S Hall, MD, ChB, PhD, FRCP
1Institut für Medizinische Biometrie und Statistik (M.P., I.R.K., A.S., A.Z.) and Medizinische Klinik II (M.P., J.E., H.S.), Universität zu Lübeck, Lübeck, Germany; Department of Health Sciences (J.R.T, C.P.N.), University of Leicester, Leicester, UK; Hudson Alpha Institute (D.A.), Huntsville, Ala; Department of Medicine (T.L.A., T.Q.), Stanford University School of Medicine, Stanford, Calif; Medizinische Klinik und Poliklinik (S.B.), Johannes-Gutenberg Universität Mainz, Universitätsmedizin, Mainz, Germany; University of Texas Health Science Center (E.B.), Human Genetics Center and Institute of Molecular Medicine, Houston, Tex; The John & Jennifer Ruddy Canadian Cardiovascular Genetics Center (L.C., R.M., R.R., A.F.R.S., G.W.), University of Ottawa, Ottawa, Ontario, Canada; Department of Biostatistics and Epidemiology (A.C.), Boston University, Boston, Mass; National Heart, Lung, and Blood Institute (A.C., C.J.O.), Framingham Heart Study, Framingham, Mass; LIGHT Research Institute (A.S.H.), Faculty of Medicine and Health, University of Leeds, Leeds, UK; The Blavatnik School of Computer Science and the Department of Molecular Microbiology and Biotechnology (E.H.), Tel-Aviv University, Tel-Aviv, Israel; International Computer Science Institute (E.H.), Berkeley, Calif; Klinik und Poliklinik für Innere Medizin II (C.H.), Universität Regensburg, Regensburg, Germany; deCODE Genetics (H.H., G.T., U.T.), 101 Reykjavik, Iceland; Science Center (R.L.), Tampere University Hospital, Tampere, Finland; Biostatistics and Epidemiology (M.L.), University of Pennsylvania, Philadelphia, Pa; Synlab Center of Laboratory Diagnostics Heidelberg (W.M.), Heidelberg, Germany; Clinical Institute of Medicine and Chemical Laboratory Diagnostics (W.M.), Medical University of Graz, Graz, Austria; Institute of Public Health, Social and Preventive Medicine (W.M.), Faculty of Medicine Mannheim, University of Heidelberg, Germany; Cardiovascular Research Center and Cardiology Division (K.M., S.K.), Massachusetts General Hospital, Boston, Mass; Center for Human Genetic Research (K.M., B.F.V., S.K.), Massachusetts General Hospital, Boston, Mass; Program in Medical and Population Genetics (K.M., B.F.V., S.K.), Broad Institute of Harvard and Massachusetts Institute of Technology, Cambridge, Mass; Cardiovascular Research Institute (M.S.B., S.E.E.), MedStar Research Institute, Washington Hospital Center, Washington, DC; Cardiology Division (C.J.O.), Massachusetts General Hospital, Boston, Mass; The Institute for Translational Medicine and Therapeutics (D.J.R., M.P.R.), School of Medicine, University of Pennsylvania, Philadelphia, Pa; The Cardiovascular Institute (D.J.R., M.P.R.), University of Pennsylvania, Philadelphia, Pa; University of Iceland (U.T.), Faculty of Medicine, 101 Reykjavik, Iceland; Department of Medicine (B.F.V.), Harvard Medical School, Boston, Mass; Department of Cardiovascular Sciences (N.J.S.), University of Leicester, Glenfield Hospital, Leicester, UK.
1,
Eran Halperin
Eran Halperin, PhD
1Institut für Medizinische Biometrie und Statistik (M.P., I.R.K., A.S., A.Z.) and Medizinische Klinik II (M.P., J.E., H.S.), Universität zu Lübeck, Lübeck, Germany; Department of Health Sciences (J.R.T, C.P.N.), University of Leicester, Leicester, UK; Hudson Alpha Institute (D.A.), Huntsville, Ala; Department of Medicine (T.L.A., T.Q.), Stanford University School of Medicine, Stanford, Calif; Medizinische Klinik und Poliklinik (S.B.), Johannes-Gutenberg Universität Mainz, Universitätsmedizin, Mainz, Germany; University of Texas Health Science Center (E.B.), Human Genetics Center and Institute of Molecular Medicine, Houston, Tex; The John & Jennifer Ruddy Canadian Cardiovascular Genetics Center (L.C., R.M., R.R., A.F.R.S., G.W.), University of Ottawa, Ottawa, Ontario, Canada; Department of Biostatistics and Epidemiology (A.C.), Boston University, Boston, Mass; National Heart, Lung, and Blood Institute (A.C., C.J.O.), Framingham Heart Study, Framingham, Mass; LIGHT Research Institute (A.S.H.), Faculty of Medicine and Health, University of Leeds, Leeds, UK; The Blavatnik School of Computer Science and the Department of Molecular Microbiology and Biotechnology (E.H.), Tel-Aviv University, Tel-Aviv, Israel; International Computer Science Institute (E.H.), Berkeley, Calif; Klinik und Poliklinik für Innere Medizin II (C.H.), Universität Regensburg, Regensburg, Germany; deCODE Genetics (H.H., G.T., U.T.), 101 Reykjavik, Iceland; Science Center (R.L.), Tampere University Hospital, Tampere, Finland; Biostatistics and Epidemiology (M.L.), University of Pennsylvania, Philadelphia, Pa; Synlab Center of Laboratory Diagnostics Heidelberg (W.M.), Heidelberg, Germany; Clinical Institute of Medicine and Chemical Laboratory Diagnostics (W.M.), Medical University of Graz, Graz, Austria; Institute of Public Health, Social and Preventive Medicine (W.M.), Faculty of Medicine Mannheim, University of Heidelberg, Germany; Cardiovascular Research Center and Cardiology Division (K.M., S.K.), Massachusetts General Hospital, Boston, Mass; Center for Human Genetic Research (K.M., B.F.V., S.K.), Massachusetts General Hospital, Boston, Mass; Program in Medical and Population Genetics (K.M., B.F.V., S.K.), Broad Institute of Harvard and Massachusetts Institute of Technology, Cambridge, Mass; Cardiovascular Research Institute (M.S.B., S.E.E.), MedStar Research Institute, Washington Hospital Center, Washington, DC; Cardiology Division (C.J.O.), Massachusetts General Hospital, Boston, Mass; The Institute for Translational Medicine and Therapeutics (D.J.R., M.P.R.), School of Medicine, University of Pennsylvania, Philadelphia, Pa; The Cardiovascular Institute (D.J.R., M.P.R.), University of Pennsylvania, Philadelphia, Pa; University of Iceland (U.T.), Faculty of Medicine, 101 Reykjavik, Iceland; Department of Medicine (B.F.V.), Harvard Medical School, Boston, Mass; Department of Cardiovascular Sciences (N.J.S.), University of Leicester, Glenfield Hospital, Leicester, UK.
1,
Christian Hengstenberg
Christian Hengstenberg
1Institut für Medizinische Biometrie und Statistik (M.P., I.R.K., A.S., A.Z.) and Medizinische Klinik II (M.P., J.E., H.S.), Universität zu Lübeck, Lübeck, Germany; Department of Health Sciences (J.R.T, C.P.N.), University of Leicester, Leicester, UK; Hudson Alpha Institute (D.A.), Huntsville, Ala; Department of Medicine (T.L.A., T.Q.), Stanford University School of Medicine, Stanford, Calif; Medizinische Klinik und Poliklinik (S.B.), Johannes-Gutenberg Universität Mainz, Universitätsmedizin, Mainz, Germany; University of Texas Health Science Center (E.B.), Human Genetics Center and Institute of Molecular Medicine, Houston, Tex; The John & Jennifer Ruddy Canadian Cardiovascular Genetics Center (L.C., R.M., R.R., A.F.R.S., G.W.), University of Ottawa, Ottawa, Ontario, Canada; Department of Biostatistics and Epidemiology (A.C.), Boston University, Boston, Mass; National Heart, Lung, and Blood Institute (A.C., C.J.O.), Framingham Heart Study, Framingham, Mass; LIGHT Research Institute (A.S.H.), Faculty of Medicine and Health, University of Leeds, Leeds, UK; The Blavatnik School of Computer Science and the Department of Molecular Microbiology and Biotechnology (E.H.), Tel-Aviv University, Tel-Aviv, Israel; International Computer Science Institute (E.H.), Berkeley, Calif; Klinik und Poliklinik für Innere Medizin II (C.H.), Universität Regensburg, Regensburg, Germany; deCODE Genetics (H.H., G.T., U.T.), 101 Reykjavik, Iceland; Science Center (R.L.), Tampere University Hospital, Tampere, Finland; Biostatistics and Epidemiology (M.L.), University of Pennsylvania, Philadelphia, Pa; Synlab Center of Laboratory Diagnostics Heidelberg (W.M.), Heidelberg, Germany; Clinical Institute of Medicine and Chemical Laboratory Diagnostics (W.M.), Medical University of Graz, Graz, Austria; Institute of Public Health, Social and Preventive Medicine (W.M.), Faculty of Medicine Mannheim, University of Heidelberg, Germany; Cardiovascular Research Center and Cardiology Division (K.M., S.K.), Massachusetts General Hospital, Boston, Mass; Center for Human Genetic Research (K.M., B.F.V., S.K.), Massachusetts General Hospital, Boston, Mass; Program in Medical and Population Genetics (K.M., B.F.V., S.K.), Broad Institute of Harvard and Massachusetts Institute of Technology, Cambridge, Mass; Cardiovascular Research Institute (M.S.B., S.E.E.), MedStar Research Institute, Washington Hospital Center, Washington, DC; Cardiology Division (C.J.O.), Massachusetts General Hospital, Boston, Mass; The Institute for Translational Medicine and Therapeutics (D.J.R., M.P.R.), School of Medicine, University of Pennsylvania, Philadelphia, Pa; The Cardiovascular Institute (D.J.R., M.P.R.), University of Pennsylvania, Philadelphia, Pa; University of Iceland (U.T.), Faculty of Medicine, 101 Reykjavik, Iceland; Department of Medicine (B.F.V.), Harvard Medical School, Boston, Mass; Department of Cardiovascular Sciences (N.J.S.), University of Leicester, Glenfield Hospital, Leicester, UK.
Prof Dr med
1,
Hilma Holm
Hilma Holm, MD
1Institut für Medizinische Biometrie und Statistik (M.P., I.R.K., A.S., A.Z.) and Medizinische Klinik II (M.P., J.E., H.S.), Universität zu Lübeck, Lübeck, Germany; Department of Health Sciences (J.R.T, C.P.N.), University of Leicester, Leicester, UK; Hudson Alpha Institute (D.A.), Huntsville, Ala; Department of Medicine (T.L.A., T.Q.), Stanford University School of Medicine, Stanford, Calif; Medizinische Klinik und Poliklinik (S.B.), Johannes-Gutenberg Universität Mainz, Universitätsmedizin, Mainz, Germany; University of Texas Health Science Center (E.B.), Human Genetics Center and Institute of Molecular Medicine, Houston, Tex; The John & Jennifer Ruddy Canadian Cardiovascular Genetics Center (L.C., R.M., R.R., A.F.R.S., G.W.), University of Ottawa, Ottawa, Ontario, Canada; Department of Biostatistics and Epidemiology (A.C.), Boston University, Boston, Mass; National Heart, Lung, and Blood Institute (A.C., C.J.O.), Framingham Heart Study, Framingham, Mass; LIGHT Research Institute (A.S.H.), Faculty of Medicine and Health, University of Leeds, Leeds, UK; The Blavatnik School of Computer Science and the Department of Molecular Microbiology and Biotechnology (E.H.), Tel-Aviv University, Tel-Aviv, Israel; International Computer Science Institute (E.H.), Berkeley, Calif; Klinik und Poliklinik für Innere Medizin II (C.H.), Universität Regensburg, Regensburg, Germany; deCODE Genetics (H.H., G.T., U.T.), 101 Reykjavik, Iceland; Science Center (R.L.), Tampere University Hospital, Tampere, Finland; Biostatistics and Epidemiology (M.L.), University of Pennsylvania, Philadelphia, Pa; Synlab Center of Laboratory Diagnostics Heidelberg (W.M.), Heidelberg, Germany; Clinical Institute of Medicine and Chemical Laboratory Diagnostics (W.M.), Medical University of Graz, Graz, Austria; Institute of Public Health, Social and Preventive Medicine (W.M.), Faculty of Medicine Mannheim, University of Heidelberg, Germany; Cardiovascular Research Center and Cardiology Division (K.M., S.K.), Massachusetts General Hospital, Boston, Mass; Center for Human Genetic Research (K.M., B.F.V., S.K.), Massachusetts General Hospital, Boston, Mass; Program in Medical and Population Genetics (K.M., B.F.V., S.K.), Broad Institute of Harvard and Massachusetts Institute of Technology, Cambridge, Mass; Cardiovascular Research Institute (M.S.B., S.E.E.), MedStar Research Institute, Washington Hospital Center, Washington, DC; Cardiology Division (C.J.O.), Massachusetts General Hospital, Boston, Mass; The Institute for Translational Medicine and Therapeutics (D.J.R., M.P.R.), School of Medicine, University of Pennsylvania, Philadelphia, Pa; The Cardiovascular Institute (D.J.R., M.P.R.), University of Pennsylvania, Philadelphia, Pa; University of Iceland (U.T.), Faculty of Medicine, 101 Reykjavik, Iceland; Department of Medicine (B.F.V.), Harvard Medical School, Boston, Mass; Department of Cardiovascular Sciences (N.J.S.), University of Leicester, Glenfield Hospital, Leicester, UK.
1,
Reijo Laaksonen
Reijo Laaksonen, MD, PhD
1Institut für Medizinische Biometrie und Statistik (M.P., I.R.K., A.S., A.Z.) and Medizinische Klinik II (M.P., J.E., H.S.), Universität zu Lübeck, Lübeck, Germany; Department of Health Sciences (J.R.T, C.P.N.), University of Leicester, Leicester, UK; Hudson Alpha Institute (D.A.), Huntsville, Ala; Department of Medicine (T.L.A., T.Q.), Stanford University School of Medicine, Stanford, Calif; Medizinische Klinik und Poliklinik (S.B.), Johannes-Gutenberg Universität Mainz, Universitätsmedizin, Mainz, Germany; University of Texas Health Science Center (E.B.), Human Genetics Center and Institute of Molecular Medicine, Houston, Tex; The John & Jennifer Ruddy Canadian Cardiovascular Genetics Center (L.C., R.M., R.R., A.F.R.S., G.W.), University of Ottawa, Ottawa, Ontario, Canada; Department of Biostatistics and Epidemiology (A.C.), Boston University, Boston, Mass; National Heart, Lung, and Blood Institute (A.C., C.J.O.), Framingham Heart Study, Framingham, Mass; LIGHT Research Institute (A.S.H.), Faculty of Medicine and Health, University of Leeds, Leeds, UK; The Blavatnik School of Computer Science and the Department of Molecular Microbiology and Biotechnology (E.H.), Tel-Aviv University, Tel-Aviv, Israel; International Computer Science Institute (E.H.), Berkeley, Calif; Klinik und Poliklinik für Innere Medizin II (C.H.), Universität Regensburg, Regensburg, Germany; deCODE Genetics (H.H., G.T., U.T.), 101 Reykjavik, Iceland; Science Center (R.L.), Tampere University Hospital, Tampere, Finland; Biostatistics and Epidemiology (M.L.), University of Pennsylvania, Philadelphia, Pa; Synlab Center of Laboratory Diagnostics Heidelberg (W.M.), Heidelberg, Germany; Clinical Institute of Medicine and Chemical Laboratory Diagnostics (W.M.), Medical University of Graz, Graz, Austria; Institute of Public Health, Social and Preventive Medicine (W.M.), Faculty of Medicine Mannheim, University of Heidelberg, Germany; Cardiovascular Research Center and Cardiology Division (K.M., S.K.), Massachusetts General Hospital, Boston, Mass; Center for Human Genetic Research (K.M., B.F.V., S.K.), Massachusetts General Hospital, Boston, Mass; Program in Medical and Population Genetics (K.M., B.F.V., S.K.), Broad Institute of Harvard and Massachusetts Institute of Technology, Cambridge, Mass; Cardiovascular Research Institute (M.S.B., S.E.E.), MedStar Research Institute, Washington Hospital Center, Washington, DC; Cardiology Division (C.J.O.), Massachusetts General Hospital, Boston, Mass; The Institute for Translational Medicine and Therapeutics (D.J.R., M.P.R.), School of Medicine, University of Pennsylvania, Philadelphia, Pa; The Cardiovascular Institute (D.J.R., M.P.R.), University of Pennsylvania, Philadelphia, Pa; University of Iceland (U.T.), Faculty of Medicine, 101 Reykjavik, Iceland; Department of Medicine (B.F.V.), Harvard Medical School, Boston, Mass; Department of Cardiovascular Sciences (N.J.S.), University of Leicester, Glenfield Hospital, Leicester, UK.
1,
Mingyao Li
Mingyao Li, PhD
1Institut für Medizinische Biometrie und Statistik (M.P., I.R.K., A.S., A.Z.) and Medizinische Klinik II (M.P., J.E., H.S.), Universität zu Lübeck, Lübeck, Germany; Department of Health Sciences (J.R.T, C.P.N.), University of Leicester, Leicester, UK; Hudson Alpha Institute (D.A.), Huntsville, Ala; Department of Medicine (T.L.A., T.Q.), Stanford University School of Medicine, Stanford, Calif; Medizinische Klinik und Poliklinik (S.B.), Johannes-Gutenberg Universität Mainz, Universitätsmedizin, Mainz, Germany; University of Texas Health Science Center (E.B.), Human Genetics Center and Institute of Molecular Medicine, Houston, Tex; The John & Jennifer Ruddy Canadian Cardiovascular Genetics Center (L.C., R.M., R.R., A.F.R.S., G.W.), University of Ottawa, Ottawa, Ontario, Canada; Department of Biostatistics and Epidemiology (A.C.), Boston University, Boston, Mass; National Heart, Lung, and Blood Institute (A.C., C.J.O.), Framingham Heart Study, Framingham, Mass; LIGHT Research Institute (A.S.H.), Faculty of Medicine and Health, University of Leeds, Leeds, UK; The Blavatnik School of Computer Science and the Department of Molecular Microbiology and Biotechnology (E.H.), Tel-Aviv University, Tel-Aviv, Israel; International Computer Science Institute (E.H.), Berkeley, Calif; Klinik und Poliklinik für Innere Medizin II (C.H.), Universität Regensburg, Regensburg, Germany; deCODE Genetics (H.H., G.T., U.T.), 101 Reykjavik, Iceland; Science Center (R.L.), Tampere University Hospital, Tampere, Finland; Biostatistics and Epidemiology (M.L.), University of Pennsylvania, Philadelphia, Pa; Synlab Center of Laboratory Diagnostics Heidelberg (W.M.), Heidelberg, Germany; Clinical Institute of Medicine and Chemical Laboratory Diagnostics (W.M.), Medical University of Graz, Graz, Austria; Institute of Public Health, Social and Preventive Medicine (W.M.), Faculty of Medicine Mannheim, University of Heidelberg, Germany; Cardiovascular Research Center and Cardiology Division (K.M., S.K.), Massachusetts General Hospital, Boston, Mass; Center for Human Genetic Research (K.M., B.F.V., S.K.), Massachusetts General Hospital, Boston, Mass; Program in Medical and Population Genetics (K.M., B.F.V., S.K.), Broad Institute of Harvard and Massachusetts Institute of Technology, Cambridge, Mass; Cardiovascular Research Institute (M.S.B., S.E.E.), MedStar Research Institute, Washington Hospital Center, Washington, DC; Cardiology Division (C.J.O.), Massachusetts General Hospital, Boston, Mass; The Institute for Translational Medicine and Therapeutics (D.J.R., M.P.R.), School of Medicine, University of Pennsylvania, Philadelphia, Pa; The Cardiovascular Institute (D.J.R., M.P.R.), University of Pennsylvania, Philadelphia, Pa; University of Iceland (U.T.), Faculty of Medicine, 101 Reykjavik, Iceland; Department of Medicine (B.F.V.), Harvard Medical School, Boston, Mass; Department of Cardiovascular Sciences (N.J.S.), University of Leicester, Glenfield Hospital, Leicester, UK.
1,
Winfried März
Winfried März
1Institut für Medizinische Biometrie und Statistik (M.P., I.R.K., A.S., A.Z.) and Medizinische Klinik II (M.P., J.E., H.S.), Universität zu Lübeck, Lübeck, Germany; Department of Health Sciences (J.R.T, C.P.N.), University of Leicester, Leicester, UK; Hudson Alpha Institute (D.A.), Huntsville, Ala; Department of Medicine (T.L.A., T.Q.), Stanford University School of Medicine, Stanford, Calif; Medizinische Klinik und Poliklinik (S.B.), Johannes-Gutenberg Universität Mainz, Universitätsmedizin, Mainz, Germany; University of Texas Health Science Center (E.B.), Human Genetics Center and Institute of Molecular Medicine, Houston, Tex; The John & Jennifer Ruddy Canadian Cardiovascular Genetics Center (L.C., R.M., R.R., A.F.R.S., G.W.), University of Ottawa, Ottawa, Ontario, Canada; Department of Biostatistics and Epidemiology (A.C.), Boston University, Boston, Mass; National Heart, Lung, and Blood Institute (A.C., C.J.O.), Framingham Heart Study, Framingham, Mass; LIGHT Research Institute (A.S.H.), Faculty of Medicine and Health, University of Leeds, Leeds, UK; The Blavatnik School of Computer Science and the Department of Molecular Microbiology and Biotechnology (E.H.), Tel-Aviv University, Tel-Aviv, Israel; International Computer Science Institute (E.H.), Berkeley, Calif; Klinik und Poliklinik für Innere Medizin II (C.H.), Universität Regensburg, Regensburg, Germany; deCODE Genetics (H.H., G.T., U.T.), 101 Reykjavik, Iceland; Science Center (R.L.), Tampere University Hospital, Tampere, Finland; Biostatistics and Epidemiology (M.L.), University of Pennsylvania, Philadelphia, Pa; Synlab Center of Laboratory Diagnostics Heidelberg (W.M.), Heidelberg, Germany; Clinical Institute of Medicine and Chemical Laboratory Diagnostics (W.M.), Medical University of Graz, Graz, Austria; Institute of Public Health, Social and Preventive Medicine (W.M.), Faculty of Medicine Mannheim, University of Heidelberg, Germany; Cardiovascular Research Center and Cardiology Division (K.M., S.K.), Massachusetts General Hospital, Boston, Mass; Center for Human Genetic Research (K.M., B.F.V., S.K.), Massachusetts General Hospital, Boston, Mass; Program in Medical and Population Genetics (K.M., B.F.V., S.K.), Broad Institute of Harvard and Massachusetts Institute of Technology, Cambridge, Mass; Cardiovascular Research Institute (M.S.B., S.E.E.), MedStar Research Institute, Washington Hospital Center, Washington, DC; Cardiology Division (C.J.O.), Massachusetts General Hospital, Boston, Mass; The Institute for Translational Medicine and Therapeutics (D.J.R., M.P.R.), School of Medicine, University of Pennsylvania, Philadelphia, Pa; The Cardiovascular Institute (D.J.R., M.P.R.), University of Pennsylvania, Philadelphia, Pa; University of Iceland (U.T.), Faculty of Medicine, 101 Reykjavik, Iceland; Department of Medicine (B.F.V.), Harvard Medical School, Boston, Mass; Department of Cardiovascular Sciences (N.J.S.), University of Leicester, Glenfield Hospital, Leicester, UK.
Prof Dr med
1,
Ruth McPherson
Ruth McPherson, MD, PhD, FRCPC
1Institut für Medizinische Biometrie und Statistik (M.P., I.R.K., A.S., A.Z.) and Medizinische Klinik II (M.P., J.E., H.S.), Universität zu Lübeck, Lübeck, Germany; Department of Health Sciences (J.R.T, C.P.N.), University of Leicester, Leicester, UK; Hudson Alpha Institute (D.A.), Huntsville, Ala; Department of Medicine (T.L.A., T.Q.), Stanford University School of Medicine, Stanford, Calif; Medizinische Klinik und Poliklinik (S.B.), Johannes-Gutenberg Universität Mainz, Universitätsmedizin, Mainz, Germany; University of Texas Health Science Center (E.B.), Human Genetics Center and Institute of Molecular Medicine, Houston, Tex; The John & Jennifer Ruddy Canadian Cardiovascular Genetics Center (L.C., R.M., R.R., A.F.R.S., G.W.), University of Ottawa, Ottawa, Ontario, Canada; Department of Biostatistics and Epidemiology (A.C.), Boston University, Boston, Mass; National Heart, Lung, and Blood Institute (A.C., C.J.O.), Framingham Heart Study, Framingham, Mass; LIGHT Research Institute (A.S.H.), Faculty of Medicine and Health, University of Leeds, Leeds, UK; The Blavatnik School of Computer Science and the Department of Molecular Microbiology and Biotechnology (E.H.), Tel-Aviv University, Tel-Aviv, Israel; International Computer Science Institute (E.H.), Berkeley, Calif; Klinik und Poliklinik für Innere Medizin II (C.H.), Universität Regensburg, Regensburg, Germany; deCODE Genetics (H.H., G.T., U.T.), 101 Reykjavik, Iceland; Science Center (R.L.), Tampere University Hospital, Tampere, Finland; Biostatistics and Epidemiology (M.L.), University of Pennsylvania, Philadelphia, Pa; Synlab Center of Laboratory Diagnostics Heidelberg (W.M.), Heidelberg, Germany; Clinical Institute of Medicine and Chemical Laboratory Diagnostics (W.M.), Medical University of Graz, Graz, Austria; Institute of Public Health, Social and Preventive Medicine (W.M.), Faculty of Medicine Mannheim, University of Heidelberg, Germany; Cardiovascular Research Center and Cardiology Division (K.M., S.K.), Massachusetts General Hospital, Boston, Mass; Center for Human Genetic Research (K.M., B.F.V., S.K.), Massachusetts General Hospital, Boston, Mass; Program in Medical and Population Genetics (K.M., B.F.V., S.K.), Broad Institute of Harvard and Massachusetts Institute of Technology, Cambridge, Mass; Cardiovascular Research Institute (M.S.B., S.E.E.), MedStar Research Institute, Washington Hospital Center, Washington, DC; Cardiology Division (C.J.O.), Massachusetts General Hospital, Boston, Mass; The Institute for Translational Medicine and Therapeutics (D.J.R., M.P.R.), School of Medicine, University of Pennsylvania, Philadelphia, Pa; The Cardiovascular Institute (D.J.R., M.P.R.), University of Pennsylvania, Philadelphia, Pa; University of Iceland (U.T.), Faculty of Medicine, 101 Reykjavik, Iceland; Department of Medicine (B.F.V.), Harvard Medical School, Boston, Mass; Department of Cardiovascular Sciences (N.J.S.), University of Leicester, Glenfield Hospital, Leicester, UK.
1,
Kiran Musunuru
Kiran Musunuru, MD, PhD, MPH
1Institut für Medizinische Biometrie und Statistik (M.P., I.R.K., A.S., A.Z.) and Medizinische Klinik II (M.P., J.E., H.S.), Universität zu Lübeck, Lübeck, Germany; Department of Health Sciences (J.R.T, C.P.N.), University of Leicester, Leicester, UK; Hudson Alpha Institute (D.A.), Huntsville, Ala; Department of Medicine (T.L.A., T.Q.), Stanford University School of Medicine, Stanford, Calif; Medizinische Klinik und Poliklinik (S.B.), Johannes-Gutenberg Universität Mainz, Universitätsmedizin, Mainz, Germany; University of Texas Health Science Center (E.B.), Human Genetics Center and Institute of Molecular Medicine, Houston, Tex; The John & Jennifer Ruddy Canadian Cardiovascular Genetics Center (L.C., R.M., R.R., A.F.R.S., G.W.), University of Ottawa, Ottawa, Ontario, Canada; Department of Biostatistics and Epidemiology (A.C.), Boston University, Boston, Mass; National Heart, Lung, and Blood Institute (A.C., C.J.O.), Framingham Heart Study, Framingham, Mass; LIGHT Research Institute (A.S.H.), Faculty of Medicine and Health, University of Leeds, Leeds, UK; The Blavatnik School of Computer Science and the Department of Molecular Microbiology and Biotechnology (E.H.), Tel-Aviv University, Tel-Aviv, Israel; International Computer Science Institute (E.H.), Berkeley, Calif; Klinik und Poliklinik für Innere Medizin II (C.H.), Universität Regensburg, Regensburg, Germany; deCODE Genetics (H.H., G.T., U.T.), 101 Reykjavik, Iceland; Science Center (R.L.), Tampere University Hospital, Tampere, Finland; Biostatistics and Epidemiology (M.L.), University of Pennsylvania, Philadelphia, Pa; Synlab Center of Laboratory Diagnostics Heidelberg (W.M.), Heidelberg, Germany; Clinical Institute of Medicine and Chemical Laboratory Diagnostics (W.M.), Medical University of Graz, Graz, Austria; Institute of Public Health, Social and Preventive Medicine (W.M.), Faculty of Medicine Mannheim, University of Heidelberg, Germany; Cardiovascular Research Center and Cardiology Division (K.M., S.K.), Massachusetts General Hospital, Boston, Mass; Center for Human Genetic Research (K.M., B.F.V., S.K.), Massachusetts General Hospital, Boston, Mass; Program in Medical and Population Genetics (K.M., B.F.V., S.K.), Broad Institute of Harvard and Massachusetts Institute of Technology, Cambridge, Mass; Cardiovascular Research Institute (M.S.B., S.E.E.), MedStar Research Institute, Washington Hospital Center, Washington, DC; Cardiology Division (C.J.O.), Massachusetts General Hospital, Boston, Mass; The Institute for Translational Medicine and Therapeutics (D.J.R., M.P.R.), School of Medicine, University of Pennsylvania, Philadelphia, Pa; The Cardiovascular Institute (D.J.R., M.P.R.), University of Pennsylvania, Philadelphia, Pa; University of Iceland (U.T.), Faculty of Medicine, 101 Reykjavik, Iceland; Department of Medicine (B.F.V.), Harvard Medical School, Boston, Mass; Department of Cardiovascular Sciences (N.J.S.), University of Leicester, Glenfield Hospital, Leicester, UK.
1,
Christopher P Nelson
Christopher P Nelson, MSc, PhD
1Institut für Medizinische Biometrie und Statistik (M.P., I.R.K., A.S., A.Z.) and Medizinische Klinik II (M.P., J.E., H.S.), Universität zu Lübeck, Lübeck, Germany; Department of Health Sciences (J.R.T, C.P.N.), University of Leicester, Leicester, UK; Hudson Alpha Institute (D.A.), Huntsville, Ala; Department of Medicine (T.L.A., T.Q.), Stanford University School of Medicine, Stanford, Calif; Medizinische Klinik und Poliklinik (S.B.), Johannes-Gutenberg Universität Mainz, Universitätsmedizin, Mainz, Germany; University of Texas Health Science Center (E.B.), Human Genetics Center and Institute of Molecular Medicine, Houston, Tex; The John & Jennifer Ruddy Canadian Cardiovascular Genetics Center (L.C., R.M., R.R., A.F.R.S., G.W.), University of Ottawa, Ottawa, Ontario, Canada; Department of Biostatistics and Epidemiology (A.C.), Boston University, Boston, Mass; National Heart, Lung, and Blood Institute (A.C., C.J.O.), Framingham Heart Study, Framingham, Mass; LIGHT Research Institute (A.S.H.), Faculty of Medicine and Health, University of Leeds, Leeds, UK; The Blavatnik School of Computer Science and the Department of Molecular Microbiology and Biotechnology (E.H.), Tel-Aviv University, Tel-Aviv, Israel; International Computer Science Institute (E.H.), Berkeley, Calif; Klinik und Poliklinik für Innere Medizin II (C.H.), Universität Regensburg, Regensburg, Germany; deCODE Genetics (H.H., G.T., U.T.), 101 Reykjavik, Iceland; Science Center (R.L.), Tampere University Hospital, Tampere, Finland; Biostatistics and Epidemiology (M.L.), University of Pennsylvania, Philadelphia, Pa; Synlab Center of Laboratory Diagnostics Heidelberg (W.M.), Heidelberg, Germany; Clinical Institute of Medicine and Chemical Laboratory Diagnostics (W.M.), Medical University of Graz, Graz, Austria; Institute of Public Health, Social and Preventive Medicine (W.M.), Faculty of Medicine Mannheim, University of Heidelberg, Germany; Cardiovascular Research Center and Cardiology Division (K.M., S.K.), Massachusetts General Hospital, Boston, Mass; Center for Human Genetic Research (K.M., B.F.V., S.K.), Massachusetts General Hospital, Boston, Mass; Program in Medical and Population Genetics (K.M., B.F.V., S.K.), Broad Institute of Harvard and Massachusetts Institute of Technology, Cambridge, Mass; Cardiovascular Research Institute (M.S.B., S.E.E.), MedStar Research Institute, Washington Hospital Center, Washington, DC; Cardiology Division (C.J.O.), Massachusetts General Hospital, Boston, Mass; The Institute for Translational Medicine and Therapeutics (D.J.R., M.P.R.), School of Medicine, University of Pennsylvania, Philadelphia, Pa; The Cardiovascular Institute (D.J.R., M.P.R.), University of Pennsylvania, Philadelphia, Pa; University of Iceland (U.T.), Faculty of Medicine, 101 Reykjavik, Iceland; Department of Medicine (B.F.V.), Harvard Medical School, Boston, Mass; Department of Cardiovascular Sciences (N.J.S.), University of Leicester, Glenfield Hospital, Leicester, UK.
1,
Mary Susan Burnett
Mary Susan Burnett, PhD
1Institut für Medizinische Biometrie und Statistik (M.P., I.R.K., A.S., A.Z.) and Medizinische Klinik II (M.P., J.E., H.S.), Universität zu Lübeck, Lübeck, Germany; Department of Health Sciences (J.R.T, C.P.N.), University of Leicester, Leicester, UK; Hudson Alpha Institute (D.A.), Huntsville, Ala; Department of Medicine (T.L.A., T.Q.), Stanford University School of Medicine, Stanford, Calif; Medizinische Klinik und Poliklinik (S.B.), Johannes-Gutenberg Universität Mainz, Universitätsmedizin, Mainz, Germany; University of Texas Health Science Center (E.B.), Human Genetics Center and Institute of Molecular Medicine, Houston, Tex; The John & Jennifer Ruddy Canadian Cardiovascular Genetics Center (L.C., R.M., R.R., A.F.R.S., G.W.), University of Ottawa, Ottawa, Ontario, Canada; Department of Biostatistics and Epidemiology (A.C.), Boston University, Boston, Mass; National Heart, Lung, and Blood Institute (A.C., C.J.O.), Framingham Heart Study, Framingham, Mass; LIGHT Research Institute (A.S.H.), Faculty of Medicine and Health, University of Leeds, Leeds, UK; The Blavatnik School of Computer Science and the Department of Molecular Microbiology and Biotechnology (E.H.), Tel-Aviv University, Tel-Aviv, Israel; International Computer Science Institute (E.H.), Berkeley, Calif; Klinik und Poliklinik für Innere Medizin II (C.H.), Universität Regensburg, Regensburg, Germany; deCODE Genetics (H.H., G.T., U.T.), 101 Reykjavik, Iceland; Science Center (R.L.), Tampere University Hospital, Tampere, Finland; Biostatistics and Epidemiology (M.L.), University of Pennsylvania, Philadelphia, Pa; Synlab Center of Laboratory Diagnostics Heidelberg (W.M.), Heidelberg, Germany; Clinical Institute of Medicine and Chemical Laboratory Diagnostics (W.M.), Medical University of Graz, Graz, Austria; Institute of Public Health, Social and Preventive Medicine (W.M.), Faculty of Medicine Mannheim, University of Heidelberg, Germany; Cardiovascular Research Center and Cardiology Division (K.M., S.K.), Massachusetts General Hospital, Boston, Mass; Center for Human Genetic Research (K.M., B.F.V., S.K.), Massachusetts General Hospital, Boston, Mass; Program in Medical and Population Genetics (K.M., B.F.V., S.K.), Broad Institute of Harvard and Massachusetts Institute of Technology, Cambridge, Mass; Cardiovascular Research Institute (M.S.B., S.E.E.), MedStar Research Institute, Washington Hospital Center, Washington, DC; Cardiology Division (C.J.O.), Massachusetts General Hospital, Boston, Mass; The Institute for Translational Medicine and Therapeutics (D.J.R., M.P.R.), School of Medicine, University of Pennsylvania, Philadelphia, Pa; The Cardiovascular Institute (D.J.R., M.P.R.), University of Pennsylvania, Philadelphia, Pa; University of Iceland (U.T.), Faculty of Medicine, 101 Reykjavik, Iceland; Department of Medicine (B.F.V.), Harvard Medical School, Boston, Mass; Department of Cardiovascular Sciences (N.J.S.), University of Leicester, Glenfield Hospital, Leicester, UK.
1,
Stephen E Epstein
Stephen E Epstein, MD
1Institut für Medizinische Biometrie und Statistik (M.P., I.R.K., A.S., A.Z.) and Medizinische Klinik II (M.P., J.E., H.S.), Universität zu Lübeck, Lübeck, Germany; Department of Health Sciences (J.R.T, C.P.N.), University of Leicester, Leicester, UK; Hudson Alpha Institute (D.A.), Huntsville, Ala; Department of Medicine (T.L.A., T.Q.), Stanford University School of Medicine, Stanford, Calif; Medizinische Klinik und Poliklinik (S.B.), Johannes-Gutenberg Universität Mainz, Universitätsmedizin, Mainz, Germany; University of Texas Health Science Center (E.B.), Human Genetics Center and Institute of Molecular Medicine, Houston, Tex; The John & Jennifer Ruddy Canadian Cardiovascular Genetics Center (L.C., R.M., R.R., A.F.R.S., G.W.), University of Ottawa, Ottawa, Ontario, Canada; Department of Biostatistics and Epidemiology (A.C.), Boston University, Boston, Mass; National Heart, Lung, and Blood Institute (A.C., C.J.O.), Framingham Heart Study, Framingham, Mass; LIGHT Research Institute (A.S.H.), Faculty of Medicine and Health, University of Leeds, Leeds, UK; The Blavatnik School of Computer Science and the Department of Molecular Microbiology and Biotechnology (E.H.), Tel-Aviv University, Tel-Aviv, Israel; International Computer Science Institute (E.H.), Berkeley, Calif; Klinik und Poliklinik für Innere Medizin II (C.H.), Universität Regensburg, Regensburg, Germany; deCODE Genetics (H.H., G.T., U.T.), 101 Reykjavik, Iceland; Science Center (R.L.), Tampere University Hospital, Tampere, Finland; Biostatistics and Epidemiology (M.L.), University of Pennsylvania, Philadelphia, Pa; Synlab Center of Laboratory Diagnostics Heidelberg (W.M.), Heidelberg, Germany; Clinical Institute of Medicine and Chemical Laboratory Diagnostics (W.M.), Medical University of Graz, Graz, Austria; Institute of Public Health, Social and Preventive Medicine (W.M.), Faculty of Medicine Mannheim, University of Heidelberg, Germany; Cardiovascular Research Center and Cardiology Division (K.M., S.K.), Massachusetts General Hospital, Boston, Mass; Center for Human Genetic Research (K.M., B.F.V., S.K.), Massachusetts General Hospital, Boston, Mass; Program in Medical and Population Genetics (K.M., B.F.V., S.K.), Broad Institute of Harvard and Massachusetts Institute of Technology, Cambridge, Mass; Cardiovascular Research Institute (M.S.B., S.E.E.), MedStar Research Institute, Washington Hospital Center, Washington, DC; Cardiology Division (C.J.O.), Massachusetts General Hospital, Boston, Mass; The Institute for Translational Medicine and Therapeutics (D.J.R., M.P.R.), School of Medicine, University of Pennsylvania, Philadelphia, Pa; The Cardiovascular Institute (D.J.R., M.P.R.), University of Pennsylvania, Philadelphia, Pa; University of Iceland (U.T.), Faculty of Medicine, 101 Reykjavik, Iceland; Department of Medicine (B.F.V.), Harvard Medical School, Boston, Mass; Department of Cardiovascular Sciences (N.J.S.), University of Leicester, Glenfield Hospital, Leicester, UK.
1,
Christopher J O’Donnell
Christopher J O’Donnell, MD, MPH
1Institut für Medizinische Biometrie und Statistik (M.P., I.R.K., A.S., A.Z.) and Medizinische Klinik II (M.P., J.E., H.S.), Universität zu Lübeck, Lübeck, Germany; Department of Health Sciences (J.R.T, C.P.N.), University of Leicester, Leicester, UK; Hudson Alpha Institute (D.A.), Huntsville, Ala; Department of Medicine (T.L.A., T.Q.), Stanford University School of Medicine, Stanford, Calif; Medizinische Klinik und Poliklinik (S.B.), Johannes-Gutenberg Universität Mainz, Universitätsmedizin, Mainz, Germany; University of Texas Health Science Center (E.B.), Human Genetics Center and Institute of Molecular Medicine, Houston, Tex; The John & Jennifer Ruddy Canadian Cardiovascular Genetics Center (L.C., R.M., R.R., A.F.R.S., G.W.), University of Ottawa, Ottawa, Ontario, Canada; Department of Biostatistics and Epidemiology (A.C.), Boston University, Boston, Mass; National Heart, Lung, and Blood Institute (A.C., C.J.O.), Framingham Heart Study, Framingham, Mass; LIGHT Research Institute (A.S.H.), Faculty of Medicine and Health, University of Leeds, Leeds, UK; The Blavatnik School of Computer Science and the Department of Molecular Microbiology and Biotechnology (E.H.), Tel-Aviv University, Tel-Aviv, Israel; International Computer Science Institute (E.H.), Berkeley, Calif; Klinik und Poliklinik für Innere Medizin II (C.H.), Universität Regensburg, Regensburg, Germany; deCODE Genetics (H.H., G.T., U.T.), 101 Reykjavik, Iceland; Science Center (R.L.), Tampere University Hospital, Tampere, Finland; Biostatistics and Epidemiology (M.L.), University of Pennsylvania, Philadelphia, Pa; Synlab Center of Laboratory Diagnostics Heidelberg (W.M.), Heidelberg, Germany; Clinical Institute of Medicine and Chemical Laboratory Diagnostics (W.M.), Medical University of Graz, Graz, Austria; Institute of Public Health, Social and Preventive Medicine (W.M.), Faculty of Medicine Mannheim, University of Heidelberg, Germany; Cardiovascular Research Center and Cardiology Division (K.M., S.K.), Massachusetts General Hospital, Boston, Mass; Center for Human Genetic Research (K.M., B.F.V., S.K.), Massachusetts General Hospital, Boston, Mass; Program in Medical and Population Genetics (K.M., B.F.V., S.K.), Broad Institute of Harvard and Massachusetts Institute of Technology, Cambridge, Mass; Cardiovascular Research Institute (M.S.B., S.E.E.), MedStar Research Institute, Washington Hospital Center, Washington, DC; Cardiology Division (C.J.O.), Massachusetts General Hospital, Boston, Mass; The Institute for Translational Medicine and Therapeutics (D.J.R., M.P.R.), School of Medicine, University of Pennsylvania, Philadelphia, Pa; The Cardiovascular Institute (D.J.R., M.P.R.), University of Pennsylvania, Philadelphia, Pa; University of Iceland (U.T.), Faculty of Medicine, 101 Reykjavik, Iceland; Department of Medicine (B.F.V.), Harvard Medical School, Boston, Mass; Department of Cardiovascular Sciences (N.J.S.), University of Leicester, Glenfield Hospital, Leicester, UK.
1,
Thomas Quertermous
Thomas Quertermous, MD
1Institut für Medizinische Biometrie und Statistik (M.P., I.R.K., A.S., A.Z.) and Medizinische Klinik II (M.P., J.E., H.S.), Universität zu Lübeck, Lübeck, Germany; Department of Health Sciences (J.R.T, C.P.N.), University of Leicester, Leicester, UK; Hudson Alpha Institute (D.A.), Huntsville, Ala; Department of Medicine (T.L.A., T.Q.), Stanford University School of Medicine, Stanford, Calif; Medizinische Klinik und Poliklinik (S.B.), Johannes-Gutenberg Universität Mainz, Universitätsmedizin, Mainz, Germany; University of Texas Health Science Center (E.B.), Human Genetics Center and Institute of Molecular Medicine, Houston, Tex; The John & Jennifer Ruddy Canadian Cardiovascular Genetics Center (L.C., R.M., R.R., A.F.R.S., G.W.), University of Ottawa, Ottawa, Ontario, Canada; Department of Biostatistics and Epidemiology (A.C.), Boston University, Boston, Mass; National Heart, Lung, and Blood Institute (A.C., C.J.O.), Framingham Heart Study, Framingham, Mass; LIGHT Research Institute (A.S.H.), Faculty of Medicine and Health, University of Leeds, Leeds, UK; The Blavatnik School of Computer Science and the Department of Molecular Microbiology and Biotechnology (E.H.), Tel-Aviv University, Tel-Aviv, Israel; International Computer Science Institute (E.H.), Berkeley, Calif; Klinik und Poliklinik für Innere Medizin II (C.H.), Universität Regensburg, Regensburg, Germany; deCODE Genetics (H.H., G.T., U.T.), 101 Reykjavik, Iceland; Science Center (R.L.), Tampere University Hospital, Tampere, Finland; Biostatistics and Epidemiology (M.L.), University of Pennsylvania, Philadelphia, Pa; Synlab Center of Laboratory Diagnostics Heidelberg (W.M.), Heidelberg, Germany; Clinical Institute of Medicine and Chemical Laboratory Diagnostics (W.M.), Medical University of Graz, Graz, Austria; Institute of Public Health, Social and Preventive Medicine (W.M.), Faculty of Medicine Mannheim, University of Heidelberg, Germany; Cardiovascular Research Center and Cardiology Division (K.M., S.K.), Massachusetts General Hospital, Boston, Mass; Center for Human Genetic Research (K.M., B.F.V., S.K.), Massachusetts General Hospital, Boston, Mass; Program in Medical and Population Genetics (K.M., B.F.V., S.K.), Broad Institute of Harvard and Massachusetts Institute of Technology, Cambridge, Mass; Cardiovascular Research Institute (M.S.B., S.E.E.), MedStar Research Institute, Washington Hospital Center, Washington, DC; Cardiology Division (C.J.O.), Massachusetts General Hospital, Boston, Mass; The Institute for Translational Medicine and Therapeutics (D.J.R., M.P.R.), School of Medicine, University of Pennsylvania, Philadelphia, Pa; The Cardiovascular Institute (D.J.R., M.P.R.), University of Pennsylvania, Philadelphia, Pa; University of Iceland (U.T.), Faculty of Medicine, 101 Reykjavik, Iceland; Department of Medicine (B.F.V.), Harvard Medical School, Boston, Mass; Department of Cardiovascular Sciences (N.J.S.), University of Leicester, Glenfield Hospital, Leicester, UK.
1,
Daniel J Rader
Daniel J Rader, MD
1Institut für Medizinische Biometrie und Statistik (M.P., I.R.K., A.S., A.Z.) and Medizinische Klinik II (M.P., J.E., H.S.), Universität zu Lübeck, Lübeck, Germany; Department of Health Sciences (J.R.T, C.P.N.), University of Leicester, Leicester, UK; Hudson Alpha Institute (D.A.), Huntsville, Ala; Department of Medicine (T.L.A., T.Q.), Stanford University School of Medicine, Stanford, Calif; Medizinische Klinik und Poliklinik (S.B.), Johannes-Gutenberg Universität Mainz, Universitätsmedizin, Mainz, Germany; University of Texas Health Science Center (E.B.), Human Genetics Center and Institute of Molecular Medicine, Houston, Tex; The John & Jennifer Ruddy Canadian Cardiovascular Genetics Center (L.C., R.M., R.R., A.F.R.S., G.W.), University of Ottawa, Ottawa, Ontario, Canada; Department of Biostatistics and Epidemiology (A.C.), Boston University, Boston, Mass; National Heart, Lung, and Blood Institute (A.C., C.J.O.), Framingham Heart Study, Framingham, Mass; LIGHT Research Institute (A.S.H.), Faculty of Medicine and Health, University of Leeds, Leeds, UK; The Blavatnik School of Computer Science and the Department of Molecular Microbiology and Biotechnology (E.H.), Tel-Aviv University, Tel-Aviv, Israel; International Computer Science Institute (E.H.), Berkeley, Calif; Klinik und Poliklinik für Innere Medizin II (C.H.), Universität Regensburg, Regensburg, Germany; deCODE Genetics (H.H., G.T., U.T.), 101 Reykjavik, Iceland; Science Center (R.L.), Tampere University Hospital, Tampere, Finland; Biostatistics and Epidemiology (M.L.), University of Pennsylvania, Philadelphia, Pa; Synlab Center of Laboratory Diagnostics Heidelberg (W.M.), Heidelberg, Germany; Clinical Institute of Medicine and Chemical Laboratory Diagnostics (W.M.), Medical University of Graz, Graz, Austria; Institute of Public Health, Social and Preventive Medicine (W.M.), Faculty of Medicine Mannheim, University of Heidelberg, Germany; Cardiovascular Research Center and Cardiology Division (K.M., S.K.), Massachusetts General Hospital, Boston, Mass; Center for Human Genetic Research (K.M., B.F.V., S.K.), Massachusetts General Hospital, Boston, Mass; Program in Medical and Population Genetics (K.M., B.F.V., S.K.), Broad Institute of Harvard and Massachusetts Institute of Technology, Cambridge, Mass; Cardiovascular Research Institute (M.S.B., S.E.E.), MedStar Research Institute, Washington Hospital Center, Washington, DC; Cardiology Division (C.J.O.), Massachusetts General Hospital, Boston, Mass; The Institute for Translational Medicine and Therapeutics (D.J.R., M.P.R.), School of Medicine, University of Pennsylvania, Philadelphia, Pa; The Cardiovascular Institute (D.J.R., M.P.R.), University of Pennsylvania, Philadelphia, Pa; University of Iceland (U.T.), Faculty of Medicine, 101 Reykjavik, Iceland; Department of Medicine (B.F.V.), Harvard Medical School, Boston, Mass; Department of Cardiovascular Sciences (N.J.S.), University of Leicester, Glenfield Hospital, Leicester, UK.
1,
Robert Roberts
Robert Roberts, MD, FRCP(C), MACC
1Institut für Medizinische Biometrie und Statistik (M.P., I.R.K., A.S., A.Z.) and Medizinische Klinik II (M.P., J.E., H.S.), Universität zu Lübeck, Lübeck, Germany; Department of Health Sciences (J.R.T, C.P.N.), University of Leicester, Leicester, UK; Hudson Alpha Institute (D.A.), Huntsville, Ala; Department of Medicine (T.L.A., T.Q.), Stanford University School of Medicine, Stanford, Calif; Medizinische Klinik und Poliklinik (S.B.), Johannes-Gutenberg Universität Mainz, Universitätsmedizin, Mainz, Germany; University of Texas Health Science Center (E.B.), Human Genetics Center and Institute of Molecular Medicine, Houston, Tex; The John & Jennifer Ruddy Canadian Cardiovascular Genetics Center (L.C., R.M., R.R., A.F.R.S., G.W.), University of Ottawa, Ottawa, Ontario, Canada; Department of Biostatistics and Epidemiology (A.C.), Boston University, Boston, Mass; National Heart, Lung, and Blood Institute (A.C., C.J.O.), Framingham Heart Study, Framingham, Mass; LIGHT Research Institute (A.S.H.), Faculty of Medicine and Health, University of Leeds, Leeds, UK; The Blavatnik School of Computer Science and the Department of Molecular Microbiology and Biotechnology (E.H.), Tel-Aviv University, Tel-Aviv, Israel; International Computer Science Institute (E.H.), Berkeley, Calif; Klinik und Poliklinik für Innere Medizin II (C.H.), Universität Regensburg, Regensburg, Germany; deCODE Genetics (H.H., G.T., U.T.), 101 Reykjavik, Iceland; Science Center (R.L.), Tampere University Hospital, Tampere, Finland; Biostatistics and Epidemiology (M.L.), University of Pennsylvania, Philadelphia, Pa; Synlab Center of Laboratory Diagnostics Heidelberg (W.M.), Heidelberg, Germany; Clinical Institute of Medicine and Chemical Laboratory Diagnostics (W.M.), Medical University of Graz, Graz, Austria; Institute of Public Health, Social and Preventive Medicine (W.M.), Faculty of Medicine Mannheim, University of Heidelberg, Germany; Cardiovascular Research Center and Cardiology Division (K.M., S.K.), Massachusetts General Hospital, Boston, Mass; Center for Human Genetic Research (K.M., B.F.V., S.K.), Massachusetts General Hospital, Boston, Mass; Program in Medical and Population Genetics (K.M., B.F.V., S.K.), Broad Institute of Harvard and Massachusetts Institute of Technology, Cambridge, Mass; Cardiovascular Research Institute (M.S.B., S.E.E.), MedStar Research Institute, Washington Hospital Center, Washington, DC; Cardiology Division (C.J.O.), Massachusetts General Hospital, Boston, Mass; The Institute for Translational Medicine and Therapeutics (D.J.R., M.P.R.), School of Medicine, University of Pennsylvania, Philadelphia, Pa; The Cardiovascular Institute (D.J.R., M.P.R.), University of Pennsylvania, Philadelphia, Pa; University of Iceland (U.T.), Faculty of Medicine, 101 Reykjavik, Iceland; Department of Medicine (B.F.V.), Harvard Medical School, Boston, Mass; Department of Cardiovascular Sciences (N.J.S.), University of Leicester, Glenfield Hospital, Leicester, UK.
1,
Arne Schillert
Arne Schillert
1Institut für Medizinische Biometrie und Statistik (M.P., I.R.K., A.S., A.Z.) and Medizinische Klinik II (M.P., J.E., H.S.), Universität zu Lübeck, Lübeck, Germany; Department of Health Sciences (J.R.T, C.P.N.), University of Leicester, Leicester, UK; Hudson Alpha Institute (D.A.), Huntsville, Ala; Department of Medicine (T.L.A., T.Q.), Stanford University School of Medicine, Stanford, Calif; Medizinische Klinik und Poliklinik (S.B.), Johannes-Gutenberg Universität Mainz, Universitätsmedizin, Mainz, Germany; University of Texas Health Science Center (E.B.), Human Genetics Center and Institute of Molecular Medicine, Houston, Tex; The John & Jennifer Ruddy Canadian Cardiovascular Genetics Center (L.C., R.M., R.R., A.F.R.S., G.W.), University of Ottawa, Ottawa, Ontario, Canada; Department of Biostatistics and Epidemiology (A.C.), Boston University, Boston, Mass; National Heart, Lung, and Blood Institute (A.C., C.J.O.), Framingham Heart Study, Framingham, Mass; LIGHT Research Institute (A.S.H.), Faculty of Medicine and Health, University of Leeds, Leeds, UK; The Blavatnik School of Computer Science and the Department of Molecular Microbiology and Biotechnology (E.H.), Tel-Aviv University, Tel-Aviv, Israel; International Computer Science Institute (E.H.), Berkeley, Calif; Klinik und Poliklinik für Innere Medizin II (C.H.), Universität Regensburg, Regensburg, Germany; deCODE Genetics (H.H., G.T., U.T.), 101 Reykjavik, Iceland; Science Center (R.L.), Tampere University Hospital, Tampere, Finland; Biostatistics and Epidemiology (M.L.), University of Pennsylvania, Philadelphia, Pa; Synlab Center of Laboratory Diagnostics Heidelberg (W.M.), Heidelberg, Germany; Clinical Institute of Medicine and Chemical Laboratory Diagnostics (W.M.), Medical University of Graz, Graz, Austria; Institute of Public Health, Social and Preventive Medicine (W.M.), Faculty of Medicine Mannheim, University of Heidelberg, Germany; Cardiovascular Research Center and Cardiology Division (K.M., S.K.), Massachusetts General Hospital, Boston, Mass; Center for Human Genetic Research (K.M., B.F.V., S.K.), Massachusetts General Hospital, Boston, Mass; Program in Medical and Population Genetics (K.M., B.F.V., S.K.), Broad Institute of Harvard and Massachusetts Institute of Technology, Cambridge, Mass; Cardiovascular Research Institute (M.S.B., S.E.E.), MedStar Research Institute, Washington Hospital Center, Washington, DC; Cardiology Division (C.J.O.), Massachusetts General Hospital, Boston, Mass; The Institute for Translational Medicine and Therapeutics (D.J.R., M.P.R.), School of Medicine, University of Pennsylvania, Philadelphia, Pa; The Cardiovascular Institute (D.J.R., M.P.R.), University of Pennsylvania, Philadelphia, Pa; University of Iceland (U.T.), Faculty of Medicine, 101 Reykjavik, Iceland; Department of Medicine (B.F.V.), Harvard Medical School, Boston, Mass; Department of Cardiovascular Sciences (N.J.S.), University of Leicester, Glenfield Hospital, Leicester, UK.
Dr rer hum biol
1,
Kari Stefansson
Kari Stefansson, MD, PhD
1Institut für Medizinische Biometrie und Statistik (M.P., I.R.K., A.S., A.Z.) and Medizinische Klinik II (M.P., J.E., H.S.), Universität zu Lübeck, Lübeck, Germany; Department of Health Sciences (J.R.T, C.P.N.), University of Leicester, Leicester, UK; Hudson Alpha Institute (D.A.), Huntsville, Ala; Department of Medicine (T.L.A., T.Q.), Stanford University School of Medicine, Stanford, Calif; Medizinische Klinik und Poliklinik (S.B.), Johannes-Gutenberg Universität Mainz, Universitätsmedizin, Mainz, Germany; University of Texas Health Science Center (E.B.), Human Genetics Center and Institute of Molecular Medicine, Houston, Tex; The John & Jennifer Ruddy Canadian Cardiovascular Genetics Center (L.C., R.M., R.R., A.F.R.S., G.W.), University of Ottawa, Ottawa, Ontario, Canada; Department of Biostatistics and Epidemiology (A.C.), Boston University, Boston, Mass; National Heart, Lung, and Blood Institute (A.C., C.J.O.), Framingham Heart Study, Framingham, Mass; LIGHT Research Institute (A.S.H.), Faculty of Medicine and Health, University of Leeds, Leeds, UK; The Blavatnik School of Computer Science and the Department of Molecular Microbiology and Biotechnology (E.H.), Tel-Aviv University, Tel-Aviv, Israel; International Computer Science Institute (E.H.), Berkeley, Calif; Klinik und Poliklinik für Innere Medizin II (C.H.), Universität Regensburg, Regensburg, Germany; deCODE Genetics (H.H., G.T., U.T.), 101 Reykjavik, Iceland; Science Center (R.L.), Tampere University Hospital, Tampere, Finland; Biostatistics and Epidemiology (M.L.), University of Pennsylvania, Philadelphia, Pa; Synlab Center of Laboratory Diagnostics Heidelberg (W.M.), Heidelberg, Germany; Clinical Institute of Medicine and Chemical Laboratory Diagnostics (W.M.), Medical University of Graz, Graz, Austria; Institute of Public Health, Social and Preventive Medicine (W.M.), Faculty of Medicine Mannheim, University of Heidelberg, Germany; Cardiovascular Research Center and Cardiology Division (K.M., S.K.), Massachusetts General Hospital, Boston, Mass; Center for Human Genetic Research (K.M., B.F.V., S.K.), Massachusetts General Hospital, Boston, Mass; Program in Medical and Population Genetics (K.M., B.F.V., S.K.), Broad Institute of Harvard and Massachusetts Institute of Technology, Cambridge, Mass; Cardiovascular Research Institute (M.S.B., S.E.E.), MedStar Research Institute, Washington Hospital Center, Washington, DC; Cardiology Division (C.J.O.), Massachusetts General Hospital, Boston, Mass; The Institute for Translational Medicine and Therapeutics (D.J.R., M.P.R.), School of Medicine, University of Pennsylvania, Philadelphia, Pa; The Cardiovascular Institute (D.J.R., M.P.R.), University of Pennsylvania, Philadelphia, Pa; University of Iceland (U.T.), Faculty of Medicine, 101 Reykjavik, Iceland; Department of Medicine (B.F.V.), Harvard Medical School, Boston, Mass; Department of Cardiovascular Sciences (N.J.S.), University of Leicester, Glenfield Hospital, Leicester, UK.
1,
Alexandre FR Stewart
Alexandre FR Stewart, PhD
1Institut für Medizinische Biometrie und Statistik (M.P., I.R.K., A.S., A.Z.) and Medizinische Klinik II (M.P., J.E., H.S.), Universität zu Lübeck, Lübeck, Germany; Department of Health Sciences (J.R.T, C.P.N.), University of Leicester, Leicester, UK; Hudson Alpha Institute (D.A.), Huntsville, Ala; Department of Medicine (T.L.A., T.Q.), Stanford University School of Medicine, Stanford, Calif; Medizinische Klinik und Poliklinik (S.B.), Johannes-Gutenberg Universität Mainz, Universitätsmedizin, Mainz, Germany; University of Texas Health Science Center (E.B.), Human Genetics Center and Institute of Molecular Medicine, Houston, Tex; The John & Jennifer Ruddy Canadian Cardiovascular Genetics Center (L.C., R.M., R.R., A.F.R.S., G.W.), University of Ottawa, Ottawa, Ontario, Canada; Department of Biostatistics and Epidemiology (A.C.), Boston University, Boston, Mass; National Heart, Lung, and Blood Institute (A.C., C.J.O.), Framingham Heart Study, Framingham, Mass; LIGHT Research Institute (A.S.H.), Faculty of Medicine and Health, University of Leeds, Leeds, UK; The Blavatnik School of Computer Science and the Department of Molecular Microbiology and Biotechnology (E.H.), Tel-Aviv University, Tel-Aviv, Israel; International Computer Science Institute (E.H.), Berkeley, Calif; Klinik und Poliklinik für Innere Medizin II (C.H.), Universität Regensburg, Regensburg, Germany; deCODE Genetics (H.H., G.T., U.T.), 101 Reykjavik, Iceland; Science Center (R.L.), Tampere University Hospital, Tampere, Finland; Biostatistics and Epidemiology (M.L.), University of Pennsylvania, Philadelphia, Pa; Synlab Center of Laboratory Diagnostics Heidelberg (W.M.), Heidelberg, Germany; Clinical Institute of Medicine and Chemical Laboratory Diagnostics (W.M.), Medical University of Graz, Graz, Austria; Institute of Public Health, Social and Preventive Medicine (W.M.), Faculty of Medicine Mannheim, University of Heidelberg, Germany; Cardiovascular Research Center and Cardiology Division (K.M., S.K.), Massachusetts General Hospital, Boston, Mass; Center for Human Genetic Research (K.M., B.F.V., S.K.), Massachusetts General Hospital, Boston, Mass; Program in Medical and Population Genetics (K.M., B.F.V., S.K.), Broad Institute of Harvard and Massachusetts Institute of Technology, Cambridge, Mass; Cardiovascular Research Institute (M.S.B., S.E.E.), MedStar Research Institute, Washington Hospital Center, Washington, DC; Cardiology Division (C.J.O.), Massachusetts General Hospital, Boston, Mass; The Institute for Translational Medicine and Therapeutics (D.J.R., M.P.R.), School of Medicine, University of Pennsylvania, Philadelphia, Pa; The Cardiovascular Institute (D.J.R., M.P.R.), University of Pennsylvania, Philadelphia, Pa; University of Iceland (U.T.), Faculty of Medicine, 101 Reykjavik, Iceland; Department of Medicine (B.F.V.), Harvard Medical School, Boston, Mass; Department of Cardiovascular Sciences (N.J.S.), University of Leicester, Glenfield Hospital, Leicester, UK.
1,
Gudmar Thorleifsson
Gudmar Thorleifsson, PhD
1Institut für Medizinische Biometrie und Statistik (M.P., I.R.K., A.S., A.Z.) and Medizinische Klinik II (M.P., J.E., H.S.), Universität zu Lübeck, Lübeck, Germany; Department of Health Sciences (J.R.T, C.P.N.), University of Leicester, Leicester, UK; Hudson Alpha Institute (D.A.), Huntsville, Ala; Department of Medicine (T.L.A., T.Q.), Stanford University School of Medicine, Stanford, Calif; Medizinische Klinik und Poliklinik (S.B.), Johannes-Gutenberg Universität Mainz, Universitätsmedizin, Mainz, Germany; University of Texas Health Science Center (E.B.), Human Genetics Center and Institute of Molecular Medicine, Houston, Tex; The John & Jennifer Ruddy Canadian Cardiovascular Genetics Center (L.C., R.M., R.R., A.F.R.S., G.W.), University of Ottawa, Ottawa, Ontario, Canada; Department of Biostatistics and Epidemiology (A.C.), Boston University, Boston, Mass; National Heart, Lung, and Blood Institute (A.C., C.J.O.), Framingham Heart Study, Framingham, Mass; LIGHT Research Institute (A.S.H.), Faculty of Medicine and Health, University of Leeds, Leeds, UK; The Blavatnik School of Computer Science and the Department of Molecular Microbiology and Biotechnology (E.H.), Tel-Aviv University, Tel-Aviv, Israel; International Computer Science Institute (E.H.), Berkeley, Calif; Klinik und Poliklinik für Innere Medizin II (C.H.), Universität Regensburg, Regensburg, Germany; deCODE Genetics (H.H., G.T., U.T.), 101 Reykjavik, Iceland; Science Center (R.L.), Tampere University Hospital, Tampere, Finland; Biostatistics and Epidemiology (M.L.), University of Pennsylvania, Philadelphia, Pa; Synlab Center of Laboratory Diagnostics Heidelberg (W.M.), Heidelberg, Germany; Clinical Institute of Medicine and Chemical Laboratory Diagnostics (W.M.), Medical University of Graz, Graz, Austria; Institute of Public Health, Social and Preventive Medicine (W.M.), Faculty of Medicine Mannheim, University of Heidelberg, Germany; Cardiovascular Research Center and Cardiology Division (K.M., S.K.), Massachusetts General Hospital, Boston, Mass; Center for Human Genetic Research (K.M., B.F.V., S.K.), Massachusetts General Hospital, Boston, Mass; Program in Medical and Population Genetics (K.M., B.F.V., S.K.), Broad Institute of Harvard and Massachusetts Institute of Technology, Cambridge, Mass; Cardiovascular Research Institute (M.S.B., S.E.E.), MedStar Research Institute, Washington Hospital Center, Washington, DC; Cardiology Division (C.J.O.), Massachusetts General Hospital, Boston, Mass; The Institute for Translational Medicine and Therapeutics (D.J.R., M.P.R.), School of Medicine, University of Pennsylvania, Philadelphia, Pa; The Cardiovascular Institute (D.J.R., M.P.R.), University of Pennsylvania, Philadelphia, Pa; University of Iceland (U.T.), Faculty of Medicine, 101 Reykjavik, Iceland; Department of Medicine (B.F.V.), Harvard Medical School, Boston, Mass; Department of Cardiovascular Sciences (N.J.S.), University of Leicester, Glenfield Hospital, Leicester, UK.
1,
Benjamin F Voight
Benjamin F Voight, PhD
1Institut für Medizinische Biometrie und Statistik (M.P., I.R.K., A.S., A.Z.) and Medizinische Klinik II (M.P., J.E., H.S.), Universität zu Lübeck, Lübeck, Germany; Department of Health Sciences (J.R.T, C.P.N.), University of Leicester, Leicester, UK; Hudson Alpha Institute (D.A.), Huntsville, Ala; Department of Medicine (T.L.A., T.Q.), Stanford University School of Medicine, Stanford, Calif; Medizinische Klinik und Poliklinik (S.B.), Johannes-Gutenberg Universität Mainz, Universitätsmedizin, Mainz, Germany; University of Texas Health Science Center (E.B.), Human Genetics Center and Institute of Molecular Medicine, Houston, Tex; The John & Jennifer Ruddy Canadian Cardiovascular Genetics Center (L.C., R.M., R.R., A.F.R.S., G.W.), University of Ottawa, Ottawa, Ontario, Canada; Department of Biostatistics and Epidemiology (A.C.), Boston University, Boston, Mass; National Heart, Lung, and Blood Institute (A.C., C.J.O.), Framingham Heart Study, Framingham, Mass; LIGHT Research Institute (A.S.H.), Faculty of Medicine and Health, University of Leeds, Leeds, UK; The Blavatnik School of Computer Science and the Department of Molecular Microbiology and Biotechnology (E.H.), Tel-Aviv University, Tel-Aviv, Israel; International Computer Science Institute (E.H.), Berkeley, Calif; Klinik und Poliklinik für Innere Medizin II (C.H.), Universität Regensburg, Regensburg, Germany; deCODE Genetics (H.H., G.T., U.T.), 101 Reykjavik, Iceland; Science Center (R.L.), Tampere University Hospital, Tampere, Finland; Biostatistics and Epidemiology (M.L.), University of Pennsylvania, Philadelphia, Pa; Synlab Center of Laboratory Diagnostics Heidelberg (W.M.), Heidelberg, Germany; Clinical Institute of Medicine and Chemical Laboratory Diagnostics (W.M.), Medical University of Graz, Graz, Austria; Institute of Public Health, Social and Preventive Medicine (W.M.), Faculty of Medicine Mannheim, University of Heidelberg, Germany; Cardiovascular Research Center and Cardiology Division (K.M., S.K.), Massachusetts General Hospital, Boston, Mass; Center for Human Genetic Research (K.M., B.F.V., S.K.), Massachusetts General Hospital, Boston, Mass; Program in Medical and Population Genetics (K.M., B.F.V., S.K.), Broad Institute of Harvard and Massachusetts Institute of Technology, Cambridge, Mass; Cardiovascular Research Institute (M.S.B., S.E.E.), MedStar Research Institute, Washington Hospital Center, Washington, DC; Cardiology Division (C.J.O.), Massachusetts General Hospital, Boston, Mass; The Institute for Translational Medicine and Therapeutics (D.J.R., M.P.R.), School of Medicine, University of Pennsylvania, Philadelphia, Pa; The Cardiovascular Institute (D.J.R., M.P.R.), University of Pennsylvania, Philadelphia, Pa; University of Iceland (U.T.), Faculty of Medicine, 101 Reykjavik, Iceland; Department of Medicine (B.F.V.), Harvard Medical School, Boston, Mass; Department of Cardiovascular Sciences (N.J.S.), University of Leicester, Glenfield Hospital, Leicester, UK.
1,
George A Wells
George A Wells, MSc, PhD
1Institut für Medizinische Biometrie und Statistik (M.P., I.R.K., A.S., A.Z.) and Medizinische Klinik II (M.P., J.E., H.S.), Universität zu Lübeck, Lübeck, Germany; Department of Health Sciences (J.R.T, C.P.N.), University of Leicester, Leicester, UK; Hudson Alpha Institute (D.A.), Huntsville, Ala; Department of Medicine (T.L.A., T.Q.), Stanford University School of Medicine, Stanford, Calif; Medizinische Klinik und Poliklinik (S.B.), Johannes-Gutenberg Universität Mainz, Universitätsmedizin, Mainz, Germany; University of Texas Health Science Center (E.B.), Human Genetics Center and Institute of Molecular Medicine, Houston, Tex; The John & Jennifer Ruddy Canadian Cardiovascular Genetics Center (L.C., R.M., R.R., A.F.R.S., G.W.), University of Ottawa, Ottawa, Ontario, Canada; Department of Biostatistics and Epidemiology (A.C.), Boston University, Boston, Mass; National Heart, Lung, and Blood Institute (A.C., C.J.O.), Framingham Heart Study, Framingham, Mass; LIGHT Research Institute (A.S.H.), Faculty of Medicine and Health, University of Leeds, Leeds, UK; The Blavatnik School of Computer Science and the Department of Molecular Microbiology and Biotechnology (E.H.), Tel-Aviv University, Tel-Aviv, Israel; International Computer Science Institute (E.H.), Berkeley, Calif; Klinik und Poliklinik für Innere Medizin II (C.H.), Universität Regensburg, Regensburg, Germany; deCODE Genetics (H.H., G.T., U.T.), 101 Reykjavik, Iceland; Science Center (R.L.), Tampere University Hospital, Tampere, Finland; Biostatistics and Epidemiology (M.L.), University of Pennsylvania, Philadelphia, Pa; Synlab Center of Laboratory Diagnostics Heidelberg (W.M.), Heidelberg, Germany; Clinical Institute of Medicine and Chemical Laboratory Diagnostics (W.M.), Medical University of Graz, Graz, Austria; Institute of Public Health, Social and Preventive Medicine (W.M.), Faculty of Medicine Mannheim, University of Heidelberg, Germany; Cardiovascular Research Center and Cardiology Division (K.M., S.K.), Massachusetts General Hospital, Boston, Mass; Center for Human Genetic Research (K.M., B.F.V., S.K.), Massachusetts General Hospital, Boston, Mass; Program in Medical and Population Genetics (K.M., B.F.V., S.K.), Broad Institute of Harvard and Massachusetts Institute of Technology, Cambridge, Mass; Cardiovascular Research Institute (M.S.B., S.E.E.), MedStar Research Institute, Washington Hospital Center, Washington, DC; Cardiology Division (C.J.O.), Massachusetts General Hospital, Boston, Mass; The Institute for Translational Medicine and Therapeutics (D.J.R., M.P.R.), School of Medicine, University of Pennsylvania, Philadelphia, Pa; The Cardiovascular Institute (D.J.R., M.P.R.), University of Pennsylvania, Philadelphia, Pa; University of Iceland (U.T.), Faculty of Medicine, 101 Reykjavik, Iceland; Department of Medicine (B.F.V.), Harvard Medical School, Boston, Mass; Department of Cardiovascular Sciences (N.J.S.), University of Leicester, Glenfield Hospital, Leicester, UK.
1,
Andreas Ziegler
Andreas Ziegler
1Institut für Medizinische Biometrie und Statistik (M.P., I.R.K., A.S., A.Z.) and Medizinische Klinik II (M.P., J.E., H.S.), Universität zu Lübeck, Lübeck, Germany; Department of Health Sciences (J.R.T, C.P.N.), University of Leicester, Leicester, UK; Hudson Alpha Institute (D.A.), Huntsville, Ala; Department of Medicine (T.L.A., T.Q.), Stanford University School of Medicine, Stanford, Calif; Medizinische Klinik und Poliklinik (S.B.), Johannes-Gutenberg Universität Mainz, Universitätsmedizin, Mainz, Germany; University of Texas Health Science Center (E.B.), Human Genetics Center and Institute of Molecular Medicine, Houston, Tex; The John & Jennifer Ruddy Canadian Cardiovascular Genetics Center (L.C., R.M., R.R., A.F.R.S., G.W.), University of Ottawa, Ottawa, Ontario, Canada; Department of Biostatistics and Epidemiology (A.C.), Boston University, Boston, Mass; National Heart, Lung, and Blood Institute (A.C., C.J.O.), Framingham Heart Study, Framingham, Mass; LIGHT Research Institute (A.S.H.), Faculty of Medicine and Health, University of Leeds, Leeds, UK; The Blavatnik School of Computer Science and the Department of Molecular Microbiology and Biotechnology (E.H.), Tel-Aviv University, Tel-Aviv, Israel; International Computer Science Institute (E.H.), Berkeley, Calif; Klinik und Poliklinik für Innere Medizin II (C.H.), Universität Regensburg, Regensburg, Germany; deCODE Genetics (H.H., G.T., U.T.), 101 Reykjavik, Iceland; Science Center (R.L.), Tampere University Hospital, Tampere, Finland; Biostatistics and Epidemiology (M.L.), University of Pennsylvania, Philadelphia, Pa; Synlab Center of Laboratory Diagnostics Heidelberg (W.M.), Heidelberg, Germany; Clinical Institute of Medicine and Chemical Laboratory Diagnostics (W.M.), Medical University of Graz, Graz, Austria; Institute of Public Health, Social and Preventive Medicine (W.M.), Faculty of Medicine Mannheim, University of Heidelberg, Germany; Cardiovascular Research Center and Cardiology Division (K.M., S.K.), Massachusetts General Hospital, Boston, Mass; Center for Human Genetic Research (K.M., B.F.V., S.K.), Massachusetts General Hospital, Boston, Mass; Program in Medical and Population Genetics (K.M., B.F.V., S.K.), Broad Institute of Harvard and Massachusetts Institute of Technology, Cambridge, Mass; Cardiovascular Research Institute (M.S.B., S.E.E.), MedStar Research Institute, Washington Hospital Center, Washington, DC; Cardiology Division (C.J.O.), Massachusetts General Hospital, Boston, Mass; The Institute for Translational Medicine and Therapeutics (D.J.R., M.P.R.), School of Medicine, University of Pennsylvania, Philadelphia, Pa; The Cardiovascular Institute (D.J.R., M.P.R.), University of Pennsylvania, Philadelphia, Pa; University of Iceland (U.T.), Faculty of Medicine, 101 Reykjavik, Iceland; Department of Medicine (B.F.V.), Harvard Medical School, Boston, Mass; Department of Cardiovascular Sciences (N.J.S.), University of Leicester, Glenfield Hospital, Leicester, UK.
Prof Dr rer nat
1,
Sekar Kathiresan
Sekar Kathiresan, MD
1Institut für Medizinische Biometrie und Statistik (M.P., I.R.K., A.S., A.Z.) and Medizinische Klinik II (M.P., J.E., H.S.), Universität zu Lübeck, Lübeck, Germany; Department of Health Sciences (J.R.T, C.P.N.), University of Leicester, Leicester, UK; Hudson Alpha Institute (D.A.), Huntsville, Ala; Department of Medicine (T.L.A., T.Q.), Stanford University School of Medicine, Stanford, Calif; Medizinische Klinik und Poliklinik (S.B.), Johannes-Gutenberg Universität Mainz, Universitätsmedizin, Mainz, Germany; University of Texas Health Science Center (E.B.), Human Genetics Center and Institute of Molecular Medicine, Houston, Tex; The John & Jennifer Ruddy Canadian Cardiovascular Genetics Center (L.C., R.M., R.R., A.F.R.S., G.W.), University of Ottawa, Ottawa, Ontario, Canada; Department of Biostatistics and Epidemiology (A.C.), Boston University, Boston, Mass; National Heart, Lung, and Blood Institute (A.C., C.J.O.), Framingham Heart Study, Framingham, Mass; LIGHT Research Institute (A.S.H.), Faculty of Medicine and Health, University of Leeds, Leeds, UK; The Blavatnik School of Computer Science and the Department of Molecular Microbiology and Biotechnology (E.H.), Tel-Aviv University, Tel-Aviv, Israel; International Computer Science Institute (E.H.), Berkeley, Calif; Klinik und Poliklinik für Innere Medizin II (C.H.), Universität Regensburg, Regensburg, Germany; deCODE Genetics (H.H., G.T., U.T.), 101 Reykjavik, Iceland; Science Center (R.L.), Tampere University Hospital, Tampere, Finland; Biostatistics and Epidemiology (M.L.), University of Pennsylvania, Philadelphia, Pa; Synlab Center of Laboratory Diagnostics Heidelberg (W.M.), Heidelberg, Germany; Clinical Institute of Medicine and Chemical Laboratory Diagnostics (W.M.), Medical University of Graz, Graz, Austria; Institute of Public Health, Social and Preventive Medicine (W.M.), Faculty of Medicine Mannheim, University of Heidelberg, Germany; Cardiovascular Research Center and Cardiology Division (K.M., S.K.), Massachusetts General Hospital, Boston, Mass; Center for Human Genetic Research (K.M., B.F.V., S.K.), Massachusetts General Hospital, Boston, Mass; Program in Medical and Population Genetics (K.M., B.F.V., S.K.), Broad Institute of Harvard and Massachusetts Institute of Technology, Cambridge, Mass; Cardiovascular Research Institute (M.S.B., S.E.E.), MedStar Research Institute, Washington Hospital Center, Washington, DC; Cardiology Division (C.J.O.), Massachusetts General Hospital, Boston, Mass; The Institute for Translational Medicine and Therapeutics (D.J.R., M.P.R.), School of Medicine, University of Pennsylvania, Philadelphia, Pa; The Cardiovascular Institute (D.J.R., M.P.R.), University of Pennsylvania, Philadelphia, Pa; University of Iceland (U.T.), Faculty of Medicine, 101 Reykjavik, Iceland; Department of Medicine (B.F.V.), Harvard Medical School, Boston, Mass; Department of Cardiovascular Sciences (N.J.S.), University of Leicester, Glenfield Hospital, Leicester, UK.
1,
Muredach P Reilly
Muredach P Reilly, MBBCH, MSCE
1Institut für Medizinische Biometrie und Statistik (M.P., I.R.K., A.S., A.Z.) and Medizinische Klinik II (M.P., J.E., H.S.), Universität zu Lübeck, Lübeck, Germany; Department of Health Sciences (J.R.T, C.P.N.), University of Leicester, Leicester, UK; Hudson Alpha Institute (D.A.), Huntsville, Ala; Department of Medicine (T.L.A., T.Q.), Stanford University School of Medicine, Stanford, Calif; Medizinische Klinik und Poliklinik (S.B.), Johannes-Gutenberg Universität Mainz, Universitätsmedizin, Mainz, Germany; University of Texas Health Science Center (E.B.), Human Genetics Center and Institute of Molecular Medicine, Houston, Tex; The John & Jennifer Ruddy Canadian Cardiovascular Genetics Center (L.C., R.M., R.R., A.F.R.S., G.W.), University of Ottawa, Ottawa, Ontario, Canada; Department of Biostatistics and Epidemiology (A.C.), Boston University, Boston, Mass; National Heart, Lung, and Blood Institute (A.C., C.J.O.), Framingham Heart Study, Framingham, Mass; LIGHT Research Institute (A.S.H.), Faculty of Medicine and Health, University of Leeds, Leeds, UK; The Blavatnik School of Computer Science and the Department of Molecular Microbiology and Biotechnology (E.H.), Tel-Aviv University, Tel-Aviv, Israel; International Computer Science Institute (E.H.), Berkeley, Calif; Klinik und Poliklinik für Innere Medizin II (C.H.), Universität Regensburg, Regensburg, Germany; deCODE Genetics (H.H., G.T., U.T.), 101 Reykjavik, Iceland; Science Center (R.L.), Tampere University Hospital, Tampere, Finland; Biostatistics and Epidemiology (M.L.), University of Pennsylvania, Philadelphia, Pa; Synlab Center of Laboratory Diagnostics Heidelberg (W.M.), Heidelberg, Germany; Clinical Institute of Medicine and Chemical Laboratory Diagnostics (W.M.), Medical University of Graz, Graz, Austria; Institute of Public Health, Social and Preventive Medicine (W.M.), Faculty of Medicine Mannheim, University of Heidelberg, Germany; Cardiovascular Research Center and Cardiology Division (K.M., S.K.), Massachusetts General Hospital, Boston, Mass; Center for Human Genetic Research (K.M., B.F.V., S.K.), Massachusetts General Hospital, Boston, Mass; Program in Medical and Population Genetics (K.M., B.F.V., S.K.), Broad Institute of Harvard and Massachusetts Institute of Technology, Cambridge, Mass; Cardiovascular Research Institute (M.S.B., S.E.E.), MedStar Research Institute, Washington Hospital Center, Washington, DC; Cardiology Division (C.J.O.), Massachusetts General Hospital, Boston, Mass; The Institute for Translational Medicine and Therapeutics (D.J.R., M.P.R.), School of Medicine, University of Pennsylvania, Philadelphia, Pa; The Cardiovascular Institute (D.J.R., M.P.R.), University of Pennsylvania, Philadelphia, Pa; University of Iceland (U.T.), Faculty of Medicine, 101 Reykjavik, Iceland; Department of Medicine (B.F.V.), Harvard Medical School, Boston, Mass; Department of Cardiovascular Sciences (N.J.S.), University of Leicester, Glenfield Hospital, Leicester, UK.
1,
Nilesh J Samani
Nilesh J Samani
1Institut für Medizinische Biometrie und Statistik (M.P., I.R.K., A.S., A.Z.) and Medizinische Klinik II (M.P., J.E., H.S.), Universität zu Lübeck, Lübeck, Germany; Department of Health Sciences (J.R.T, C.P.N.), University of Leicester, Leicester, UK; Hudson Alpha Institute (D.A.), Huntsville, Ala; Department of Medicine (T.L.A., T.Q.), Stanford University School of Medicine, Stanford, Calif; Medizinische Klinik und Poliklinik (S.B.), Johannes-Gutenberg Universität Mainz, Universitätsmedizin, Mainz, Germany; University of Texas Health Science Center (E.B.), Human Genetics Center and Institute of Molecular Medicine, Houston, Tex; The John & Jennifer Ruddy Canadian Cardiovascular Genetics Center (L.C., R.M., R.R., A.F.R.S., G.W.), University of Ottawa, Ottawa, Ontario, Canada; Department of Biostatistics and Epidemiology (A.C.), Boston University, Boston, Mass; National Heart, Lung, and Blood Institute (A.C., C.J.O.), Framingham Heart Study, Framingham, Mass; LIGHT Research Institute (A.S.H.), Faculty of Medicine and Health, University of Leeds, Leeds, UK; The Blavatnik School of Computer Science and the Department of Molecular Microbiology and Biotechnology (E.H.), Tel-Aviv University, Tel-Aviv, Israel; International Computer Science Institute (E.H.), Berkeley, Calif; Klinik und Poliklinik für Innere Medizin II (C.H.), Universität Regensburg, Regensburg, Germany; deCODE Genetics (H.H., G.T., U.T.), 101 Reykjavik, Iceland; Science Center (R.L.), Tampere University Hospital, Tampere, Finland; Biostatistics and Epidemiology (M.L.), University of Pennsylvania, Philadelphia, Pa; Synlab Center of Laboratory Diagnostics Heidelberg (W.M.), Heidelberg, Germany; Clinical Institute of Medicine and Chemical Laboratory Diagnostics (W.M.), Medical University of Graz, Graz, Austria; Institute of Public Health, Social and Preventive Medicine (W.M.), Faculty of Medicine Mannheim, University of Heidelberg, Germany; Cardiovascular Research Center and Cardiology Division (K.M., S.K.), Massachusetts General Hospital, Boston, Mass; Center for Human Genetic Research (K.M., B.F.V., S.K.), Massachusetts General Hospital, Boston, Mass; Program in Medical and Population Genetics (K.M., B.F.V., S.K.), Broad Institute of Harvard and Massachusetts Institute of Technology, Cambridge, Mass; Cardiovascular Research Institute (M.S.B., S.E.E.), MedStar Research Institute, Washington Hospital Center, Washington, DC; Cardiology Division (C.J.O.), Massachusetts General Hospital, Boston, Mass; The Institute for Translational Medicine and Therapeutics (D.J.R., M.P.R.), School of Medicine, University of Pennsylvania, Philadelphia, Pa; The Cardiovascular Institute (D.J.R., M.P.R.), University of Pennsylvania, Philadelphia, Pa; University of Iceland (U.T.), Faculty of Medicine, 101 Reykjavik, Iceland; Department of Medicine (B.F.V.), Harvard Medical School, Boston, Mass; Department of Cardiovascular Sciences (N.J.S.), University of Leicester, Glenfield Hospital, Leicester, UK.
FMedSci
1,
Heribert Schunkert
Heribert Schunkert
1Institut für Medizinische Biometrie und Statistik (M.P., I.R.K., A.S., A.Z.) and Medizinische Klinik II (M.P., J.E., H.S.), Universität zu Lübeck, Lübeck, Germany; Department of Health Sciences (J.R.T, C.P.N.), University of Leicester, Leicester, UK; Hudson Alpha Institute (D.A.), Huntsville, Ala; Department of Medicine (T.L.A., T.Q.), Stanford University School of Medicine, Stanford, Calif; Medizinische Klinik und Poliklinik (S.B.), Johannes-Gutenberg Universität Mainz, Universitätsmedizin, Mainz, Germany; University of Texas Health Science Center (E.B.), Human Genetics Center and Institute of Molecular Medicine, Houston, Tex; The John & Jennifer Ruddy Canadian Cardiovascular Genetics Center (L.C., R.M., R.R., A.F.R.S., G.W.), University of Ottawa, Ottawa, Ontario, Canada; Department of Biostatistics and Epidemiology (A.C.), Boston University, Boston, Mass; National Heart, Lung, and Blood Institute (A.C., C.J.O.), Framingham Heart Study, Framingham, Mass; LIGHT Research Institute (A.S.H.), Faculty of Medicine and Health, University of Leeds, Leeds, UK; The Blavatnik School of Computer Science and the Department of Molecular Microbiology and Biotechnology (E.H.), Tel-Aviv University, Tel-Aviv, Israel; International Computer Science Institute (E.H.), Berkeley, Calif; Klinik und Poliklinik für Innere Medizin II (C.H.), Universität Regensburg, Regensburg, Germany; deCODE Genetics (H.H., G.T., U.T.), 101 Reykjavik, Iceland; Science Center (R.L.), Tampere University Hospital, Tampere, Finland; Biostatistics and Epidemiology (M.L.), University of Pennsylvania, Philadelphia, Pa; Synlab Center of Laboratory Diagnostics Heidelberg (W.M.), Heidelberg, Germany; Clinical Institute of Medicine and Chemical Laboratory Diagnostics (W.M.), Medical University of Graz, Graz, Austria; Institute of Public Health, Social and Preventive Medicine (W.M.), Faculty of Medicine Mannheim, University of Heidelberg, Germany; Cardiovascular Research Center and Cardiology Division (K.M., S.K.), Massachusetts General Hospital, Boston, Mass; Center for Human Genetic Research (K.M., B.F.V., S.K.), Massachusetts General Hospital, Boston, Mass; Program in Medical and Population Genetics (K.M., B.F.V., S.K.), Broad Institute of Harvard and Massachusetts Institute of Technology, Cambridge, Mass; Cardiovascular Research Institute (M.S.B., S.E.E.), MedStar Research Institute, Washington Hospital Center, Washington, DC; Cardiology Division (C.J.O.), Massachusetts General Hospital, Boston, Mass; The Institute for Translational Medicine and Therapeutics (D.J.R., M.P.R.), School of Medicine, University of Pennsylvania, Philadelphia, Pa; The Cardiovascular Institute (D.J.R., M.P.R.), University of Pennsylvania, Philadelphia, Pa; University of Iceland (U.T.), Faculty of Medicine, 101 Reykjavik, Iceland; Department of Medicine (B.F.V.), Harvard Medical School, Boston, Mass; Department of Cardiovascular Sciences (N.J.S.), University of Leicester, Glenfield Hospital, Leicester, UK.
Prof Dr med
1;
on behalf of the CARDIoGRAM Consortium1