Abstract
Background
The P140 phosphopeptide issued from the spliceosomal U1-70K small nuclear ribonucleoprotein protein displays protective properties in MRL/lpr lupus-prone mice. It binds both major histocompatibility class II (MHCII) and HSC70/Hsp73 molecules. P140 peptide increases MRL/lpr peripheral blood lymphocyte apoptosis and decreases autoepitope recognition by T cells.
Objective
To explore further the mode of action of P140 peptide on HSC70+ antigen-presenting cells.
Methods
P140 biodistribution was monitored in real time using an imaging system and by fluorescence and electron microscopy. Fluorescence activated cell sorting and Western blotting experiments were used to evaluate the P140 effects on autophagic flux markers.
Results
P140 fluorescence accumulated especially in the lungs and spleen. P140 peptide reduced the number of peripheral and splenic T and B cells without affecting these cells in normal mice. Remaining MRL/lpr B cells responded normally to mitogens. P140 peptide decreased the expression levels of HSC70/Hsp73 chaperone and stable MHCII dimers, which are both increased in MRL/lpr splenic B cells. It impaired refolding properties of chaperone HSC70. In MRL/lpr B cells, it increased the accumulation of the autophagy markers p62/SQSTM1 and LC3-II, consistent with a downregulated lysosomal degradation during autophagic flux.
Conclusion
The study results suggest that after P140 peptide binding to HSC70, the endogenous (auto)antigen processing might be greatly affected in MRL/lpr antigen-presenting B cells, leading to the observed decrease of autoreactive T-cell priming and signalling via a mechanism involving a lysosomal degradation pathway. This unexpected mechanism might explain the beneficial effect of P140 peptide in treated MRL/lpr mice.
Introduction
Systemic lupus erythematosus is a multifactorial (multigenic) and highly polymorphic systemic autoimmune disorder. It affects multiple organs, including skin, muscle, joints and vital internal organs such as kidneys and heart. Current pharmacological treatments are largely palliative and result in non-specific immunosuppression. The goal today is to design more specific and effective treatments.
P140 peptide is a 21-mer fragment of the spliceosomal U1-70K small nuclear ribonucleoprotein protein that significantly ameliorates clinical and biological manifestations in autoimmune patients with systemic lupus erythematosus and enhances survival in lupus-prone mice.1 2 This peptide contains a phosphoserine residue at position 140, a modification that specifically occurs at an early stage of apoptosis, before the cleavage of the C-terminal part of the protein by caspase-3 and the dephosphorylation of other serine residues by a PP1 phosphatase-mediated mechanism.3 Originally thought to act solely via the modulation of intracellular signalling induced by αβ autoreactive T-cell receptor (TCR) engagement,2 4 P140 was unexpectedly found to interact selectively with the constitutive heat-shock HSC70/Hsp73 protein.5 By a granzyme-B and caspase-dependent mechanism, it induces in Fas-deficient MRL/lpr lupus mice apoptosis of B cells and CD4, CD8 or double negative (DN) αβTCR+/CD4/CD8/B220+ T lymphocytes through a regulatory circuit involving γδT cells.5 Intravenous P140 peptide administration into young MRL/lpr mice thus causes T- and B-cell egress from peripheral blood but does not affect T-cell priming, and does not interfere with the ability of P140-treated mice to resist to an infectious viral challenge.6 P140 also downregulates the expression of programmed death 1 (PD-1/CD279) receptor, a molecule of the CD28 family, which is overexpressed at the surface of MRL/lpr CD4 T cells.5 P140 peptide thus displays various effects on the immune system that probably interfere with several, yet unidentified, molecular pathways leading finally to a decreased T-cell autoreactivity and to significantly lower levels of autoantibodies to native DNA.
In the postulated P140 mode of action, binding to the chaperone HSC70 protein seems to be central. The implication of or any defect of the HSC70 pathway in regulating immune functions in lupus is not documented. It is not known either if HSC70-chaperoned molecules, such as other Hsps and major histocompatibility complex (MHC) molecules, are affected by P140–HSC70 binding. To address these major questions, we evaluated the effect of P140 administration on the expression of HSC70 and MHCII molecules, and studied the possible influence of P140 on autophagosomal pathways implicated in the endogenous MHCII loading. Since HSC70 is involved in certain phases of these pathways, we expected that binding of P140 to HSC70 might alter the delivery of intracellular material into the endosomal/lysosomal pathway and therefore thwart the final presentation of self-antigens to autoreactive T cells.
Methods
Synthetic peptides and biochemical assays
Peptides were assembled and purified as described.1 5 The P140 peptide was also synthesised with a cysteine residue added at its N-terminus. The luciferase renaturation assay was as described7 8 using either intact or heat-denatured luciferase and rabbit reticulocyte lysate in the presence of increasing concentrations of P140 peptide. Luciferase activity was determined using Bright-glo reagent (Promega, Charbomières, France ).
In vivo experiments and cell studies
Protection experiments and clinical monitoring have been described previously.1 All experimental protocols were carried out with the approval of the local Institutional Animal Care and Use Committee (CREMEAS). The renal pathology was evaluated by measuring proteinuria levels and observing vasculitis corresponding to the area of cell infiltrates. For in vivo imaging, Alexa Fluor633-labelled P140 was injected through the retro-orbital venous sinus of anaesthetised mice. Mice were immediately transferred into the cabinet of the NightOwl Bioimager (Berthold Technologies, Thoiry, France ) and images were taken periodically for 3 h. Mice were then killed to collect organs for final fluorescence acquisition and validation of biodistribution. Signal intensity was evaluated using the Indigo software.
Histology was carried out on frozen section of organs collected from MRL/lpr mice that received rhodamine-labelled P140 intravenously. For co-localisation experiments, spleen sections were permeabilised and incubated with either fluorescein isothiocyanate (FITC)-labelled anti-B220 antibody or biotin-labelled anti-CD3e antibodies, followed by Alexa Fluor647-labelled streptavidin, or biotin-labelled anti-MOMA-1/CD169 antibodies specific for metallophilic macrophages, followed by Alexa Fluor488-labelled streptavidin. Other antibodies tested were: biotin-labelled anti-CD11b and biotin-labelled anti-F4/80 antibodies.
Immunofluorescence and immunoelectron microscopy experiments were performed on peripheral blood lymphocytes (PBLs) and splenocytes collected from MRL/lpr mice that had received Alexa Fluor488-labelled P140 intravenously 1 h before examination. Some experiments used splenocytes incubated for 1 h at 37°C with 5 µM Alexa Fluor488-labelled P140. HSC70 and MHCII molecules were revealed with specific antibodies (clone 1B5; Stressgen (Enzo Life Sciences, Villeurbanne, France) and 2G9; BD Biosciences (Le Pont de Claix, France), respectively). Fluorescence activated cell sorting analysis was as described previously.5 The antibodies were anti-HSC70 antibody labelled with R-phycoerythrin (clone 1B5) and anti-IA/IE antibody labelled with FITC (clone 2G9). For specific analysis of cellular subtypes, antibodies used were: anti-CD3e-FITC, anti-CD3e-allophycocyanin, anti-B220-PerCP, anti-B220-allophycocyanin, anti-CD4-allophycocyanin, anti-CD8-allophycocyanin, anti-CD11b-PerCP and anti-Gr-1-FITC. HSC70 and MHCII levels were expressed as the ratio of relative mean fluorescence intensity for specific antibody/mean fluorescence intensity for isotype-matched control antibody.
For measuring lymphocyte proliferation, MRL/lpr and CBA/J mice (four to five/group) were given P140 peptide or ScP140 (100 µg/mouse) or saline only, daily for 10 consecutive days via the intraperitoneal route. Ciclosporin A (20 mg/kg/day) and prednisolone (feeding at 4 mg/kg/day) were tested in parallel. A lymphocyte proliferation assay was performed as described previously6 using 3×105 cells/well.
Statistics
Statistical analyses were performed using parametric (Student t test) and non-parametric (Mann–Whitney U) test. For all tests, p<0.05 was considered statistically significant.
Results
In vivo P140 peptide effects and biodistribution
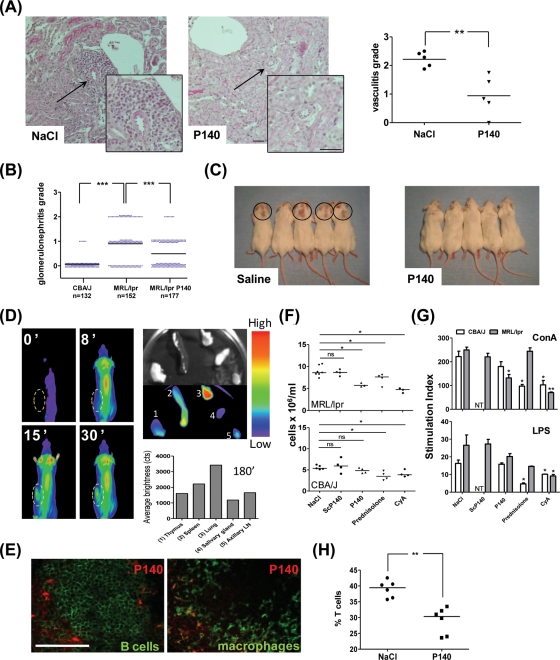
We reported previously that intravenous administration of P140 to MRL/lpr mice significantly improves their clinical and biological manifestations and prolongs their survival.1 We show here that P140 significantly decreases vasculitis with fewer perivascular inflammatory infiltrates in kidneys (figure 1A; p=0.0079), slows down glomerulonephritis (figure 1B; p<0.0001) and reduces the appearance of dermatitis (figure 1C).
Figure 1.
In vivo effect and biodistribution of P140 peptide in MRL/lpr mice. (A, B) MRL/lpr mice received saline alone or 100 µg P140 peptide/injection/mouse on four occasions. Kidneys were collected from 25-week-old MRL/lpr mice and fixed in formalin. Haematoxylin and eosin-stained kidney paraffin sections were scored for (A) vasculitis (n=5/group; p=0.0046 between the two groups) and (B) glomerulonephritis grade (five mice/group, corresponding to 132–177 glomeruli). (C) Observation of skin lesions in both groups of 16-week-old mice. (D) In vivo biodistribution of Alexa Fluor633-labelled P140 peptide following retro-orbital injection of 100 µg peptide in NaCl (complete data in online supplementary figure S1). The yellow circle designates the position of the spleen. At the end of the experiment (180 min), organs were collected and fluorescence was measured in the isolated organs (1, thymus; 2, spleen; 3, lung; 4, salivary gland; 5, axillary lymph node). (E) Spleen sections were co-stained with antibodies to B220 and MOMA-1/CD169 to identify B cells and marginal zone metallophilic macrophages, respectively. (F) Peripheral blood lymphocyte (PBL) counts measured in 10-week-old MRL/lpr and CBA/J mice (n=3–4 mice tested individually/group) that received at daily intervals 10 intraperitoneal injections of P140 (100 µg/mouse), ScP140 (100 µg/mouse), ciclosporin A (20 mg/kg) or saline or that received prednisolone orally at a 4 mg/kg dose. (G) Effect of P140 overdosage on the ability of PBLs to respond to T- and B-cell mitogens. Cultures for 48 h in the presence of 3 µg/ml concanavalin A (ConA) or 5 µg/ml; lipopolysaccharide (LPS). NT, not tested. (H) P140 peptide administration reduces the percentage of total CD3 T cells among splenocytes. Splenocytes from 6-week-old MRL/lpr mice were collected 3 days after a single intraperitoneal injection of 200 µg P140 or saline (n=6/group). Representative results of two independent experiments are shown. *p<0.05, **p<0.01, ***p<0.001. NS, not significant (Mann–Whitney U test in panels A, B, F and H; Student t test in panel G). Bars, 100 µm.
P140 biodistribution after intravenous administration to MRL/lpr mice was monitored in real time by bioimaging in living mice. Alexa Fluor633-labelled P140 accumulated particularly in the lungs and spleen, a distribution that was not observed with an unrelated peptide (figure 1D; online supplementary figure S1; similar results were obtained in 6- and 12-week-old MRL/lpr mice). Fluorescence in the spleen was detectable 10–15 min after P140 injection and remained for at least 90–120 min. Similar results were observed in MRL/lpr mice that received rhodamine-labelled P140 intravenously (supplementary figure S2A). In the spleen, fluorescence was mainly localised in the lymphoid white pulp, mostly in the mantle and marginal zones (supplementary figure S2B). It co-localised essentially with splenic B cells and resident macrophages (figure 1E) as well as with CD11b- and F4/80-positive cells. The macrophage F4/80 antigen is absent from macrophages located in T-cell areas of the spleen and lymph nodes (LNs). Thus, P140 preferentially homes in organs enriched in activated B cells and, more generally, in antigen-presenting cells (APCs).
P140 administration significantly decreased peripheral hypercellularity in MRL/lpr mice (supplementary figure S3; no effect was seen with the ScP140 scrambled analogue). B cells, T cells (including γδT cells and DN T cells) and granulocytes/monocytes were affected. In CBA/J control mice, however, even an overdosage of P140 did not produce any observable effect while, in contrast, the glucocorticoid prednisolone, which is active in MRL/lpr mice,9 and immunosuppressant ciclosporin A induced a decrease of PBL counts in both CBA/J and MRL/lpr mice (figure 1F). Peripheral B and T cells from non-treated MRL/lpr mice and remaining peripheral B and T cells from P140-treated mice responded equally well ex vivo to B-cell mitogen and slightly less well (p=0.024) to T-cell mitogen (figure 1G). No effect of P140 was observed in CBA/J mice. This was not the case for ciclosporin A, which significantly affected the abilities of peripheral B and T cells from MRL/lpr and CBA/J mice to respond to mitogens, and prednisolone, which affected the abilities of CBA/J B and T cells to respond to mitogens. In the spleen, the proportion of T cells (including activated T cells) was significantly decreased after P140 treatment (p=0.0022; figure 1H). The percentage of B cells remained unchanged.
HSC70 and MHCII overexpression in MRL/lpr mice is reduced after P140 treatment
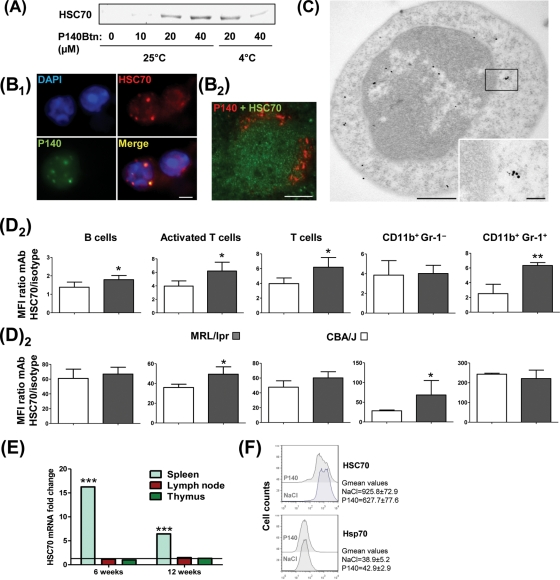
P140 binds to HSC70 protein both at 25°C and 4°C (figure 2A), suggesting that P140 recognises both intracellular and cell surface-expressed HSC70 (result obtained with splenocytes, LN cells and PBLs). Fluorescent staining of P140 and HSC70 co-localises in the cytoplasm of freshly isolated, permeabilised MRL/lpr PBLs 1 h after intravenous administration of Alexa Fluor488-labelled P140 (figure 2B1). This co-localisation was confirmed by immunofluorescence and immunoelectron microscopy (figure 2B2, C), and was mainly cytoplasmic.
Figure 2.
P140 peptide interacts with HSC70 and, in vivo, diminishes HSC70 overexpression in MRL/lpr lymphocytes. (A) Pull-down experiments showing the interaction of P140 peptide with HSC70 at 25°C and 4°C (representative results of five independent experiments). (B1) Co-localisation in permeabilised MRL/lpr dissociated splenocytes of P140 peptide and HSC70 examined 1 h after retro-orbital injection of 100 µg Alexa Fluor488-labelled P140 in NaCl. (B2) Co-localisation of P140 and HSC70 on spleen sections from MRL/lpr mice that received intravenously 200 µg rhodamine-labelled P140. (C) Co-localisation of P140 and HSC70 staining shown by immunoelectron microscopy (example with a single cell). HSC70, 15 nm gold particles; P140, 6 nm gold particles. (D) Fluorescence activated cell sorting analysis showing HSC70 expression at the surface of (D1) or intracellularly (D2) in different spleen cell subsets from CBA/J and 12-week-old MRL/lpr mice (n=5): B cells (B220+CD3), activated T cells included double negative T cells (B220+CD3), naïve T cells (B220–CD3), granulocytes/macrophages (CD11b+Gr-1+). HSC70 levels were expressed as the mean fluorescence intensity (MFI) for specific antibody/MFI for isotype-matched control antibody (Ab) ratio. Error bars represent SD. Representative results of at least two independent experiments. (E) Levels of HSC70 mRNA detected by qRT-PCR in lymph node, splenic and thymic cells from 6- and 12-week-old MRL/lpr mice compared with CBA/J mice. Mean of two experiments in triplicate, normalised to actin-β expression. (F) Intracellular staining of HSC70 and Hsp70 in splenic B cells collected from untreated or P140-treated (six times at daily intervals; 100 µg/injection/mouse) 6-week-old female MRL/lpr mice. *p<0.05, **p<0.01, ***p<0.001 (Student t test). Bars = 100 µm, except in (C) where bars = 500 and 100 nm in the picture and inset, respectively.
HSC70 is present at the surface of non-permeabilised MRL/lpr and CBA/J peripheral B and T cells (notably B220+/TCRβ+ activated T cells and DN T cells)5 as well as on splenic MRL/lpr B220–/CD3/CD11b+/MAC-1/Gr-1– monocytes/macrophages and B220–/CD3/CD11b+/Gr-1+ granulocytes (figure 2D1). In the spleen, surface and intracellular HSC70 expression levels were raised in MRL/lpr mice (figure 2D and supplementary figure S4; observed also in LN cells, notably at the surface). The HSC70 expression increase observed in the spleen of MRL/lpr mice appeared to be correlated with an increased HSC70 mRNA expression in MRL/lpr splenocytes (figure 2E; not visualised in LN cells and thymocytes). Interestingly, in MRL/lpr mice that received intravenous injections of 100 µg P140 daily over 6 days, HSC70 expression in splenic B cells was decreased by 32% (figure 2F; expression of inducible Hsp70/Hsp72 protein was not affected).
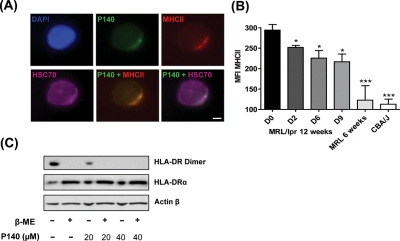
Interaction of HSC70 with antigen is an important component of the MHCII antigen processing pathway.12–14 In accordance with observations showing that in B cells HSC70 associates with HLA-DRB1*0401,10 and that HSC70 and MHCII molecules are associated in B-cell exosomes, for example,15 we found that HSC70 and MHCII molecules staining co-localises in permeabilised MRL/lpr splenocytes (figure 3A and supplementary figure S5). After in vitro incubation, co-localisation of P140 and MHCII molecules staining was observed in 25% of cells, and co-localisation of P140, HSC70 and MHCII molecules staining was visualised in 5–10% of cells. Interestingly, we found that after daily intraperitoneal injections of P140 into 12-week-old MRL/lpr mice, overexpression of MHCII molecules in spleen B cells significantly decreased (figure 3B). We thus examined further the effect of P140 on the stability of functional MHC molecules using Raji cells.13 In a dose-dependent manner, P140 hampered the formation of stable HLA-DRαβ dimers in these lymphoblast-like cells (figure 3C; MHCII monomer expression remained unaffected). Thus, P140 associates with both HSC70 and MHCII molecules and decreases directly or indirectly their surface expression on splenic B cells.
Figure 3.
P140 peptide interacts with major histocompatibility class II (MHCII) molecules and diminishes their in vivo expression. (A) Representative fluorescence microscopy pictures revealing the co-localisation, in permeabilised MRL/lpr dissociated splenocytes, of P140, HSC70 and MHCII staining after a 1 h incubation at 37°C (bar = 5 µm). See other examples in online supplementary figure S5. (B) Expression of MHCII molecules measured by fluorescence activated cell sorting analysis after daily P140 intraperitoneal administrations. MHCII expression measured in spleen B cells from 12-week-old MRL/lpr mice at days 0, 2, 6 and 9 after injection, and compared with the expression level in those cells collected from 6-week-old MRL/lpr and CBA/J mice (three mice/group tested individually). Error bars represent SD. Differences versus 12-week-old MRL/lpr mice at day 0 were determined using the Student t test. *p<0.05, ***p<0.001. MFI, mean fluorescence intensity. (C) P140 alters the stability of MHCII dimers in human lymphoma B cells. Raji cells were incubated for 24 h with P140 and then lysed with or without β-mercaptoethanol. Cell lysates were analysed by Western immunoblotting for expression of MHCII DRαβ dimers or MHCII DRα chain, compared with actin-β control. Representative results of two independent experiments.
P140 alters autophagic processes in MRL/lpr B cells
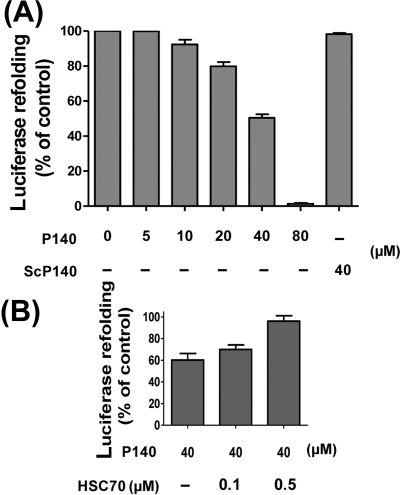
It has been shown earlier in B-cell lines that manipulating APC expression of HSC70 modifies the presentation of epitopes derived from intracellular proteins but not from exogenous antigens to CD4 T cells.14 Since HSC70 is involved in autophagy pathways, notably in chaperone-mediated autophagy,14 16–18 leading to endogenous MHCII loading, we explored this mechanism, hypothesising that P140 might disrupt normal functions of HSC70 in this catabolic pathway. First, using a refolding test of heat-denatured luciferase by rabbit reticulocyte lysate,7 8 we examined in vitro whether P140 could hamper folding properties of the HSC70 molecule. At 40 and 80 µM, P140 inhibited luciferase refolding by 50.2% and 100%, respectively (figure 4A; ScP140 showed no effect). This effect was reversed by adding an excess of exogenous HSC70 (figure 4B).
Figure 4.
P140 peptide alters folding properties of HSC70. (A) Luciferase was denatured at 40°C for 30 min and then refolded during 1 h at 30°C with rabbit reticulocyte lysate in the presence or absence of increasing concentrations of P140 or ScP140. The results (expressed as a percentage) compare activity of renatured luciferase in the presence or absence of peptide. (B) The addition of increasing amounts of HSC70 counterbalances P140 peptide inhibition of luciferase refolding in a dose-dependent manner. Mean (SD) of two independent measurements.
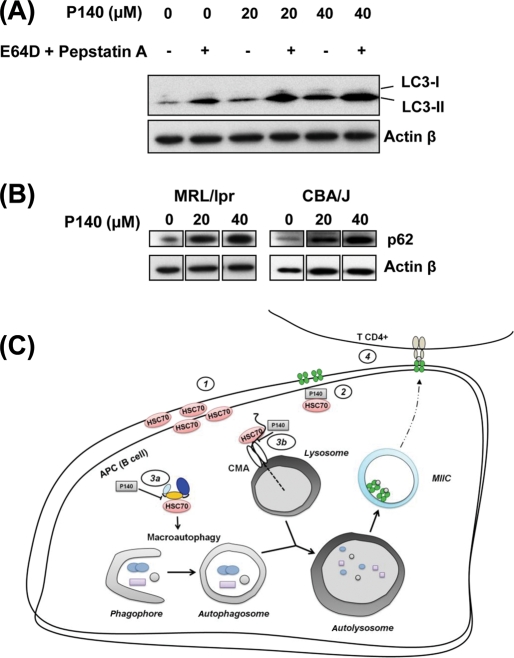
Next, we examined P140-treated cells for altered expression of lysosomal or autophagy pathways markers. We studied the conversion in MRL/lpr cells of the soluble form of microtubule-associated protein-1 light chain 3 (LC3-I), a mammalian homologue of yeast Atg8, into the hydrophobic, mostly membrane-associated form LC3-II, which is enriched in the autophagic vacuole fraction but is rapidly degraded by lysosomal proteases when the autophagic flux is intense.19 We observed that splenic B cells purified from 8-week-old MRL/lpr mice exhibited almost undetectable levels of LC3-I and rather low LC3-II levels, which were dramatically enhanced in the presence of lysosomal protease inhibitors E64D (calpain and cathepsin B inhibitor) and pepstatin A (cathepsin D inhibitor) (figure 5A). This reflects the existence of a constitutive autophagic flux in resting MRL/lpr B cells that can be blocked by specific inhibitors before the autolysosome formation, at the fused autophagosome–lysosome step.19 In the presence of P140, the basal accumulation of LC3-II expression was increased in a P140 concentration-dependent manner, and strikingly, the autophagic flux was proportionally less intense (figure 5A). This suggests that in MRL/lpr B cells, P140 downregulates autophagic flux at the autolysosome stage. Interestingly, this stage is also affected by E64D and pepstatin A, for example, as well as by chloroquine,20 21 an antimalarial drug used in lupus treatment.
Figure 5.
P140 peptide alters the autophagic pathway by slowing down the lysosomal activity in MRL/lpr B cells. (A) Western immunoblotting analysis with anti-LC3-II antibodies reveals increased appearance of lipidated LC3-II in B cells after 24 h of in vitro P140 treatment. Representative results of two independent experiments. (B) Accumulation of autophagy-selective substrate p62 visualised by Western immunoblotting in MRL/lpr or CBA/J B cells treated in vitro with P140. Representative results of two independent experiments. (C) A model by which P140 peptide interacts with HSC70, alters its properties and influences activation of autoreactive lymphocytes in lupus. (1) HSC70 is overexpressed both at the surface and intracellularly on lupus antigen-presenting cells (APC; B cells). (2) P140 hampers/inhibits chaperone properties of HSC70 protein. P140 (3a) disturbs a complex of co-chaperones involved in the regulation of macroautophagy (putative) and slows down the autophagic flux, and/or (3b) affects chaperone-mediated autophagy (CMA) leading to the alteration of (auto)antigens processing by lysosomal hydrolysis. (4) Subsequent delivery of antigens into MIIC compartments for class II presentation is altered and class II MHC molecules are destabilised, therefore compromising autoreactive CD4 T-cell response in a beneficial pathway for the P40-treated individual.
This result was confirmed by measuring the levels of p62 (SQSTM1/sequestosome-1), one of the specific substrates that are degraded through the autophagy-lysosomal pathway. p62 is selectively incorporated into autophagosomes via direct interaction with LC3.22 23 In a peptide dose-dependent manner, p62 accumulated in P140-treated MRL/lpr B cells (figure 5B; the same effect, although at a lower extent, observable in CBA/J B cells), again consistent with an effect of P140 on the steady-state autophagy in these cells.
Discussion
We present evidence here that P140 seems to interfere with a signalling pathway engaged in lysosomal degradation, leading to instability of MHCII molecules supposed to present endogenous antigens to autoreactive T cells in murine lupus. After intravenous injection into MRL/lpr mice, P140 concentrates mainly in the lungs and the spleen, and in the latter it co-localises essentially with B cells and resident macrophages of the lymphoid white pulp. Its localisation (if any) is poor in LNs and thymus. This suggests that P140 might possess a certain degree of specificity for targeting some but not all tissues/organs containing HSC70+ cells, and within these tissues and organs, again, it seems to target certain, but not all, HSC70+ cell subsets.
We found that HSC70, which is present at the surface of splenic and LN MRL/lpr and CBA/J B cells, T cells (activated, non-activated and DN T cells) and granulocytes/monocytes is progressively overexpressed in MRL/lpr spleen and LN cells. Intravenous administration of P140 into lupus-prone MRL/lpr mice leads to a decreased expression of HSC70. MHCII molecule levels are also diminished. These data reveal that after downstream P140 binding to HSC70, a series of molecular events take place affecting the APC functions of MRL/lpr B cells, leading finally to a decreased expression or stability of MHCII molecules and a potential reduction of autoreactive T-cell signalling and priming.
Importantly, normal lymphocytes seem to be saved from this process, possibly because the density of HSC70 molecules is insufficient at the surface of normal APCs. The number of peripheral cells is not affected in P140-treated CBA/J mice, and B and T cells proliferate normally in response to specific mitogens. We also found that the remaining B and T cells from P140-treated mice responded normally to mitogens. These data add to earlier studies showing that P140-treated and untreated MRL/lpr mice mount equivalent immune responses to a flu virus challenge.6
HSC70 protein displays numerous housekeeping chaperoning functions. It is notably involved in nascent protein folding, prevention of protein aggregation under stress conditions, modulation of the assembly/disassembly of protein complexes and stabilisation of RNA messengers. HSC70 protein acts with a series of co-chaperones including Nag-4, CHIP, hip, Bcl-2-associated athanogene BAG-1, BAG-3 and Hsp40. Studies have suggested that depending on the cytokine environment, HSC70 preferentially associates with certain of these co-chaperones leading to different effects. We found that P140 inhibits the ability of HSC70 to refold denatured protein. It might therefore disrupt certain interactions of HSC70 with cofactors and chaperones. Future studies should provide insight into this topic and determine if this is relevant to some effects described in this study and lupus phenotype.
HSC70 is central in protein translocation across membranes and lysosomal transport of proteins.24 25 It plays a pivotal role in chaperone-mediated autophagy in acting as a chaperone for unfolded substrate protein in association with the membrane receptor Lamp2a.14 16–18 26 In certain conditions, such as an elevation of co-chaperone BAG-3 level, HSC70 can also associate with p62.27 Proper turnover of p62 by autophagy is critical, notably to prevent spontaneous aggregate formation.23 28–31 This protein is regarded as a key signalling player in the cell survival/death balance. Thus, certainly the most remarkable information raised in this work, is the striking effect of P140 on lysosomal degradation and class II antigen presentation (figure 5C). P140 directly impairs the molecular chaperoning properties of HSC70 by altering its ability to promote proper folding of polypeptide. In MRL/lpr B cells, P140 increases the accumulation of the autophagy markers p62/SQSTM1 and LC3-II, consistent with a downregulation of lysosomal degradation at the autolysosome stage or with a lysosomal dysfunction. Moreover, the expression of stable MHCII molecules is dramatically affected. This might explain our earlier observations showing a decreased T-cell response to various T-cell autoepitopes in P140-treated MRL/lpr mice6 32 and the absence of lupus patient's PBL proliferation ex vivo in the presence of P140.4
Autophagy processes have not been explored in lupus. Although much has still to be determined to fully understand the impact of this complex pathway in MHCII presentation, inflammation and central or peripheral tolerance processes, our studies show that a peptide that can modulate lysosomal degradation can be beneficial in MRL/lpr mice by reducing autoreactive T-cell and B-cell activation. Of importance, P140 peptide exerts its effects without affecting normal B and T cells, possibly thanks to the expression levels of HSC70, which seems to act as a sensor receptor on MRL/lpr B cells.
Acknowledgments
The authors are indebted to Monique Duval for valuable assistance. The authors acknowledge the Imaging facility (Plateforme Inter-Unités de Microscopie et d'Imagerie Cellulaire Strasbourg-Esplanade).
Footnotes
Funding This research was funded by the Centre National de la Recherche Scientifique (CNRS), Région Alsace and ImmuPharma France.
Competing interests None.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Monneaux F, Lozano JM, Patarroyo ME, et al. T cell recognition and therapeutic effect of a phosphorylated synthetic peptide of the 70K snRNP protein administered in MR/lpr mice. Eur J Immunol 2003;33:287–96 [DOI] [PubMed] [Google Scholar]
- 2.Muller S, Monneaux F, Schall N, et al. Spliceosomal peptide P140 for immunotherapy of systemic lupus erythematosus: results of an early phase II clinical trial. Arthritis Rheum 2008;58:3873–83 [DOI] [PubMed] [Google Scholar]
- 3.Dieker J, Cisterna B, Monneaux F, et al. Apoptosis-linked changes in the phosphorylation status and subcellular localization of the spliceosomal autoantigen U1-70K. Cell Death Differ 2008;15:793–804 [DOI] [PubMed] [Google Scholar]
- 4.Monneaux F, Hoebeke J, Sordet C, et al. Selective modulation of CD4+ T cells from lupus patients by a promiscuous, protective peptide analog. J Immunol 2005;175:5839–47 [DOI] [PubMed] [Google Scholar]
- 5.Page N, Schall N, Strub JM, et al. The spliceosomal phosphopeptide P140 controls the lupus disease by interacting with the HSC70 protein and via a mechanism mediated by gammadelta T cells. PLoS ONE 2009;4:e5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monneaux F, Parietti V, Briand JP, et al. Importance of spliceosomal RNP1 motif for intermolecular T-B cell spreading and tolerance restoration in lupus. Arthritis Res Ther 2007;9:R111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freeman BC, Myers MP, Schumacher R, et al. Identification of a regulatory motif in Hsp70 that affects ATPase activity, substrate binding and interaction with HDJ-1. EMBO J 1995;14:2281–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terada K, Kanazawa M, Bukau B, et al. The human DnaJ homologue dj2 facilitates mitochondrial protein import and luciferase refolding. J Cell Biol 1997;139:1089–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mae T, Nemoto K, Sugawara Y, et al. Therapeutic studies of the combination of deoxyspergualin and prednisolone in MRL/lpr mice with advanced lupus-like disease. J Antibiot 1994;47:90–4 [DOI] [PubMed] [Google Scholar]
- 10.Auger I, Escola JM, Gorvel JP, et al. HLA-DR4 and HLA-DR10 motifs that carry susceptibility to rheumatoid arthritis bind 70-kD heat shock proteins. Nat Med 1996;2:306–10 [DOI] [PubMed] [Google Scholar]
- 11.Panjwani N, Akbari O, Garcia S, et al. The HSC73 molecular chaperone: involvement in MHC class II antigen presentation. J Immunol 1999;163:1936–42. [PubMed] [Google Scholar]
- 12.Srivastava P. Roles of heat-shock proteins in innate and adaptive immunity. Nat Rev Immunol 2002;2:185–94 [DOI] [PubMed] [Google Scholar]
- 13.Houlihan JL, Metzler JJ, Blum JS. HSP90alpha and HSP90beta isoforms selectively modulate MHC class II antigen presentation in B cells. J Immunol 2009;182:7451–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou D, Li P, Lin Y, et al. Lamp-2a facilitates MHC class II presentation of cytoplasmic antigens. Immunity 2005;22:571–81 [DOI] [PubMed] [Google Scholar]
- 15.Buschow SI, van Balkom BW, Aalberts M, et al. MHC class II-associated proteins in B-cell exosomes and potential functional implications for exosome biogenesis. Immunol Cell Biol 2010;88:1–6 [DOI] [PubMed] [Google Scholar]
- 16.Kon M, Cuervo AM. Chaperone-mediated autophagy in health and disease. FEBS Lett 2010;584:1399–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crotzer VL, Blum JS. Autophagy and its role in MHC-mediated antigen presentation. J Immunol 2009;182:3335–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Münz C. Enhancing immunity through autophagy. Annu Rev Immunol 2009;27:423–49 [DOI] [PubMed] [Google Scholar]
- 19.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell 2010;140:313–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science 2004;306:990–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartleben B, Gödel M, Meyer-Schwesinger C, et al. Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J Clin Invest 2010;120:1084–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bjørkøy G, Lamark T, Brech A, et al. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol 2005;171:603–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Komatsu M, Waguri S, Koike M, et al. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell 2007;131:1149–63 [DOI] [PubMed] [Google Scholar]
- 24.Beckmann RP, Mizzen LE, Welch WJ. Interaction of Hsp 70 with newly synthesized proteins: implications for protein folding and assembly. Science 1990;248:850–4 [DOI] [PubMed] [Google Scholar]
- 25.Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci 2005;62:670–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nedjic J, Aichinger M, Mizushima N, et al. Macroautophagy, endogenous MHC II loading and T cell selection: the benefits of breaking the rules. Curr Opin Immunol 2009;21:92–7 [DOI] [PubMed] [Google Scholar]
- 27.Arndt V, Dick N, Tawo R, et al. Chaperone-assisted selective autophagy is essential for muscle maintenance. Curr Biol 2010;20:143–8 [DOI] [PubMed] [Google Scholar]
- 28.Moscat J, Diaz-Meco MT. p62 at the crossroads of autophagy, apoptosis, and cancer. Cell 2009;137:1001–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu HB, Kielczewska A, Rozek A, et al. Sequestosome-1/p62 is the key intracellular target of innate defense regulator peptide. J Biol Chem 2009;284:36007–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Komatsu M, Ichimura Y. Physiological significance of selective degradation of p62 by autophagy. FEBS Lett 2010;584:1374–8 [DOI] [PubMed] [Google Scholar]
- 31.Nakamura K, Kimple AJ, Siderovski DP, et al. PB1 domain interaction of p62/sequestosome 1 and MEKK3 regulates NF-kappaB activation. J Biol Chem 2010;285:2077–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monneaux F, Parietti V, Briand JP, et al. Intramolecular T cell spreading in unprimed MRL/lpr mice: importance of the U1-70k protein sequence 131-151. Arthritis Rheum 2004;50:3232–8 [DOI] [PubMed] [Google Scholar]