ABSTRACT
Treatment algorithms and survival for patients with metastatic colorectal cancer have changed dramatically over the past decade, largely due to the advent of molecularly targeted agents. The lessons we have learned with the integration of bevacizumab and cetuximab/panitumumab into standard therapy is that meaningful clinical end points can be achieved, and more patients with metastatic colorectal cancer are being cured or kept alive with a reasonable quality of life due to these new agents. As we enter this second decade of “modern therapy” for metastatic colorectal cancer, an ever-increasing number of new agents aimed at a variety of targets believed to promote cancer cell growth are being tested in clinical trials, and dozens of studies of novel targeted therapies are ongoing. Moreover, during the next decade, we can expect to see an explosion of new agents that will likely improve clinical outcomes further. This review focuses on molecularly targeted agents that are being used regularly in the treatment of colorectal cancer and highlights a number of new agents/targets that are being explored and appear promising in phase I or early phase II trials.
Colorectal cancer remains the second leading cause of cancer death in the United States.1 Survival for patients with metastatic colorectal cancer, however, has improved dramatically over the past decade. In the mid 1990s, the median overall survival (OS) for patients with metastatic colon cancer treated with a 5-fluorouracil (5-FU)-based regimen was only about 12 months.2 With the addition of irinotecan and oxaliplatin, OS increased to approximately 18 months,3 but it has really been the addition of biologic agents that led to a substantial jump in OS, which approaches 30 months in some studies.4
Along with markedly improved OS, a corresponding leap in response rates has occurred, increasing the number of patients oncologists can reconsider as candidates for metastasectomy with potential curative intent. The dramatic benefits seen with biologic agents have spurred a number of ongoing studies examining the benefits of these agents in the adjuvant setting. This review focuses on molecularly targeted agents that are being used regularly in the treatment of colorectal cancer and highlights a number of new agents/targets that are being explored and appear promising in phase I or early phase II trials.
BEVACIZUMAB AND THE ANTIANGIOGENIC AGENTS
Bevacizumab
Researchers have recognized for decades that tumor growth requires the recruitment of new blood vessels (angiogenesis), a process that does not occur in the normal, healthy adult except in the context of wound repair, tissue remodeling (such as during menstruation), or inflammation.5 Angiogenesis is a multistep process that involves vasodilation, enhanced vessel permeability, stromal degradation, and endothelial cell proliferation and migration, resulting in the formation of a new or extended capillary.6 In neoplastic tissues, this highly regulated process is disordered, resulting in leaky, tortuous vessels that branch excessively. Microcirculation is inefficient, rendering the area hypoxic and acidotic, and creating higher hydrostatic pressures in the local stroma (which hampers diffusion of chemotherapy agents to the target tissues).
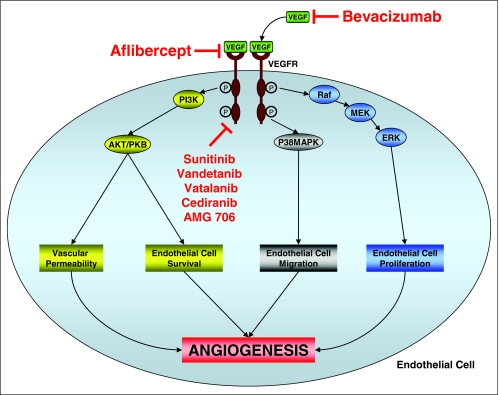
The process of angiogenesis can be regulated by a number of growth factors and their cognate receptors such as platelet-derived growth factor, fibroblast growth factor, and transforming growth factor alpha (Figure 1). The most studied pathway, however, involves vascular endothelial of growth factors (VEGFs) and their receptors (VEGFRs).7
Figure 1.
The vascular endothelial growth factor (VEGF) signaling pathway
The VEGF family of growth factors is composed of six members, VEGF-A through E, and placenta growth factor-1 and -2, with VEGF-A (commonly referred to simply as VEGF) being the most prominent mediator of angiogenesis.7 VEGFs are soluble growth factors secreted by tumor cells and stromal cells that act by binding to the extracellular domain of the VEGFRs. The intracellular domain of these receptors contains catalytic tyrosine kinase domains. Binding to the VEGFs results in the activation of a number of intracellular signaling cascades that result in endothelial cell survival, proliferation, migration, differentiation, and increased vascular permeability. It has been established that the level of VEGF expression likely also plays an important role in determining the pace and breadth of the development of metastases, given that overexpression of VEGF correlates with tumor progression and a worse overall prognosis in colorectal cancer.8,9
In 1971, Judah Folkman hypothesized that the development of an agent that prevents angiogenesis could have dramatic implications for cancer treatment.10 While it took several decades to understand the underlying biology, that hypothesis is beginning to bear fruit, to the clinical benefit of patients. A number of antiangiogenesis agents have been approved or are undergoing clinical testing. The first such drug approved was bevacizumab, a monoclonal antibody directed against VEGF-A. The presumed benefit of such an agent was that it would inhibit angiogenesis and thus prevent tumor growth, and while this may be at least partially true, bevacizumab as a single agent only induces minimal response rates.11
The true benefit of bevacizumab was realized when used in conjunction with cytotoxic chemotherapy and may be due to an additive suppression of tumor cell growth and induction of apoptosis. Bevacizumab also “normalizes” tumor blood vessel architecture and decreases intratumoral hydrostatic pressure, thus enhancing the delivery of anticancer agents to the tumor.12
Bevacizumab was first approved based on its ability to prolong survival in patients with metastatic colorectal cancer. In a pivotal trial, Hurwitz et al demonstrated increased response rates (RR), progression-free survival (PFS), and OS when bevacizumab was combined with the IFL regimen (irinotecan, 5-FU bolus, and leucovorin) in patients with previously untreated metastatic colorectal cancer.13 IFL later proved to be an inferior regimen and is no longer used, but the addition of bevacizumab to chemotherapy has consistently increased RR and survival rates in most regimens tested, including in second-line and even third-line therapy. In fact the recently reported BRiTE registry data demonstrate that patients who continued bevacizumab throughout treatment had a significantly greater OS compared with those in whom bevacizumab had been discontinued (31.8 vs.19.9 months, HR 0.48, P <.001).4
One of the benefits of better RR in patients with metastatic colorectal cancer has been that an increasing number of patients with liver metastases are rendered resectable with the intent to cure. Concerns arose that the use of angiogenesis inhibitors could complicate postoperative wound healing in patients undergoing hepatic resection. However, two studies (one retrospective review by Kesmodel14 from M D Anderson Cancer Center and one prospective, nonrandomized phase II study by Gruenberger15 from Vienna, Austria) have clearly demonstrated no increase in the rates of wound healing as a complication of surgery in patients undergoing partial hepatectomies who had received prior bevacizumab. It should be noted that in both studies, patients had been off bevacizumab therapy for at least 1 month prior to surgery. This simple precaution is a logical guideline to follow in this setting.
However, there have been notable exceptions to the benefits of adding bevacizumab to chemotherapy. First, in patients with previously untreated metastatic colorectal cancer, the addition of bevacizumab to FOLFOX (oxaliplatin/5-FU [bolus and continuous infusion]/leucovorin) failed to increase RR or OS over FOLFOX alone, though there was an improved PFS benefit.16 Second, the recently presented C08 trial failed to demonstrate any benefit from the addition of bevacizumab to FOLFOX in the adjuvant setting, in patients with resected non-metastatic CRC.17
Bevacizumab is not without side effects. The most serious potential toxicities observed with the use of bevacizumab include gastrointestinal perforation (1.5%) and arterial thromboses, including myocardial infarctions and strokes (2.5% above baseline).18 Approximately 25% of patients will also develop hypertension, with 10% requiring medical therapy.
Other Antiangiogenesis Agents and Targets
Several other antiangiogenesis agents are currently in development. Aflibercept is a recombinant fusion molecule of the human VEGF receptor extracellular domain and the Fc portion of human IgG1, and is a potent inhibitor of VEGF. Aflibercept demonstrated single-agent activity in a phase II trial in 51 patients with refractory metastatic colorectal cancer, with a disease control rate at 4 months of approximately 30%.19 Several phase I trials have proven this agent to be safe when combined with standard chemotherapy, and trials assessing efficacy of combination therapy are ongoing.
Several oral agents are also in development, though for reasons that are unclear, the oral VEGFR antagonists do not seem to add much benefit to standard chemotherapy. Vatalanib (PTK/ZK222584) is an inhibitor of VEGFR-1, -2, and -3–mediated angiogenesis and reduces tumor growth and metastasis in preclinical models. CONFIRM-1, a randomized phase III trial of FOLFOX vs. FOLFOX/vatalanib, failed to demonstrate any significant improvement in progression-free survival with vatalanib, though response rates were slightly higher in patients treated with vatalanib.20
Sunitinib is an oral multi-tyrosine kinase inhibitor targeting the VEGFRs, PDGFR, c-KIT, RET, and LT3 that is approved for use in patients with renal cell cancer. However, in a phase II trial in refractory patients, only 1 of 84 patients responded (1.1%) while 13 had stable disease for at least 6 months.21 In a randomized phase II study of irinotecan, cetuximab, and either bevacizumab or sunitinib, the RR (8% vs. 0%), PFS (8.7 vs. 3.2 months), and OS (12.1 vs. 8.7 months) were significantly higher in patients who received bevacizumab, while the addition of sunitinib was moderately more toxic.
Additionally, in a phase II study of FOLFIRI (irinotecan/5-FU [bolus and continuous infusion]/leucovorin) with or without vandetanib, no significant benefit was seen with the addition of vandetanib. Additional antiangiogenic agents, include cediranib (AZD2171) and AMG 706, which have both demonstrated stable disease in patients with metastatic colorectal cancer in phase I trials, are now being studied in phase II trials.22,23
EGFR ANTAGONISTS
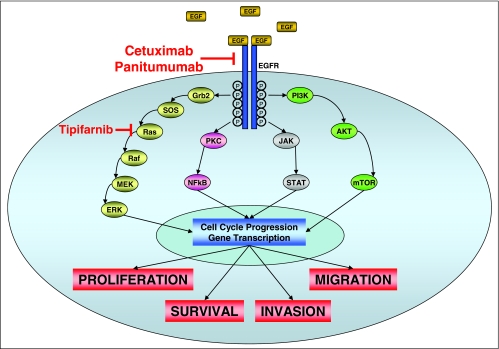
The epidermal growth factor receptor (EGFR) is a member of the HER family of receptor tyrosine kinases that includes the EGFR itself (ErbB1/HER1), ErbB2 (HER2/neu), ErbB3 (HER3), and ErbB4 (HER4).24 These proteins are classic membrane-bound tyrosine kinase receptors, whose activation is typically ligand dependent, with the principal ligands being EGF and TGF-α (Figure 2). Receptor activation results in homo- or heterodimerization and autophosphorylation of c-terminal tyrosine residues. Receptor activation enables the docking of cytoplasmic proteins that bind to specific phosphotyrosine residues, and initiate several cell signaling pathways. These pathways include the Ras-Raf-MAPK pathway, the PI3K-AKT pathway, the protein kinase C pathway, the STAT pathway, and the src kinase pathway, all of which play important roles in tumor cell proliferation, invasion, migration, and inhibition of apoptosis. EGFR activation does not initiate linear downstream pathway signaling, but rather can activate multiple pathways that cross-connect intracellularly.25
Figure 2.
The endothelial growth factor receptor (EGFR) signaling pathway
Anti-EGFR therapies include monoclonal antibodies (mAbs) that recognize the EGFR and small molecule inhibitors of EGFR tyrosine kinase activity (TKIs).25 The mAb cetuximab prevents receptor dimerization through steric inhibition of the extracellular domain. Cetuximab also promotes receptor internalization and degradation without receptor activation, resulting in receptor down-regulation and reduced cell surface expression levels of EGFR. Cetuximab also blocks the transport of EGFR into the nucleus, thus inhibiting any direct affects on DNA transcription and/or repair. Finally, cetuximab has the potential to kill target cells by mediating antibody-dependent, cell-mediated cytotoxicity (ADCC) and complement fixation. The TKIs are competitive inhibitors of adenosine triphosphate (ATP). They block the enzymatic activity of the intracellular domain of EGFR.
Cetuximab and panitumumab are the two monoclonal antibodies to EGFR approved for the treatment of colorectal cancer. Based on the pivotal BOND 1 study, cetuximab was approved by the US Food and Drug Administration (FDA) in February 2004 in combination with irinotecan in irinotecan-refractory disease, or as a single agent in patients intolerant to irinotecan.26 In patients who had progressed on or were refractory to prior irinotecan-containing regimens, cetuximab induced a response in 11% of patients with a median time to progression (TTP) of 1.5 months when used alone. Furthermore, 23% of patients responded to a combination of cetuximab plus irinotecan, with a median TTP of 4.1 months. In another trial the efficacy of single-agent cetuximab was demonstrated in the third line setting, resulting in a 10% response rate.27
Panitumumab obtained FDA approval based on a similar study, where 8% of patients with EGFR-expressing colorectal cancer whose disease had progressed on fluoropyrimidine-, oxaliplatin-, and irinotecan-containing chemotherapy regimens.28
Despite the clinical activities and safety data of the anti-EGFR antibodies in colorectal cancer, TKIs as single agents showed minimal activity in metastatic colorectal cancer. In patients with metastatic colorectal cancer treated with a combination of a TKI and fluoropyrimidine-, oxaliplatin-, and irinotecan-based regimens, the clinical response rate ranged from 24% to 74% in phase II studies. However, TKIs were found to increase grade 3/4 toxicities and some of the trials had to be closed prematurely due to adverse effects. To confirm the clinical benefit of adding TKIs to chemotherapy, a phase III study of chemotherapy plus bevacizumab with or without erlotinib in metastatic colorectal cancer is ongoing, with a target accrual of 640 patients.
Moreover, the benefits of first line anti-EGFR therapy have shown great promise. Phase II data of cetuximab combined with irinotecan or oxaliplatin have demonstrated RR as high as 77%. However, a large phase II trial of panitumumab plus standard chemotherapy with or without bevacizumab revealed a worse outcome in patients who received panitumumab.29 Thus, the role of anti-EGFR agents in first-line therapy of metastatic colorectal cancer is still unclear, and several large studies are ongoing to address that question.
Similarly, the role of cetuximab in adjuvant treatment of colorectal cancer is being evaluated with two randomized phase III clinical trials, NCCTG/INT N0147 and PETACC-8, and in neoadjuvant therapy for rectal cancer in the EXPERT-C clinical trial. The anti-EGFR mAbs have been very well tolerated, but more than 80% of patients will develop an acneiform rash. Surprisingly, the RR, time to progression (TTP) and OS increase with an increasing grade of skin toxicity, clarifying development of the acneiform rash as a surrogate marker of efficacy. By contrast, EGFR expression levels show no correlation with response to therapy.
Perhaps the most intriguing development in predicting response to anti-EGFR mAb therapy has been the exploration of the role of mutant k-ras as a predictor of response. K-ras is a small serine-threonine kinase that is farnesylated and inserted into the cell membrane. It is activated just downstream of activation of the EGFR (or other TK receptors) and propagates further signaling events. Researchers have retrospectively evaluated patient tumor samples from two large studies, one using cetuximab and one using panitumumab both as single agents in refractory disease, for the mutational status of the k-ras gene.
Lievre et al identified a k-ras mutation in 27% of patients, with a response rate of 0% in tumors with mutated k-ras vs. 40% in tumors with wild type k-ras and a median OS of 10.1 vs. 14.3 months, respectively.30 Similarly, Amado et al identified k-ras mutations in 43% of patients with, again, no patients responding who had mutated k-ras vs. 17% of patients with wild type k-ras responding.31 As a result of these findings virtually all clinical trials using the anti-EGFR mAbs have been put on hold pending amendments to take k-ras mutational status into account. K-ras mutation testing is commercially available and we are routinely assessing k-ras mutational status to guide therapeutic decisions.
Finally, attempts to target k-ras specifically in metastatic colorectal cancer have not demonstrated much clinical activity. Tipifarnib is a farnesyl transferase inhibitor that demonstrated no single agent activity in a phase II study.32 In the race to develop novel therapies for metastatic colorectal cancer, agents that act to inhibit cell signaling downstream of k-ras are being tested actively, and such agents (including tipifarnib) are likely to be most effective when used in combination with other cytotoxic and anti-EGFR/anti-angiogenesis agents.
NOVEL THERAPIES
mTOR Inhibitors
Mammalian Target of Rapamycin (mTOR) is a multifunctional serine-threonine kinase member of the phosphatidyl inositol 3' kinase family.33 mTOR is activated in response to growth stimuli such as nutrients and/or growth factors and the growth factor receptors. Stimulation of mTOR results in the phosphorylation of translational regulation factors such as eukaryotic initiation factor 4E-binding protein and p70s6 kinase. These events stimulate cell growth and proliferation. By contrast, mTOR inhibition leads to cell cycle arrest in late G1 through down-regulation of cyclin/CDK complexes, and accumulation of the cell cycle inhibitor p27. mTOR inhibitors also block proliferation of endothelial and vascular smooth muscle cells, thus inhibiting angiogenesis. Finally, inhibition of mTOR can induce apoptosis.
The mTOR inhibitors are analogs of rapamycin and act by binding to the immunophilin FK506/rapamycin-binding protein, which binds to mTOR and inhibits its function. Preclinical models have revealed the efficacy of the mTOR inhibitors, particularly in the absence of the PTEN tumor suppressor gene, and the mTOR inhibitor temsirolimus is FDA approved for the treatment of patients with poor prognosis metastatic renal cell.34
There have been several early studies of the mTOR inhibitors suggesting some benefit in patients with metastatic colorectal cancer. In one phase I trial of RAD-001, another mTOR inhibitor, one partial response was seen in a patient with colorectal cancer (lasting 5.3 months with a disease control period 9 months).35 In the subsequent phase II study, a disease control rate (defined as RR plus rate of stable disease) of 25%, with an OS of 5.9 months was achieved. The combination was somewhat toxic, though with fatigue, cytopenias and nausea/vomiting/dehydration being the main adverse events.
The mTOR inhibitors will likely be most effective when combined with traditional chemotherapy, and in one phase I clinical trial, the combination of RAD-001 with 5-fluorouracil in a refractory colorectal cancer patient population resulted in one PR lasting 7.4 months.36 Several phase I and phase II studies of the mTOR inhibitors in combination with chemotherapy are currently under way.
Protein Kinase C Antagonists
Protein Kinase C (PKC) is a large family of serine/threonine kinases involved in a variety of cellular processes.37 Most notably here, PKC is activated downstream from the receptor tyrosine kinases such as the EGFR and VEGFRs, and activation of PKC is considered a key part of the cell signaling cascade that leads to tumor growth and survival. PKC expression and/or activity is also elevated in many cancer types, including colorectal cancer.
Enzastaurin is a potent selective serine/threonine kinase inhibitor that targets PKC as well as the PI3K/AKT and GSK3. Enzastaurin has proven to be safe either as a single agent or in combination with 5-FU or bevacizumab,38–40 but most intriguing was a disease control rate of 53%, with no significant toxicities in the enzastaurin plus bevacizumab study. Currently, a phase II trial of enzastaurin plus bevacizumab plus 5-FU is ongoing and showing clinical promise.
Src
The nonreceptor tyrosine kinase Src was the first identified proto-oncogene. Src is a nonreceptor tyrosine kinase that plays a critical role in cancer cell proliferation and invasion, angiogenesis, and the regulation of apoptosis.41 Src activation also occurs downstream from a number of cell signaling pathways, including growth factor receptor activation. Enhanced Src activity is observed in 80% of colorectal cancers, and very high levels of Src activity correlate with the metastatic phenotype, poor prognosis, and resistance to chemotherapy.
AZD0530 is an orally active inhibitor of Src that has demonstrated pre-clinical activity against colon cancer metastases in vivo, and is now undergoing phase II testing in patients with metastatic colorectal cancer. In the phase I trial, 81 patients were tested, 28 of whom had colorectal cancer.42 Eleven patients were on study for >12 weeks, five of whom had colorectal cancer, demonstrating some promise of activity, at least of disease stabilization in this patient population. Furthermore, a study of the approved Src inhibitor dasatinib in combination with FOLFOX and erbitux is ongoing and seems promising.
Kinesin Spindle Protein
Mitotic kinesins, such as KSP are involved in the establishment and function of the mitotic spindle, providing the propulsive forces required to separate centrosomes during prophase.43 Mitotic kinesins are preferentially expressed in proliferating cells, and thus are an attractive molecular target for anticancer therapy.44 Ispinesib (SB-715992) is a potent and selective small molecule inhibitor of KSP. Two phase I studies of ispinesib have been performed with the dose limiting toxicities being related to neutropenia.45,46 Taken together, a very heavily pretreated population of 20 patients with metastatic colorectal cancer were treated, and stable disease lasting at least 3 months was achieved in 2 patients. Phase II studies of ispinesib as a single agent in metastatic colorectal cancer are ongoing, but surprisingly, no studies combining ispinesib with colorectal-cancer –directed chemotherapy have been performed.
CONCLUSIONS
The treatment algorithms and the expectations for patients with metastatic colorectal cancer have changed dramatically over the past decade, owing greatly to the addition of molecularly targeted agents. As we enter this second decade of “modern therapy” for metastatic colorectal cancer, an ever-increasing number of new agents aimed at a variety of targets believed to promote cancer cell growth are being tested in clinical trials. These targets include other tyrosine kinases such as FLT3, the histone deacetylase inhibitors, the hypomethylating agents, and differentiating agents such as PPAR gamma agonists and delta/notch antagonists.
All of these agents have demonstrated some degree of activity preclinically (which highlights one of the major pitfalls of using such preclinical models), and many of them have demonstrated a hint (or more) of activity in colon cancer patients who have been enrolled in phase I studies. As a result, dozens of clinical trials of novel targeted therapies are ongoing. Typically these are single-agent studies, often designed to demonstrate some degree of clinical activity in refractory patients in order to move forward into first- or second-line treatment.
Alternatively, many phase II studies directly assess the efficacy of the novel agent in combination with standard therapy. In truth, many of these agents will ultimately prove ineffective in the treatment of metastatic colorectal cancer. However, the lessons we have learned over the past decade with the integration of bevacizumab and cetuximab/panitumumab into standard therapy for patients with metastatic colorectal cancer is that meaningful clinical end points can be achieved, and more patients are being cured, or kept alive with a reasonable quality of life due to these new agents. In the next decade we will likely see an explosion of new agents that will likely improve clinical outcomes further.
Footnotes
Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
REFERENCES
- 1. American Cancer Society: Cancer Facts and Figures 2010. Atlanta, Ga: American Cancer Society, 2010 [Google Scholar]
- 2. Saltz LB: Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med 343:905–914, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Goldberg RM: A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 22:23–30, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Grothey A: Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE). J Clin Oncol 26:5326–5334, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Kerbel RS: Tumor angiogenesis. N Engl J Med 358:2039–49, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tassi E, Wellstein A: The angiogenic switch molecule, secreted FGF-binding protein, an indicator of early stages of pancreatic and colorectal adenocarcinoma. Semin Oncol 33:S50–S56, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hicklin DJ, Ellis LM: Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol 23:1011–1027, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Cascinu S, Staccioli MP, Gasparini G, et al. : Expression of vascular endothelial growth factor can predict event-free survival in stage II colon cancer. Clin Cancer Res 6:2803–2807, 2000 [PubMed] [Google Scholar]
- 9. Lee JC, Chow NH, Wang ST, et al. : Prognostic value of vascular endothelial growth factor expression in colorectal cancer patients. Eur J Cancer 36:748–753, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Folkman J: Tumor angiogenesis: therapeutic implications. N Engl J Med 285:1182–1186, 1971 [DOI] [PubMed] [Google Scholar]
- 11. Gordon MS, Margolin K, Talpaz M, et al. : Phase I safety and pharmacokinetic study of recombinant human anti-vascular endothelial growth factor in patients with advanced cancer. J Clin Oncol 19:843–850, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Ellis LM: Mechanisms of action of bevacizumab as a component of therapy for metastatic colorectal cancer. Semin Oncol 33:S1–S7, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Hurwitz H, Fehrenbacher L, Novotny W, et al. : Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350:2335–2342, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Kesmodel SB, Ellis LM, Lin E, et al. : Complication rates following hepatic surgery in patients receiving neoadjuvant bevacizumab (BV) for colorectal cancer (CRC) liver metastases. 2007 Gastrointestinal Cancers Symposium, 2007 (abstr 234) [Google Scholar]
- 15. Gruenberger B, Tamandl D, Schueller J, et al. : Bevacizumab, capecitabine, and oxaliplatin as neoadjuvant therapy for patients with potentially curable metastatic colorectal cancer. J Clin Oncol 26:1830–1835, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Saltz LB, Clarke S, Diaz-Rubio E, et al. : Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 26:2013–2019, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Wolmark N, Yothers G, O'Connell MJ, et al. : A phase III trial comparing mFOLFOX6 to mFOLFOX6 plus bevacizumab in stage II or III carcinoma of the colon: results of NSABP Protocol C-08. J Clin Oncol 27:, 2009. (abstr LBA4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Prat A, Casado E, Cortes J: New approaches in angiogenic targeting for colorectal cancer. World J Gastroenterol 13:5857–5866, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tang P, Cohen SJ, Bjarnason GA, et al. : Phase II trial of aflibercept (VEGF Trap) in previously treated patients with metastatic colorectal cancer (MCRC): a PMH phase II consortium trial. J Clin Oncol 26: 2008. (May 20 suppl; abstr 4027) [Google Scholar]
- 20. Hecht JR, Trarbach T, Jaeger E, et al. : A randomized, double-blind, placebo-controlled, phase III study in patients (Pts) with metastatic adenocarcinoma of the colon or rectum receiving first-line chemotherapy with oxaliplatin/5-fluorouracil/leucovorin and PTK787/ZK 222584 or placebo (CONFIRM-1) 2005 ASCO Annual Meeting Proceedings. J Clin Oncol 23:16S, 2005. (abstr LBA3) [Google Scholar]
- 21. Saltz LB, Rosen LS, Marshall JL, et al. : Phase II trial of sunitinib in patients with metastatic colorectal cancer after failure of standard therapy. J Clin Oncol 25:4793–4799, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Rosen LS, Kurzrock R, Mulay M, et al. : Safety, pharmacokinetics, and efficacy of AMG 706, an oral multikinase inhibitor, in patients with advanced solid tumors. J Clin Oncol 25:2369–76, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Drevs J, Siegert P, Medinger M, et al. : Phase I clinical study of AZD2171, an oral vascular endothelial growth factor signaling inhibitor, in patients with advanced solid tumors. J Clin Oncol 25:3045–3054, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Pishvaian MJ, Wang D, He R, Marshall JL: Epidermal Growth Factor Inhibitors, Encyclopedia of Cancer, Springer Berlin Heidelberg, 2008 [Google Scholar]
- 25. Marshall J: Clinical implications of the mechanism of epidermal growth factor receptor inhibitors. Cancer 107:1207–1218, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Cunningham D, Humblet Y, Siena S, et al. : Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351:337–345, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Saltz LB, Meropol NJ, Loehrer PJ, Sr., et al. : Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol 22:1201–1208, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Gibson TB, Ranganathan A, Grothey A: Randomized phase III trial results of panitumumab, a fully human anti-epidermal growth factor receptor monoclonal antibody, in metastatic colorectal cancer. Clin Colorectal Cancer 6:29–31, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Hecht JR, Mitchell E, Chidiac T, et al. : Interim results from PACCE: irinotecan (Iri)/bevacizumab (bev) ± panitumumab (pmab) as first-line treatment (tx) for metastatic colorectal cancer (mCRC), 2008 Gastrointestinal Cancers Symposium, 2008 (abstr 279) [Google Scholar]
- 30. Lievre A, Bachet JB, Boige V, et al. : KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol 26:374–379, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Amado RG, Wolf M, Peeters M, et al. : Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 26:1626–1634, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Whitehead RP, McCoy S, Macdonald JS, et al. : Phase II trial of R115777 (NSC #70818) in patients with advanced colorectal cancer: a Southwest Oncology Group study. Invest New Drugs 24:335–341, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Abraham RT, Gibbons JJ: The mammalian target of rapamycin signaling pathway: twists and turns in the road to cancer therapy. Clin Cancer Res 13:3109–3114, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Hudes G, Carducci M, Tomczak P, et al. : Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 356:2271–2281, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Tabernero J, Rojo F, Calvo E, et al. : Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a phase I tumor pharmacodynamic study in patients with advanced solid tumors. J Clin Oncol 26:1603–1610, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Punt CJ, Boni J, Bruntsch U, et al. : Phase I and pharmacokinetic study of CCI-779, a novel cytostatic cell-cycle inhibitor, in combination with 5-fluorouracil and leucovorin in patients with advanced solid tumors. Ann Oncol 14:931–937, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Mackay HJ, Twelves CJ: Targeting the protein kinase C family: are we there yet? Nat Rev Cancer 7:554–562, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Camidge DR, Gail Eckhardt S, Gore L, et al. : A phase I safety, tolerability, and pharmacokinetic study of enzastaurin combined with capecitabine in patients with advanced solid tumors. Anticancer Drugs 19:77–84, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Carducci MA, Musib L, Kies MS, et al. : Phase I dose escalation and pharmacokinetic study of enzastaurin, an oral protein kinase C beta inhibitor, in patients with advanced cancer. J Clin Oncol 24:4092–4099, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Resta LP, Ermisch S, Collins C, et al. : Phase I study of enzastaurin (ENZ) and bevacizumab (BV) in patients with advanced cancer. J Clin Oncol 26(May 20 suppl): 2008. (abstr 3529) [Google Scholar]
- 41. Summy JM, Gallick GE: Treatment for advanced tumors: SRC reclaims center stage. Clin Cancer Res 12:1398–1401, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Tabernero J, Cervantes A, Hoekman K, et al. : Phase I study of AZD0530, an oral potent inhibitor of Src kinase: first demonstration of inhibition of Src activity in human cancers. J Clin Oncol 25:18S, 2007. (abstr 3520) [Google Scholar]
- 43. Wood KW, Cornwell WD, Jackson JR: Past and future of the mitotic spindle as an oncology target. Curr Opin Pharmacol 1:370–377, 2001 [DOI] [PubMed] [Google Scholar]
- 44. Luo L, Parrish CA, Nevins N, et al. : ATP-competitive inhibitors of the mitotic kinesin KSP that function via an allosteric mechanism. Nat Chem Biol 3:722–726, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Chu QS, Holen KD, Rowinsky ED, et al. : Phase I trial of novel kinesin spindle protein (KSP) inhibitor SB-715992 IV Q 21 days. J Clin Oncol 22:14S, 2004. (abstr 2078) [Google Scholar]
- 46. Burris HA, Lorusso P, Jones S, et al. : Phase I trial of novel kinesin spindle protein (KSP) inhibitor SB-715992 IV days 1, 8, 15 q 28 days. J Clin Oncol 22:128, 2004. (abstr 2004) [Google Scholar]