Abstract
OBJECTIVES
Factors that determine disease severity in nonalcoholic fatty liver disease (NAFLD) are unclear, but exercise is a recommended treatment. We evaluated the association between physical activity intensity and histological severity of NAFLD.
METHODS
We conducted a retrospective analysis of adults with biopsy-proven NAFLD enrolled in the NASH CRN (Nonalcoholic Steatohepatitis Clinical Research Network). Using self-reported time spent in physical activity, we classified participants as inactive or as meeting the US guidelines for either moderate or vigorous exercise. Histology was reviewed by a central pathology committee. Frequency and odds of steatohepatitis (NASH) and advanced fibrosis were compared between subjects who either met or did not meet exercise recommendations, and by the total amount of exercise per week.
RESULTS
A total of 813 adults (males = 302, females = 511) with NAFLD were included, with a mean age of 48 years. Neither moderate-intensity exercise nor total exercise per week was associated with NASH or stage of fibrosis. Meeting vigorous recommendations was associated with a decreased adjusted odds of having NASH (odds ratio (OR): 0.65 (0.43–0.98)). Doubling the recommended time spent in vigorous exercise, as is suggested for achieving additional health benefits, was associated with a decreased adjusted odds of advanced fibrosis (OR: 0.53 (0.29–0.97)).
CONCLUSIONS
These data support an association of vigorous but not moderate or total exercise with the severity of NAFLD. Optimal doses of exercise by duration and intensity for the prevention or treatment of NASH have not been established; however, intensity may be more important than duration or total volume.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is increasingly recognized as an important public health problem. It is the most common chronic liver disease in the United States (1,2).
From epidemiological surveys, it is estimated that up to 30% of the general population has NAFLD (1–5). The full spectrum of NAFLD ranges from isolated steatosis to nonalcoholic steatohepatitis (NASH), fibrosis, and cirrhosis. Obesity and insulin resistance are well-established risk factors for NAFLD; how ever, the pathogenesis of NAFLD is incompletely understood and factors that determine disease severity remain unclear.
Exercise is a major component of treatment for NAFLD as recommended by the American Gastroenterological Association (6) and by the American Association for the Study of Liver Diseases. These recommendations are based on the relationship of NAFLD to obesity and insulin resistance. However, there is a lack of published data on the effectiveness of exercise in treating NAFLD. Ueno et al. evaluated the effects of a diet and exercise (walking and jogging) intervention in 15 subjects with NAFLD. At 3 months follow-up, intervention subjects showed a significant decrease in steatosis but no change in fibrosis (7). In a cross-sectional study of 37 subjects with biopsy-proven NAFLD, Krasnoff et al. did not find a significant association between current physical activity level volume and histological severity of NAFLD. However, they did note that peak VO2 was greater in subjects with milder disease, suggesting a potential role for exercise intensity (8). Other studies that have examined the role of physical activity in NAFLD were limited by the use of surrogate markers for NAFLD (liver biochemistry or imaging markers of steatosis) and/or by the lack of liver histology (9–18). However, it is the presence of steatohepatitis and the stage of fibrosis that are most important to clinical outcome. Information on the relationship between physical activity and histological severity of NAFLD is thus limited. As there are currently no noninvasive markers that can adequately replace biopsy evaluation for the presence and severity of the lesions of NASH, data with biopsy findings are important to improve the understanding of the association between NAFLD histology and physical activity.
As shown in Table 1, federal guidelines from the US Department of Health and Human Services and the US Department of Agriculture recommend that adults engage in ≥150 min of moderate-intensity physical activity per week, 75 min of vigorous-intensity physical activity per week, or a combination, to improve and maintain health (19). Doubling these amounts is recommended to produce additional health benefits. Controversy remains over the role of exercise intensity vs. total volume, and dose–response relationships differ across health outcomes (19). In the context of these guidelines, we evaluated the association of exercise intensity and total volume of physical activity with histological severity of NAFLD using data obtained from a large multicenter study, namely the NASH Clinical Research Network (NASH CRN). We hypothesized that a lower frequency of NASH and a lesser stage of fibrosis would be found for subjects who met the moderate or vigorous recommendations for physical activity.
Table 1.
DHHS and USDA recommendations for physical activity in adults
| Moderate physical activity (minutes a week) | Vigorous physical activity (minutes a week) | |
|---|---|---|
| Minimum targets | ≥150 | ≥75 |
| Targets for more extensive health benefits | ≥300 | ≥150 |
DHHS, Department of Health and Human Services; USDA, US Department of Agriculture.
DHHS and USDA recommendations for physical activity in adults can be met by achieving ≥150 min a week of moderate physical activity or ≥75 min a week of vigorous physical activity. To achieve additional and more extensive health benefits, the guidelines recommend increasing time spent in moderate physical activity to ≥300 min a week or increasing time spent in vigorous physical activity to ≥150 min a week.
METHODS
Subjects
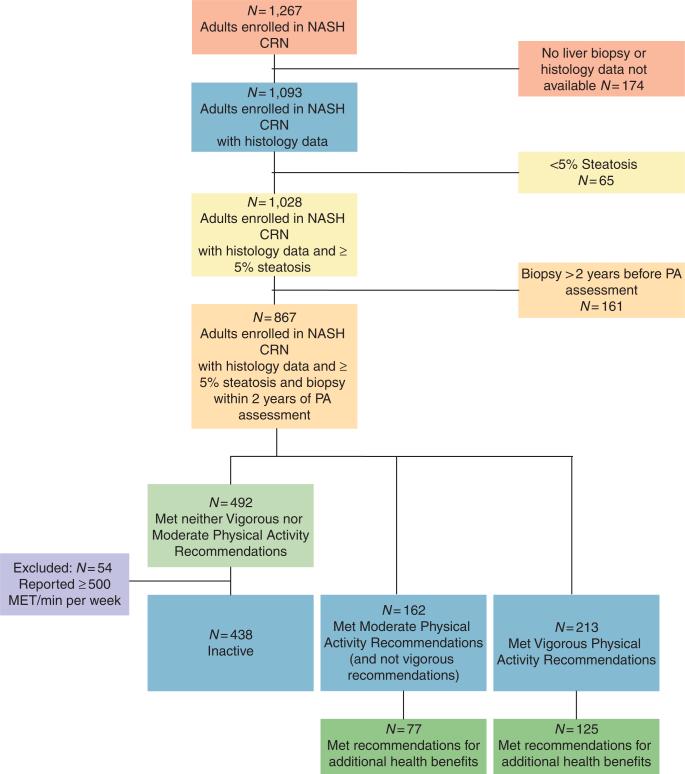
Details on the NASH CRN have been reported previously (20,21). For these analyses, we included baseline data obtained from adults enrolled in two NASH CRN studies: (i) an observational cohort study, the NAFLD Database (20) and (ii) a clinical trial, Pioglitazone vs. Vitamin E vs. Placebo for the Treatment of Non-Diabetic Patients With Nonalcoholic Steatohepatitis (PIVENS) (NCT00063622) (22). Study protocols were approved by all participating center Institutional Review Boards and by an independent Data and Safety Monitoring Board. Each participant provided written informed consent. A flowchart showing both inclusion and exclusion criteria is provided in Figure 1. We included participants who were ≥18 years of age with liver biopsies available for central reading that were obtained within 2 years of enrollment, who had ≥5% steatosis with or without any inflammation or fibrosis. Participants without liver biopsy or central histology review (n = 435), steatosisin <5% of hepatocytes (n = 49), or biopsy >2 years from study enrollment (n = 132) were excluded from theanalyses.
Figure 1.
Inclusion and exclusion flow chart. MET, metabolic equivalent; NASH CRN, Nonalcoholic Steatohepatitis Clinical Research Network. PA, physical activity.
Measures
Anthropometrics
Information on demographics was obtained and anthropometrics measured as reported previously (23).
Histology
The NASH CRN Pathology Committee centrally reviewed all liver biopsy slides obtained from participants. Steatosis was scored according to amount (%). A diagnosis of NAFLD required the presence of ≥5% steatosis. The histological features of NAFLD and NASH were assessed according to the validated system published by the Central Pathology Committee (24). In this system, lesions of active disease are scored separately from fibrosis. These lesions include steatosis, ballooning, and lobular inflammation. Fibrosis is staged as follows: (i) stage 1a and 1b with zone 3 perisinusoidal fibrosis, mild (requiring trichrome stain), and moderate (not requiring trichrome stain), respectively; (ii) stage 1c is portal/periportal only; (iii) stage 2 with zone 3 perisinusoidal plus periportal fibrosis; (iv) stage 3 is bridging fibrosis; and (v) stage 4 is cirrhosis. After the determination of specific features, diagnostic determinations were assigned as follows: biopsies were categorized as definitely not steatohepatitis, borderline steatohepatitis (zone 1 or zone 3 pattern), and definite steatohepatitis. These determinations were made independently of lesion scores, and were based on pattern of injury, as well as the presence and amount of individual lesions (24).
Physical activity
Leisure-time physical activity was measured by self-report using the physical activity questionnaire from the National Health and Nutrition Examination Survey (25). Participants reported time spent per week in specified recreational activities; a free-text field was also available for participants to report additional recreational activities. The following 19 recreational activities (plus free-text responses) were considered: swimming, jogging, running, brisk walking, bicycling (hills), bicycling (flat surfaces), hiking/climbing, aerobics, dancing, calisthenics, weight lifting, using a treadmill or step machine, golfing, singles tennis, doubles tennis, basketball, football, and soccer. Activities were assigned metabolic equivalent (MET) values based on a standard reference (26). One (1) MET is the rate of energy expenditure while at rest. Activities with MET values between 3 and 5.9 were classified as moderate intensity, and activities with MET values ≥6 were classified as vigorous intensity. MET minutes per week was calculated as: MET value×minutes spent per week in activity.
Participants were categorized into the following groups based on federal recommendations (19): (i) inactive (did not meet recommendations for moderate or vigorous); (ii) moderate (met moderate but not vigorous recommendations); (iii) vigorous (met vigorous +/− moderate recommendations). For discrete classification, subjects who had ≥500 MET minutes per week but did not meet either moderate or vigorous recommendations were excluded (n = 54). Subjects in groups 2 and 3 were further categorized into group 2a (those who met the recommendations for additional health benefits for moderate-intensity exercise) and group 3a (those who met the recommendations for additional health benefits for vigorous-intensity exercise).
Data analysis
Descriptive statistics were reported. A χ2 test was used for categorical data. For continuous data, analysis of variance and Tukey's post hoc test were used to compare normally distributed characteristics between groups. The Kruskal–Wallis test was used to compare nonnormally distributed characteristics between the groups, and the Mann–Whitney test was used for pairwise comparisons in the event the Kruskal–Wallis test was significant. The unadjusted association between meeting moderate and vigorous physical activity recommendations, meeting the recommendations for additional health benefits, the association between MET minutes per week (categorized as no MET minutes per week, >0 and <500 MET minutes per week, and ≥500 MET minutes per week), odds of NASH, and odds of advanced fibrosis (no fibrosis, mild to moderate, bridging fibrosis, or cirrhosis) were evaluated using a χ2 test. The conditional odds of definite NASH and of advanced fibrosis were evaluated using multinomial logistic regression. All models used the same adjustment variables: age, gender, education, income, body mass index, and glucose. To make these variables more relevant, they were categorized as shown in Table 2 (e.g., body mass index was categorized as overweight, mild obesity, moderate obesity, or severe obesity). Glucose was log transformed. Pearson's and deviance goodness-of-fit tests were used to assess model adequacy. The significance associated with the ability of meeting recommendations to distinguish between NASH and fibrosis categories was not evaluated, unless an overall significant (P<0.10)relationship betweenmeeting recommendations and NASH/advanced fibrosis was observed in the likelihood ratio test. We checked for multicollinearity among factors by ensuring that all s.e. for the b coefficients were <2.0. A P-value of <0.05 was defined as significant for individual factors in the logistic models. Post hoc, a χ2 test for trend was performed to assess whether the decreased odds for NASH observed for the increasing amount of vigorous physical activity was statistically significant.
Table 2.
Demographic characteristics and levels of exercise intensity
| All subject | Inactive (%)a | Moderate (%)b | Vigorous (%)c | P value* | |
|---|---|---|---|---|---|
| All subjects | 813 | 438 (54) | 162 (20) | 213 (26) | |
| Age, n (%), years | |||||
| < 40 | 199 | 98 (49) | 32 (16) | 69 (35) | 0.021 |
| 40 – 60 | 485 | 271 (56) | 98 (20) | 116 (24) | |
| >60 | 129 | 69 (53) | 32 (25) | 28 (22) | |
| Gender, n (%) | |||||
| Female | 511 | 298 (58) | 90 (18) | 123 (24) | 0.004 |
| Male | 302 | 140 (46) | 72 (24) | 90 (30) | |
| Education, n (%) | |||||
| Less than high school | 75 | 55 (73)d,e | 14 (19)f | 6 (8)f | < 0.001 |
| High school graduate | 179 | 115 (64) | 25 (14) | 39 (22) | |
| Some college | 301 | 162 (54) | 63 (21) | 76 (25) | |
| Greater than or equal to a Bachelors degree | 257 | 106 (41) | 60 (23) | 91 (35) | |
| Income, n (%), $ | |||||
| < 29,999 | 115 | 79 (69)d,e | 14 (12)f | 22 (19)f | < 0.001 |
| 30,000–49,000 | 127 | 84 (66) | 20 (16) | 23 (18) | |
| ≥50,000 | 351 | 164 (47) | 80 (23) | 107 (30) | |
| BMI, n (%), kg/m2 | |||||
| ≤29.9 | 222 | 98 (44)e | 43 (19)e | 81 (36)d,f | < 0.001 |
| 30–34.9 | 252 | 135 (54) | 45 (18) | 72 (29) | |
| 35–39.9 | 197 | 112 (57) | 45 (23) | 40 (20) | |
| ≥40 | 140 | 91 (65) | 29 (21) | 20 (14) | |
| Diabetic, n (%) | 204 (25) | 129 (63)e | 40 (20) | 35 (17)f | 0.002 |
| Nondiabetic | 609 (75) | 309 (51) | 122 (20) | 178 (29) |
ANOVA, analysis of variance; BMI, body mass index.
P value is from comparison of inactive, moderate, and vigorous groups, and from ANOVA (continuous data; pairwise comparisons were conducted using the Tukey test) or χ2 test (categorical variables).
Subjects not meeting moderate or vigorous physical activity recommendations.
≥150-min moderate-intensity physical activity per week.
≥75-min vigorous-intensity physical activity per week.
Significantly different from the moderate group.
Significantly different from the vigorous group.
Significantly different from the inactive group.
As the timing of liver biopsy and/or participation in a clinical trial could potentially be associated with changes in physical activity, we performed three sets of sensitivity analysis. The first two analyses were predicated upon the experience that most lifestyle changes do not persist beyond 3–6 months: (i) sensitivity analysis no. 1 excluded participants who underwent the physical activity assessment 1–90 days after biopsy and (ii) sensitivity analysis no. 2 excluded participants who underwent the physical activity assessment 1–180 days after biopsy. As participants in a clinical trial with more frequent visits might be more likely to increase their physical activity than participants in an observational cohort study, sensitivity analysis no. 3 excluded participants from the PIVENs clinical trial.
RESULTS
Subject characteristics and distribution of physical activity levels
A total of 1,267 adults were enrolled in the NASH CRN; of these, 813 met the inclusion/exclusion criteria and were included in the analysis (Figure 1). The mean age was 48±12 years and 63% were female. Overall, 73% of participants were obese, including 31% mild, 24% moderate, and 17% severe. Type 2 diabetes was present in 25%. The histological diagnosis was NAFLD without NASH in 21% (170/813), borderline NASH in 20% (161/813), and definite NASH in 59% patients (482/813). Fibrosis was absent in 25% (202/813), mild to moderate in 48% (387/813), and advanced (bridging fibrosis or cirrhosis) in 27% patients (216/813).
The most commonly reported activity level was inactive (54%, 438/813). Of those participants who were inactive, 57% (251/438) reported no time spent in recreational physical activity and 43% (187/438) reported participation in some recreational physical activity but not enough to meet either of the recommendations.
Moderate physical activity recommendations were met by 20% patients (162/813). Brisk walking was the most common moderate-intensity activity. Among participants who met moderate activity recommendations, the median (interquartile range) hours per week of moderate physical activity was 4.5 (3.3–7.0). The median (interquartile range) total MET hours per week was 19.0 (13.3–30.0), and the median (interquartile range) total hours spent per week in any physical activity was 5.0 (3.5–7.5). Among participants who met moderate activity recommendations, 48% (77/162) also met recommendations for additional health benefits for moderate-intensity exercise.
Vigorous physical activity recommendations were met by 26% patients (213/813). Overall, 51% (109/213) of subjects in this group met both moderate and vigorous recommendations, whereas 49% (104/213) met vigorous recommendations and not moderate recommendations. Running on a treadmill and/or using a step machine were the most common vigorous-intensity activities reported. Of subjects who met vigorous recommendations, the median (interquartile range) hours per week spent in vigorous physical activity was 3.0 (2.0–4.0). The median (interquartile range) total MET hours per week was 31.6 (20.2–51.0), and the median (interquartile range) total hours spent per week in any physical activity was 6.0 (3.3–9.0). Among participants who met vigorous activity recommendations, 59% (125/213) also met recommendations for additional health benefits for vigorous-intensity exercise.
Table 2 displays the activity categories—inactive, moderate, and vigorous—according to the demographic characteristics of this cohort. Women were more likely to be inactive (58%) than men (46%). Vigorous physical activity was reported significantly more often in younger subjects and in those with more years of education and higher income. Subjects with a lower body mass index and who were not diabetic were also more likely to achieve vigorous recommendations (but not moderate recommendations) compared with those with a higher body mass index or with type 2 diabetes.
Table 3 displays laboratory values by activity group. No significant between-group differences were found for lipids, ALT, or AST. Meeting vigorous recommendations was associated with lower γ-glutamyl transferase levels compared with being inactive. Meeting vigorous recommendations was associated with significantly lower glucose and insulin values compared with being inactive.
Table 3.
Laboratory values of subjects by exercise intensity
| All subjects (n= 813) | Inactive (N = 438)a | Moderate (N = 162)b | Vigorous (N = 213)c | P value* | |
|---|---|---|---|---|---|
| ALT median (IQR), Units/l | 61 (40–91) | 62 (40–93) | 60 (38–91) | 61 (41–89) | 0.646 |
| AST median (IQR), Units/l | 44 (31–63) | 44 (32–68) | 45 (31–62) | 42 (31–58) | 0.190 |
| GGT, median (IQR), Units/l | 47 (29–83) | 50 (32–89) | 45 (29–71) | 45 (28–74)d | 0.027 |
| Triglycerides, median (IQR), mg/dl | 149 (106–209) | 149 (108–209) | 165 (104–226) | 140 (99–195) | 0.108 |
| High-density lipoprotein, median (IQR), mg/dl | 43 (36–50) | 42 (36–50) | 41 (35–53) | 44 (37–53) | 0.208 |
| Low-density lipoprotein, median (IQR), mg/dl | 119 (95–143) | 121 (97–147) | 117 (92–137) | 117 (93–140) | 0.093 |
| Glucose, median (IQR), mg/dl | 97 (86–112) | 99 (87–117) | 97 (86–110) | 94 (84–106)d | 0.001 |
| Insulin, median (IQR), mUnits/l | 18 (12–28) | 19 (13–28) | 19 (11–30) | 14 (9–24)d | < 0.001 |
| NASH, n (%) | 0.005 | ||||
| Definitely not NASH | 170 (21) | 85 (19) | 26 (16) | 59 (28) | |
| Borderline (zone 3) | 153 (19) | 71 (16) | 45 (28) | 37 (17) | |
| Borderline (zone 1) | 8 (1) | 6 (1) | 1 (1) | 1 (1) | |
| Definitely NASH | 482 (59) | 276 (63) | 90 (56) | 116 (54)d |
ALT, alanine transaminase; AST, aspartate transaminase; GGT, γ-glutamyl transferase; IQR, interquartile range; NASH, nonalcoholic steatohepatitis.
P value is from comparison of inactive, moderate, and vigorous groups from the Kruskal–Wallis test, pairwise comparisons were conducted using the Mann–Whitney test; P value for the presence of NASH from χ2 test.
Subjects not meeting moderate or vigorous physical activity recommendations.
≥150-min moderate-intensity physical activity per week.
≥75-min vigorous-intensity physical activity per week.
Significantly different from inactive group.
Association between recommendations and NAFLD severity
Steatohepatitis
The distribution of the diagnostic categories of NASH (not NASH, borderline zone 3, borderline zone 1, and definite NASH) by activity group (inactive, moderate, and vigorous) is shown in Table 3. There was no significant (P=0.42) difference in the adjusted odds of having definite NASH between subjects who met recommendations for moderate activity and those who were inactive (odds ratio (OR): 1.24 (0.73, 2.11), or between individuals who met the recommendations for additional health benefits for moderate activity and those who were inactive (OR: 1.46 (95% confidence interval (95% CI): 0.68–3.10), P=0.33).
Subjects who met vigorous activity recommendations had a significantly (P=0.04) decreased adjusted odds of having NASH than did those who did not meet vigorous activity recommendations (OR: 0.65 (95% CI: 0.43–0.98)) (Table 4). There was no significant interaction between gender and meeting vigorous recommendations (P=0.14); therefore, an interaction term wasnot included in the model. The adjusted odds of NASH was even lower in participants who met the additional health benefits standard for vigorous activity (OR: 0.56 (0.34–0.90) P=0.02). The χ2 test for trend demonstrated a significant association between increasing levels of vigorous physical activity (i.e., from inactive behavior, meeting vigorous physical activity recommendations to meeting additional vigorous physical activity recommendations) and a decreasing odds for NASH (χ2=6.52, P<0.05).
Table 4.
Unadjusted and adjusted odds for NASH and advanced fibrosis based on meeting physical activity recommendations
| Moderate target met |
Vigorous target met |
||||
|---|---|---|---|---|---|
| Odds | Minimum | More Extensive | Minimum | More extensive | |
| NASHa | Unadjusted | 1.01 (0.62, 1.66) | 1.1 (0.56, 2.2) | 0.62 (0.42, 0.90) | 0.57 (0.36, 0.90) |
| Adjusted | 1.24 (0.73, 2.1) | 1.46 (0.68, 3.1) | 0.65 (0.43, 0.98) | 0.56 (0.34, 0.90) | |
| Advanced fibrosisb | Unadjusted | 1.1 (0.65, 1.8) | 1.1 (0.55, 2.0) | 0.53 (0.34, 0.82) | 0.41 (0.23, 0.72) |
| Adjusted | 1.2 (0.69, 2.1) | 1.1 (0.53, 2.3) | 0.75 (0.46, 1.2) | 0.53 (0.29, 0.97) | |
BMI, body mass index; NASH, nonalcoholic steatohepatitis.
Odds ratio (95% confidence interval) derived from multinomial logistic regression.
The adjusted odds ratios were adjusted for age, gender, income, BMI, and glucose.
Statistically significant odds ratios and confidence intervals are in bold font for emphasis.
Definite steatohepatitis vs. not steatohepatitis.
Advanced fibrosis vs. no fibrosis
Sensitivity analyses yielded similar results as the main analyses. In all three analyses, meeting moderate activity recommendations was not associated with any difference in the odds for NASH, whereas meeting vigorous activity recommendations was associated with a significant decrease in the adjusted odds for NASH. We observed for sensitivity analysis no. 1: (excluding subjects with activity assessment 1–90 days after biopsy) moderate (OR: 1.4, 95% CI: 0.67–3.09) and vigorous (OR: 0.54, 95% CI: 0.31–0.94); For sensitivity analysis no.2 : (excluding subjects with activity assessment 1–180 days after biopsy) moderate (OR: 0.92, 95% CI: 0.33–2.56) and vigorous (OR: 0.51, 95% CI: 0.26–0.99); and for sensitivity analysis no. 3: (excluding participants in PIVENs) moderate (OR: 1.27, 95% CI: 0.69–2.33) and vigorous (OR: 0.61, 95% CI: 0.38–0.98).
Advanced fibrosis
Neither meeting moderate activity recommendations (P=0.90) nor recommendations for additional health benefits for moderate activity (P=0.51) was associated with degree of fibrosis. In contrast, meeting vigorous activity recommendations was associated with a significantly (P=0.005) lower odds of advanced fibrosis compared with no fibrosis (OR: 0.53 (0.34–0.82)) in univariate analysis. After adjustment, however, this association lost significance. Notably, meeting the additional vigorous activity recommendations was associated with a significantly lower odds of advanced fibrosis vs. no fibrosis (OR: 0.53 (0.29–0.97)) even after adjustment (Table 4).
MET minutes per week
There was no significant (P=0.15) difference in the frequency of NASH between participants with no MET minutes per week (63%), >0 and <500 MET minutes per week (62%), and ≥500 MET minutes per week (55%), nor was there a significant (P=0.19) difference in the frequency of advanced fibrosis between participants with no MET minutes per week (25%), >0 and <500 MET minutes per week (33%), and ≥500 MET minutes per week (25%).
DISCUSSION
In a large multicenter study with patients from across the United States, we assessed the cross-sectional relationship between meeting or exceeding US national guidelines for physical activity and histological severity of NAFLD. There was no association between meeting moderate-activity guidelines and histological severity of NAFLD. In contrast, meeting the minimum guidelines for vigorous activity was associated with a significant reduction in the adjusted odds of having NASH. Furthermore, exceeding the vigorous-activity guidelines, which is recommended for additional health benefits, was associated with a decreased odds of fibrosis.
These data, based on a large population with biopsy-proven NAFLD and a careful examination of liver histology, substantially extend the knowledge base of physical activity and severity of NAFLD. Two cross-sectional studies have evaluated the association between physical activity or fitness level and the presence or absence of suspected NAFLD based on liver biochemistry (elevated AST, ALT) and/or liver imaging (ultrasonography or computed tomography). These technologies are limited both by being unreliable for the detection of mild steatosis and by their inability to assess for the presence of steatohepatitis or stage of fibrosis. Zelber-Sagi et al. (18), found that subjects with suspected NAFLD (identified by ultrasonography) reported a lower weekly duration of exercise than did those without suspected NAFLD. In a study by Church et al. (9), adults with suspected NAFLD had a lower cardiorespiratory fitness level than did those without suspected NAFLD. Other studies have examined changes in liver biochemistry in response to multimodal interventions incorporating both diet and exercise in subjects with suspected NAFLD. In a study by Suzuki et al. (12), 348 subjects with elevated ALT were identified and counseled on exercise and nutrition. Beginning or continuing an exercise routine was associated with a greater change in ALT compared with performing no exercise. A similar study by Kim et al., included subjects identified by ultrasonography during a regular health checkup as having NAFLD. Those subjects were advised to increase physical activity and to reduce calorie intake (17). At 5-year follow-up, a greater proportion of subjects exercising ≥3 times a week had a decrease in ultrasonographic abnormalities than did those who exercised <3 times a week. St George et al. (15,16), randomized subjects with suspected NAFLD (ALT >30 for males or >19 for females) to one of three different intensity counseling groups (low intensity: three sessions; moderate: six sessions; moderate plus: six sessions plus phone follow-up) or to a control group. Subjects who increased their physical activity by at least 60 min (n=85), or who maintained their physical activity at ≥150 min per week (n=29) had the greatest improvements in liver biochemistry than did those who were inactive (n=25). In another small study (n=16) that included subjects with biopsy-proven NASH, subjects received diet counseling and were encouraged to walk or jog for 30 min a day (11). Improvements were seen in weight and serum aminotransferases; however, post-treatment histology was not assessed.
We expected to find that both moderate and vigorous physical activities were inversely associated with disease severity. However, the finding that only vigorous-intensity physical activity was associated with histological severity is consistent with a large body of literature on all-cause mortality, cardiovascular disease, and colon cancer (27,28). Epidemiological studies on exercise intensity and cardiovascular outcomes are better developed for men than for women. In men, the preponderance of evidence suggests that physical activity of vigorous intensity, but not of moderate intensity, is associated with a decreased frequency of coronary heart disease (29,30). For example, The Health Professionals’ Follow-up Study, with a sample of 44,452 men and 12 years follow-up, demonstrated that, adjusted for exercise volume, vigorous but not moderate physical activity was associated with a decreased risk for myocardial infarction (31). In addition to cardiovascular disease, large long-term studies of men have shown that vigorous- but not moderate-intensity physical activity was associated with decreased risk for all-cause mortality (32–34). Less is known about the effect of exercise intensity on health outcomes in women because there are fewer published data and the available studies have much shorter follow-up duration. However, a large study reported a similar risk reduction for coronary events for moderate and vigorous activities (35). Thus, data obtained from men suggest important differences between exercise intensity and risk for coronary heart disease, whereas more data with longer follow-up are required in women. In the current study, no gender interaction was found in the analysis of meeting vigorous recommendations and odds of NASH.
The biological basis for observed differences in the association between moderate and vigorous physical activities and the severity of NAFLD are not known. One potential explanation is the effect of exercise on AMP-activated protein kinase (AMP-kinase), a regulator of intracellular energy metabolism. AMP-kinase activation in the liver increases fatty acid oxidation and decreases glucose production (36). Activation of AMP-kinase leads to phosphorylation of many downstream targets that regulate mitochondrial biogenesis and hepatic gluconeogenesis (37). Furthermore, AMP-kinase not only regulates energy but may also have a key role in hepatic fibrogenesis, as it has been shown to suppress hepatic stellate cell proliferation. Under normal conditions, AMP-kinase is activated when the ratio of AMP is greater than ATP. Exercise can increase the AMP-to-ATP ratio; however, only vigorous activity results in a large enough shift of the AMP-to-ATP ratio required to activate AMP-kinase. Thus, observed differences in the association between exercise intensity and histological severity could be due to differences in the ability of exercise to modulate cellular pathways controlling metabolism, inflammation, and matrix deposition (38).
The multicenter design of the NASH CRN makes these results generalizable to adults in the United States with a clinical (i.e., biopsy-proven) diagnosis of NAFLD. An additional strength of this study was the inclusion of liver histology on all patients with a rigorous, standardized, central biopsy review. A limitation was the cross-sectional nature of this study; thus, these data do not establish a causal relationship between exercise and disease severity. In addition, self-report of physical activity may result in error and misclassification. In particular, better reliability and validity has been demonstrated for recall of vigorous- vs. moderate-intensity activity (39–41). The present measure was limited to leisure-time physical activity, and it is likely activities in occupation, transport, and household domains that were not assessed are more likely to be moderate than vigorous (26). Measurement limitations and misclassification due to reporting bias could have reduced power to detect associations with moderate physical activity. Furthermore, this analysis included a large sample of individuals with NASH, but without cirrhosis, who qualified for the PIVENS trial. However, sensitivity analyses excluding the clinical trial participants (no. 3) showed similar results. Longitudinal studies with objective measures of physical activity and randomized clinical trials are required to further examine the relationship between physical activity intensity and histological severity in individuals with NAFLD. Moderate exercise may still be beneficial over time, if only by attenuating further weight gain, but longitudinal studies are necessary to address this possibility.
In conclusion, we found an inverse relationship between vigorous-intensity physical activity and NAFLD severity. Moderate-intensity physical activity and total volume of physical activity were not related to outcomes. Thus, intensity may be an important dimension of physical activity to consider when counseling patients and planning interventions. Intervention studies with objective measures of physical activity are required to confirm the differential effects of vigorous compared with moderate physical activity on NAFLD severity.
Supplementary Material
Acknowledgments
Financial support: The Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) is supported by the National Institute of Diabetes and Digestive and Kidney Diseases grants U01DK061718, U01DK061728, U01DK061731, U01DK061732, U01DK061734, U01DK061737, U01DK061738, U01DK061730, U01DK061713, and the National Institute of Child Health and Human Development. This study is supported in part by the Intramural Research Program of the National Cancer Institute. Other grant supports include the National Institutes of Health General Clinical Research Centers or Clinical and Translational Science Awards: UL1RR024989, M01RR000750, ULRR02413101, M01RR000827, UL1RR02501401, and M01RR000065.
Footnotes
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/ajg
CONFLICT OF INTEREST
Guarantors of the article: Jeffrey B. Schwimmer, MD and Kristin D. Kistler, PhD.
Potential competing interests: None.
REFERENCES
- 1.Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98:960–7. doi: 10.1111/j.1572-0241.2003.07486.x. [DOI] [PubMed] [Google Scholar]
- 2.Kim WR, Brown RS, Jr, Terrault NA, et al. Burden of liver disease in the United States: summary of aworkshop. Hepatology. 2002;36:227–42. doi: 10.1053/jhep.2002.34734. [DOI] [PubMed] [Google Scholar]
- 3.Ioannou GN, Boyko EJ, Lee SP. The prevalence and predictors of elevated serum aminotransferases activity in the United States in 1999–2002. Am J Gastroenterol. 2006;101:76–82. doi: 10.1111/j.1572-0241.2005.00341.x. [DOI] [PubMed] [Google Scholar]
- 4.Bell BP, Manos MM, Zaman A, et al. The epidemiology of newly diagnosed chronic liver disease in gastroenterology practices in the United States: results from population-based surveillance. Am J Gastroenterol. 2008;103:2727–36. doi: 10.1111/j.1572-0241.2008.02071.x. [DOI] [PubMed] [Google Scholar]
- 5.Lazo M, Clark JM. The epidemiology of nonalcoholic fatty liver disease: a global perspective. Semin Liver Dis. 2008;28:339–50. doi: 10.1055/s-0028-1091978. [DOI] [PubMed] [Google Scholar]
- 6.American Gastroenterological Association American Gastroenterological Association medical position statement: nonalcoholic fatty liver disease. Gastroenterology. 2002;123:1702–4. doi: 10.1053/gast.2002.36569. [DOI] [PubMed] [Google Scholar]
- 7.Ueno T, Sugawara H, Sujaku K, et al. Therapeutic effects of restricted diet and exercise in obese patients with fatty liver. J Hepatol. 1997;27:103–7. doi: 10.1016/s0168-8278(97)80287-5. [DOI] [PubMed] [Google Scholar]
- 8.Krasnoff JB, Painter PL, Wallace JP, et al. Health-related fitness and physical activity in patients with nonalcoholic fatty liver disease. Hepatology. 2008;47:1158–65. doi: 10.1002/hep.22137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Church TS, Kuk JL, Ross R, et al. Association of cardiorespiratory fitness, body mass index, and waist circumference to nonalcoholic fatty liver disease. Gastroenterology. 2006;130:2023–30. doi: 10.1053/j.gastro.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 10.Chen SM, Liu CY, Li SR, et al. Effects of therapeutic lifestyle program on ultrasound-diagnosed nonalcoholic fatty liver disease. J Chin Med Assoc. 2008;71:551–8. doi: 10.1016/S1726-4901(08)70168-0. [DOI] [PubMed] [Google Scholar]
- 11.Kugelmas M, Hill DB, Vivian B, et al. Cytokines and NASH: a pilot study of the effects of lifestyle modification and vitamin E. Hepatology. 2003;38:413–9. doi: 10.1053/jhep.2003.50316. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki A, Lindor K, St Saver J, et al. Effect of changes on body weight and lifestyle in nonalcoholic fatty liver disease. J Hepatol. 2005;43:1060–6. doi: 10.1016/j.jhep.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Baba SC, Alexander G, Kalyani B, et al. Effect of exercise and dietary modification on serum aminotransferase levels in patients with nonalcoholic. J Gastroenterol Hepatol. 2006;21:191–8. doi: 10.1111/j.1440-1746.2005.04233.x. [DOI] [PubMed] [Google Scholar]
- 14.Akyuz F, Demir K, Ozdil S, et al. The effects of rosiglitazone, metformin, and diet with exercise in nonalcoholic fatty liver disease. Dig Dis Sci. 2007;52:2359–67. doi: 10.1007/s10620-006-9145-x. [DOI] [PubMed] [Google Scholar]
- 15.St George A, Bauman A, Johnston A, et al. The independent effects of physical activity in patients with non-alcoholic fatty liver disease. Hepatology. 2009;50:68–76. doi: 10.1002/hep.22940. [DOI] [PubMed] [Google Scholar]
- 16.St George A, Bauman A, Johnston A, et al. Effect of lifestyle intervention in patients with abnormal liver enzymes and metabolic risk factors. J Gastroenterol Hepatol. 2009;24:399–407. doi: 10.1111/j.1440-1746.2008.05694.x. [DOI] [PubMed] [Google Scholar]
- 17.Kim HK, Park JY, Lee KU, et al. Effect of body weight and lifestyle changes on long-term course of nonalcoholic fatty liver disease in Koreans. Am J Med Sci. 2009;337:98–102. doi: 10.1097/MAJ.0b013e3181812879. [DOI] [PubMed] [Google Scholar]
- 18.Zelber-Sagi S, Nitzan-Kaluski D, Goldsmith R, et al. Role of leisure-time physical activity in nonalcoholic fatty liver disease: a population-based study. Hepatology. 2008;48:1791–8. doi: 10.1002/hep.22525. [DOI] [PubMed] [Google Scholar]
- 19.US Dept. of Health and Human Services . Physical Activity Guidelines for Americans. US Dept. of Health and Human Services; Washington, DC: [Google Scholar]
- 20.Nonalcoholic Steatohepatitis Clinical Research Network Hepatology. 2003;37:244. doi: 10.1002/hep.510370203. [DOI] [PubMed] [Google Scholar]
- 21.Brunt EM, Kleiner DE, Wilson LA, et al. NASH Clinical Research Network. Portal chronic inflammation in nonalcoholic fatty liver disease (NAFLD): a histologic marker of advanced NAFLD-Clinicopathologic correlations from the nonalcoholic steatohepatitis clinical research network. Hepatology. 2009;49:809–20. doi: 10.1002/hep.22724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–85. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.David K, Kowdley KV, Unalp A, et al. NASH CRN Research Group. Quality of life in adults with nonalcoholic fatty liver disease: baseline data from the nonalcoholic steatohepatitis clinical research network. Hepatology. 2009;49:1904–12. doi: 10.1002/hep.22868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 25.National Center for Health Statistics. National Center for Health Statistics . Vital Health Stat.1 1[32]. 1994. NHANES III reference manuals and reports {CD-ROM} US Department of Health and Human Services; Public Health Service, CDC; Washington, DC: Hyattsville, Maryland: 1996. Plan and Operation of the Third National Health and Nutrition Examination Survey, 1988–1994. [Google Scholar]
- 26.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 27.Kesaniemi YK, Danforth E, Jr, Jensen MD, et al. Dose-response issues concerning physical activity and health: an evidence-based symposium. Med Sci Sports Exerc. 2001;33:S351–8. doi: 10.1097/00005768-200106001-00003. [DOI] [PubMed] [Google Scholar]
- 28.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American college of sports medicine and the American heart association. Med Sci Sports Exerc. 2007;39:1423–34. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 29.Sesso HD, Paffenbarger RS, Jr, Lee IM. Physical activity and coronary heart disease in men: The Harvard Alumni Health Study. Circulation. 2000;102:975–80. doi: 10.1161/01.cir.102.9.975. [DOI] [PubMed] [Google Scholar]
- 30.Swain DP, Franklin BA. Comparison of cardioprotective benefits of vigorous versus moderate intensity aerobic exercise. Am J Cardiol. 2006;97:141–7. doi: 10.1016/j.amjcard.2005.07.130. [DOI] [PubMed] [Google Scholar]
- 31.Tanasescu M, Leitzman MF, Rimm EB, et al. Exercise type and intensity in relation to coronary heart disease in men. JAMA. 2002;288:1994–2000. doi: 10.1001/jama.288.16.1994. [DOI] [PubMed] [Google Scholar]
- 32.Yu S, Yarnell JW, Sweetnam PM, et al. Caerphilly study. What level of physical activity protects against premature cardiovascular death? The Caerphilly study. Heart. 2003;89:502–6. doi: 10.1136/heart.89.5.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee IM, Paffenbarger RS., Jr Associations of light, moderate, and vigorous intensity physical activity with longevity. Am J Epidemiol. 2000;151:293–9. doi: 10.1093/oxfordjournals.aje.a010205. [DOI] [PubMed] [Google Scholar]
- 34.Lee IM, Hsieh CC, Paffenbarger RS., Jr Exercise intensity and longevity in men. The Harvard Alumni Health Study. JAMA. 1995;273:1179–84. [PubMed] [Google Scholar]
- 35.Manson JE, Hu FB, Rich-Edwards JW, et al. A prospective study of walking as compared with vigorous exercise in the prevention of coronary heart disease. N Engl J Med. 1999;341:650–8. doi: 10.1056/NEJM199908263410904. [DOI] [PubMed] [Google Scholar]
- 36.Kemp BE, Mitchelhill KI, Stapleton D, et al. Dealing with energy demand: the AMP-activated protein kinase. Trends Biochem Sci. 1999;24:22–5. doi: 10.1016/s0968-0004(98)01340-1. [DOI] [PubMed] [Google Scholar]
- 37.Houten SM, Auwerx J. PGC-1α: turbocharging mitochondria. Cell. 2004;119:5–7. doi: 10.1016/j.cell.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 38.Richter EA, Ruderman NB. AMPK and the biochemistry of exercise: implications for human health and disease. Biochemistry. 2009;418:261–75. doi: 10.1042/BJ20082055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sallis JF, Saelens BE. Assessment of physical activity by self-report: status, limitations, and future directions. Res Q Exerc Sport. 2000;71:S1–14. [PubMed] [Google Scholar]
- 40.Slattery ML, Jacobs DR., Jr Assessment of ability to recall physical activity of several years ago. Ann Epidemiol. 1995;5:292–6. doi: 10.1016/1047-2797(94)00095-b. [DOI] [PubMed] [Google Scholar]
- 41.Blair SN, Dowda M, Pate RR, et al. Reliability of long-term recall of participation in physical activity by middle-aged men and women. Am J Epidemiol. 1991;133:266–75. doi: 10.1093/oxfordjournals.aje.a115871. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.