Abstract
The role of inhibition in sensory cortical map plasticity is not well understood. Here we tested whether inhibition contributes to expression of receptive field plasticity in developing rat somatosensory (S1) cortex. In normal rats, microiontophoresis of gabazine (SR 95531), a competitive γ-aminobutyric acid (GABA)-A receptor antagonist, preferentially disinhibited surround whisker responses relative to principal whisker responses, indicating that GABAA inhibition normally acts to sharpen whisker tuning. Plasticity was induced by transiently depriving adolescent rats of all but one whisker; this causes layer 2/3 (L2/3) receptive fields to shift away from the deprived principal whisker and toward the spared surround whisker. In units with shifted receptive fields, gabazine preferentially disinhibited responses to the deprived principal whisker, unlike in controls, suggesting that GABAA inhibition was acting to preferentially suppress these responses relative to spared whisker responses. This effect was not observed for L2/3 units that did not express receptive field plasticity or in layer 4, where receptive field plasticity did not occur. Thus GABAA inhibition promoted expression of sensory map plasticity by helping to sharpen receptive fields around the spared input.
INTRODUCTION
Sensory experience produces long-term changes in cortical sensory maps that are known to involve experience-dependent modification of excitatory cortical circuits (Allen et al. 2003; Darian-Smith and Gilbert 1994, 1995; Finnerty et al. 1999; Heynen et al. 2003; Lowel and Singer 1992; Takahashi et al. 2003; Trachtenberg and Stryker 2001). In contrast, the role of cortical inhibition in plasticity is less clear (Calford 2002; Jacobs and Donoghue 1991; Jones 1993; Rajan 2001). Inhibitory conductances suppress spiking responses, refine temporal features of spike trains, and often sharpen sensory receptive fields from the broader tuning of subthreshold excitatory inputs (Kelly et al. 1999; Kyriazi et al. 1996b, 1998; Miller et al. 2001; Wehr and Zador 2003). This latter process is referred to as inhibitory sharpening of receptive fields. How inhibitory sharpening works on the cellular level is unclear: it could reflect either preferential recruitment of inhibitory inputs by nonoptimal stimuli (e.g., classical lateral inhibition) or the existence of inhibitory conductances that are broadly tuned or co-tuned with excitation, which would preferentially inhibit the weakest excitatory inputs because these are most readily suppressed below spike threshold (Heeger 1992; Miller et al. 2001; Priebe and Ferster 2005; Wehr and Zador 2003).
How inhibition contributes to cortical map plasticity is not well understood. Inhibition is generally hypothesized to regulate the induction of map plasticity by enabling or disabling experience-dependent plasticity mechanisms within excitatory circuits. Thus maturation of inhibition may define critical periods in visual cortex (Hensch and Stryker 2004), and changes in inhibitory tone may enable plasticity after sensory denervation or deprivation in adult sensory cortex (Calford and Tweedale 1988; Dykes 1997; Fuchs and Salazar 1998; Rajan 1998, 2001; Rosier et al. 1995; Tremere et al. 2001). Less studied is the possibility that inhibitory sharpening of receptive fields contributes directly to expression of receptive field plasticity by preferentially suppressing responses to deprived or behaviorally inappropriate inputs, thus sharpening receptive fields around spared, behaviorally appropriate inputs (Zheng and Knudsen 1999, 2001). To examine this possibility, we have studied how inhibitory sharpening contributes to deprivation-induced whisker map plasticity in rat primary somatosensory (S1) cortex.
In the normal whisker map in rat S1, each whisker is represented in layer 4 (L4) by a cluster of neurons called a barrel, with the barrels forming an isomorphic map of the contralateral whisker array (Woolsey and Van der Loos 1970). L4 excitatory neurons receive direct thalamic input and project densely to layer 2/3 (L2/3) neurons within the same radial column, termed the barrel column. L4 and L2/3 neurons respond most strongly to deflection of the whisker corresponding to their barrel column, termed the principal whisker (PW) and less strongly to deflection of surrounding whiskers (SWs), thus forming an orderly map of whisker receptive fields across S1. These whisker receptive fields are strongly shaped by PW-and SW-evoked GABAergic inhibition (Brecht et al. 2003; Derdikman et al. 2003; Kyriazi et al. 1996a,b, 1998; Moore and Nelson 1998; Simons and Carvell 1989; Wilent and Contreras 2004).
Plucking or trimming all but a single whisker (univibrissa experience) for several days or weeks in adolescent rats causes rapid changes in L2/3 receptive fields but minimal changes in L4 (Fox 2002). During plasticity, L2/3 neurons in deprived columns lose responses to their deprived PW (Glazewski and Fox 1996). This process reflects, in part, deprivation-induced long-term depression at excitatory L4–L2/3 synapses, which reduces PW-evoked excitatory inputs to L2/3 neurons (Allen et al. 2003). Here we demonstrate that inhibitory sharpening of receptive fields also promotes expression of receptive field plasticity in L2/3 neurons.
The contribution of inhibition to shaping whisker receptive fields was estimated during extracellular recording by comparing receptive fields before and during iontophoretic application of the GABAA-receptor antagonist gabazine (SR 95531). This technique allows estimation of the net effects of inhibition on receptive field shape but does not reveal the underlying cellular mechanisms for inhibitory sharpening. Results showed that in normal rats, inhibition sharpened whisker tuning by preferentially suppressing SW responses. In L2/3 neurons the receptive fields of which had been shifted substantially by univibrissa experience, inhibition acted instead to preferentially suppress responses to the deprived PW, thus sharpening tuning around the spared SW. Thus inhibitory sharpening of receptive fields exists both before and after whisker deprivation and contributes to expression of receptive field changes during whisker-map plasticity. This effect may reflect either experience-induced changes in the magnitude or timing of whisker-evoked inhibitory conductances or stable inhibition that consistently acts to amplify changes in excitatory inputs.
METHODS
Animals and deprivation paradigm
Procedures were approved by the University of California San Diego Institutional Animal Care and Use Committee. Recordings were made from 16 Long-Evans rats (either sex), postnatal age (P) 30–40. Seven rats had all but the D1 whisker plucked unilaterally from the right side of the face, beginning at P13 (“D1-spared”). Plucking was performed under isoflurane anesthesia (2.5% in oxygen, 2 l/min) every other day for 14–21 days. Prior to the recording session, whiskers were allowed to regrow for 4–7 days (to 5- to 10-mm length) to allow whisker-evoked responses to be measured. Six sham-deprived rats and three naïve rats served as controls.
Surgical preparation
On the day of recording, anesthesia was induced with urethan (Sigma-Aldrich, 1.5 g/kg ip, 20% in saline), and atropine sulfate (1 mg/kg) and lactated Ringers (3 ml) were administered intraperitoneally. The scalp was anesthetized with lidocaine hydrochloride, and the skull exposed and a small head bolt attached posterior to lambda. A craniotomy (2 mm diam) was made over the barrel cortex, 5 mm lateral and 2.5 mm caudal of bregma. The dura was removed, and the exposed cortex was covered with warm saline. Body temperature was maintained at 37°C by a homeothermic heating pad (Harvard Apparatus, Holliston, MA).
Recordings and iontophoresis
After surgery, animals were transferred to the recording apparatus, and the head bolt was secured to a stereotactic holder. Anesthesia was maintained with supplemental doses of urethan (10% of the initial dose, ip) whenever corneal or limb withdrawal reflexes were brisk, whisker movements were observed, or breathing rate exceeded 120/ min. To confirm the stage of anesthesia, the electrocorticogram (ECoG) was measured in three animals and contained dominant frequencies in the 4- to 6-Hz range (n = 13 measurements). This ECoG pattern, together with reflex state and breathing rate, are consistent with Guedel stage III-3/III-2 anesthesia (Friedberg et al. 1999).
Initial mapping penetrations were made with glass-insulated single-barrel carbon fiber microelectrodes (Armstrong-James and Millar 1979) to locate the D2 barrel column, which was the site of all pharmacological measurements. In control rats, the D2 column was identified operationally during mapping by the presence of recording sites at which the D2 whisker evoked the largest magnitude and shortest latency responses. In D1-spared rats, the D2 column was identified operationally by position relative to the physiologically identified D1 column and by the presence of D2-dominated receptive fields in L4, where receptive field plasticity does not occur (see results). In all cases, recording site location was confirmed by histological recovery of marking lesions.
Combined iontophoresis and recording electrodes were fashioned from five-barrel glass capillaries (A-M Systems, Carlsborg, WA) containing a 7-µm carbon fiber in the central recording barrel. After pulling on a vertical micropipette puller (Stoelting, Wood Dale, IL), the pipette tip was cut to a total diameter of 12–20 µm (single iontophoresis barrel diameter: 1–3 µm). Electrical contact with the carbon fiber was made with 0.9% NaCl. Two or three outer barrels were filled for iontophoresis with gabazine (SR 95531, 3 mM, pH 4.0; Tocris, Ellisville, MO, dissolved in 0.9% NaCl), and remaining barrels were filled with 0.9% NaCl, one of which was used for current balancing. A microiontophoresis system (Dagan 6400 Advanced, Minneapolis, MN) generated and monitored ejection (3–30 nA) and retention currents (−5 to −15 nA).
Recordings were made every 80–150 µm in penetrations perpendicular to the cortical surface, i.e., within a single-barrel column, at microdrive depths of 200–950 µm below the pia. These depths correspond to L2 to L4 (Celikel et al. 2004) (see also following text). Recordings were restricted to sites with whisker-evoked spikes the amplitude of which was more than four times background noise by visual inspection of the recording trace. Each penetration contained one to four recording sites. Amplitude or rate of spontaneous activity was not a selection criterion for recording sites.
Recordings were preamplified (1,000× gain, DAM-50, WPI, Sarasota, FL), band-pass filtered (0.5–6 kHz, Krohn-Hite 3364, Brockton, MA), further amplified (5×, Brownlee 410, San Jose, CA) and digitized at 32 kHz using a 12-bit data acquisition board (National Instruments, Austin, TX) and custom software written in Igor (Wave-metrics, Lake Oswego, OR). Whisker deflections were applied using three independent, calibrated, computer-controlled piezoelectic bi-morph actuators (Piezo Systems, Cambridge, MA) that allowed interleaved, independent deflection of D1–D3 whiskers. Each actuator carried a lightweight plastic tube into which a whisker was inserted. Whisker deflection consisted of 2° ramp-and-hold deflections applied 5 mm from the whisker base (4-ms rise/fall time, upward direction, 200-ms duration, beginning 100 ms after sweep onset).
Measurement of whisker tuning and iontophoresis protocol
At each recording site, an initial whisker tuning curve (“predrug condition”) was collected consisting of 100 repetitions each of D1–D3 whisker deflection (randomly interleaved, 1 Hz). Retention currents (−5 to −15 nA) were applied to all gabazine-containing barrels during the predrug tuning curve. Gabazine was then applied at an ejection current (range: 3–30 nA; mean: 10.8 ± 6.3; mean ± SD) that caused multiunit responses to the D1 whisker to increase approximately three- to fourfold. There was no difference in ejection currents between control and deprived animals (P = 0.1; unpaired t-test). No bursting or epileptiform activity was induced at these ejection currents. A second tuning curve was collected during gabazine application (data collection began 1–3 min after ejection onset when whisker-evoked responses were significantly elevated and lasted 5 min). Retention currents (−5 to −15 nA) were then applied, and tuning was reassessed periodically until recovery was achieved (usually within 5–30 min; defined by return of multiunit responses to <1.5 times predrug response levels for at least one whisker). Sites that failed to recover were excluded from analysis.
Histology
After recording, focal electrolytic lesions (3–4 µA DC current, 10 s, tip negative) were made at at least four penetration sites at a depth of 700–950 µm below the pia, corresponding to our operational definition of L4 (see following text). Animals were deeply anesthetized with an overdose of urethan, decapitated, and the head was fixed (3–5 days) in 4% paraformaldehyde and 10% sucrose in 0.1 M phosphate buffer. The cortex was removed, flattened, postfixed for 0.5–2 days in 4% paraformaldehyde and 30% sucrose in 0.1 M phosphate buffer, and cut in 50-µm-thick sections parallel to the pia. Sections were processed for cytochrome oxidase histology to visualize the barrels (Wong-Riley 1979). Lesion sites were reconstructed relative to barrel boundaries using Neurolucida software (Micro-brightfield, Williston, VT).
Column and laminar identity of recording sites
Based on lesion reconstructions, each penetration was classified as being within the half of the D2 barrel column nearest D1 or the half nearest D3. This was done because D1-sparing produces different amounts of plasticity in these regions (Fox 2002; Glazewski and Fox 1996). Laminar identity of recording sites was determined from microdrive depths with 150–650 µm below the pia corresponding to L2/3 and 700–950 µm corresponding to L4. These depths were determined by latency analysis and lesion recovery in a previous study (Celikel et al. 2004) and were verified here by the recovery of 45/48 lesions made at 700–950 µm depth in L4 as characterized by cytochrome oxidase staining.
Spike sorting and data analysis
Spikes with positive amplitudes ≥8 SD above background noise (measured in the predrug condition) were selected for spike sorting. Sorting was performed using an algorithm developed by Fee et al. (1996) and implemented in Matlab by S. Mehta and D. Kleinfeld (Dept. of Physics, UCSD). This algorithm identifies spike clusters in multi-unit signals even in the presence of realistic, anisotropic noise, and can be used with both single-electrode (Celikel et al. 2004) and multi-electrode recordings (Fee et al. 1996). Single units were excluded if >1% of spikes exhibited interspike intervals <1 ms. (Our sampling method allowed detection of spikes as little as 0.5 ms apart.).
Response magnitude was defined as the number of spikes per stimulus occurring within 100 ms of stimulus onset and was not corrected for spontaneous firing, which was very low [0.01 ± 0.02 (SD) spikes per 100 ms during predrug; n = 256 units]. Onset latency was calculated from peristimulus time histograms (PSTHs) as the first of two consecutive 1-ms bins containing ≥2 SD above the mean firing rate in the 100 ms before stimulus onset. Response offset was defined as the time, within 100 ms of stimulus onset, of the final PSTH bin containing significant evoked firing (≥2 SD above mean spontaneous firing) that preceded three consecutive bins with firing below this threshold.
The D1-dominance index (D1-di) was calculated as a measure of the relative responsiveness of a neuron to D1 and D2 whiskers (Fox 1992). D1-di was defined as the response magnitude to D1 whisker deflection divided by the sum of responses to D1 and D2 whisker deflections. D1-di >0.5 indicates that D1 is the dominant whisker, whereas D1-di <0.5 indicates that the D2 whisker is dominant. A critical value of P < 0.05 was required for significance in statistical tests. All error values are SE otherwise specified.
RESULTS
Receptive field plasticity induced by D1 sparing
Whisker map plasticity was induced using a standard manipulation in which all whiskers but D1 were plucked, unilaterally, for 14–21 days, starting at P13. At this age, univibrissa experience drives receptive field plasticity in L2/3 but not L4, indicating an intracortical locus for plasticity (Fox 1992; Foeller and Feldman 2004; Glazewski and Fox 1996). Plasticity was assessed after a short period of whisker regrowth by measuring whisker receptive fields of L4 and L2/3 single units in the half of the D2 barrel column nearest D1, where plasticity is reported to be maximal (Fox 1992; Glazewski and Fox 1996). Receptive fields at this location were compared between D1-spared rats (n = 7, aged P32–39 at time of recording) and control rats with normal whisker experience (n = 6, aged P30–40). Receptive field data were obtained primarily from predrug recordings in gabazine iontophoresis experiments and from one additional control animal in which iontophoresis was not performed.
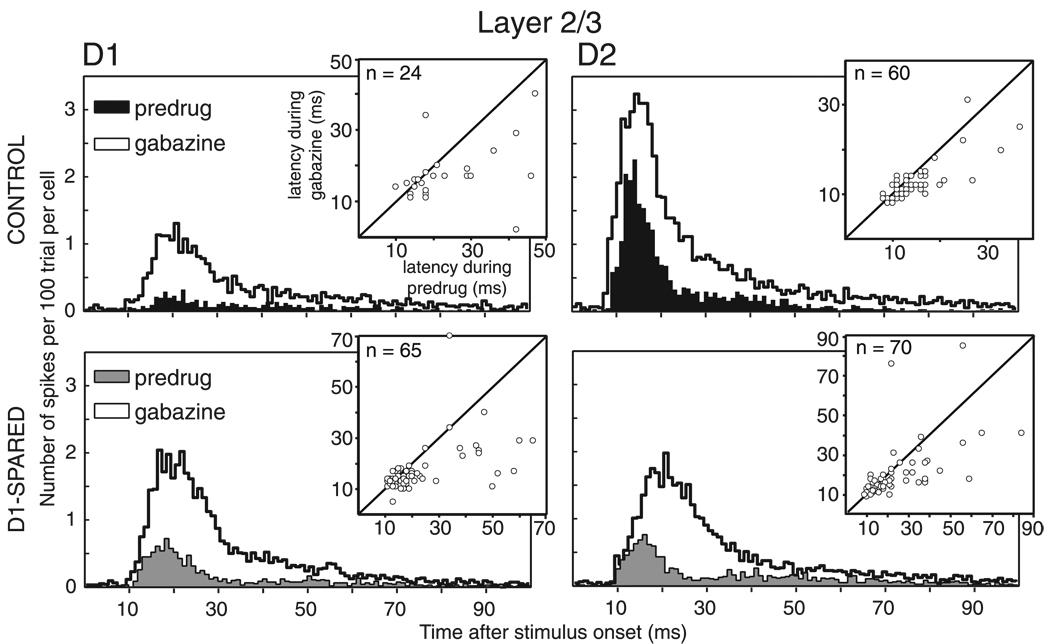
In control rats, single L2/3 units at this location (n = 98 units, 20 recording sites, 8 penetrations) responded more strongly to deflection of the D2 whisker (0.28 ± 0.02 spikes/ stimulus) than to surrounding D1 and D3 whiskers (0.12 ± 0.02 and 0.06 ± 0.02 spikes/stimulus, respectively) (Fig. 1, A and B). For 87 of 98 L2/3 units, D2 evoked the strongest response. In L4, similar tuning was observed, with D1–D3 eliciting 0.13 ± 0.03, 0.29 ± 0.04, and 0.04 ± 0.02 spikes/ stimulus, respectively, and D2 evoking the strongest response for 50 of 56 units (9 recording sites, 6 penetrations). Our use of strict spike-sorting criteria resulted in relatively low response magnitudes, consistent with recent studies using tetrode or whole cell recording to isolate single-cell responses to single whisker deflections (Brecht and Sakmann 2002; Petersen et al. 2003). The slightly stronger response to D1 than D3 is consistent with recording locations in the half of the D2 column nearest D1 (Glazewski and Fox 1996).
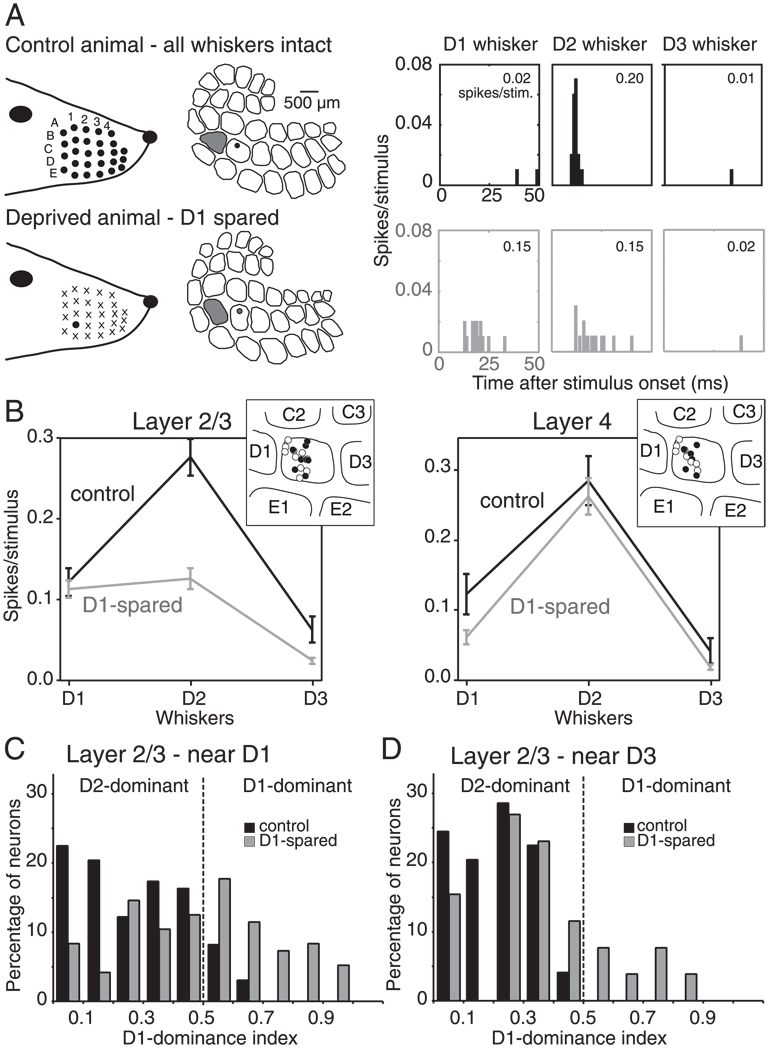
FIG. 1.
Whisker receptive field plasticity with univibrissa experience. A: whisker responses for example single units in the D2 barrel column of a control (top) and D1-spared (bottom) rat. Peristimulus time histograms (PSTHs) show strong responses to the principal whisker D2 in the control rat but relatively weak responses to the deprived D2 whisker and abnormally strong responses to the spared neighboring whisker D1 in the D1-spared rat. Numbers show spikes/stimulus calculated during 100 ms after stimulus onset. Recording sites were closely matched and are indicated as dots in the reconstructed barrel map. The D1 column is shaded gray. B: mean whisker tuning curves for all single units in layer 2/3 (L2/3) and layer 4 (L4) of control and D1-spared animals. The tuning curves show responses only to D1–D3 whiskers and thus represent only partial whisker receptive fields for these units. Bars are SE. Insets: the distribution of recording sites, relative to barrel boundaries, for control (filled symbols) and D1-spared animals (open symbols). C and D: distribution of the D1-dominance index for L2/3 single units in the half of the D2 barrel column closest to D1 (C) and the half closest to D3 (D). Values of D1 dominance index >0.50 indicate that the D1 whisker elicits a stronger response than the D2 whisker.
In D1-spared animals, L2/3 neurons at the same anatomical location had substantially altered receptive fields (Fig. 1, A and B; n = 96 units, 20 recording sites, 11 penetrations). The deprived D2 whisker evoked only 0.13 ± 0.01 spikes/stimulus, significantly less than in control rats (P < 0.001, t-test), consistent with previous findings that deprivation reduces responses to deprived principal whiskers (Glazewski and Fox 1996; Skibinska et al. 2000). In other studies using longer periods of deprivation and/or older animals, D1 sparing was also found to increase single-unit responses to the neighboring, spared whisker (Fox 1992; Glazewski and Fox 1996; Wallace and Fox 1999). However, this component of plasticity was absent with the deprivation duration and animal ages studied here. A small proportion of units exhibited abnormally strong responses to the spared D1 whisker (e.g., Fig. 1A), but on average, D1 responses were not different from control rats (0.11 ± 0.01 vs. 0.12 ± 0.02 spikes/stimulus in controls; P = 0.66, t-test; Fig. 1B). Thus the main effect of D1 sparing was to decrease responses in L2/3 to the deprived D2 principal whisker so that neurons became more dominated by the spared D1 whisker. We term this change in the shape of the whisker tuning curve “receptive field plasticity.”
In contrast, L4 units showed no receptive field plasticity, with D1–D3 whiskers eliciting 0.06 ± 0.01, 0.26 ± 0.03, and 0.02 ± 0.00 spikes/stimulus, respectively, in D1-spared animals (n = 54, 9 recording sites, 8 penetrations). These values were not different from control animals (all P ≥ 0.05, t-test). The lack of plasticity in L4 is consistent with previous studies (Fox 1992; Glazewski and Fox 1996) and confirms that receptive field plasticity in L2/3 reflects plasticity of intracortical circuits rather than impaired whisker mechanics or damage to the D2 follicle.
To quantify receptive field plasticity on a single neuron basis, we calculated a standard measure of each neuron’s relative responsiveness to the D1 and D2 whiskers, the D1-dominance index (D1-di; see methods) (Fox 1992). In control rats, 89% of L2/3 units located in the half of the D2 column closest to D1 had D1-di <0.50, indicating that D2 was the dominant whisker, whereas only 8% had D1-di >0.50 (Fig. 1C). In contrast, in D1-spared animals, the distribution of D1-di was shifted significantly toward higher values, and 46% of L2/3 units at this location had D1-di >0.50, indicating that the spared D1 whisker evoked a stronger response than the deprived D2 whisker (Fig. 1C). Across all L2/3 units, D1-di increased from 0.26 ±0.18 (SD) in control animals to 0.48 ± 0.26 in D1-spared animals (P < 0.0001, t-test). The D1-di was not correlated with the deprivation duration (P = 0.4, Spearman test).
We also assessed plasticity in the half of the D2 column nearest D3, where D1 sparing has been reported to induce less plasticity (Fig. 1D). In control animals, L2/3 units at this location (n = 49) had mean D1-di values of 0.20 ± 0.13, and no units had D1-di values of >0.50. In D1-spared animals, L2/3 units in this location (n = 26) had mean D1-di values of 0.36 ± 0.23, significantly different from control (P < 0.0002, t-test), but less dominated by D1 than units in the half of D2 closest to D1 in the same animals (P < 0.04, t-test). Thus receptive field plasticity occurred at this location but was less pronounced than in the half of D2 nearest D1, consistent with previous findings (Fox 1992; Glazewski and Fox 1996). To test the role of GABAergic inhibition in receptive field plasticity, we focused exclusively on the half of the D2 column nearest D1.
Gabazine iontophoresis
To measure how GABAA conductances shaped receptive fields, we iontophoretically applied the GABAA-receptor antagonist gabazine at the site of recording, and compared whisker tuning before (predrug), during (Gabazine), and 5–30 min after gabazine application (recovery) (Fig. 2A). Ejection currents were adjusted at each site to provide substantial but nonepileptiform disinhibition of whisker-evoked responses (see methods).
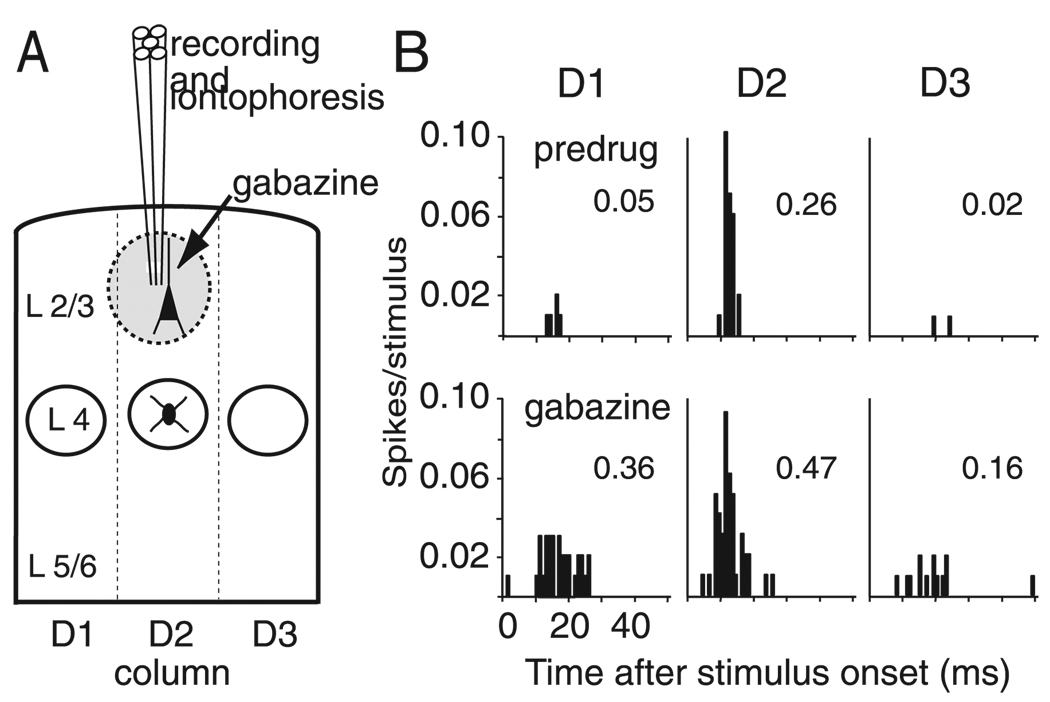
FIG. 2.
Gabazine iontophoresis. A: schematic of extracellular recording and local iontophoresis using a multibarrel electrode, for a recording site in L2/3. Gabazine is estimated to have a maximum effective spread, illustrated by the shaded circle, of ~350 µm, which is less than the width of a barrel column (see Fig. 7). B: whisker responses of a representative L2/3 single unit from a control animal before (predrug) and during gabazine iontophoresis. Each panel shows PSTHs for responses to D1, D2, or D3 whisker deflection. Numbers show spikes/stimulus calculated during 100 ms after stimulus onset. Note that gabazine disproportionately increases responses to surround whiskers (D1 and D3) relative to the principal whisker (D2).
Gabazine increased the magnitude and duration of whisker-evoked responses (Fig. 2B). In addition, it increased spontaneous firing: in control rats, spontaneous firing of L2/3 units increased from 0.01 ± 0.02 (SD) spikes in the 100 ms before stimulus onset in the predrug condition to 0.05 ± 0.02 spikes in the gabazine condition, and spontaneous firing of L4 units increased from 0.01 ± 0.02 to 0.04 ± 0.06 spikes (P < 0.0001, paired t-test). In D1-spared rats, gabazine increased spontaneous firing from 0.01 ± 0.02 to 0.06 ± 0.06 spikes/100 ms in L2/3 units and from 0.01 ± 0.02 to 0.05 ± 0.06 spikes in L4 units (P < 0.0001, paired t-test). The increase in spontaneous firing in D1-spared animals was identical to control animals for both layers (all P = 0.9).
Effect of gabazine on whisker tuning in L2/3 of control rats
In control animals, gabazine disinhibited D1 (surround) whisker responses more strongly than D2 (principal) whisker responses. A single example is shown in Fig. 2B, and data from 65 single units (12 recording sites, 5 penetrations) are shown in Fig. 3A. On average, D1 and D2 whiskers evoked 0.06 ± 0.01 and 0.25 ± 0.02 spikes/stimulus, respectively, during the predrug condition. With gabazine, D1 and D2 responses increased to 0.28 ± 0.03 and 0.58 ± 0.04 spikes/stimulus. This represents a 4.7-fold increase in the mean response to D1 and a 2.3-fold increase in the mean response to D2 compared with the predrug condition (Fig. 3A).
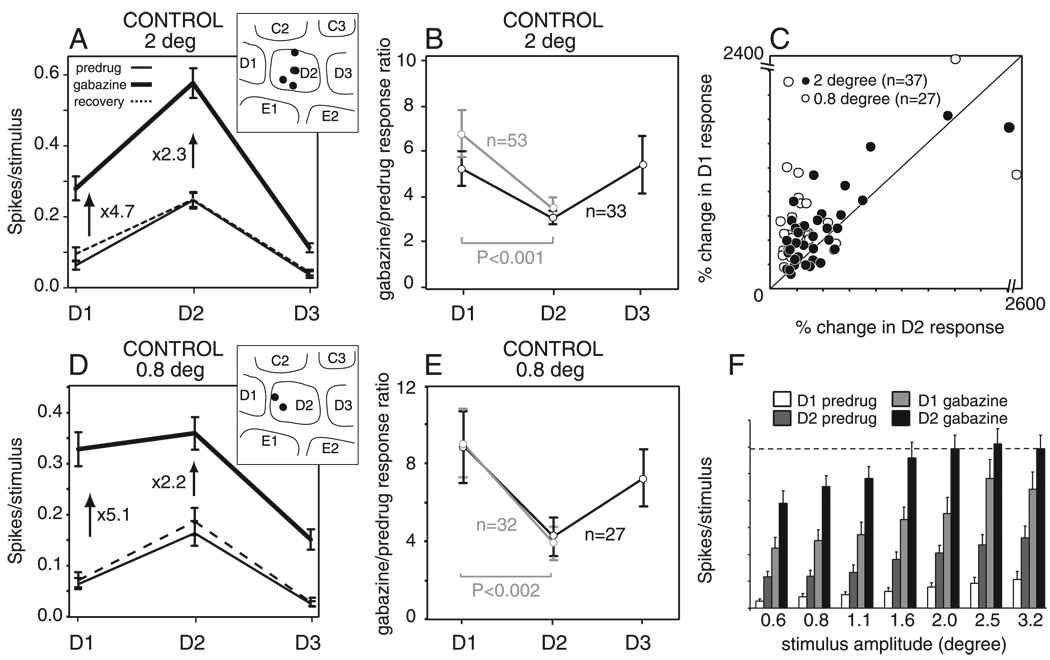
FIG. 3.
Effect of gabazine on whisker tuning curves in layer 2/3 in control animals. A and D: mean whisker receptive fields for deflection amplitude of 2 (A) and 0.8 (D) degrees before, during, and after gabazine iontophoresis. Numbers show the fractional increase in mean response produced by gabazine. Bars are SE. Insets: recording site locations for all units in each population. B and E: quantification of increase in whisker responses by gabazine as calculated by the gabazine/predrug response ratio (see text) for all units with non-0 predrug responses. Black, all units with nonzero predrug responses to D1–D3. Gray, all units with non-0 predrug responses to D1 and D2. Bars are SE. Two (B) and 0.8 (E) degrees deflection amplitude. C: relative disinhibition of D1 and D2 whisker responses by gabazine for neurons with D1 and D2 predrug response magnitude ≥0.02 spikes/stimulus. F: rate-amplitude relationship in control units (n = 33). The dashed line indicates mean saturation level of the D2 response during gabazine. Bars are SE. Note that A, F, and D are derived from separate data sets, thus the precise magnitude of D1 and D2 responses are slightly different between these data sets.
We calculated, for each neuron, the fractional increase in D1–D3 whisker responses that was produced by gabazine (gabazine/predrug response ratio; Fig. 3B). For all neurons with nonzero predrug responses to all three whiskers (n = 33, black lines), gabazine increased D2 responses by a factor of 3.1 ± 0.3 but increased D1 and D3 responses of the same neurons by a larger fraction, 5.3 ± 0.8 and 5.4 ± 1.3, respectively. A similar preferential disinhibition of D1 responses was observed when additional neurons with nonzero predrug responses to D1 and D2, but no predrug responses to D3, were included in the analysis (n = 53, gray lines; Fig. 3B). The preferential disinhibition of D1 relative to D2 responses was significant (P < 0.001, n = 53; paired t-test).
The preferential disinhibition of D1 responses was highly reproducible across units (Fig. 3C). For this analysis, we considered only units with ≥0.02 spikes/stimulus predrug response magnitude (n = 37) to minimize distortions from a small denominator in the gabazine/predrug ratio (see following text and Fig. 6). The gabazine/predrug ratio was larger for D1 responses than for D2 responses in 27 (73%) of 37 units (Fig. 3C, black points above the diagonal). This preferential disinhibition of D1 responses was significant across this population (P < 0.02, paired t-test).
FIG. 6.
Effect of gabazine in L4. A and B: effect of gabazine on mean whisker tuning curves in control (A) and in D1-spared (B) animals. Numbers denote the fractional increase in mean spike count. Insets: recording sites for each population of units. C and D: quantification of gabazine effect by gabazine/predrug response ratio for all units with nonzero predrug responses. Black, all units with non-0 predrug responses to D1–D3. Gray, all units with non-0 predrug responses to D1 and D2. C: control animals. D: D1-spared animals. E: comparison of disinhibition of D1 and D2 whisker responses for individual neurons. F: change in the D1-dominance index by gabazine, for individual units in control and D1-spared animals. Gabazine preferentially disinhibited D1 responses and increased the D1-di for most units in control and D1-spared animals.
This nonuniform disinhibition across the receptive field was not due to saturation of D2 responses during gabazine application. To assess response saturation, we presented a range of whisker deflection amplitudes to 33 L2/3 units in three control rats. Gabazine strongly disinhibited all whisker responses. D2 responses during gabazine varied significantly across stimulus amplitudes and units [2-factor ANOVA; F(38,192) = 15.9, r2 = 0.76; stimulus amplitude: F(6,192) = 9.7, P < 0.0001; unit: F(32,192) = 17.1, P < 0.0001]. The Tukey-Kramer HSD test for multiple comparisons between groups showed that mean D2 responses in gabazine significantly increased between 0.6 and 1.6° deflection amplitudes but were not significantly different between 1.6 and 3.2°, indicating that responses became saturated at 1.6°. The saturation level (mean response for 1.6–3.2°) was 0.59 spikes per stimulus (Fig. 3F). Thus during the 2° whisker deflections reported in the preceding text (Fig. 3, A–C), D2 whisker responses were likely to have been saturated during gabazine application. To determine whether this saturation was responsible for the preferential disinhibition of D1 responses, we re-measured gabazine’s effects using 0.8° deflections, where responses were significantly weaker than at 2° (P < 0.05, Tukey-Kramer HSD test) and therefore not saturated on average during gabazine application. With these smaller deflections, gabazine still disinhibited the mean D1 response across neurons by 5.1-fold, and the mean D2 response by only 2.2-fold (n = 36; Fig. 3D). Calculating the gabazine/ predrug response ratio separately for each neuron showed that gabazine disinhibited D1 responses by a factor of 9.0 ± 1.7, significantly more than D2 responses (3.9 ± 0.9; P < 0.002, n = 32, gray lines, paired t-test; Fig. 3E), and that this preferential disinhibition was highly consistent across neurons (Fig. 3C). Thus preferential disinhibition of D1 responses was not due to response saturation.
We interpret the preferential disinhibition of SW responses by gabazine to indicate that GABAA conductances in L2/3 normally suppress SW responses more than PW responses, thereby sharpening whisker tuning (see discussion). However, another explanation for preferential disinhibition of SW responses is that gabazine depolarized neurons, which, assuming a linear Vm-spike rate relationship, would generate an equal, additive increase in spikes for all whiskers (i.e., a DC shift or iceberg effect on whisker tuning) (Martin 1988). To assess whether an iceberg model could explain these results, we calculated for each unit a DC-shifted version of the predrug whisker tuning curve that represented the maximal possible DC shift that could have occurred with gabazine (this DC-shifted curve was constrained by the requirement that no point exceeds the measured gabazine tuning curve). This DC shift could not explain the large increase in SW responses with gabazine because D1 responses in the DC-shifted tuning curves (0.14 ± 0.01 spikes/stimulus) were significantly weaker than the actual D1 responses measured during gabazine for the same units (0.28 ± 0.03; P < 0.001, n = 65, paired t-test). Across units, the DC-shifted tuning curves accounted for only 57 ± 4% of the actual fractional increase in D1 responses with gabazine. This analysis indicates that a DC shift alone cannot explain the data and that gabazine preferentially disinhibits SW responses, relative to PW responses, even when a possible DC shift is taken into account.
A second alternative interpretation is that use of a ratio measure of disinhibition exaggerated the magnitude of disinhibition for SW responses because these responses often had initially low, near-zero magnitudes. To test whether this accounted for our results, we recalculated the effect of gabazine using unsorted, multiunit data in which each recording site contained at least three (typically ~5) single units. These sites exhibited substantially stronger predrug responses for all whiskers, including SWs. However, gabazine still preferentially disinhibited SW responses at these sites (P < 0.02, Wilcoxon test, n = 12), indicating that preferential disinhibition of SW responses was not due to bias from very low predrug responses (see supplemental material, Fig. S1)1.
Effect of gabazine on whisker tuning in L2/3 of D1-spared rats
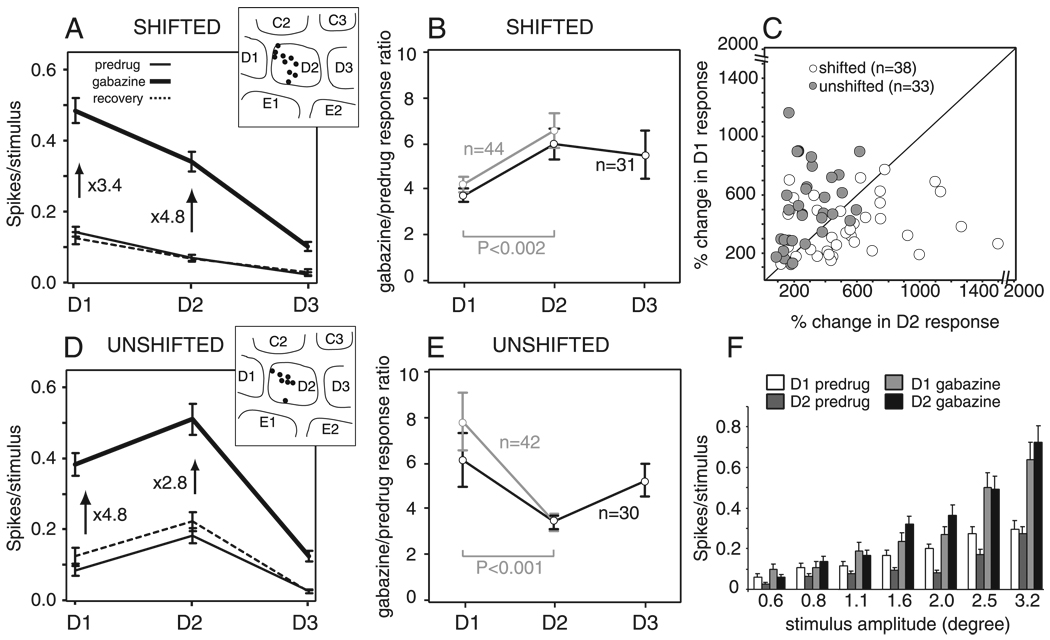
The effect of gabazine was altered in D1-spared animals. Gabazine was applied to 96 single units in L2/3 (20 recording sites, 11 penetrations), with all penetrations localized by lesion recovery to the half of the D2 column nearest D1. Whisker deflections of 2° were used, because these did not cause response saturation even during gabazine application in deprived rats (Fig. 4F). Units were divided into two groups based on predrug whisker receptive fields. The first group (D1-di ≥0.50; n = 48; termed “shifted units”) contained neurons that were dominated by the D1 whisker (mean D1-di = 0.69, range: 0.50–1.0). Because units with D1-di >0.50 are extremely rare at this location in control animals (Fig. 1C), these units are likely to have shifted their receptive field during whisker deprivation. The second group (D1-di <0.50; n = 48; termed “unshifted units”) was still dominated by the D2 whisker (mean D1-di = 0.27, range: 0.0–0.48) as in control rats.
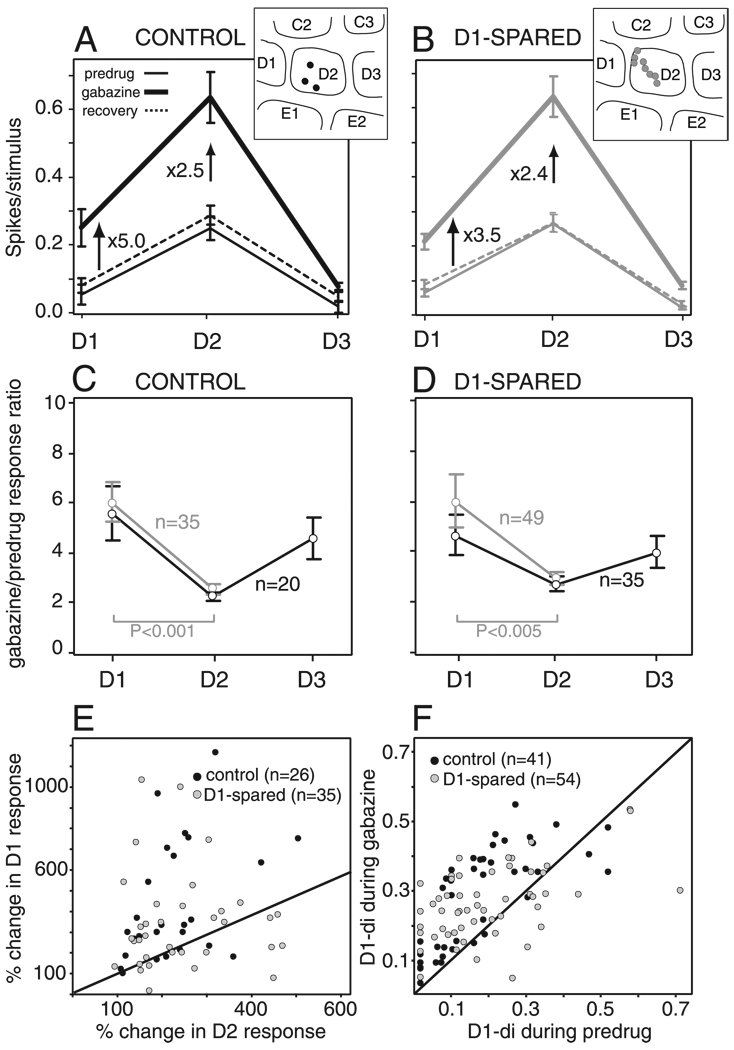
FIG. 4.
Effect of gabazine on whisker tuning curves in layer 2/3 in D1-spared animals. A and D: mean whisker receptive fields, calculated across all units with shifted (A) and unshifted (D) receptive fields in D1-spared animals, before, during, and after gabazine iontophoresis. Numbers show the fractional increase in mean response produced by gabazine. Bars are SE. Insets: recording site locations for all units in each population. B and E: quantification of increase in whisker responses by gabazine, as calculated by the gabazine/predrug response ratio (see text) for all units with non-0 predrug responses. Black, all units with nonzero predrug responses to D1–D3. Gray, all units with nonzero predrug responses to D1 and D2. B: shifted receptive fields. E: unshifted receptive fields. Bars are SE. C: relative disinhibition of D1 and D2 whisker responses by gabazine for all neurons with D1 and D2 predrug response magnitude ≥0.02 spikes/stimulus. F: rate-amplitude relationship in shifted units (n = 23). Bars are SE.
When applied to L2/3 units with shifted receptive fields, gabazine preferentially disinhibited D2 (principal whisker) responses (Fig. 4, A and B), rather than D1 whisker responses as in control animals (Fig. 3, A and B). Averaged across all shifted units, gabazine increased mean D2 responses by 4.8-fold and D1 responses by only 3.4-fold (Fig. 4A, n = 48). To determine whether D2 responses were preferentially disinhibited within individual units, we calculated the gabazine/predrug response ratio for D1–D3 whiskers, for each unit with nonzero predrug responses to all three whiskers (n = 31 units). We found that gabazine increased D1 responses by a factor of 3.7 ± 0.3 but increased D2 responses by a factor of 6.0 ± 0.6 (Fig. 4B). Preferential disinhibition of D2 responses was also observed when additional neurons with no predrug responses to D3 were included in the analysis (n = 44, gray lines; Fig. 4B). This preferential disinhibition of D2 versus D1 responses was significant (P < 0.002, n = 44; paired t-test) and was highly consistent across units, with 27 (71%) of 38 cells with >0.02 spikes/stimulus in the predrug condition showing greater fractional disinhibition of D2 responses than D1 responses (white points below the diagonal in Fig. 4C). The effects of gabazine on whisker tuning could not be explained by a DC shift in whisker responses.
In contrast, L2/3 units with unshifted receptive fields (D1-di <0.5, n = 48) exhibited the same effect of gabazine as units in control animals. Mean tuning curves, calculated across all unshifted units, showed that gabazine preferentially enhanced D1 (surround whisker) responses. Across units, gabazine increased the mean D1 response by a factor of 4.8 but increased the mean D2 response by only a factor of 2.8 (Fig. 4D). When gabazine/predrug ratios were calculated for individual units with nonzero predrug responses to D1–D3, it was found that gabazine increased D1 responses by a factor of 6.1 ± 1.2, whereas D2 responses were increased by only a factor of 3.4 ± 0.4 (n = 30; Fig. 4E). Significant preferential disinhibition of D1 responses was also observed when additional neurons with no predrug responses to D3 were included in the analysis (n = 42; gray lines, paired t-test, P < 0.001; Fig. 4E) and was not due to the DC shift alone caused by gabazine. Preferential disinhibition of D1 responses was consistent across units, with 27 (82%) of 33 cells with >0.02 spikes/stimulus in the predrug condition showing preferential disinhibition of D1 (gray points above the diagonal in Fig. 4C; P < 0.0001, paired t-test). Thus the functional effects of GABAA conductances on whisker tuning were altered in L2/3 units with shifted receptive fields, relative to control animals. This effect was not seen in units with unshifted receptive fields.
To determine the robustness of these findings, we also analyzed the effect of gabazine for the entire population of shifted and nonshifted units in D1-spared animals, considered as a single undivided group. Gabazine/predrug ratios for D1 and D2 responses were 4.9 ± 0.6 and 4.7 ± 0.4, respectively, for units with nonzero predrug responses to all whiskers (n = 61) and were 6.0 ± 0.4 and 5.0 ± 0.4, respectively, when additional units lacking predrug responses to D3 were included (n = 86; no significant difference between D1 and D2 responses, P = 0.2, paired t-test). This relatively equal disinhibition of D1 and D2 responses was in contrast to control rats, where gabazine preferentially disinhibited D1 responses. These results indicate that abnormal effect of gabazine in deprived animals was a real result of D1-sparing and was not due artifactually to the division of units into shifted and nonshifted groups.
Verification of findings using D1 dominance index
Because the denominator of the gabazine/predrug response ratio is the predrug response to a single whisker, this ratio may exaggerate the magnitude of disinhibition for responses with initially low, near-zero magnitudes. Therefore we routinely excluded units with very weak predrug responses (<0.02 spikes/stimulus) from the analyses in Figs. 3C and 4C. As reported in the preceding text, significant differential inhibition of D1 and D2 responses was observed with this ratio measure, and plasticity still reversed this differential inhibition (Figs. 3C and 4C). In addition, recalculation of gabazine effects on multiunit sites, which exhibit substantially stronger predrug responses to all whiskers, showed the same trends as the single units, again suggesting that differential disinhibition was not an artifact of low baseline responses (see supplemental material, Fig. S1).
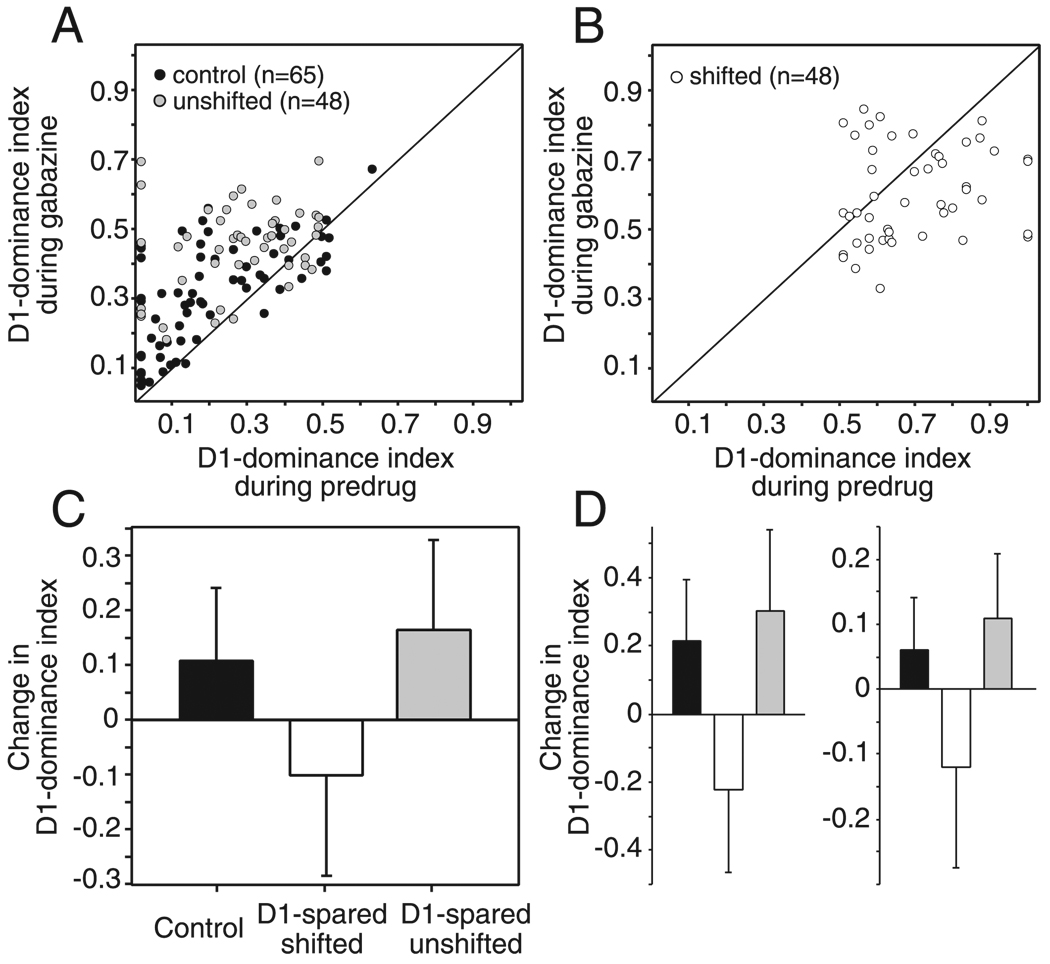
An alternative way of quantifying the effect of gabazine on whisker tuning is to use the D1 dominance index (D1-di). This metric exhibits less bias from initial response magnitude, because the denominator is relatively large—the sum of the responses to the D1 and D2 whiskers. For single units in control rats, gabazine significantly increased D1-di values, by a mean of 0.11 ± 0.13 (SD; P < 0.0005, paired t-test; n = 65 units; all units included regardless of predrug response magnitude; Fig. 5, A and C). This increase indicates that gabazine shifted whisker tuning toward the D1 whisker, consistent with the preferential disinhibition of D1 (surround whisker) responses.
FIG. 5.
Quantification of gabazine’s effect using D1 dominance index (D1-di). A and B: effect of gabazine on D1-di for control, unshifted (A) and shifted (B) units. Points above the diagonal indicate that gabazine increased the D1-di and thus preferentially disinhibited D1 (surround) whisker responses. Points below the diagonal indicate that gabazine decreased the D1-di and thus preferentially disinhibited D2 (principal) whisker responses. C: mean change in the D1-di across all control, shifted, and unshifted units. Bars are SD. D: mean change in the D1-dominance index for 20% of units in each group with the strongest (left) and weakest (right) predrug responses. Changes in the index are significant for all groups (1-tailed Wilcoxon). Bars are SD.
In contrast, units with shifted receptive fields in D1-spared animals showed the opposite effect, with gabazine significantly decreasing D1-di values by 0.10 ± 0.18 (P < 0.001; n = 48), indicating a shift in whisker tuning toward the D2 whisker (Fig. 5, B and C). Units with unshifted receptive fields in D1-spared animals behaved similarly to control units, with gabazine producing an increase in D1-di by 0.16 ± 0.16 (P < 0.0001; n = 48; Fig. 5, A and C). The change in D1-di by gabazine was not correlated with the deprivation duration (P = 0.6, Spearman test).
These differences between control, shifted, and unshifted units were not due to any differences in predrug response magnitude between groups because similar effects were observed for the units with strongest and weakest predrug responses in each group (Fig. 5D). Thus multiple analyses showed that gabazine preferentially disinhibited surround whisker responses for units in control animals and preferentially disinhibited principal whisker responses for shifted units in D1-spared animals.
Effect of gabazine in L4
In L4, where receptive field plasticity did not occur (Fig. 1B), plucking had no effect on the normal inhibitory sharpening of receptive fields (Fig. 6). In control animals, gabazine was applied to 41 units in the half of the D2 column closest to D1. During the predrug period, D1 and D2 whiskers elicited 0.05 ± 0.02 and 0.25 ± 0.03 spikes/stimulus, respectively. During gabazine, D1 and D2 responses increased to 0.25 ± 0.05 and 0.63 ± 0.07 spikes/stimulus, respectively, which represents a 5.0- and 2.5-fold increase in mean D1 and D2 responses, respectively (Fig. 6A).
When gabazine/predrug ratios were calculated for individual units (n = 20) with nonzero predrug responses to D1–D3, it was found that gabazine increased D2 responses by a factor of 2.3 ± 0.2, whereas D1 and D3 responses were increased more, by factors of 5.6 ± 1.1 and 4.6 ± 0.8, respectively (n = 20; Fig. 6C). Preferential disinhibition of D1 responses was also observed when additional neurons with nonzero predrug responses to D1 and D2, but no predrug responses to D3, were included in the analysis (P < 0.001, n = 35 units, gray line, paired t-test; Fig. 6C). In addition, preferential disinhibition of D1 responses was reliable across units: 20 (77%) of 26 units with >0.02 spikes/stimulus showed a preferential disinhibition of D1 responses (Fig. 6E; black points above the diagonal). Thus gabazine preferentially disinhibited D1 responses in L4 of control animals, similar to L2/3 of control animals (Fig. 3) and previous reports (Kyriazi et al. 1996b, 1998).
Gabazine’s effect was unchanged in deprived animals. During the predrug condition, D1 and D2 whiskers elicited 0.06 ± 0.01 and 0.26 ± 0.03 spikes/stimulus, respectively (n = 54). Gabazine increased D1 and D2 responses to 0.21 ± 0.02 and 0.63 ± 0.06 spikes/stimulus, respectively, an increase in mean responses of 3.5- and 2.4-fold, respectively (Fig. 6B). In individual units with nonzero predrug responses to all three whiskers, gabazine increased D2 responses by a factor of 2.7 ± 0.3, whereas D1 and D3 responses were increased more, by factors of 4.6 ± 0.8 and 4.0 ± 0.6, respectively (n = 35; Fig. 6D). When additional neurons with no predrug responses to D3 were included in the analysis, similar preferential disinhibition of D1 responses was found (P < 0.005, n = 49 units, gray line, paired t-test; Fig. 6D). Considered individually, most units (22 of 35 units with predrug responses >0.02 spikes/stimulus) showed preferential disinhibition of D1 responses (gray points above the diagonal in Fig. 6E).
The preferential disinhibition of D1 responses in control and D1-spared animals was confirmed by analyzing the D1-di metric. In control animals, gabazine significantly increased the D1-di by 0.1 ± 0.1 (SD; P < 0.0001, n = 41), consistent with a preferential increase of D1 responses. An identical effect occurred in deprived animals (D1-di increased by 0.1 ± 0.1, P < 0.003, n = 54; Fig. 6F). Thus gabazine preferentially disinhibited D1 responses in L4, independent of the sensory experience of the animal.
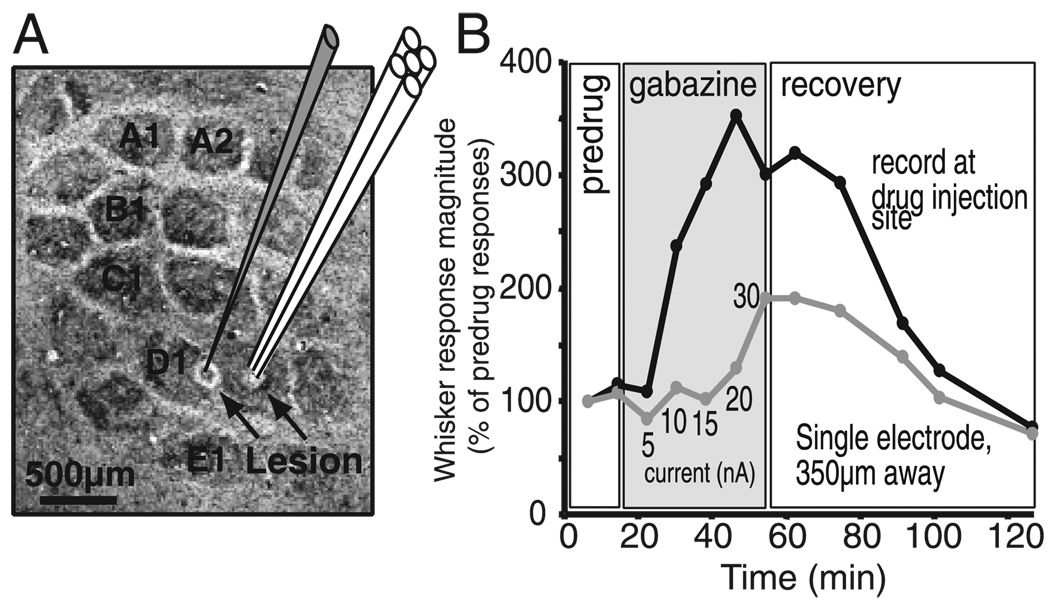
Diffusion extent of gabazine
To determine if the effects of gabazine were due primarily to blockade of GABAA receptors near the iontophoresis/recording site or by diffusion into neighboring columns, we measured the functional extent of gabazine diffusion in one experiment (Fig. 7). Gabazine was applied iontophoretically, in incrementally increasing ejection currents, through a multibarrel electrode in L4 in the center of the D2 column. Multiunit responses to simultaneous deflection of the D1 and D2 whiskers were recorded both from that electrode and, at the same time, from a second recording electrode in L4 at the edge of the D1 barrel, at a lateral distance of 350 µm from the iontophoresis pipette. Electrode placement was confirmed by lesion recovery (Fig. 7A).
FIG. 7.
Diffusion extent of gabazine. A: schematic of the diffusion experiment, showing location of the multibarrel recording/iontophoresis electrode in the center of the D2 barrel and the separate recording electrode, located 350 µm away at the edge of the D1 barrel. Both electrode tips were in L4. Background, cytochrome oxidase-stained flattened section through L4, showing marking lesions recovered after the experiment. B: whisker response magnitude for multiunit clusters at the iontophoresis recording site and the separate recording site 350 µm away as a function of time during the experiment. Gabazine was applied with increasing ejection currents (5–30 nA) at the iontophoresis site. Ejection currents ≤15 nA caused pronounced disinhibition at the local site, but no disinhibition at the distant site.
Gabazine application at >5 nA ejection currents caused large, rapid increases in whisker-evoked responses at the iontophoresis site. In contrast, whisker responses were largely unchanged for currents ≤ 15 nA at the site 350 µm away and only relatively modestly increased (less than twofold) for 20-to 30-nA currents. Recovery was slow because of the long (40 min) duration of gabazine application in this experiment (as opposed to 5–10 min in the standard protocol). Thus these data indicate that short-duration, low gabazine ejection currents (≤ 15 nA for ~ 10 min) as used in 88% of the recordings in this study, caused substantial disinhibition only locally near the site of iontophoresis and not in neighboring columns.
Effects of gabazine on temporal response characteristics
GABAA-receptor blockade also prolonged the duration of whisker responses (Fig. 8), consistent with previous reports (Kyriazi et al. 1998). This was due primarily to a significant prolongation of response offset (see methods). In L2/3, gabazine application increased median response offset by 6–10 ms for D1 and D2 responses in control and D1-spared rats (P < 0.03 for each whisker and type of experience; Wilcoxon test; Fig. 8). In L4, gabazine prolonged median response offsets by 6–7 ms (P < 0.01 for each whisker and type of experience except D1 responses in deprived animals, for which P = 0.06).
FIG. 8.
Effects of gabazine on response time course. Population PSTHs for responses to D1 and D2 whiskers in L2/3 of control (top; n = 65) and D1-spared (bottom; n = 96) animals. Population PSTHs were calculated by summing nonnormalized PSTHs from individual neurons. Bin width is 1 ms. Insets: comparison of onset latency before (predrug) and during gabazine application.
Gabazine application also reduced median onset latencies, in contrast to a previous study (Kyriazi et al. 1998), suggesting that inhibition normally suppresses early excitation of S1 neurons. This was particularly apparent in L2/3, where gabazine significantly reduced onset latencies for both D1 and D2 whisker responses, both in control and deprived animals (Table 1, Fig. 8, insets). In L4, the tendency to reduce onset latency was only significant for D2 responses in deprived rats. These reductions in response latency were not due to artifactual overestimation of latency for weakly responding units because latency reduction was uncorrelated with predrug spike count (data not shown).
TABLE 1.
Gabazine-induced changes in median onset latency
| L2/3 |
L4 |
|||
|---|---|---|---|---|
| Control, ms | Deprived, ms | Control, ms | Deprived, ms | |
| No gabazine | ||||
| D1 | 18(24) | 17(65) | 15.5(16) | 15(23) |
| D2 | 12(60) | 18(70) | 10(41) | 10(51) |
| With gabazine | ||||
| D1 | 16.5(24)* | 14(65)** | 14(16) | 15(23) |
| D2 | 11(60)** | 15.5(70)*** | 9(41) | 10(51)† |
P < 0.005,
P < 0.0001, and
P < 0.0005 relative to no-gabazine condition, Wilcoxon test.
P < 0.01, Wilcoxon test, relative to no-gabazine condition. This significant difference reflects a small but consistent decrease in latency with gabazine, with mean onset latency decreasing from 12.3 ms before gabazine to 11.4 ms during gabazine (P < 0.05, using paired t-test).
DISCUSSION
General interpretation of gabazine effects
In this study, we interpret the differential disinhibition of PW and SW responses by gabazine as evidence that GABAA inhibitory conductances (either in the recorded neuron or the local network) differentially suppressed these responses in the intact network, before gabazine was applied. This interpretation has been used in many studies (e.g., Foeller et al. 2001; Kyriazi et al. 1996b; Zheng and Knudsen 1999) and requires that the extent of disinhibition was not determined trivially by response saturation during gabazine application. In our data, differential disinhibition of PW and SW responses was robust even for weak, sub-saturating responses in control rats and univibrissa rats, indicating that this effect was not due to saturation (Figs. 3 and 4). Another alternative interpretation is that gabazine depolarizes neurons, thus producing a generalized, additive increase in whisker-evoked responses across the entire receptive field (an iceberg effect). However, the preferential enhancement of SW responses in our data exceeded the enhancement predicted by such an additive mechanism. Thus we interpret the differential disinhibition of PW and SW responses to indicate that inhibitory conductances differentially suppress these responses, and thus that inhibition helps shape whisker tuning, both before and after whisker map plasticity.
A third concern is that the use of ratio-based measures of disinhibition, together with the relatively low predrug response magnitudes in our data, exaggerate the disinhibition of the weakest responses. Strict spike-sorting criteria indeed kept our response magnitudes low compared with prior studies using peak amplitude- or template-based spike sorting methods (e.g., Armstrong-James et al. 1992; Ego-Stengel et al. 2005; Glazewski and Fox 1996), but consistent with studies using more selective tetrode and whole cell recording techniques (Brecht and Sakmann 2002; Petersen et al. 2003). Three analyses indicate that preferential disinhibition of specific whisker responses was not due to low response magnitude. First, similar results were obtained for multiunit data, which had stronger predrug responses (Fig. S1). Second, similar results were also obtained using the D1-di, which shows less bias to exaggerate weak predrug responses (Fig. 5). Third, identical effects were observed for single units with the lowest and highest predrug response rates (Fig. 5D).
Importantly, these results only describe the net effect of inhibition in shaping receptive fields and not the absolute magnitude of inhibitory potentials or conductances evoked by specific whiskers. For example, preferential disinhibition of SW responses by gabazine in control rats indicates that SW responses were suppressed proportionately more by inhibition than PW responses and thus that inhibition had the net effect of narrowing receptive fields around the PW. This result does not mean that SWs elicited more inhibitory conductance than the PW. Instead preferential suppression of SW responses could reflect the fact that SW inputs elicit weak excitatory potentials, which are readily suppressed below spike threshold by even modest amounts of inhibition. Thus we distinguish below between the net functional effect of inhibition on whisker tuning, which can be inferred from these data, and the magnitude of inhibitory potentials or conductances, which cannot.
Inhibitory sharpening of receptive fields in control animals
In control animals, gabazine preferentially disinhibited surround whisker responses, thereby broadening whisker receptive fields. This effect was reflected in the D1-di, which increased significantly with gabazine, indicating that when inhibition was blocked, tuning broadened to include more surround D1 whisker responses. We interpret these results to indicate that GABAergic conductances on L4 and L2/3 neurons normally act to preferentially suppress surround whisker responses and sharpen whisker receptive fields. This finding is consistent with previous studies in S1 (Kelly et al. 1999; Kyriazi et al. 1996b, 1998; Simons and Carvell 1989) and other cortical areas (Foeller et al. 2001; Miller et al. 2001; Sompolinsky and Shapley 1997; Wang et al. 2002; Wehr and Zador 2003).
How inhibitory conductances sharpen whisker tuning is not clear. L4 and L2/3 neurons receive tonic and whisker-evoked inhibition from local interneurons (Brumberg et al. 1996; Bruno and Simons 2002; Douglas and Martin 2004; Porter et al. 2001; Simons and Carvell 1989; Swadlow and Gusev 2002; Welker et al. 1993). In a classical lateral inhibition model, nonpreferred (SW) inputs are hypothesized to evoke a larger inhibitory conductance than preferred (PW) inputs, leading to preferential suppression of SW responses. Alternatively, inhibitory conductance may be untuned, broadly tuned, or co-tuned with excitatory inputs. The existence of any of these patterns of inhibitory conductance would preferentially suppress spiking responses to weak (SW) excitatory inputs, relative to strong (PW) excitatory inputs, because weak inputs are more readily reduced below spike threshold by either subtractive or divisive inhibition (Anderson et al. 2000; Heeger 1992; Miller et al. 2001; Wehr and Zador 2003). Thus in these models, inhibition and the spike threshold act together to sharpen the tuning of the cell’s spiking output around the whisker that elicits the strongest excitatory synaptic input. The current data do not distinguish between these models, although whole cell recording experiments in vivo argue against the lateral inhibition model (Brecht et al. 2003; Moore and Nelson 1998).
Receptive field plasticity and the effect of inhibition in univibrissa rats
Following univibrissa experience, many L2/3 neurons showed decreased responses to the deprived PW, consistent with previous descriptions of whisker map plasticity (Glazewski and Fox 1996). However, we did not observe a second, previously reported effect of univibrissa experience, an increase in responses to the spared SW whisker (Glazewski and Fox 1996). This discrepancy may reflect the fact that potentiation of spared whisker responses required ≥20 days of univibrissa experience in prior studies, whereas most rats (6/7) in our study were plucked <20 days (Glazewski and Fox 1996). Alternatively, this effect may be less robust in 2-wk-old rats, compared with the 1- and 4-wk-old rats studied previously (Fox 1992; Glazewski and Fox 1996).
For L2/3 units the receptive fields of which were shifted substantially away from the PW by univibrissa experience, gabazine preferentially disinhibited responses to the deprived PW, opposite to its effect in controls (Fig. 4). As a result, gabazine application tended to restore the D1-dominance index of shifted units toward values observed in control animals (Fig. 5). We interpret these results to indicate that in shifted units, GABAA conductances preferentially suppressed PW responses, rather than SW responses as in controls, and therefore that inhibition helped to sharpen whisker tuning around the spared SW, thereby promoting the receptive field shift away from the deprived PW. This finding is consistent with early studies of monocular deprivation and strabismus, in which deprived eye responses could be restored by application of the GABAA-receptor antagonist bicuculline (Mower et al. 1984; Sillito et al. 1981). In contrast, the effect of gabazine on receptive fields was unaltered for L2/3 units with unshifted receptive fields and in L4, where receptive field plasticity did not take place. For these units, gabazine preferentially disinhibited surround whisker responses, as in control rats, suggesting that inhibition continued to sharpen receptive fields around the PW. Thus inhibition opposed receptive field plasticity in units with unshifted receptive fields but enhanced plasticity in units with shifted receptive fields. Similar results have been reported during learning-related plasticity in the owl sound-localization system, where inhibitory shaping opposes receptive field plasticity at early time points during learning, but promotes plasticity at later time points, after substantial receptive field plasticity has occurred (Zheng and Knudsen 1999, 2001).
Possible mechanisms for altered inhibition during plasticity
Two models may account for the altered functional effects of inhibition in units with shifted receptive fields. In the first model, PW deprivation may trigger lasting changes in inhibitory circuits that alter whisker-specific recruitment of inhibition. For example, after deprivation the magnitude of deprived PW-evoked inhibitory conductance may increase in S1 neurons, thus increasing inhibitory suppression of PW responses and promoting the receptive field shift toward the spared SW. Substantial precedent exists for experience-dependent (Fuchs and Salazar 1998; Gierdalski et al. 2001; Hendry and Jones 1986; Knott et al. 2002; Lech et al. 2001; Micheva and Beaulieu 1997; Wellman et al. 2002) and activity-dependent plasticity of GABAergic synapses and circuits as is implicit in this model (Chevaleyre and Castillo 2003; Gaiarsa et al. 2002; Kandler 2004; Kittler and Moss 2003; Komatsu and Iwakiri 1993; Marsicano et al. 2002; Marty and Llano 1995; Woodin et al. 2003).
An alternate model is that the magnitude of whisker-evoked inhibitory conductances is entirely unchanged by deprivation and that instead inhibition consistently acts to sharpen tuning around the whisker with the strongest excitatory synaptic input. In this model, any experience-dependent changes in the tuning of excitatory input to a neuron would be amplified in the cell’s spiking output by the nonlinear effects of inhibition. For example, whisker deprivation is known to weaken the excitatory L4 synaptic inputs that drive PW responses in L2/3 pyramidal cells (Allen et al. 2003). As PW-evoked excitation is reduced, existing levels of inhibition would be expected to more effectively suppress the remaining PW responses below spike threshold (Anderson et al. 2000; Heeger 1992; Miller et al. 2001; Wehr and Zador 2003). Thus inhibition would promote plasticity by amplifying the effects of experience-induced changes in excitatory input, even if the magnitude of whisker-evoked inhibitory conductances were entirely unchanged by deprivation.
Our data also provide additional evidence that whisker deprivation does alter whisker-evoked excitatory input to L2/3 neurons. Whisker receptive fields measured during gabazine application are likely to reflect the net tuning of excitatory inputs to L2/3 neurons (Zheng and Knudsen 1999). In control animals, whisker receptive fields during gabazine were peaked at the D2 whisker, suggesting that the D2 whisker provided maximal excitatory input to cells in the D2 column (Brecht et al. 2003; Moore and Nelson 1998). In deprived animals, however, receptive fields during gabazine were dominated equally by D1 and D2 whiskers (0.43 ± 0.02 and 0.43 ± 0.03 spikes/stimulus, respectively), suggesting that deprivation had decreased PW (D2)-evoked excitation relative to SW-evoked excitation. Thus these data are consistent with whole cell recordings showing that deprivation weakens excitatory L4–L2/3 synapses, which are known to mediate PW responses (Allen et al. 2003).
It is important to note that in these experiments deprivation was performed during a major phase of intracortical synaptogenesis for excitatory and inhibitory circuits. From P10 to 15 synapse density increases fivefold in S1, and adult density is reached only at P30 (Micheva and Beaulieu 1996). In addition, during this period dendritic spines are highly motile and turn over rapidly, and deprivation can trigger unique, large-scale reorganization of L2/3 receptive fields (Lendvai et al. 2000; Stern et al. 2001). Thus the mechanisms for plasticity at these ages may be different from those in younger and older animals.
General models for inhibition in plasticity
In most models of cortical plasticity, the primary role of inhibition is to enable or disable activity-dependent modification of excitatory synapses, which is hypothesized to ultimately drive receptive field and map plasticity (Chowdhury and Rasmusson 2002; Dykes 1997; Jacobs and Donoghue 1991; Massie et al. 2003; Rosier et al. 1995; Tremere et al. 2001; but see also Jones 1993; Rajan 1998, 2001). For example, during development, maturation of inhibition may gate the onset of critical periods for plasticity by enabling spiking patterns that allow long-term potentiation, long-term depression, and similar plasticity mechanisms to be induced at excitatory synapses (Hensch 2004). Similarly, inhibition has been proposed to gate deafferentation- and lesion-induced plasticity in adult brain: In deafferented cortical regions, decreased inhibition allows weaker excitatory input to be unmasked, enabling subsequent activity-dependent strengthening of excitatory circuits (Dykes 1997).
Our data support an additional role for inhibition in cortical plasticity: inhibition appears to contribute directly to the final expression of receptive field plasticity by preferentially suppressing responses to deprived sensory inputs and sharpening receptive fields around spared, behaviorally relevant inputs. Thus acute blockade of inhibition causes neuronal receptive fields that were altered by plasticity to preferentially re-express these responses and shift back toward the neural tuning observed in control animals. The changes in inhibitory sharpening of receptive fields with plasticity may reflect deprivation-induced changes within cortical inhibitory circuits themselves (Jones 1993) or may paradoxically reflect stable, unchanging inhibitory circuits that consistently act to amplify experience-dependent changes in excitatory inputs to cortical neurons. Additional cellular and synapse-level studies will be required to distinguish between these models and determine the precise cellular mechanisms involved.
Supplementary Material
ACKNOWLEDGMENTS
We thank B. Gaese, C. Kapfer, M. Kössl, M. Scanziani, and Feldman lab members for critically reading the manuscript.
GRANTS
This study was supported by the Deutsche Forschungsgemeinschaft (FO 344/2-1).
Footnotes
The Supplementary Material for this article (a figure) is available online at http://jn.physiology.org/cgi/content/full/00553.2005/DC1.
REFERENCES
- Allen CB, Celikel T, Feldman DE. Long-term depression induced by sensory deprivation during cortical map plasticity in vivo. Nat Neurosci. 2003;6:291–299. doi: 10.1038/nn1012. [DOI] [PubMed] [Google Scholar]
- Anderson JS, Carandini M, Ferster D. Orientation tuning of input conductance, excitation, and inhibition in cat primary visual cortex. J Neurophysiol. 2000;84:909–926. doi: 10.1152/jn.2000.84.2.909. [DOI] [PubMed] [Google Scholar]
- Armstrong-James M, Fox K, Das-Gupta A. Flow of excitation within rat barrel cortex on striking a single vibrissa. J Neurophysiol. 1992;68:1345–1358. doi: 10.1152/jn.1992.68.4.1345. [DOI] [PubMed] [Google Scholar]
- Armstrong-James M, Millar J. Carbon fiber microelectrodes. J Neurosci Methods. 1979;1:279–287. doi: 10.1016/0165-0270(79)90039-6. [DOI] [PubMed] [Google Scholar]
- Brecht M, Roth A, Sakmann B. Dynamic receptive fields of reconstructed pyramidal cells in layers 3 and 2 of rat somatosensory barrel cortex. J Physiol. 2003;553:243–265. doi: 10.1113/jphysiol.2003.044222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecht M, Sakmann B. Dynamic representation of whisker deflection by synaptic potentials in spiny stellate and pyramidal cells in the barrels and septa of layer 4 rat somatosensory cortex. J Physiol. 2002;543:49–70. doi: 10.1113/jphysiol.2002.018465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumberg JC, Pinto DJ, Simons DJ. Spatial gradients and inhibitory summation in the rat whisker barrel system. J Neurophysiol. 1996;76:130–140. doi: 10.1152/jn.1996.76.1.130. [DOI] [PubMed] [Google Scholar]
- Bruno RM, Simons DJ. Feedforward mechanisms of excitatory and inhibitory cortical receptive fields. J Neurosci. 2002;22:10966–10975. doi: 10.1523/JNEUROSCI.22-24-10966.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calford MB. Dynamic representational plasticity in sensory cortex. Neuroscience. 2002;111:709–738. doi: 10.1016/s0306-4522(02)00022-2. [DOI] [PubMed] [Google Scholar]
- Calford MB, Tweedale R. Immediate and chronic changes in responses of somatosensory cortex in adult flying-fox after digit amputation. Nature. 1988;332:446–448. doi: 10.1038/332446a0. [DOI] [PubMed] [Google Scholar]
- Celikel T, Szostak VA, Feldman DE. Modulation of spike timing by sensory deprivation during induction of cortical map plasticity. Nat Neurosci. 2004;7:534–541. doi: 10.1038/nn1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron. 2003;38:461–472. doi: 10.1016/s0896-6273(03)00235-6. [DOI] [PubMed] [Google Scholar]
- Chowdhury SA, Rasmusson DD. Effect of GABAB receptor blockade on receptive fields of raccoon somatosensory cortical neurons during reorganization. Exp Brain Res. 2002;145:150–157. doi: 10.1007/s00221-002-1130-9. [DOI] [PubMed] [Google Scholar]
- Darian-Smith C, Gilbert CD. Axonal sprouting accompanies functional reorganization in adult cat striate cortex. Nature. 1994;368:737–740. doi: 10.1038/368737a0. [DOI] [PubMed] [Google Scholar]
- Darian-Smith C, Gilbert CD. Topographic reorganization in the striate cortex of the adult cat and monkey is cortically mediated. J Neurosci. 1995;15:1631–1647. doi: 10.1523/JNEUROSCI.15-03-01631.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derdikman D, Hildesheim R, Ahissar E, Arieli A, Grinvald A. Imaging spatiotemporal dynamics of surround inhibition in the barrels somatosensory cortex. J Neurosci. 2003;23:3100–3105. doi: 10.1523/JNEUROSCI.23-08-03100.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas RJ, Martin KA. Neuronal circuits of the neocortex. Annu Rev Neurosci. 2004;27:419–451. doi: 10.1146/annurev.neuro.27.070203.144152. [DOI] [PubMed] [Google Scholar]
- Dykes RW. Mechanisms controlling neuronal plasticity in somatosensory cortex. Can J Physiol Pharmacol. 1997;75:535–545. [PubMed] [Google Scholar]
- Ego-Stengel V, Mello e Souza T, Jacob V, Shulz DE. Spatiotemporal characteristics of neuronal sensory integration in the barrel cortex of the rat. J Neurophysiol. 93:1450–1467. doi: 10.1152/jn.00912.2004. [DOI] [PubMed] [Google Scholar]
- Fee MS, Mitra PP, Kleinfeld D. Automatic sorting of multiple unit neuronal signals in the presence of anisotropic and non-Gaussian variability. J Neurosci Methods. 1996;69:175–188. doi: 10.1016/S0165-0270(96)00050-7. [DOI] [PubMed] [Google Scholar]
- Finnerty GT, Roberts LS, Connors BW. Sensory experience modifies the short-term dynamics of neocortical synapses. Nature. 1999;400:367–371. doi: 10.1038/22553. [DOI] [PubMed] [Google Scholar]
- Foeller E, Feldman DE. Synaptic basis for developmental plasticity in somatosensory cortex. Curr Opin Neurobiol. 2004;14:89–95. doi: 10.1016/j.conb.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Foeller E, Vater M, Kossl M. Laminar analysis of inhibition in the gerbil primary auditory cortex. J Assoc Res Otolaryngol. 2001;2:279–296. doi: 10.1007/s101620010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K. A critical period for experience-dependent synaptic plasticity in rat barrel cortex. J Neurosci. 1992;12:1826–1838. doi: 10.1523/JNEUROSCI.12-05-01826.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K. Anatomical pathways and molecular mechanisms for plasticity in the barrel cortex. Neuroscience. 2002;111:799–814. doi: 10.1016/s0306-4522(02)00027-1. [DOI] [PubMed] [Google Scholar]
- Friedberg MH, Lee SM, Ebner FF. Modulation of receptive field properties of thalamic somatosensory neurons by the depth of anesthesia. J Neurophysiol. 1999;81:2243–2252. doi: 10.1152/jn.1999.81.5.2243. [DOI] [PubMed] [Google Scholar]
- Fuchs JL, Salazar E. Effects of whisker trimming on GABA(A) receptor binding in the barrel cortex of developing and adult rats. J Comp Neurol. 1998;395:209–216. [PubMed] [Google Scholar]
- Gaiarsa JL, Caillard O, Ben-Ari Y. Long-term plasticity at GABAergic and glycinergic synapses: mechanisms and functional significance. Trends Neurosci. 2002;25:564–570. doi: 10.1016/s0166-2236(02)02269-5. [DOI] [PubMed] [Google Scholar]
- Gierdalski M, Jablonska B, Siucinska E, Lech M, Skibinska A, Kossut M. Rapid regulation of GAD67 mRNA and protein level in cortical neurons after sensory learning. Cereb Cortex. 2001;11:806–815. doi: 10.1093/cercor/11.9.806. [DOI] [PubMed] [Google Scholar]
- Glazewski S, Fox K. Time course of experience-dependent synaptic potentiation and depression in barrel cortex of adolescent rats. J Neurophysiol. 1996;75:1714–1729. doi: 10.1152/jn.1996.75.4.1714. [DOI] [PubMed] [Google Scholar]
- Heeger DJ. Normalization of cell responses in cat striate cortex. Vis Neurosci. 1992;9:181–197. doi: 10.1017/s0952523800009640. [DOI] [PubMed] [Google Scholar]
- Hendry SH, Jones EG. Reduction in number of immunostained GABAergic neurons in deprived-eye dominance columns of monkey area 17. Nature. 1986;320:750–753. doi: 10.1038/320750a0. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period regulation. Annu Rev Neurosci. 2004;27:549–579. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- Hensch TK, Stryker MP. Columnar architecture sculpted by GABA circuits in developing cat visual cortex. Science. 2004;303:1678–1681. doi: 10.1126/science.1091031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heynen AJ, Yoon BJ, Liu CH, Chung HJ, Huganir RL, Bear MF. Molecular mechanism for loss of visual cortical responsiveness following brief monocular deprivation. Nat Neurosci. 2003;6:854–862. doi: 10.1038/nn1100. [DOI] [PubMed] [Google Scholar]
- Jacobs KM, Donoghue JP. Reshaping the cortical motor map by unmasking latent intracortical connections. Science. 1991;251:944–947. doi: 10.1126/science.2000496. [DOI] [PubMed] [Google Scholar]
- Jones EG. GABAergic neurons and their role in cortical plasticity in primates. Cereb Cortex. 1993;3:361–372. doi: 10.1093/cercor/3.5.361-a. [DOI] [PubMed] [Google Scholar]
- Kandler K. Activity-dependent organization of inhibitory circuits: lessons from the auditory system. Curr Opin Neurobiol. 2004;14:96–104. doi: 10.1016/j.conb.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Kelly MK, Carvell GE, Kodger JM, Simons DJ. Sensory loss by selected whisker removal produces immediate disinhibition in the somatosensory cortex of behaving rats. J Neurosci. 1999;19:9117–9125. doi: 10.1523/JNEUROSCI.19-20-09117.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, Moss SJ. Modulation of GABAA receptor activity by phosphorylation and receptor trafficking: implications for the efficacy of synaptic inhibition. Curr Opin Neurobiol. 2003;13:341–347. doi: 10.1016/s0959-4388(03)00064-3. [DOI] [PubMed] [Google Scholar]
- Knott GW, Quairiaux C, Genoud C, Welker E. Formation of dendritic spines with GABAergic synapses induced by whisker stimulation in adult mice. Neuron. 2002;34:265–273. doi: 10.1016/s0896-6273(02)00663-3. [DOI] [PubMed] [Google Scholar]
- Komatsu Y, Iwakiri M. Long-term modification of inhibitory synaptic transmission in developing visual cortex. Neuroreport. 1993;4:907–910. doi: 10.1097/00001756-199307000-00017. [DOI] [PubMed] [Google Scholar]
- Kyriazi H, Carvell GE, Brumberg JC, Simons DJ. Laminar differences in bicuculline methiodide’s effects on cortical neurons in the rat whisker/barrel system. Somatosens Mot Res. 1998;15:146–156. doi: 10.1080/08990229870871. [DOI] [PubMed] [Google Scholar]
- Kyriazi HT, Carvell GE, Brumberg JC, Simons DJ. Effects of baclofen and phaclofen on receptive field properties of rat whisker barrel neurons. Brain Res. 1996a;712:325–328. doi: 10.1016/0006-8993(95)01562-0. [DOI] [PubMed] [Google Scholar]
- Kyriazi HT, Carvell GE, Brumberg JC, Simons DJ. Quantitative effects of GABA and bicuculline methiodide on receptive field properties of neurons in real and simulated whisker barrels. J Neurophysiol. 1996b;75:547–560. doi: 10.1152/jn.1996.75.2.547. [DOI] [PubMed] [Google Scholar]
- Lech M, Skibinska A, Kossut M. Delayed upregulation of GABA(A) alpha1 receptor subunit mRNA in somatosensory cortex of mice following learning-dependent plasticity of cortical representations. Brain Res Mol Brain Res. 2001;96:82–86. doi: 10.1016/s0169-328x(01)00271-6. [DOI] [PubMed] [Google Scholar]
- Lendvai B, Stern E, Chen B, Svoboda K. Experience-dependent plasticity of dendritic spines in the developing rat barrel cortex in vivo. Nature. 2000;404:876–881. doi: 10.1038/35009107. [DOI] [PubMed] [Google Scholar]
- Lowel S, Singer W. Selection of intrinsic horizontal connections in the visual cortex by correlated neuronal activity. Science. 1992;255:209–212. doi: 10.1126/science.1372754. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Martin KA. The Wellcome Prize lecture. From single cells to simple circuits in the cerebral cortex. Q J Exp Physiol. 1988;73:637–702. doi: 10.1113/expphysiol.1988.sp003190. [DOI] [PubMed] [Google Scholar]
- Marty A, Llano I. Modulation of inhibitory synapses in the mammalian brain. Curr Opin Neurobiol. 1995;5:335–341. doi: 10.1016/0959-4388(95)80046-8. [DOI] [PubMed] [Google Scholar]
- Massie A, Cnops L, Smolders I, Van Damme K, Vandenbussche E, Vandesande F, Eysel UT, Arckens L. Extracellular GABA concentrations in area 17 of cat visual cortex during topographic map reorganization following binocular central retinal lesioning. Brain Res. 2003;976:100–108. doi: 10.1016/s0006-8993(03)02717-3. [DOI] [PubMed] [Google Scholar]
- Micheva KD, Beaulieu C. Quantitative aspects of synaptogenesis in the rat barrel field cortex with special reference to GABA circuitry. J Comp Neurol. 1996;373:340–354. doi: 10.1002/(SICI)1096-9861(19960923)373:3<340::AID-CNE3>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Micheva KD, Beaulieu C. Development and plasticity of the inhibitory neocortical circuitry with an emphasis on the rodent barrel field cortex: a review. Can J Physiol Pharmacol. 1997;75:470–478. [PubMed] [Google Scholar]
- Miller KD, Pinto DJ, Simons DJ. Processing in layer 4 of the neocortical circuit: new insights from visual and somatosensory cortex. Curr Opin Neurobiol. 2001;11:488–497. doi: 10.1016/s0959-4388(00)00239-7. [DOI] [PubMed] [Google Scholar]
- Moore CI, Nelson SB. Spatio-temporal subthreshold receptive fields in the vibrissa representation of rat primary somatosensory cortex. J Neurophysiol. 1998;80:2882–2892. doi: 10.1152/jn.1998.80.6.2882. [DOI] [PubMed] [Google Scholar]
- Mower GD, Christen WG, Burchfiel JL, Duffy FH. Microiontophoretic bicuculline restores binocular responses to visual cortical neurons in strabismic cats. Brain Res. 1984;309:168–172. doi: 10.1016/0006-8993(84)91024-2. [DOI] [PubMed] [Google Scholar]
- Petersen CC, Hahn TT, Mehta M, Grinvald A, Sakmann B. Interaction of sensory responses with spontaneous depolarization in layer 2/3 barrel cortex. Proc Natl Acad Sci USA. 2003;100:13638–13643. doi: 10.1073/pnas.2235811100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JT, Johnson CK, Agmon A. Diverse types of interneurons generate thalamus-evoked feedforward inhibition in the mouse barrel cortex. J Neurosci. 2001;21:2699–2710. doi: 10.1523/JNEUROSCI.21-08-02699.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priebe NJ, Ferster D. Direction selectivity of excitation and inhibition in simple cells of the cat primary visual cortex. Neuron. 2005;45:133–145. doi: 10.1016/j.neuron.2004.12.024. [DOI] [PubMed] [Google Scholar]
- Rajan R. Receptor organ damage causes loss of cortical surround inhibition without topographic map plasticity. Nat Neurosci. 1998;1:138–143. doi: 10.1038/388. [DOI] [PubMed] [Google Scholar]
- Rajan R. Plasticity of excitation and inhibition in the receptive field of primary auditory cortical neurons after limited receptor organ damage. Cereb Cortex. 2001;11:171–182. doi: 10.1093/cercor/11.2.171. [DOI] [PubMed] [Google Scholar]
- Rosier AM, Arckens L, Demeulemeester H, Orban GA, Eysel UT, Wu YJ, Vandesande F. Effect of sensory deafferentation on immunoreactivity of GABAergic cells and on GABA receptors in the adult cat visual cortex. J Comp Neurol. 1995;359:476–489. doi: 10.1002/cne.903590309. [DOI] [PubMed] [Google Scholar]
- Sillito AM, Kemp JA, Blakemore C. The role of GABAergic inhibition in the cortical effects of monocular deprivation. Nature. 1981;291:318–320. doi: 10.1038/291318a0. [DOI] [PubMed] [Google Scholar]
- Simons DJ, Carvell GE. Thalamocortical response transformation in the rat vibrissa/barrel system. J Neurophysiol. 1989;61:311–330. doi: 10.1152/jn.1989.61.2.311. [DOI] [PubMed] [Google Scholar]
- Skibinska A, Glazewski S, Fox K, Kossut M. Age-dependent response of the mouse barrel cortex to sensory deprivation: a 2-deoxyglucose study. Exp Brain Res. 2000;132:134–138. doi: 10.1007/s002210000341. [DOI] [PubMed] [Google Scholar]
- Sompolinsky H, Shapley R. New perspectives on the mechanisms for orientation selectivity. Curr Opin Neurobiol. 1997;7:514–522. doi: 10.1016/s0959-4388(97)80031-1. [DOI] [PubMed] [Google Scholar]
- Stern EA, Maravall M, Svoboda K. Rapid development and plasticity of layer 2/3 maps in rat barrel cortex in vivo. Neuron. 2001;31:305–315. doi: 10.1016/s0896-6273(01)00360-9. [DOI] [PubMed] [Google Scholar]
- Swadlow HA, Gusev AG. Receptive-field construction in cortical inhibitory interneurons. Nat Neurosci. 2002;5:403–404. doi: 10.1038/nn847. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Svoboda K, Malinow R. Experience strengthening transmission by driving AMPA receptors into synapses. Science. 2003;299:1585–1588. doi: 10.1126/science.1079886. [DOI] [PubMed] [Google Scholar]
- Trachtenberg JT, Stryker MP. Rapid anatomical plasticity of horizontal connections in the developing visual cortex. J Neurosci. 2001;21:3476–3482. doi: 10.1523/JNEUROSCI.21-10-03476.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremere L, Hicks TP, Rasmusson DD. Role of inhibition in cortical reorganization of the adult raccoon revealed by microiontophoretic blockade of GABA(A) receptors. J Neurophysiol. 2001;86:94–103. doi: 10.1152/jn.2001.86.1.94. [DOI] [PubMed] [Google Scholar]
- Wallace H, Fox K. Local cortical interactions determine the form of cortical plasticity. J Neurobiol. 1999;41:58–63. [PubMed] [Google Scholar]
- Wang J, McFadden SL, Caspary D, Salvi R. Gamma-aminobutyric acid circuits shape response properties of auditory cortex neurons. Brain Res. 2002;944:219–231. doi: 10.1016/s0006-8993(02)02926-8. [DOI] [PubMed] [Google Scholar]
- Wehr M, Zador AM. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature. 2003;426:442–446. doi: 10.1038/nature02116. [DOI] [PubMed] [Google Scholar]
- Welker E, Armstrong-James M, Van der Loos H, Kraftsik R. The mode of activation of a barrel column: response properties of single units in the somatosensory cortex of the mouse upon whisker deflection. Eur J Neurosci. 1993;5:691–712. doi: 10.1111/j.1460-9568.1993.tb00534.x. [DOI] [PubMed] [Google Scholar]
- Wellman CL, Arnold LL, Garman EE, Garraghty PE. Acute reductions in GABAA receptor binding in layer IV of adult primate somatosensory cortex after peripheral nerve injury. Brain Res. 2002;954:68–72. doi: 10.1016/s0006-8993(02)03343-7. [DOI] [PubMed] [Google Scholar]
- Wilent WB, Contreras D. Synaptic responses to whisker deflections in rat barrel cortex as a function of cortical layer and stimulus intensity. J Neurosci. 2004;24:3985–3998. doi: 10.1523/JNEUROSCI.5782-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res. 1979;171:11–28. doi: 10.1016/0006-8993(79)90728-5. [DOI] [PubMed] [Google Scholar]
- Woodin MA, Ganguly K, Poo MM. Coincident pre- and postsynaptic activity modifies GABAergic synapses by postsynaptic changes in Cl-transporter activity. Neuron. 2003;39:807–820. doi: 10.1016/s0896-6273(03)00507-5. [DOI] [PubMed] [Google Scholar]
- Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 1970;17:205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]
- Zheng W, Knudsen EI. Functional selection of adaptive auditory space map by GABAA-mediated inhibition. Science. 1999;284:962–965. doi: 10.1126/science.284.5416.962. [DOI] [PubMed] [Google Scholar]
- Zheng W, Knudsen EI. Gabaergic inhibition antagonizes adaptive adjustment of the owl’s auditory space map during the initial phase of plasticity. J Neurosci. 2001;21:4356–4365. doi: 10.1523/JNEUROSCI.21-12-04356.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.