Abstract
Background
Because iodine deficiency can influence background rates of thyroid disease or modify radiation dose–response relationships, we compiled descriptive data on iodine status among participants in a Belarusian–American screening study who were exposed in childhood to radioiodine fallout from the Chornobyl nuclear accident. We have used the data from two consecutive screening cycles to examine whether indicators of iodine status changed before and after documented government initiatives to improve iodine intake.
Methods
Urinary iodine concentrations in spot samples and prevalence of diffuse goiter by palpation were assessed in 11,676 exposed subjects who were 18 years or younger at the time of the accident on April 26, 1986, and were screened beginning 11 years later in connection with the Belarus–American Thyroid Study. Data for the first (January 1997–March 2001) and second (April 2001–December 2004) screening cycles, which largely correspond to time periods before and after official iodination efforts in 2000/2001, were compared for the cohort overall as well as by oblast of residence (i.e., state) and type of residency (urban/rural).
Results
Median urine iodine levels among cohort members increased significantly in the later period (111.5 μg/L) compared to the earlier (65.3 μg/L), with the cycle 2 level in the range defined as adequate iodine intake by the World Health Organization. During the same period, a significant decline in diffuse goiter prevalence was also observed. In both cycles, urinary iodine levels were lower in rural than in urban residents. Urinary iodine levels, but not rates of goiter, varied by oblast of residence. In both periods, adjusted median urine iodine concentrations were similar in Gomel and Minsk oblasts, where ∼89% of cohort members resided, and were lowest in Mogilev oblast. Yet Mogilev oblast and rural areas showed the most marked increases over time.
Conclusions
Trends in urinary iodine concentrations and prevalence of diffuse goiter by palpation suggest that iodination efforts in Belarus were successful, with benefits extending to the most iodine-deficient populations. Iodine status should be considered when evaluating thyroid disease risk in radioiodine-exposed populations since it can change over time and may influence rates of disease and, possibly, dose–response relationships.
Introduction
The nuclear plant accident at Chornobyl (Chernobyl) on April 26, 1986, exposed the populations of areas in Ukraine, Belarus, and the Russian Federation to radioactive iodines—principally, Iodine-131 (131 I) (1), which concentrates in the thyroid gland. According to available historical data, the areas affected by Chornobyl fallout were mildly or moderately deficient in stable iodine (2). Because iodine deficiency can increase radioiodine uptake to the thyroid (2,3) and, by stimulating thyroid cellular activity, affect response to radioiodine dose (4), there has been concern that the risk of radiation-related thyroid cancer and other thyroid disease would be higher in iodine-deficient areas. Two of the three studies published to date suggest that insufficient iodine intake may be a modifying factor in the development of thyroid cancer (5–7). A case–control study (5) based largely on post-Chornobyl cancers from Belarus estimated a threefold higher odds ratio of thyroid cancer at 1 gray (Gy) in subjects whose residence at the time of the accident was in settlements with the lowest level of soil iodine; in addition, use of iodine supplementation was associated with significantly reduced risk of thyroid cancer. Similarly, an ecological study in contaminated regions of Russia (6) estimated an excess relative risk for thyroid cancer at 1 Gy to be twofold higher in areas with median urinary iodine levels in 1996 indicative of severe iodine deficiency (<20 μg/L) compared to essentially uncontaminated areas. In contrast, a large thyroid screening study of exposed young people in Ukraine (7) found no evidence of significant effect modification by iodine status at the time of the initial examination (1998–2000). With the issue unresolved, it remains important to consider background information on iodine deficiency in exposed populations when assessing associations between exposure to 131 I and thyroid disease.
Firm data are lacking on iodine status in regions of Belarus during the year of the Chornobyl accident (2). An historical report from Belarusian scientists describes an increasing rate of diffuse goiter in the decades from 1970 to 1990 but does not give a specific figure for 1986 (8). Beginning in 1990, surveys of urinary iodine excretion and thyroid enlargement in the Chornobyl area were undertaken by several international groups. A study of 1680 children in Belarus during 1990–1994 (9) found a 35% prevalence of goiter in some affected areas; the overall frequency of urinary iodine levels indicative of severe iodine deficiency was 10%. In a 1991 study of diffuse goiter diagnosed using ultrasonography-based thyroid volumes and age-specific norms (10), rates of 18% and 22% were reported for Belarusian children from Gomel (N = 19,273) and Mogilev (N = 23,581) oblasts, respectively. A 1995–1998 survey of 11,562 children from Belarus (11) reported median urinary iodine levels of 79.8 μg/L in Gomel and 49.0 μg/L in Mogilev oblasts, with corresponding palpation-based prevalence rates for diffuse goiter of 23% and 44%.
Little is known about iodine status in Chornobyl-affected areas after the initiation of state iodination programs in 2000/2001. Findings from a thyroid screening study in Ukraine based on pre-/postprogram comparisons of median urinary iodine levels, a measure highly sensitive to recent changes in iodine intake, have been described (12). The objective of this report is to present data on iodine status from a parallel Belarus–American screening study (BelAm) using measurements from two screening cycles corresponding to time periods before and after governmental decrees aimed at reducing iodine deficiency. In addition to urinary iodine concentrations, the prevalence of diffuse goiter at screening is included to reflect iodine status over a longer time period.
Materials and Methods
Approximately a decade after the nuclear plant accident in 1986, we assembled two cohorts of individuals exposed to 131 I from Chornobyl fallout as children or adolescents in Belarus (∼12,000 subjects) and the contaminated northern regions of Ukraine (∼13,000 subjects) (7). We measured urinary iodine concentrations in spot samples collected from cohort members during standardized screening examinations of the thyroid gland designed to identify cases of thyroid cancer and other thyroid diseases. In each cohort, we have examined median urinary iodine levels for two screening cycles in time periods before and after government programs to improve iodine intake through food and supplements. In addition to patterns by time period, we evaluated iodine status by place of residence and type of residency (urban or rural). Intake of salt, foods, and preparations with high concentrations of iodine were also considered.
The BelAm cohort
Details of the methods of both the Belarus study and the parallel study in Ukraine are available in Stezhko et al. (13). In brief, the cohort in Belarus was assembled using a database of thyroid radioactivity measurements taken within 2 months after the Chornobyl accident. From the database, we identified 38,543 individuals who were born in Belarus between April 26, 1968, and April 26, 1986, and attempted to trace them using a variety of sources. These included address bureaus at the oblast and raion levels (administrative units similar to a U.S. state and county, respectively), military registration offices, local departments of education and public health, as well as medical establishments in the localities where individuals lived in April–May 1986. Nearly 5% (n = 1,804) of the potential study subjects were ineligible (incorrect age, died, incarcerated, moved out of the country, etc.) and 20,526 (53.3%) could not be traced. Of the 16,213 individuals who were traced and invited to participate in the study, 11,970 (73.8%) subjects responded and came for screening during the first cycle of examination.
After exclusion of an additional 294 individuals found to be ineligible due to incorrect age (n = 114), incorrect identification (n = 20), lack of thyroid tissue resulting from primary thyroid gland aplasia (n = 10), or no measurements of urinary iodine concentrations (n = 150), there were 11,676 subjects available for analysis in cycle 1 (January 1997–March 2001). This included three subjects who were screened in 1996 and 65 cohort members who had initial examinations in 2002–2003. Of the subjects screened in the first cycle, 9,563 (81.9%) returned for the second screening cycle (April 2001–December 2004).
Local institutional review boards in Belarus and the United States approved the study protocol, and signed informed consent was obtained from either study subjects or their guardians.
Screening procedures
The screening protocol included, among other components, a medical history, thyroid palpation by an endocrinologist and an ultrasonographer, and an ultrasonography examination to be performed after palpation by the ultrasonographer. Data on demographic characteristics and residential histories were gathered by questionnaire. We also asked participants to report their dietary intake of iodine: specifically, whether they did or did not use iodized salt; consume foods rich in iodine (defined as seaweed, herring, or other ocean fish); or take iodine-containing vitamins. Spot samples of urine were collected from study subjects in plastic containers. Since most cohort members traveled from their residences to the central screening facility in Minsk, urine samples were not fasting.
Urine iodine determination
A 3-mL aliquot of each sample was transferred in tightly capped plastic tubes barcode-labeled with a unique identifying number. Specimens were stored in a refrigerator before transfer to the Central Laboratory, where they were maintained at −18°C until analysis. Iodine concentrations were measured by the photometric method using the Sandell-Kolthoff reaction as modified by Dunn (14), and expressed as μg/L. The analytical sensitivity of the assay is 10 μg/L. Strict internal quality control was maintained by plotting results of repeat analysis of two pooled urine samples on Levey-Jennings charts for detection of out-of-control runs. Any run with a control value out of range was repeated. The method of urinary iodine determination was certified at the Laboratory of Pediatrics of the Free University of Brussels (Belgium).
Diffuse goiter
The grade of diffuse goiter was determined at screening based on palpation by an endocrinologist, and was recorded as Grade 0 (no enlargement), Grade 1 (thyroid gland enlargement that is not visible with neck in a neutral position), and Grade 2 (visible thyroid gland enlargement), with Grades 1 and 2 combined for the purpose of analysis. Clinicians carrying out the palpation examinations were given a standardized training program involving demonstration by a clinical supervisor followed by observation of trial examinations and correction of technique as indicated. Trainees were certified only after independent examination of 10 or more subjects, with and without thyroid abnormalities, produced results consistent with those of the trainer. Re-certifications were carried out periodically and at the discretion of the supervisor.
Statistical analysis
A major objective of the analysis was to examine median urinary iodine concentration by time period (first and second cycle) for the cohort overall and separately for the three Belarusian oblasts with large numbers of resident subjects at the time of examination (Gomel, Minsk, and Mogilev) as well as for a grouping labeled “Other” comprised of the remaining three Belarusian oblasts, where few cohort members resided (Brest, Grodno, and Vitebsk). To evaluate possible regional variation in iodine deficiency within Gomel, Minsk, and Mogilev oblasts, we calculated urinary iodine levels separately for cities and for the two constituent raions with the most resident cohort members. The distribution of urine iodine levels by type of residency was also examined, with urban/rural status defined using the classification of territories and settlements in Belarus (15) applied to the subject's place of residence at the time of first screening. According to these criteria, residence in a city, large town or village, or settlement with some urban features was considered urban. In addition, we evaluated intake of iodized salt, iodine-containing foods, and supplements by oblast and time period. We also compared median urinary concentrations of iodine by oblast and time period with the World Health Organization (WHO) criteria for assessing iodine status in populations (16). These define adequate iodine nutrition as ≥100 μg/L, mild iodine deficiency as 50–99 μg/L, moderate iodine deficiency as 20–49 μg/L, and severe iodine deficiency as 0–19 μg/L.
The distribution of urinary iodine concentrations was skewed to the right but approximated the normal distribution after loge transformation. Transformed geometric means for the total cohort and separately for oblasts and raions were therefore very close to the group medians. Medians are more intuitive central estimates of the continuously distributed urinary iodine concentration. Therefore, in our analyses we estimated geometric means but refer to them as medians throughout the text. The median (geometric mean) urine iodine levels and 95% confidence intervals for each group studied were computed using the LSMEANS option in SAS PROC GLM. Where indicated, models were adjusted for age, sex, and urban/rural status, with the adjusted urinary iodine levels standardized to the cohort's overall distribution of age, gender, and urban/rural status.
The cycle 2 median urine iodine values were compared to cycle 1 using a paired analysis of variance with adjustment for the factors mentioned above, as well as for within-person correlations. Standard chi-square methods were used to compare reported intake of iodized salt and iodine-containing foods and supplements as well as the distribution of urinary iodine concentrations.
Additional goals of the analysis were to evaluate the prevalence rates of diffuse goiter based on palpation at screening for each oblast category and time period and to compare the rates to WHO's 1994 population guidelines for assessing iodine status (17). According to WHO criteria, a frequency of diffuse goiter on palpation of <5% indicates adequate iodine nutrition, whereas prevalence rates of 5%–19.9% reflect mild iodine deficiency, rates of 20%–29.9% represent moderate iodine deficiency, and goiter prevalence of 30% or greater is indicative of severe iodine deficiency.
All statistical tests were two-sided and considered statistically significant at p < 0.05. Statistical analyses were conducted using SAS software V9.2 (SAS Institute, Cary, NC).
Results
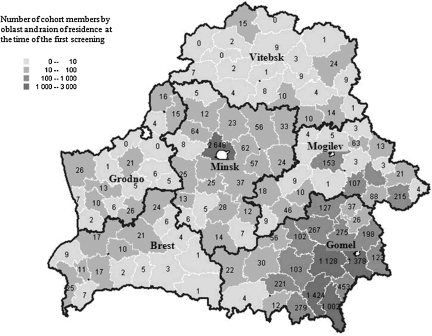
The cohort consisted of residents of Belarus who were 0–18 years old at the time of the Chornobyl accident and had been measured for thyroid radioactivity in the 2 months following. Figure 1 illustrates the geographical distribution of BelAm cohort members by oblast and raion at the time of first screening. The oblast of residence at screening and at the time of the accident are highly correlated (≥80% agreement). At both times, the great majority of subjects (∼89%) lived in Minsk and Gomel oblasts. At the start of the study, 60.3% of the cohort resided in urban areas. The median age of subjects at first screening was 21.3 years with a range from age 10.9 to 33.4 years. Women comprised 51.2% of the cohort.
FIG. 1.
Number of Belarus–American cohort members by oblast and raion at the time of the first screening cycle (January 1997–March 2001). Shading indicates four size categories: 0–10; 10–100; 100–1000; 1000–3000.
For the Belarus cohort as a whole, the age–sex–urbanicity-adjusted median urinary iodine levels for cycle 1 are 65.3 μg/L; the median concentrations for Gomel and Minsk oblasts are 68.5 and 68.7 μg/L, respectively (Table 1). However, the median urine iodine levels for Mogilev oblast in particular and for the Other Oblast grouping are considerably lower, reflecting a greater degree of iodine deficiency.
Table 1.
Median Values (Geometric Means) and 95% Confidence Intervals of Urinary Iodine Concentration According to Place of Residence in the First (January 1997–March 2001) and Second (April 2001–December 2004) Screening Cycles
| |
|
First cycle |
Second cycle |
|
||
|---|---|---|---|---|---|---|
| Study oblast | Regiona | n (%) | Median (95% CI),bμg/L | n (%) | Median (95% CI),bμg/L | p-Value for heterogeneity between cycles |
| Total cohort | 11,676 | 65.3 (64.2–66.5) | 9563 | 111.5 (109.6–113.3) | <0.0001 | |
| Gomel | Total | 7174 (61.4) | 68.5 (66.2–70.9) | 5850 (61.2) | 112.8 (110.4–115.2) | <0.0001 |
| Gomel city | 1133 (15.8) | 70.5 (66.6–74.6) | 970 (16.6) | 113.0 (106.9–119.4) | ||
| Braginski | 989 (13.8) | 55.9 (52.8–59.3) | 759 (13.0) | 96.7 (91.0–102.6) | ||
| Hoinikski | 1400 (19.5) | 101.9 (97.2–106.8) | 1114 (19.0) | 130.5 (124.5–136.9) | ||
| p-Valuec for heterogeneity within oblast | <0.0001 | <0.0001 | ||||
| Minsk | Total | 3142 (26.9) | 68.7 (67.2–70.3) | 2683 (28.0) | 117.2 (113.6–121.0) | <0.0001 |
| Minsk city | 2534 (80.6) | 84.7 (81.3–88.3) | 2128 (79.3) | 131.0 (125.9–136.4) | ||
| Minski | 56 (1.8) | 115.2 (60.3–220.2) | 50 (1.9) | 196.0 (103.7–370.3) | ||
| Chervenski | 57 (1.8) | 59.7 (34.0–104.9) | 46 (1.7) | 176.0 (100.2–308.9) | ||
| p-Valuec for heterogeneity within oblast | 0.0002 | 0.48 | ||||
| Mogilev | Total | 904 (7.7) | 38.6 (36.3–41.0) | 708 (7.4) | 90.5 (85.3–96.1) | <0.0001 |
| Mogilev city | 122 (9.0) | 40.2 (32.4–50.0) | 98 (9.5) | 109.2 (89.9–132.6) | ||
| Kostiukovitchski | 215 (15.8) | 48.1 (41.7–55.5) | 169 (16.4) | 87.4 (76.8–99.4) | ||
| Cherikovski | 131 (9.6) | 25.5 (21.2–30.6) | 106 (10.3) | 68.8 (58.2–81.3) | ||
| p-Valuec for heterogeneity within oblast | <0.0001 | 0.009 | ||||
| Otherd | Total | 456 (3.9) | 46.0 (42.2–50.2) | 322 (3.4) | 108.2 (99.1–118.1) | <0.0001 |
City and raions with the largest number of study participants.
Adjusted for age, sex, and urban/rural status.
p-Value for heterogeneity among the raions and cities listed in the table.
Includes Brest (n = 163), Vitebsk (n = 131), and Grodno (n = 162) oblasts, where the number of participants was too small for independent analyses.
CI, confidence interval.
For the cohort as a whole and for each oblast category, significant increases in urinary iodine concentrations were observed in cycle 2 (Table 1). A comparison of the overall median levels in cycle 1 and cycle 2 demonstrates a relative increase in median urine concentrations of >70% (p < 0.0001).
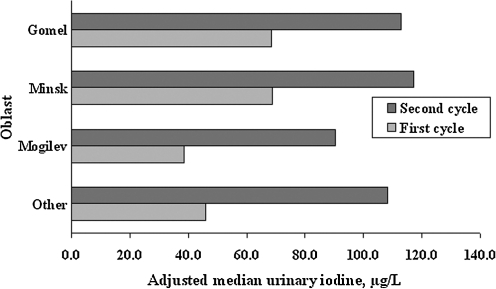
Figure 2, which illustrates by oblast the significant shift to higher values at the time of the second screening, shows that the relative increase was greatest for the oblasts with the lowest median urine iodine concentrations at cycle 1. By the time of the second screening, the median urinary iodine levels in Belarus were in excess of 100 μg/L in every oblast category except Mogilev.
FIG. 2.
Bar graph showing median urine iodine concentrations by oblast category for cycle 1 (January 1997–March 2001) and cycle 2 (April 2001–December 2004). The category “Other” includes Brest, Vitebsk, and Grodno oblasts. Median values adjusted for age, sex, and urban/rural status are significantly higher in the later time period.
The adjusted medians for Gomel and Mogilev oblast residents showed significant regional differences in both screening cycles (Table 1). In Minsk oblast, there was significant regional variation in urinary iodine in cycle 1, but the difference was based on the levels in two raions with very few subjects (n = 56 and n = 57), and no significant regional variation is observed in cycle 2 (p = 0.46).
The age- and sex-adjusted urinary iodine data presented in Table 2 show a significant urban/rural difference, with higher levels in urban areas in all oblasts studied and in both screening cycles. Although we observed significant increases in cycle 2 compared to cycle 1 in both urban and rural populations, the upward temporal trend in adjusted median urine iodine concentrations was more pronounced in rural areas (an 86% increase in rural vs. a 67.5% increase in urban areas, p < 0.0001). For the cohort overall, rates of diffuse goiter by palpation are significantly lower in cycle 2 compared to cycle 1 (in rural areas: 7.2% vs. 17.2%, and in urban areas: 5.6% vs. 16.0%), but there is no urban/rural difference in either time period (not shown).
Table 2.
Median Values (Geometric Means) and 95% Confidence Intervals of Urinary Iodine Concentration (μg/L) According to Type of Residency, by Oblast and Cycle
| |
|
|
Study oblast |
|||||
|---|---|---|---|---|---|---|---|---|
| |
Total cohort |
Gomel |
Minsk |
Mogilev and othera |
||||
| Type of residency | First cycle | Second cycle | First cycle | Second cycle | First cycle | Second cycle | First cycle | Second cycle |
| Rural | 50.6 (49.3–52.1) | 94.2 (91.7–96.7) | 56.7 (54.8–58.6) | 98.1 (95.0–101.4) | 40.5 (36.8–44.8) | 85.4 (77.7–93.9) | 31.7(29.4–34.2) | 80.3(74.4–86.6) |
| Urban | 75.9 (74.2–77.6) | 123.9 (121.4–126.5) | 77.1 (74.8–79.4) | 121.8 (118.5–125.1) | 83.1 (80.3–86.0) | 131.2 (127.2–135.4) | 48.9 (45.7–52.4) | 106.7 (100.0–113.9) |
Medians and 95% CIs were adjusted for age and sex. p-Values for heterogeneity by type of residency for the total cohort and for each oblast individually in both cycles <0.0001.
Includes Brest, Vitebsk, and Grodno oblasts.
In all study oblasts, questionnaire-based reports indicated substantially increased use of iodized salt, intake of food rich in iodine, and consumption of multivitamins from the first to the second cycle (Table 3). For the cohort overall, use of iodized salt increased by 40.9%, reported intake of iodine-rich foods (seaweed, herring, or other ocean fish) increased by 22.4%, and use of multivitamins was also reported more frequently in the second cycle.
Table 3.
Dietary Intake of Iodized Salt, Foods Rich in Iodine, and Multivitamins in the First and Second Screening Cycles
| |
Study Oblast |
|||||
|---|---|---|---|---|---|---|
| |
Gomel |
Minsk |
Mogilev and othera |
|||
| Reported dietary intake | First cycle | Second cycle | First cycle | Second cycle | First cycle | Second cycle |
| Iodized salt, n (%) Yes | 2783 (38.8) | 4195 (71.7) | 1427 (45.4) | 2028 (75.6) | 561 (41.2) | 779 (75.6) |
| Foods rich in iodine, n (%) Yes | 1602 (22.3) | 5211 (89.1) | 688 (21.9) | 2314 (86.2) | 331 (24.3) | 847 (82.2) |
| Multivitamins, n (%) Yes | 527 (7.4) | 507 (8.7) | 478 (15.2) | 893 (33.3) | 47 (3.5) | 186 (18.1) |
p-Values of heterogeneity by oblast in cycle 1 < 0.001; in cycle 2 ≤ 0.003; p-values of heterogeneity between two cycles for each oblast: all p-values ≤0.004.
Includes Brest, Vitebsk, and Grodno oblasts.
Table 4 presents data comparing the frequency distributions of unadjusted urinary iodine concentrations by oblast and time period according to the categories defined by WHO (17). At the time of the first screening, the percentage of subjects with iodine levels in urine indicative of severe iodine deficiency according to WHO criteria (<20 μg/L) was 11.1% for the cohort as a whole and ranged from 23.2% in Mogilev/Other oblasts to 10.4% in Gomel oblast and 7.6% in Minsk oblast. In cycle 2, the percentages in the severely iodine-deficient category dropped to 4.6% in Mogilev/Other oblasts, 3.4% in Gomel oblast, and 2.1% in Minsk oblast. Gomel and Minsk oblasts began cycle 1 with similar proportions of subjects whose urinary iodine levels were indicative of adequate iodine intake (34.7% and 39.5%, respectively), approximately twice the proportion (18.9%) of iodine-sufficient subjects observed in Mogilev/Other oblasts. In cycle 2 the proportion of subjects with adequate iodine intake (58.6% overall) increased across all oblasts, with the increase most pronounced (∼2.6-fold) in the Mogilev/Other category.
Table 4.
Description of Study Oblasts in Terms of World Health Organization Criteria for Iodine Deficiency in the First and Second Screening Cycles
| |
Study oblast |
|||||
|---|---|---|---|---|---|---|
| |
Gomel |
Minsk |
Mogilev and othera |
|||
| Factor | First cycle | Second cycle | First cycle | Second cycle | First cycle | Second cycle |
| Iodine levels (μg/L) falling in the categories,bn (%) | ||||||
| <20 | 747 (10.4) | 197 (3.4) | 239 (7.6) | 56 (2.1) | 315 (23.2) | 47 (4.6) |
| 20–49 | 1849 (25.8) | 722 (12.3) | 754 (24.0) | 254 (9.5) | 468 (34.4) | 181 (17.6) |
| 50–99 | 2090 (29.1) | 1547 (26.4) | 909 (28.9) | 654 (24.4) | 320 (23.5) | 304 (29.5) |
| 100–299 | 2201 (30.7) | 2923 (50.0) | 1083 (34.5) | 1449 (54.0) | 237 (17.4) | 428 (41.6) |
| 300+ | 287 (4.0) | 461 (7.9) | 157 (5.0) | 270 (10.0) | 20 (1.5) | 70 (6.8) |
| p-Value of heterogeneity by study oblast in both cycles: <0.0001 | ||||||
| Goiter by palpation,cn (%) | ||||||
| No | 6051 (84.6) | 5411 (93.3) | 2579 (82.2) | 2522 (94.8) | 1096 (80.8) | 958 (93.5) |
| Yesd | 1103 (15.4) | 385 (6.7) | 556 (17.8) | 137 (5.2) | 261 (19.2) | 66 (6.5) |
| p-Value of heterogeneity by study oblast in both cycles: <0.0001 | ||||||
Includes Brest, Vitebsk, and Grodno oblasts.
Since the World Health Organization criteria regarding goiter prevalence and iodine intake are based on data not adjusted for sex, age, and urban/rural status, unadjusted urinary iodine concentration values were used in the table.
Numbers may not add up to total because of missing values: n = 30 for cycle 1 and 84 for cycle 2.
Goiter grades 1 and 2 combined.
The data in Table 4 on prevalence of diffuse goiter by palpation (Grade >0) are consistent with this temporal trend, with the rates for cycle 2 showing a striking decline in each of the oblasts studied (15.4% to 6.7% in Gomel, 17.8% to 5.2% in Minsk, and 19.2% to 6.5% in the Mogilev/Other grouping).
Discussion
In this report describing the iodine status of the cohort of young people exposed to Chornobyl fallout in Belarus, the most striking observations related to temporal trends. Over the two time periods examined—a combined total of 8 calendar years—median urine iodine concentration for the cohort as a whole increased significantly from 65.3 to 111.5 μg/L, in a range considered by WHO to represent adequate iodine intake. The prevalence of goiter, reflecting iodine status over a longer period than urinary iodine concentrations, decreased almost threefold, to a rate of 6.1% for the cohort overall, close to the 5% prevalence WHO defines as indicating iodine sufficiency. Thus, the temporal changes in these two population measures of iodine status are consistent with one another and coincide with public health programs to improve iodine intake that were undertaken during this time period.
In March 2000, a governmental decree by the Chief Sanitary Doctor of the Ministry of Health (N11) mandated that all table salt should be iodized using potassium iodate, rather than the less stable potassium iodide used previously, at a concentration of 40.0 ± 15.0 mg/kg of salt. In addition, a nation-wide program was mounted to educate the population concerning the benefits of iodized salt. However, according to republican law, markets in Belarus had to stock both noniodized and iodized salt and it was left to consumers to make the choice. As a result, reported use of iodized salt in cycle 2, while substantially increased, did not reach the level of 100%. In April 2001, the Belarus Council of Ministers issued a decree (N484) mandating the use of iodized salt in the production of processed foods.
The increases in dietary intake of iodized salt and other iodine-containing products were observed in all parts of Belarus, suggesting that these official iodinization initiatives were effective throughout the country. Although the governmental approaches were presumably easier to implement for urbanites, the impact of the program reached rural populations as well, due in part to construction of new shops supplying iodized processed foods to rural areas. In fact, the upward trend in urinary iodine levels from the first to the second time period was more marked in rural than in urban residents.
In both cycles 1 and 2, the lowest levels of urinary iodine were found in Mogilev oblast. The low levels at cycle 1 may reflect the iodine content in local soil and water. A map of stable iodine in soil based on measurements made in the 1960s (18) indicates that, historically, the Mogilev area had levels at the lowest end of the range (0.56–0.94 mg/kg). Gomel oblast had a wide range of soil iodine levels up to and including 5.0–18.2 mg/kg, and measurements in Minsk city/oblast showed soil iodine content in an intermediate range. The lower levels at cycle 2 probably result principally from the low baseline levels since implementation of the government's iodine program was intended to be uniform across oblasts. As mentioned above, in spite of its low median urine iodine levels in cycle 1, Mogilev showed a large relative increase in median levels compared to other oblast categories at the time of cycle 2.
The descriptive study reported here has several strengths, including a large sample size, data on many factors of interest, and a standardized approach to urine collection and handling as well as repeated measurements over a time span of eight years. A limitation is the reliance on nonfasting spot urine samples. The gold standard for estimating individual iodine excretion has been a 24-hour urine collection. For large studies, however, such an approach is untenable. So long as the study sample is sufficiently large, the median figure for the spot urinary iodine concentrations is a reliable measure for application to populations since, with large numbers, the variation in daily iodine intake and urinary volume is leveled out (19). Andersen et al. (20) have estimated that a precision range of ± 5% requires 200–500 spot urine samples per subgroup for confidence intervals corresponding to 95% and 80%, respectively, whereas a precision range of ± 10% requires from 50 to 100 spot samples per subgroup. According to these guidelines, our urinary iodine data based on spot samples are sufficiently reliable for the descriptive analyses we have carried out.
Urinary iodine concentration reflects only current iodine status. To provide a better indication of iodine nutrition over an extended period, we analyzed data on prevalence of diffuse goiter by palpation. In the absence of extensive training, estimation of diffuse goiter by palpation can be subject to both inter-examiner variation as well as overdiagnosis of small thyroid enlargements, particularly in populations with mild iodine deficiency (21,22). However, given the extensive, standardized program of training and certification our examiners received, we consider the goiter rates reported here to be sufficiently reliable and valid.
Thyroid enlargement can also be determined based on ultrasonography examination (23). Although sonographic measurements are considered more precise than thyroid palpation, they are also somewhat subjective and can be affected by measurement error and variation in the shape of the thyroid lobes (24). Moreover, interpretation of goiter prevalence based on such estimates requires age-appropriate normative data from local controls (25), ideally children with adequate iodine intake (26). Since there are no widely accepted referent data for our young population, we had reservations about utilizing ultrasonography-based measures of thyroid enlargement. Nonetheless, we did conduct an analysis to examine whether, within each oblast, the distribution of absolute thyroid volumes categorized by quintiles from low to high was associated with the adjusted median urine iodine concentrations. None of the oblasts studied showed a trend toward lower levels of median urine iodine with increasing thyroid size, either in cycle 1 or cycle 2 (data not shown). While urinary iodine concentrations represent current iodine status, increases in thyroid volume from insufficient iodine intake may resolve more slowly when iodine intake improves (2). In addition, because the vast majority of our goiters were Grade 1, it is possible the lack of association could partly reflect the limited range in thyroid size. When we split the thyroid volume data into two categories [low vs. high using the criteria from Rasmussen et al. (23)], we observed a significant association between enlarged thyroid volume and reduced urine iodine levels, particularly in Minsk and Mogilev oblasts (not shown). The association was no longer significant in cycle 2, after the introduction of government iodination programs.
The urinary iodine findings reported here for Belarus can be compared with those we published previously based on measurements of 11,926 subjects in a parallel screening study in Ukraine (12). Although the iodination programs and/or implementation may have been different in Ukraine and Belarus, in general terms the results were similar. Iodine concentration levels increased significantly in the time period following government initiatives; median concentrations varied by place of residence, were higher in urban than rural areas, and increased more rapidly in rural regions; reported dietary intake of iodine-containing products rose significantly in the second screening cycle. However, the study area in Ukraine was more iodine deficient at the start, with a cycle 1 median urine iodine level of 41.7 μg/L compared to 65.3 μg/L in Belarus, and improvements in iodine intake over time were more modest (an increase of ∼14% in median urine iodine levels vs. an increase of >70% in Belarus). As a result, by the time of the second screening in Ukraine, none of the study regions (Zhytomyr, Chernihiv, and Kyiv oblasts as well as Kyiv City) and only a small percentage of the cohort (18%) had urinary iodine levels indicative of iodine sufficiency, although the period of follow-up was shorter than in Belarus.
Earlier surveys of iodine excretion and goiter prevalence in the Chornobyl region beginning 5 years after the accident also found the affected areas of Ukraine to be more iodine deficient than Belarus (9–11). In a 1991 study of diffuse goiter diagnosed using ultrasonography-based thyroid volumes (10), rates in the Kyiv (54%) and Zhytomyr (38%) regions of Ukraine were more than double those in the Gomel (18%) and Mogilev (22%) regions of Belarus. Under these circumstances, more time and sustained effort may be required to bring widespread iodine sufficiency to northern Ukraine.
Differences in iodine status should be borne in mind when interpreting results from studies of 131 I exposure from the accident at Chornobyl and estimated risk of thyroid cancer among exposed children and adolescents. Historical evidence based on iodine content in soil (18) and reported trends in populations [reviewed in (2) and (27)] as well as data gathered 5–10 years after the accident (9–11) and those presented here appear to indicate that heavily contaminated Gomel oblast seems to have a lower level of iodine deficiency than some other parts of Belarus. At the same time, the strongest evidence to date for a modifying effect of stable iodine on radiation risk of thyroid cancer comes from a study (5) in which 68% of total cases were drawn from Gomel, and iodine status for individual subjects was based on the content in soil in the settlement of residence at the time of the accident. The important issue of joint effects on thyroid cancer risk from iodine deficiency and radioiodine exposure deserves to be pursued.
Conclusions
In summary, the principal findings from our analyses of iodine status in Belarus—namely, the substantial temporal shift upward in urinary iodine concentrations and a corresponding decline in prevalence of diffuse goiter between the two screening cycles—appear to be largely attributable to government initiatives aimed at promoting iodination during this time period. The increases in dietary intake of iodine-containing foods and preparations reported in cycle 2 also support this view. Based on median urinary iodine concentrations, the Belarus cohort overall had become iodine sufficient by the time of the cycle 2 screening examinations. However, evaluation of regional differences at the oblast level identified the Mogilev area as an exception. In addition, residents of rural areas have also not yet achieved iodine sufficiency. Although participants from Mogilev oblast and rural areas in general have shown notable improvement, further efforts will be required to bring adequate iodine intake to these population subgroups. The significant temporal changes in urinary iodine levels and prevalence of diffuse goiter observed in our study have implications for analysis of radiation-related risks of thyroid diseases in Belarus.
Acknowledgments
This research was supported by the Intramural Research Program of the U.S. National Cancer Institute, NIH, DHHS, and the Department of Energy, with the U.S. Nuclear Regulatory Commission providing initial funds for purchase of equipment. The study team owes a special debt of gratitude to our eminent collaborator, the late Dr. Jacob Robbins, who gathered experts in the field for a workshop devoted to discussion of the measurement and role of iodine nutrition in a study population exposed to radioiodines. We are grateful as well to the late Dr. Daniel Fink, who provided guidance, oversight, and quality assurance of laboratory testing for iodine content in the urine samples collected from our study subjects. We also thank Miriam Ishak for her contributions to work on this topic during a summer internship in 2006.
Disclosure Statement
None of the authors of this article have any financial interests that would pose an actual or potential conflict of interest with the material presented herein.
References
- 1.Minenko VF. Ulanovsky AV. Drozdovitch VV. Shemiakina EV. Gavrilin YI. Khrouch VT. Shinkarev SM. Voillequé PG. Bouville A. Anspaugh LR. Luckyanov N. Individual thyroid dose estimates for a case-control study of Chernobyl-related thyroid cancer among children of Belarus—part II. Contributions from long-lived radionuclides and external radiation. Health Phys. 2006;90:312–327. doi: 10.1097/01.HP.0000183761.30158.c1. [DOI] [PubMed] [Google Scholar]
- 2.Robbins J. Dunn JT. Bouville A. Kravchenko VI. Lubin J. Petrenko S. Sullivan KM. VanMiddlesworth L. Wolff J. Iodine nutrition and the risk from radioactive iodine: a workshop report in the Chernobyl long-term follow-up study. Thyroid. 2001;11:487–491. doi: 10.1089/105072501300176444. [DOI] [PubMed] [Google Scholar]
- 3.Gembicki M. Stozharov AN. Arinchin AN. Moschik KV. Petrenko S. Khmara IM. Baverstock K. Iodine deficiency in Belarusian children as a possible factor stimulating the irradiation of the thyroid gland during the Chernobyl catastrophe. Environ Health Perspect. 1997;105(Suppl 6):1487–1490. doi: 10.1289/ehp.97105s61487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu XH. Chen GG. Vlantis AC. van Hasselt CA. Iodine mediated mechanisms and thyroid carcinoma. Crit Rev Clin Lab Sci. 2009;46:302–318. doi: 10.3109/10408360903306384. [DOI] [PubMed] [Google Scholar]
- 5.Cardis E. Kesminiene A. Ivanov V. Malakhova I. Shibata Y. Khrouch V. Drozdovitch V. Maceika E. Zvonova I. Vlassov O. Bouville A. Goulko G. Hoshi M. Abrosimov A. Anoshko J. Astakhova L. Chekin S. Demidchik E. Galanti R. Ito M. Korobova E. Lushnikov E. Maksioutov M. Masyakin V. Nerovnia A. Parshin V. Parshkov E. Piliptsevich N. Pinchera A. Polyakov S. Shabeka N. Suonio E. Tenet V. Tsyb A. Yamashita S. Williams D. Risk of thyroid cancer after exposure to 131I in childhood. J Natl Cancer Inst. 2005;97:724–732. doi: 10.1093/jnci/dji129. [DOI] [PubMed] [Google Scholar]
- 6.Shakhtarin VV. Tsyb AF. Stepanenko VF. Orlov MY. Kopecky KJ. Davis S. Iodine deficiency, radiation dose, and the risk of thyroid cancer among children and adolescents in the Bryansk region of Russia following the Chernobyl power station accident. Int J Epidemiol. 2003;32:584–591. doi: 10.1093/ije/dyg205. [DOI] [PubMed] [Google Scholar]
- 7.Tronko MD. Howe GR. Bogdanova TI. Bouville AC. Epstein O. Brill AB. Likhtarev I. Fink DJ. Markov VV. Greenebaum E. Olijnyk VA. Masnyk IJ. Shpak VM. McConnell RJ. Tereshchenko VP. Robbins J. Zvinchuk OV. Zablotska LB. Hatch M. Luckyanov NK. Ron E. Thomas TL. Voillequé PG. Beebe GW. 2006 A cohort study of thyroid cancer, other thyroid diseases after the Chornobyl accident: thyroid cancer in Ukraine detected during first screening. J Natl Cancer Inst. 98:897–903. doi: 10.1093/jnci/djj244. [DOI] [PubMed] [Google Scholar]
- 8.Kholodova EA. Federova LP. Epidemiology of endemic goiter in Belarus. IDD Newsl. 1992;9:2–3. [Google Scholar]
- 9.Mityukova TA. Astakhova LN. Asenchyk LD. Orlov MM. VanMiddlesworth L. Urinary iodine excretion in Belarus children. Eur J Endocrinol. 1995;133:216–217. doi: 10.1530/eje.0.1330216. [DOI] [PubMed] [Google Scholar]
- 10.Ashizawa K. Shibata Y. Yamashita S. Namba H. Hoshi M. Yokoyama N. Izumi M. Nagataki S. Prevalence of goiter and urinary iodine excretion levels in children around Chernobyl. J Clin Endocrinol Metab. 1997;82:3430–3433. doi: 10.1210/jcem.82.10.4285. [DOI] [PubMed] [Google Scholar]
- 11.Arinchin AN. Gembicki M. Moschik K. Skalyzhenko A. Khmara I. Korytko N. Petrenko S. Gomolko N. Balakleevskaya V. Laptenok S. Bertollini R. Goiter prevalance and urinary iodine excretion in Belarus children born after the Chernobyl accident. IDD Newsl. 2000;16:7–9. [Google Scholar]
- 12.Tronko M. Kravchenko V. Fink D. Hatch M. Turchin V. McConnell R. Shpak V. Brenner A. Robbins J. Lusanchuk I. Howe G. Iodine excretion in regions of Ukraine affected by the Chornobyl accident: experience of the Ukrainian-American cohort study of thyroid cancer and other thyroid diseases. Thyroid. 2005;15:1291–1297. doi: 10.1089/thy.2005.15.1291. [DOI] [PubMed] [Google Scholar]
- 13.Stezhko VA. Buglova EE. Danilova LI. Drozd VM. Krysenko NA. Lesnikova NR. Minenko VF. Ostapenko VA. Petrenko SV. Polyanskaya ON. Rzheutski VA. Tronko MD. Bobylyova OO. Bogdanova TI. Epshtein OV. Kairo IA. Kostin OV. Likhtarev IA. Markov VV. Oliynik VA. Shpak VM. Tereshchenko VP. Zamotayeva GA. Beebe GW. Bouville AC. Brill AB. Burch JD. Fink DJ. Greenebaum E. Howe GR. Luckyanov NK. Masnyk IJ. McConnell RJ. Robbins J. Thomas TL. Voillequé PG. Zablotska LB. 2004 A cohort study of thyroid cancer, other thyroid diseases after the Chornobyl accident: objectives, design, methods. Radiat Res. 161:481–492. doi: 10.1667/3148. [DOI] [PubMed] [Google Scholar]
- 14.Dunn JT. Crutchfield HE. Gutekunst R. Dunn AD. Methods for Measuring Iodine in Urine. International Council for Control of Iodine Deficiency Disorders; Netherlands. 1993. pp. 18–27. [Google Scholar]
- 15.Center for Medical Technologies, Informatization, Administration and Management of Health. Classification of Territories and Settlements: TERSON, part 2, Minsk, Belarus. 1993.
- 16.Delange F. de Benoist B. Burgi H. Determining median urinary iodine concentration that indicates adequate iodine intake at population level. Bull World Health Organ. 2002;80:633–636. [PMC free article] [PubMed] [Google Scholar]
- 17.Delange FM. Dunn JT. Iodine deficiency. In: Braverman LE, editor; Utiger RD, editor. Werner & Ingbar's The Thyroid: A Fundamental and Clinical Text. 9th. Lippincott Williams & Wilkins; Philadelphia, PA: 2005. pp. 264–287. [Google Scholar]
- 18.Lozovsky LN. Stable iodine in soil in Belarus in 1960s. In: Lupinovitch IS, editor; Dubikovsky GP, editor. Microelements in the Soils of BSSR and Effectiveness of Fertilizers. BGU, Minsk; Belarus: 1970. p. 224. [Google Scholar]
- 19.Vejbjerg P. Knudsen N. Perrild H. Laurbereg P. Andersen S. Rasmussen LB. Ovesen L. Jorgensen T. Estimation of iodine intake from various urinary iodine measurements in population studies. Thyroid. 2009;19:1281–1286. doi: 10.1089/thy.2009.0094. [DOI] [PubMed] [Google Scholar]
- 20.Andersen S. Karmisholt J. Pedersen KM. Laurberg P. Reliability of studies of iodine intake and recommendation for number of samples in groups and individuals. Br J Nutrition. 2008;99:813–818. doi: 10.1017/S0007114507842292. [DOI] [PubMed] [Google Scholar]
- 21.Zimmermann M. Saad A. Hess S. Torresani T. Chaouki N. Thyroid ultrasound compared with World Health Organization 1960 and 1994 palpation criteria for determination of goiter prevalence in regions of mild and severe iodine deficiency. Eur J Endocrinol. 2000;143b:727–731. doi: 10.1530/eje.0.1430727. [DOI] [PubMed] [Google Scholar]
- 22.Vitti P. Martino E. Aghini-Lombardi F. Rago T. Antonangeli L. Maccherini D. Nanni P. Loviselli A. Balestrieri A. Araneo G. Pinchera A. Thyroid volume measurement by ultrasound in children as a tool for the assessment of mild iodine deficiency. J Clin Endocrinol Metab. 1994;79:600–603. doi: 10.1210/jcem.79.2.8045982. [DOI] [PubMed] [Google Scholar]
- 23.Rasmussen LB. Ovesen L. Bulow I. Jorgensen T. Knudsen N. Laurberg P. Perrild H. Relations between various measures of iodine intake and thyroid volume, thyroid nodularity, and serum thyroglobulin. Am J Clin Nutr. 2002;76:1069–1076. doi: 10.1093/ajcn/76.5.1069. [DOI] [PubMed] [Google Scholar]
- 24.Zimmermann MB. Molinari L. Spehl M. Weidinger-Toth J. Podoba J. Hess S. Delange F. Toward a consensus on reference values for thyroid volume in iodine-replete schoolchildren: results of a workshop on inter-observer and inter-equipment variation in sonographic measurement of thyroid volume. Eur J Endocrinol. 2001;144:213–220. doi: 10.1530/eje.0.1440213. [DOI] [PubMed] [Google Scholar]
- 25.Foo LC. Zulfiqar A. Nafikudin M. Fadzil MT. Asmah AS. Local versus WHO/International Council for Control of Iodine Deficiency Disorders-recommended thyroid volume reference in the assessment of iodine deficiency disorders. Eur J Endocrinol. 1999;140:491–497. doi: 10.1530/eje.0.1400491. [DOI] [PubMed] [Google Scholar]
- 26.Zimmermann MB. Hess SY. Molinari L. de Benoist B. Delange F. Braverman LE. Fujieda K. Ito Y. Jooste PL. Moosa K. Pearce EN. Pretell EA. Shishiba Y. New reference values for thyroid volume by ultrasound in iodine-sufficient schoolchildren: a World Health Organization/Nutrition for Health and Development Iodine Deficiency Study Group Report. Am J Clin Nutr. 2004;79:231–237. doi: 10.1093/ajcn/79.2.231. [DOI] [PubMed] [Google Scholar]
- 27.Vanmiddlesworth L. Iodine nutrition in the Chernobyl area before and after the nuclear accident. Int Congr Ser. 2002;1234:163–168. [Google Scholar]