Abstract
Background
Although hereditary nonmedullary thyroid cancer is recognized as a distinct and isolated familial syndrome, the precise prevalence and genetic basis are poorly understood. Moreover, whether familial nonmedullary thyroid cancer (FNMTC) has a more aggressive clinical behavior is controversial. The objectives of this study were to determine the prevalence of FNMTC, and compare the extent of disease and tumor somatic genetic alteration in patients with familial and sporadic papillary thyroid cancer.
Methods
The main study entry criterion was patients who had a thyroid nodule that required a clinical evaluation with fine-needle aspiration biopsy and or thyroidectomy. A family history questionnaire was used to determine the presence of familial and sporadic thyroid cancer. Thyroid nodule fine-needle aspiration biopsy samples and tumor tissue at the time of thyroidectomy were used to test for somatic genetic mutations (BRAF V600E, NRAS, KRAS, NTRK1, RET/PTC1, and RET/PTC3).
Results
There were 402 patients with 509 thyroid nodules enrolled in the study. The prevalence of FNMTC was 8.8% in all patients with thyroid cancer and 9.4% in patients with only papillary thyroid cancer. None of the patients with FNMTC had another familial cancer syndrome. There was no significant difference in gender, tumor size, lymph node metastasis, and overall stage between sporadic and familial cases of thyroid cancer. Patients with FNMTC were younger at diagnosis than patients with sporadic papillary thyroid cancer (p < 0.002). Seventy-nine of the 504 thyroid nodules had somatic genetic mutations (29 BRAF V600E, 29 NRAS, 8 KRAS, 1 NTRK1, 4 RET/PTC1, and 8 RET/PTC3). There was no significant difference in the number or type of somatic mutations between sporadic and hereditary cases of papillary thyroid cancer.
Conclusions
We found a higher prevalence of FNMTC in patients with papillary thyroid cancer than previously reported. Patients with FNMTC present at a younger age. Somatic mutations and extent of disease are similar in sporadic and FNMTC cases.
Introduction
Familial nonmedullary thyroid cancer (FNMTC) may occur as a minor component of familial cancer syndromes (Gardner's, Cowden's disease, Carney complex type 1, Werner syndrome, and McCune-Albright syndrome) or as the predominate feature (1). Most cases of FNMTC are papillary thyroid cancer. FNMTC has an autosomal dominant pattern of inheritance. The estimated frequency of FNMTC ranges from 3.2% to 6.2% among all thyroid cancer cases, but the precise prevalence is unknown (2–4). Moreover, with the increasing incidence of thyroid cancer, FNMTC may be more common today than previously thought (5).
Some investigators have reported higher rates of multicentric tumors, lymph node metastasis, vascular invasion, and local invasion in FNMTC than sporadic cases (6–9). Aggressive disease may be more common in the index case and in families with 3 or more affected members (10). In contrast, several investigators have, however, observed no difference in disease aggressiveness in FNMTC versus sporadic cases (11–13). Determining whether FNMTC is more aggressive has important clinical ramifications. For example, is screening in at risk family members needed for FNMTC, at what age, and in whom should screening be utilized? What screening test should be used (physical examination, thyroid ultrasonography)? Should aggressive treatment be used in affected individuals?
Multigenerational kindred and population-based studies have established that FNMTC is an authentic hereditary syndrome. Unfortunately, the susceptibility genes that lead to FNMTC have not been identified to allow for early genetic screening. Several investigators have demonstrated that common somatic mutations in the mitogen signaling pathway do not occur as germline mutations in patients with FNMTC (14,15). However, it is unclear if the molecular features of sporadic versus FNMTC cases are similar or distinct.
Given the limitations of our understanding about FNMTC, we studied the prevalence, extent of disease, and somatic genetic alteration profile in unselected patients being evaluated for a thyroid nodule.
Materials and Methods
Patients
We recruited 402 subjects to participate in the study from June 2006 to July 2008 at the University of California, San Francisco (UCSF). The trial was approved by the Committee on Human Research at UCSF and registered with clinicaltrials.gov. The main study entry criterion was patients who had a thyroid nodule that required a clinical evaluation with fine-needle aspiration biopsy and or thyroidectomy. This study cohort was part of a clinical trial to validate molecular markers of thyroid cancer.
Family history questionnaire
A family history questionnaire was used as part of an intake form in all patients being evaluated for a thyroid nodule in outpatient clinics. The questionnaire asked, “Has any relative ever been treated for one of these problems?” and to check no or yes if adrenal tumor, pituitary tumor, Cushing's disease, Zollinger Ellison syndrome, hyperparathyroidism, multiple endocrine neoplasia, diabetes, or thyroid tumor applied. The questionnaire asked if yes applied to check mother, father, sister, brother, daughter, son, grandmother, grandfather, aunt, uncle, cousin, in-law, or other if they applied. In those patients who reported a history of thyroid disorder in any family member, the type of disease, the family tree, and affected individuals were reviewed. In unknown cases of the exact nature of the thyroid disorder or affected members, patients were followed up to clarify the history once medical records were made available and reviewed.
Cases of FNMTC were defined as when two or more first-degree relatives were affected with thyroid cancer of follicular cell origin and were found to have thyroid cancer. All cases of thyroid cancer were confirmed by histology. All the cases of FNMTC in this study cohort were papillary thyroid cancer. Cases of thyroid cancer without any family history were defined as sporadic disease.
RNA extraction and cDNA synthesis
Total RNA was extracted from the thyroid tumor tissue procured for research at the time of thyroidectomy or biopsy and frozen at −80°C using the TRIzol (Invitrogen, Inc., Carlsbad, CA) reagent according to the manufacturer's protocol. One microgram of total RNA was reverse transcribed using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA).
Detection of somatic genetic alterations
Detection of hotspot mutations in BRAF, KRAS, and NRAS
The samples were tested for BRAFV600E mutation, and KRAS and NRAS hotspot mutations in codons 12–13 and 61 by PCR amplification and automated direct DNA sequencing as previously described (16). Samples were then prepared with reverse primers for each hotspot mutation and sequenced using the ABI BigDye v3.1 dye terminator sequencing chemistry with the ABI PRISM 3730 × l capillary DNA analyzer. The sequences were analyzed using Mutation Surveyor v3.10 (SoftGenetics, State College, PA).
Detection of RET/PTC1, RET/PTC3, and NTRK1 rearrangements
The presence of RET/PTC1, RET/PTC3, and NTRK1 rearrangements in thyroid nodule samples were tested using nested PCR as previously described (16). Gel electrophoresis was used to determine the presence of the specific rearrangement. Positive controls for RET/PTC1 and RET/PTC3 were kindly provided by Dr. Yuri Nikiforov (University of Pittsburgh), and prior positive samples were used for NTRK1 rearrangement confirmed with nested PCR.
Statistical analyses
Data are presented as number and mean ± standard deviation. Demographic, clinicopathologic, and tumor genotype data were compared between sporadic and hereditary cases of papillary thyroid cancer using the χ2-test and Mann–Whitney test for categorical and nonparametric data, respectively.
Results
The demographic and clinicopathologic characteristics of the study cohort are summarized in Table 1. There were 402 patients with 509 thyroid nodules enrolled in the study. All patients completed the questionnaire and 48 patients (11%) reported a family history of a thyroid disorder in 2 or more first-degree relatives. In 30 of 48 patients, the family history was specifically for a thyroid neoplasm. In the remaining, a family history of chronic lymphocytic thyroiditis and Graves' disease was reported. In 12 cases of all thyroid cancer diagnoses (8%), a positive family history of thyroid cancer was reported in 2 or more family members. Three of the families had three family members affected with thyroid cancer. All the thyroid cancer cases were classic papillary thyroid cancer. Thus, the overall prevalence of FNMTC in this cohort was 8.8% among all patient with thyroid cancer and 9.4% among patients with papillary thyroid cancer.
Table 1.
Demographic and Clinical Characteristics of Study Cohort
| Characteristics | Number |
|---|---|
| Age (years) | |
| Mean ± SD | 51 ± 15 |
| Median, range | 49, 16–94 |
| Family history of thyroid disease | |
| Yes | 48 |
| History of head and neck irradiation | |
| Yes | 37 |
| Type of malignant thyroid neoplasm | |
| Conventional/classic papillary thyroid cancer | 113 |
| Follicular variant of papillary thyroid cancer | 14 |
| Follicular thyroid cancer | 8 |
| Anaplastic thyroid cancer | 1 |
| TNM stage of malignant thyroid neoplasm | 136 |
| I | 91 |
| II | 24 |
| III | 16 |
| IV | 5 |
We found patients with FNMTC presented at a significantly younger age (average 5 years) than patients with sporadic cases of thyroid cancer (p < 0.002) (Table 2). There was no significant difference in gender, tumor size, tumor multicentricity, lymph node metastasis, and overall TNM stage between sporadic and familial case (two or more affected members) of thyroid cancer. We also age-matched (±2 years) and gender-matched sporadic and familial cases (3:1 matched) of thyroid cancer, but there was no significant difference in extent of disease (tumor size, tumor multicentricity, extrathyroidal invasion, lymph node, or distant metastasis) between groups.
Table 2.
Clinical and Pathologic Features in Sporadic Versus Familial Nonmedullary Thyroid Cancer Cases
| Sporadic | HNMTC | p-Value | |
|---|---|---|---|
| Age (years) | 48 | 43 | <0.002 |
| Gender | |||
| Women (%) | 73 | 81 | 0.49 |
| Ethnicity/race | |||
| White (%) | 65 | 87 | 0.31 |
| Tumor size (cm) | 3.2 | 2.6 | 0.25 |
| Lymph node metastasis | |||
| N1 (%) | 34 | 25 | 0.52 |
| TNM Stage | |||
| I (%) | 63 | 100 | 0.065 |
All cases were patients with papillary thyroid cancer.
The median follow-up time was 19 months for the entire study cohort. Ninety-six percent of patients were disease-free at last follow-up with no deaths due to thyroid cancer. We found no difference in the disease-free survival between the sporadic and FNMTC groups, but the follow-up time is relatively short.
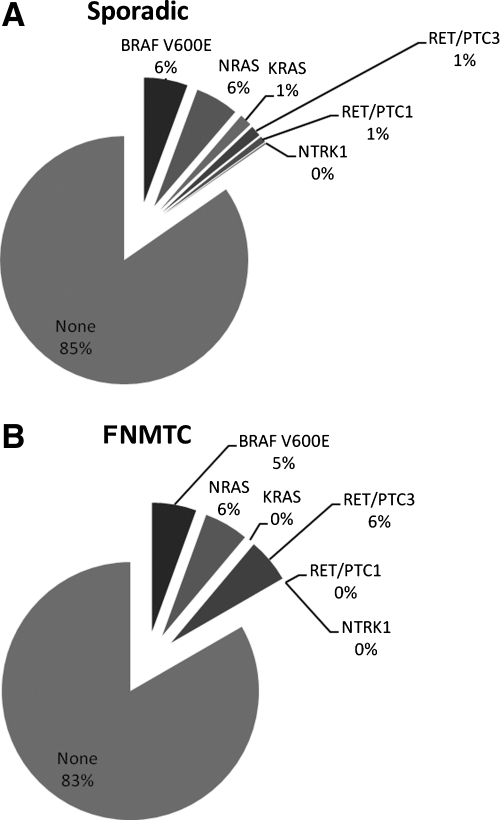
In 79 of the 504 thyroid nodule samples, a somatic genetic mutation was detected: 29 BRAF V600E, 29 NRAS, 8 KRAS, 1 NTRK1, 4 RET/PTC1, and 8 RET/PTC3. There was no significant difference in the number or type of somatic mutations between sporadic and FNMTC cases (Fig. 1). The positive mutation rate was 25% in sporadic and 19% in FNMTC cases.
FIG. 1.
Distribution of type of somatic mutation among sporadic (A) and familial cases (B) per thyroid nodule analyzed. There was no significant difference in the type or number of mutations between sporadic and FNMTC cases when analyzed by the number of thyroid nodules or thyroid cancers, or by the number of patients. FNMTC, familial nonmedullary thyroid cancer.
Discussion
To our knowledge, this is the first prospective study evaluating prevalence, and extent of disease and tumor genotype of sporadic versus FNMTC. We found a relatively high prevalence of FNMTC (8.8% among all thyroid cancer cases and 9.4% among papillary thyroid cancer cases) and that these patients present at a younger age. There was a similar somatic mutation profile and extent of disease observed between sporadic and FNMTC cases.
The higher prevalence of FNMTC observed in our study than previous estimates from retrospective studies may be due to several reasons. The high prevalence of FNMTC may have been truly underappreciated in previous studies due to the retrospective nature and or lack of screening for familial disease. Alternatively, the dramatic increase in incidence of thyroid cancer may result in the identification of higher number of familial cases, but prevalence would not be expected to be significantly altered. Also, the definition used to establish a diagnosis of FNMTC could greatly influence disease prevalence. Especially because FNMTC cases in which only two first-degree family members are affected may in fact be sporadic cases in up to 62% of cases based on probability estimate calculations (17). We recognize that there is a limitation of using the criterion for FNMTC when two first-degree family members are affected. The family histories reported in our study cohort, however, would make sporadic disease unlikely in many of the cases. For example, 6 of 9 families with 2 first-degree relatives had early age of presentation (<30 years in both members), male-to-male transmission, and siblings with papillary thyroid cancer. We also recognize that the study cohort may not be representative of the general population and be at a higher risk of having familial disease because the study was done at a referral center. To minimize this effect, the entry criteria excluded patients who had recurrent or persistent thyroid disease and or neoplasm.
We believe that the high prevalence of FNMTC observed in our study emphasis that routine questionnaire administration and inclusion of a thorough family history in the initial evaluation could help identify family members at risk of having and or developing thyroid cancer. There is no good clinical evidence and cost-effectiveness analysis to recommend screening, nor for that matter at what age to start or what screening test to use. Nonetheless, such practice recommendation or studies to address these issues could only be done if a reliable figure in the prevalence of the disease is established to determine the need and strategy for undertaking such studies.
Although FNMTC is an authentic entity, the aggressiveness of disease as compared to sporadic disease is controversial (1). FNMTC has been associated with higher rates of multicentric tumors, lymph node metastasis, vascular invasion, local invasion, and persistent and recurrent disease (6,7,9). Even cases of papillary thyroid cancer microcarcinoma that usually have an indolent course in the setting of FNMTC have been reported to have more aggressive tumor phenotype (7). In contrast, we found no difference in extent of disease at presentation even when matching patients by age and gender. Patients with FNMTC did present at a younger age for clinical evaluation. This is likely due to awareness of thyroid disease in affected families, thus prompting earlier clinical evaluation of other family members (18).
The susceptibility genes responsible for FNMTC have not been identified and several candidate loci implicated in FNMTC associated with other familial syndromes have not been found to have a role in the much more common isolated cases of FNMTC (1). All the cases of FNMTC identified in our cohort were not associated with any known familial cancer syndrome and were found to have classic papillary thyroid cancer on histologic examination. We expected the FNMTC cases to have a different rate of somatic mutations than sporadic cases because if there is a responsible germline genetic alteration that predisposes to FNMTC, the presence of somatic mutations may not be crucial to tumor initiation and or progression. Our findings, however, suggest a relatively similar rate and type of somatic mutations in sporadic and FNMTC cases. Cavaco et al. also performed tumor genotyping for RAS and BRAF mutations in 8 Portuguese families with 27 cases of thyroid cancer (15). They found that 7 of 27 were positive for BRAF V600E and 5 of 27 were positive for RAS (2 NRAS and 3 HRAS) mutations. Our results for BRAF V600E and NRAS mutations in FNMTC cases are similar to the results of Cavaco et al. but also included sporadic cases and additional somatic mutations common in nonmedullary thyroid cancer, and were performed prospectively. These findings taken together suggest that FNMTC cases may be molecularly heterogeneous as in sporadic cases and with no clear genotype–phenotype distinction based on hereditary predisposition.
In summary, we found a relatively high prevalence of FNMTC when routine prospective screening was used and patients presented at a significantly younger age for clinical evaluation. The common somatic mutations in thyroid cancer and extent of disease between sporadic versus FNMTC cases are similar.
Disclosure Statement
The authors declare that no competing financial interests exist.
References
- 1.Kebebew E. Hereditary non-medullary thyroid cancer. World J Surg. 2008;32:678–682. doi: 10.1007/s00268-007-9312-z. [DOI] [PubMed] [Google Scholar]
- 2.Pal T. Vogl FD. Chappuis PO. Tsang R. Brierley J. Renard H. Sanders K. Kantemiroff T. Bagha S. Goldgar DE. Narod SA. Foulkes WD. Increased risk for nonmedullary thyroid cancer in the first degree relatives of prevalent cases of nonmedullary thyroid cancer: a hospital-based study. J Clin Endocrinol Metab. 2001;86:5307–5312. doi: 10.1210/jcem.86.11.8010. [DOI] [PubMed] [Google Scholar]
- 3.Hemminki K. Dong C. Familial relationships in thyroid cancer by histo-pathological type. Int J Cancer. 2000;85:201–205. [PubMed] [Google Scholar]
- 4.Ron E. Kleinerman RA. LiVolsi VA. Fraumeni JF., Jr. Familial nonmedullary thyroid cancer. Oncology. 1991;48:309–311. doi: 10.1159/000226948. [DOI] [PubMed] [Google Scholar]
- 5.Davies L. Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 6.Grossman RF. Tu SH. Duh QY. Siperstein AE. Novosolov F. Clark OH. Familial nonmedullary thyroid cancer., An emerging entity that warrants aggressive treatment. Arch Surg. 1995;130:892–897. doi: 10.1001/archsurg.1995.01430080094015. discussion 898–899. [DOI] [PubMed] [Google Scholar]
- 7.Lupoli G. Vitale G. Caraglia M. Fittipaldi MR. Abbruzzese A. Tagliaferri P. Bianco AR. Familial papillary thyroid microcarcinoma: a new clinical entity. Lancet. 1999;353:637–639. doi: 10.1016/S0140-6736(98)08004-0. [DOI] [PubMed] [Google Scholar]
- 8.Alsanea O. Wada N. Ain K. Wong M. Taylor K. Ituarte PH. Treseler PA. Weier HU. Freimer N. Siperstein AE. Duh QY. Takami H. Clark OH. Is familial non-medullary thyroid carcinoma more aggressive than sporadic thyroid cancer? A multicenter series. Surgery. 2000;128:1043–1050. doi: 10.1067/msy.2000.110848. discussion 1050–1041. [DOI] [PubMed] [Google Scholar]
- 9.Uchino S. Noguchi S. Kawamoto H. Yamashita H. Watanabe S. Yamashita H. Shuto S. Familial nonmedullary thyroid carcinoma characterized by multifocality and a high recurrence rate in a large study population. World J Surg. 2002;26:897–902. doi: 10.1007/s00268-002-6615-y. [DOI] [PubMed] [Google Scholar]
- 10.Triponez F. Wong M. Sturgeon C. Caron N. Ginzinger DG. Segal MR. Kebebew E. Duh QY. Clark OH. Does familial non-medullary thyroid cancer adversely affect survival? World J Surg. 2006;30:787–793. doi: 10.1007/s00268-005-0398-x. [DOI] [PubMed] [Google Scholar]
- 11.Leprat F. Bonichon F. Guyot M. Trouette H. Trojani M. Vergnot V. Longy M. Belleannee G. de Mascarel A. Roger P. Familial non-medullary thyroid carcinoma: pathology review in 27 affected cases from 13 French families. Clin Endocrinol (Oxf) 1999;50:589–594. doi: 10.1046/j.1365-2265.1999.00687.x. [DOI] [PubMed] [Google Scholar]
- 12.Maxwell EL. Hall FT. Freeman JL. Familial non-medullary thyroid cancer: a matched-case control study. Laryngoscope. 2004;114:2182–2186. doi: 10.1097/01.mlg.0000149454.91005.65. [DOI] [PubMed] [Google Scholar]
- 13.Loh KC. Familial nonmedullary thyroid carcinoma: a meta-review of case series. Thyroid. 1997;7:107–113. doi: 10.1089/thy.1997.7.107. [DOI] [PubMed] [Google Scholar]
- 14.Xing M. The T1799A BRAF mutation is not a germline mutation in familial nonmedullary thyroid cancer. Clin Endocrinol (Oxf) 2005;63:263–266. doi: 10.1111/j.1365-2265.2005.02332.x. [DOI] [PubMed] [Google Scholar]
- 15.Cavaco BM. Batista PF. Martins C. Banito A. do Rosario F. Limbert E. Sobrinho LG. Leite V. Familial non-medullary thyroid carcinoma (FNMTC): analysis of fPTC/PRN, NMTC1, MNG1 and TCO susceptibility loci and identification of somatic BRAF and RAS mutations. Endocr Relat Cancer. 2008;15:207–215. doi: 10.1677/ERC-07-0214. [DOI] [PubMed] [Google Scholar]
- 16.Moses W. Weng J. Khanafshar E. Duh QY. Clark OH. Kebebew E. Multiple genetic alterations in papillary thyroid cancer are associated with younger age at presentation. J Surg Res. 2009;160:179–183. doi: 10.1016/j.jss.2009.05.031. [DOI] [PubMed] [Google Scholar]
- 17.Charkes ND. On the prevalence of familial nonmedullary thyroid cancer in multiply affected kindreds. Thyroid. 2006;16:181–186. doi: 10.1089/thy.2006.16.181. [DOI] [PubMed] [Google Scholar]
- 18.Capezzone M. Marchisotta S. Cantara S. Busonero G. Brilli L. Pazaitou-Panayiotou K. Carli AF. Caruso G. Toti P. Capitani S. Pammolli A. Pacini F. Familial non-medullary thyroid carcinoma displays the features of clinical anticipation suggestive of a distinct biological entity. Endocr Relat Cancer. 2008;15:1075–1081. doi: 10.1677/ERC-08-0080. [DOI] [PubMed] [Google Scholar]