Abstract
The authors previously reported on an active, young male with normal coronaries who sustained an acute myocardial infarction (AMI). The acute cause was a coronary thrombus; however, the cause of this thrombus and a definitive diagnosis remained elusive for 18 months until a new series of events, including symptoms of breathlessness, dizziness and collapse led to acute hospital admission. CT scan revealed numerous deep venous thromboses in the right leg and bilateral pulmonary emboli (PE). Acute pharmacological thrombolysis eliminated breathlessness and significantly reduced the risk of mortality. Clinical consensus suggests a coagulopathy, requiring indefinite treatment with Warfarin. In young individuals presenting with AMI, lifestyle, personal, family and clinical history should be considered and coronary artery disease should not be assumed until further tests have eliminated coagulopathy. In those presenting with breathlessness and a history which includes AMI, a CT scan is indicated to eliminate concerns of venous thromboembolism generally and PE specifically where untreated survival times are short.
Background
In 2009 we reported a case of a 45-year-old, physically active male of mixed race (Mother Caucasian, Father Indian) who presented to A&E with an acute myocardial infarction (AMI). Upon transfer to a specialist cardiac unit, coronary angiography revealed a large in situ thrombosis in the right coronary artery (figure 1). The patient was treated in the standard way with thrombus aspiration and anticoagulation and no obstructive plaque was identified. Follow-up angiographies at 24 h and 4 months with intravascular ultrasound were normal with no evidence of atheromatous disease, healed dissection or a ruptured plaque. Cardiac MRI with gadolinium imaging confirmed a near transmural myocardial infarction (MI) in the inferoposterior wall, with a total of 16% of the right ventricle myocardium replaced with scar. The overall systolic function was normal.1 Subsequent clinical events have arisen since then that shed further light on the underlying cause.
Figure 1.
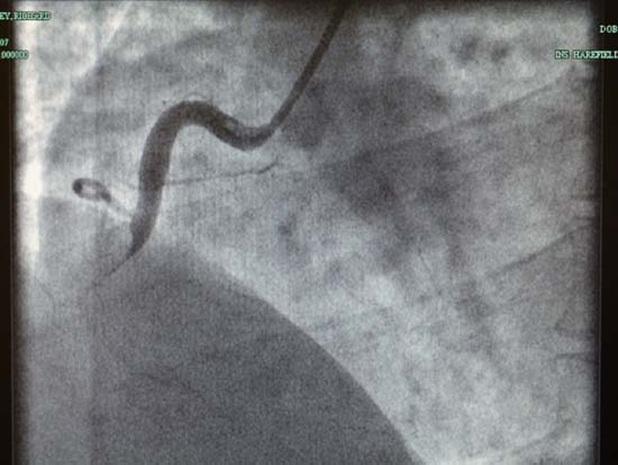
Angiography film showing in situ thrombosis in the right coronary artery.
Case presentation
In May 2009, 20 months after AMI and following generally good health including regular vigorous exercise, the patient consulted his GP for breathlessness and light-headedness on very mild exertion. He also complained of an intermittent chesty, non-expectorant cough at rest which had been apparent for 3 weeks. Examination of the chest with a stethoscope and palpation revealed no obvious additional signs. Assuming an upper respiratory tract infection (URTI), the patient was prescribed a 7 day course of antibiotics (Amoxicillin, 3 × 500 mg per day).
At the end of May the patient had to travel for work to Seattle, USA; a plane journey of 9 h, and 2 weeks later returned also on a 9 h plane journey. A week later he travelled, in a 7 h car journey, to Glasgow, returning by the same mode of transport 3 days later. By the end of June, dizziness and breathlessness had worsened and the patient attended A&E. Additional signs included an elevated resting heart rate of 95–105 bpm for the previous 3 weeks (habitual norm for this active patient being 60–65 bpm). A plain x-ray, 12-lead ECG and full blood count were carried out. The A&E consultant found nothing of note and stated that C reactive protein levels were elevated but blood count seemed normal. The patient was sent home with another 7-day course of antibiotics (Amoxicillin, 3 × 500 mg per day) and told to see his GP in the next few days.
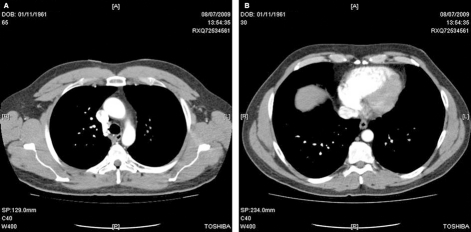
On seeing his GP a few days later he was told to take a week off work and to be as inactive as possible. Six days later the patient experienced aching at the top of his right inside thigh. The following morning the whole leg ached, felt slightly swollen and was hotter to the touch than his left leg. At 10:30 the patient collapsed at home. Upon regaining consciousness he telephoned emergency services and an ambulance conveyed him to the local hospital. On admission a 12 lead ECG revealed a large S wave in lead I and a large Q wave and inverted T wave in lead III (figure 2). Echocardiography demonstrated a dilated right atrium and right ventricle with an estimated pulmonary artery pressure of 65 mm Hg. A CT scan revealed bilateral pulmonary emboli (PE) and confirmed a dilated right atrium and ventricle (figure 3).
Figure 2.
12-lead ECG showing large S waves in lead I and large Q waves and inverted T waves in lead III suggestive of PE or acute inferior wall MI.
Figure 3.
(A) CT scan. (B) The top image is around the T7 level and shows emboli in pulmonary arteries and a dilated right atrium and ventricle. The bottom image shows emboli in lung arterioles at the T4 level.
Treatment
A bolus (50 mg) of Rapilysin (alteplase) was given to achieve thrombolysis in accordance with British Thoracic Society Guidelines.2 Sixty minutes after administration, the patient reported a cessation of symptoms of breathlessness and after two nights on a coronary care ward he was discharged. For the next 7 days self-administered injections of Fragmin (low molecular weight heparin) were used before initiation of indefinite anticoagulation therapy to a target INR of 2.0–3.0 using daily oral Warfarin.
Routine thrombophilia screening proved negative and follow-up investigation was undertaken to screen more specifically for lupus and antiphospholipid (Hughes) syndrome both of which also proved negative. The patient does, however, fulfil a number of the revised (Sapporo) classification criteria for Hughes syndrome3 including a clinical criterion of one or more episodes of arterial, venous or small vessel thrombosis in any organ. In addition, he has experienced signs such as fatigue, headache and gastrointestinal tract disturbance. However, the absence of laboratory criteria (presence of any of three specific antibodies) precludes definitive diagnosis. Hence, consensus among clinicians is that the patient has a coagulopathy which may be Hughes syndrome or a very similar condition.
Discussion
Immediately following the first presentation in September 2007 with AMI, and upon follow-up 24 h and 4 month angiography, diagnosis remained elusive. Even in cases where overt coronary artery disease (CAD) is absent, treatment is conservative and assumes CAD is the likely cause.4 Care is warranted with this approach and the complete family and personal history together with recent clinical history of the individual should be considered. In the present case this young male had no family history of note and an absence of modifiable risk factors (ie, the patient was very active, normal BMI, non-smoker, moderate drinker in the absence of hypercholesterolaemia and hypertension). This history suggests the need to search for an alternative underlying cause. Arguably, the appearance of intracardiac thrombus on the right side of the heart in an individual under 45 years of age suggests antiphospholipid syndrome (APLS).5 This could, therefore, warrant testing specifically for APLS and more generally for conditions that are pathognomonic for hypercoagulability.
In some individuals sudden death is the first and only sign of venous thromboembolism (VTE).6 PE is responsible for 10% of sudden deaths among hospital admissions and 50% of those who also have electromechanical dissociation or asystole on ECG.7 Prior AMI is associated with a 20% increased risk for VTE8 and the risk is increased 11-fold in the presence of an URTI.9 With this in mind, a CT scan may have been indicated following the first presentation of breathlessness in the present case, but this did not occur until after acute collapse, 8 weeks later. ECG is not a definitive test for PE but the recognised SIQIIITIII pattern (S wave in lead I and a large Q wave and inverted T wave in lead III) suggests either PE, or acute inferior wall MI and so warrants closer examination.
Learning points.
-
▶
An habitually active, young (<65 years) individual, with neither a remarkable cardiovascular disease risk factor profile nor prior history of CAD, who sustains an AMI should be screened for VTE.
-
▶
Presentation of symptoms associated with an increased risk of PE (breathlessness and or an URTI), in an individual with a history of AMI should indicate a comprehensive examination for VTE, that is, a CT scan.
-
▶
Patients with a coagulopathy, or with symptoms of breathlessness that are unexplained, should not travel on ‘long haul’ flights or travel in any way that results in immobilisation for more than 2 h at a time.
-
▶
PE carries with it a significant risk of morbidity and mortality. With respect to morbidity the current patient has an extensively damaged right ventricle and suboptimal pulmonary function that currently limits functionality and that could deteriorate in the medium term and lead to impaired lifestyle, reduced quality of life and increased healthcare costs in the long term.
-
▶
Arguably, many of these consequences could have been avoided with appropriate diagnosis and therapy following the index case presentation.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Whyte G, Godfrey R, O’Hanlon R, et al. Acute myocardial infarction in the presence of normal coronaries and the absence of risk factors in a young, life-long exerciser. BMJ Case Reports 2009: published online 25 May 2009, doi: 10.1136/bcr.07.2008.0384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.BTS Guidelines British Thoracic Society guidelines for management of suspected acute pulmonary embolism. Thorax 2003;58: 470–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006;4:295–306 [DOI] [PubMed] [Google Scholar]
- 4.Chandrasekaran B, Kurbaan AS. Myocardial infarction with angiographically normal coronary arteries. J R Soc Med 2002;95:398–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turiel M, Muzzupappa S, Gottardi B, et al. Evaluation of cardiac abnormalities and embolic sources in primary antiphospholipid syndrome by transesophageal echocardiography. Lupus 2000;9:406–12 [DOI] [PubMed] [Google Scholar]
- 6.Sandler DA, Martin JF. Autopsy proven pulmonary embolism in hospital patients: are we detecting enough deep vein thrombosis? J R Soc Med 1989;83:198–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Courtney DM, Sasser HC, Pincus CL, et al. Pulseless electrical activity with witnessed arrest as a predictor of sudden death from massive pulmonary embolism in outpatients. Resuscitation 2001;49:265–72 [DOI] [PubMed] [Google Scholar]
- 8.Anderson FA, Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation 2003;107(23 Suppl 1):I9–16 [DOI] [PubMed] [Google Scholar]
- 9.Smeeth L, Cook C, Thomas S, et al. Risk of deep vein thrombosis and pulmonary embolism after acute infection in a community setting. Lancet 2006;367:1075–9 [DOI] [PubMed] [Google Scholar]