Abstract
Among obesity-prone individuals, metabolic state may interact with diet in determining body composition. We tested the hypotheses that, among 103 weight-reduced women over 1 year, (i) insulin sensitivity would be positively associated with change in %fat; (ii) this association would be modulated by dietary glycemic load (GL); and (iii) changes in fat distribution would be related to indexes of glucose metabolism. Insulin sensitivity, glucose effectiveness, fasting and postchallenge insulin and glucose, and glucose tolerance were assessed during intravenous glucose tolerance test (IVGTT). Changes in %fat and fat distribution were examined using dual-energy X-ray absorptiometry and computed tomography. Dietary GL was assessed on 67 women using food records. On average, women showed a +5.3 ± 3.0% change in %fat over 1 year, with the magnitude of this change being greater in relatively insulin sensitive women (+6.0 ± 0.4%, mean ± s.e.m.) than in relatively insulin resistant women (+4.4 ± 0.4 kg; P < 0.05). Women who were relatively insulin sensitive and who consumed a higher GL diet showed a +6.8 ± 0.7% change in %fat, which was greater than those who were less insulin sensitive, regardless of diet (P < 0.05), but did not differ from women who were relatively insulin sensitive and who consumed a lower GL diet (P = 0.105). Changes in intra-abdominal and deep subcutaneous abdominal fat were inversely associated with the postchallenge decline in serum glucose. In conclusion, greater insulin sensitivity may predispose to adiposity among weight reduced women, an effect that may be ameliorated by a lower GL diet. The potential association between indexes of glucose disposal and changes in fat distribution warrants further study.
INTRODUCTION
Success with weight loss and weight loss maintenance is highly variable, with many individuals failing to achieve long-term weight maintenance (1). It is possible that individual differences in metabolic phenotype contribute to variation in propensity to obesity and success with weight loss efforts. In support of this hypothesis, insulin sensitivity and other indexes of insulin secretion or action have been associated with changes in body weight or composition (2–13). Although it is not clear if indexes of glucose metabolism mediate changes in energy balance or fuel partitioning, or serve as a marker for a given metabolic phenotype, further knowledge of these relationships may be useful for understanding risk for obesity and for developing means of successful weight loss maintenance.
Limited data suggest that individual differences in insulin sensitivity are associated with energy balance among obesity-prone individuals. Among 10 previously overweight women, the degree of improvement in insulin sensitivity with weight loss predicted the amount of weight they subsequently regained, such that women with more improved insulin sensitivity gained more weight (14). Similarly among Pima Indians, a population prone to obesity, greater insulin sensitivity was predictive of greater weight gain over time (2), although in some cases this relationship was not independent of other measures related to glucose metabolism (15). The provocative possibility that insulin sensitivity is related to propensity to weight gain within obesity-prone individuals bears further investigation.
Results from several studies have suggested that the relationship between indexes of glucose metabolism and weight change may be modulated by dietary factors (11,12,16–18). Among 21 obese women, relatively insulin sensitive subjects were more successful with weight loss when they consumed a diet with a relatively greater carbohydrate content (16). In contrast, relatively insulin resistant subjects lost more weight on a lower carbohydrate diet. Because insulin sensitivity improves with weight loss (19), the optimal diet for weight loss maintenance is not clear. Information is needed on the role of diet quality in weight loss maintenance among weight-reduced individuals, and its potential interaction with metabolic phenotype.
Inherent aspects of glucose metabolism may affect not only gain of adipose tissue, but also the distribution of the fat mass that is gained. For example, use of the peroxisome proliferator–activated receptor-γ agonist family of insulin-sensitizing agents results in selective gains in subcutaneous adipose tissue (20). It is not clear to what extent individual variability in insulin sensitivity or other indexes of glucose metabolism affect changes in adipose tissue distribution over time. However, knowledge of such relationships is desirable because adipose tissue in the intra-abdominal and deep subcutaneous abdominal spaces is more closely linked to impairment in metabolic health than is adipose tissue deposited in peripheral and superficial abdominal subcutaneous regions (21,22).
This longitudinal study was conducted to identify independent associations of indexes of glucose metabolism with changes in body composition and fat distribution among weight-reduced women, and to examine the potential modulating role of dietary glycemic load (GL), a measure that reflects both carbohydrate quantity and quality (23). We hypothesized that relatively more insulin sensitive women would show a greater increase in %fat; women who consumed a diet relatively high in GL would show a greater gain in %fat; and women who displayed more favorable indexes of glucose metabolism would deposit relatively less deep abdominal fat.
METHODS AND PROCEDURES
Participants and study protocol
Participants were 103 healthy premenopausal women who enrolled in a study designed to examine metabolic factors that predispose to weight gain. All participants were derived from a previous weight loss intervention (24). For the purposes of this study, “baseline” was taken as post-weight-loss, at which time all participants had a BMI of <25 kg/m2. All women had a family history of overweight in at least one first-degree relative. None used medications that affect body composition or metabolism. All were nonsmokers and reported experiencing menses at regular intervals.
The parent study originally enrolled 228 women, 141 of whom completed the weight loss intervention, and 117 of whom also completed a 1-year post-weight-loss follow-up evaluation. Criteria for inclusion in this study were completion of the post-weight-loss “baseline” visit with valid insulin sensitivity data, and completion of the 1-year follow-up visit (Figure 1). Because only participants having insulin sensitivity data were included, 14 women were excluded. The 103 women included in this study lost 11.95 ± 2.38 kg during the weight loss intervention phase of the parent study.
Figure 1.
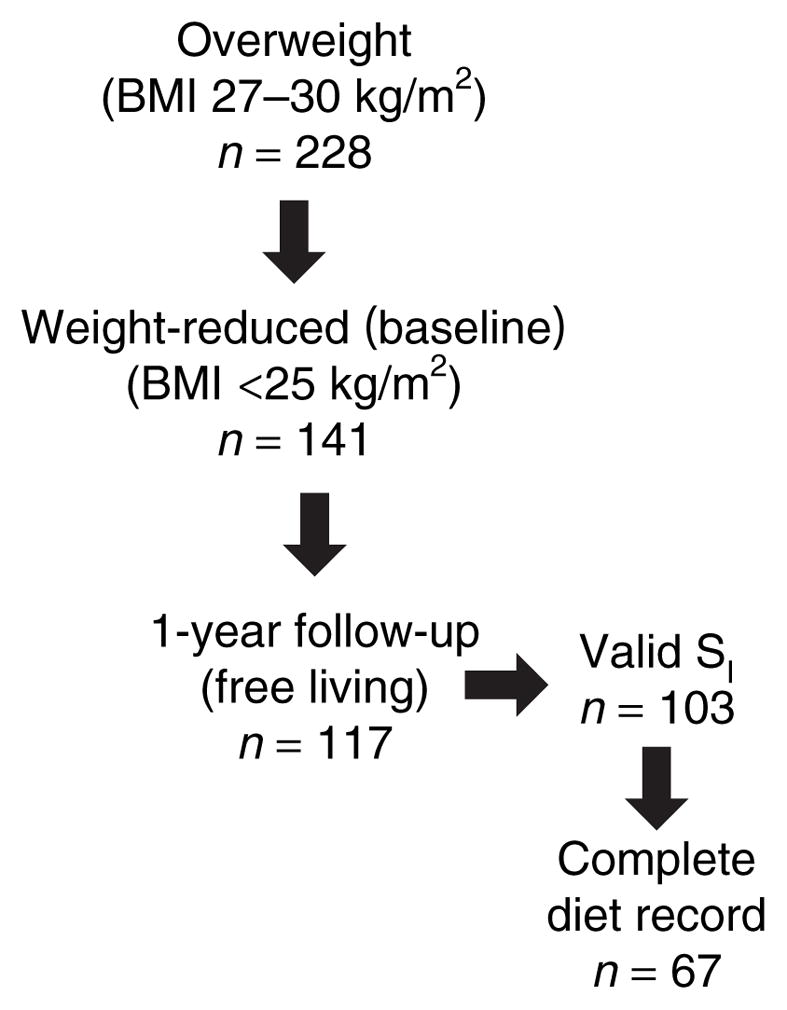
Flow diagram for study design. Of the 117 women who completed the 1-year follow-up evaluation, 103 had valid insulin sensitivity test data from the weight-reduced evaluation, and 67 of these 103 completed and returned the diet record.
Prior to baseline testing, all participants were placed on a weight-maintenance diet for 4 weeks. Diets consisted of ~22% of energy from fat, 23% from protein, and 55% from carbohydrate. At the end of the 4-week weight-maintenance period, body composition, fat distribution, and metabolic outcomes were assessed under controlled conditions during an in-patient stay at the General Clinical Research Center. All testing was conducted within 12 days of menses. Women then entered a 1-year follow-up period where they were encouraged to maintain their reduced weight status, and were offered nutrition education classes and in some cases access to a gym facility. At the conclusion of the 1-year follow-up period, body composition, fat distribution, and metabolic outcomes were assessed after weight-maintenance dietary control, as described for baseline testing.
The study was approved by the institutional review board for Human Use at the University of Alabama at Birmingham. All women provided informed consent before participating in the study.
Body composition and fat distribution
Body composition (% fat) was assessed with (dual-energy X-ray absorptiometry; Lunar Prodigy, GE-Lunar, Madison, WI). Subjects were scanned in light clothing while lying flat on their backs with arms at their sides. Change in %fat over 1 year was the main outcome for the study. Intra-abdominal adipose tissue (IAAT) and subcutaneous abdominal adipose tissue (SAAT) were analyzed by computed tomography scanning (25) with a HiLight/Advantage Scanner (General Electric, Milwaukee, WI). SAAT was further subdivided into superficial SAAT and deep (DSAAT) compartments (26). Subjects were scanned in the supine position with arms stretched above their heads. A 5 mm scan at the level of the umbilicus was taken. Scans were analyzed for cross-sectional area (cm2) of adipose tissue using the density contour program with Hounsfield units for adipose tissue set at −190 to −30. All scans were analyzed by the same individual. The CV for repeat cross-section analysis of scans among 40 subjects is <2%.
Measures of glucose metabolism
Measures of glucose metabolism were assessed on an in-patient basis in the General Clinical Research Center after an overnight fast with an insulin-modified, frequently-sampled intravenous glucose tolerance test (IVGTT). Prior to testing, flexible intravenous catheters were placed in the antecubital spaces of both arms. Three, 2.0 ml blood samples were taken over a 20-min period for determination of basal glucose and insulin (the average of the values was used for basal “fasting” concentrations). At time “0,” glucose (50% dextrose; 11.4 g/m2) was administered intravenously. Insulin (0.02 U/kg, Humulin; Eli Lilly, Indianapolis, IN) was injected at 20 min postglucose injection. Blood samples (2.0 ml) were then collected at the following times (min) relative to glucose administration: 2, 3, 4, 5, 6, 8, 10, 12, 15, 19, 20, 21, 22, 24, 26, 28, 30, 35, 40, 45, 50, 55, 60, 70, 80, 100, 120, 140, 180. Sera were stored at −85 °C.
Glucose and insulin values were entered into the MINMOD computer program (version 3, Richard N. Bergman) for determination of the insulin sensitivity index (SI) and glucose effectiveness (SG) (27). SI is a composite measure of insulin-stimulated glucose uptake and insulin suppression of endogenous glucose production, and as such reflects “whole body” insulin sensitivity. It is defined as the increase of the fractional turnover of glucose per unit increase of insulin concentration. SG is a measure of glucose-dependent glucose disposal, defined as fractional glucose turnover at basal insulin. The acute insulin response to glucose (AIRg) was calculated as the incremental insulin area-under-the-curve from minutes 0 to 10 following glucose injection using the trapezoidal method. Other measures derived from the IVGTT were intravenous glucose tolerance (KG), which is the rate of decline of glucose in %/minute from minutes 8 to 19 following glucose injection; glucose nadir (mg/dl), which is the lowest point to which serum glucose declined following glucose injection; and glucose decline (mg/dl), which is the absolute value of the change in glucose from baseline to nadir. These measures were included because low glucose concentration may drive weight gain via its effect on hunger.
Diet
Information on habitual diet was collected using 4-day food records. Participants were asked to complete the records at the 1-year time point, prior to the metabolic evaluation. Food records were analyzed using the Nutrition Data System for Research software (Nutrition Coordinating Center, University of Minnesota, MN, database versions 4.04_32 and 4.05_33, release date 2001–2002) (28). Only food records with at least 3 of the 4 days completed were used for analysis, and the days were averaged for mean nutrient intake. Because not all women returned the records, dietary information was available on 68 women. Mean daily dietary GL based on glucose reference, a measure that reflects both carbohydrate quantity and quality (23), was used as an independent variable in statistical analysis.
Laboratory analyses
All analyses were conducted in the Core Laboratory of University of Alabama at Birmingham’s General Clinical Research Center and Clinical Nutrition Research Center. Glucose was measured using an Ektachem DT II System (Johnson and Johnson Clinical Diagnostics, Rochester, NY). In the Core laboratory, this analysis has a mean intra-assay CV of 0.61%, and a mean inter-assay CV of 2.56%. Insulin was assayed in duplicate 100 μl aliquots using double-antibody radioimmunoassay (Linco Research Inc., St. Charles, MO). In the Core laboratory, this assay has a sensitivity of 3.35 μIU/ml, a mean intra-assay CV of 3.49%, and a mean inter-assay CV of 5.57%.
Statistical analysis
Descriptive statistics were generated for all variables of interest. Insulin measures were log10 transformed prior to statistical analysis to ensure a normal distribution. All statistical tests were two-sided, were performed using a type I error rate of 0.05, and were conducted using SAS (version 9.2; SAS Institute, Cary, NC).
Pearson partial correlation analysis was used to examine associations between IVGTT outcomes following weight loss and changes in %fat and fat distribution over the 1-year follow-up period, adjusting for ethnicity and, where relevant, change in %fat. Subsequently, multiple linear regression analysis was used to identify variables that were independently related to 1-year gain in %fat and indexes of abdominal fat. All IVGTT-derived measures (SI, fasting insulin, AIRg, SG, KG, glucose decline, glucose nadir) were tested, with ethnicity included as a covariate. Final models were derived showing only those variables that were significantly and independently related to the dependent variable. Relevant covariates identified through this procedure were used in subsequent analyses.
Subsequently, subjects were divided into groups based on median SI and median dietary GL. Analysis of covariance, adjusting for ethnicity and fasting insulin (relevant covariates identified in analyses described above), was used to examine the associations of SI and GL with gain in % fat.
RESULTS
Subject characteristics are shown in Table 1. By design, all women were BMI <25 kg/m2 (weight reduced) at the time of metabolic testing. Ethnic composition was 44% European American and 56% African American. African Americans had lower SI and higher AIRg (P < 0.001).
Table 1.
Descriptive statistics (mean ± s.d.) on the entire group (n = 103) and on women upon whom dietary data were available (n = 67)
| Baseline (all women) | Baseline (women with dietary data) | 1 year (all women) | |
|---|---|---|---|
| Age (years) | 34.6 ± 6.3 | 34.2 ± 6.5 | – |
| Ethnicity (EA/AA) | 46/57 | 31/36 | – |
| Body weight (kg) | 65.3 ± 6.2 | 64.6 ± 6.0 | 71.1 ± 7.9 |
| BMI (kg/m2) | 23.8 ± 1.0 | 23.8 ± 1.0 | 25.9 ± 2.0 |
| Percent fat | 33.1 ± 4.6 | 33.0 ± 4.6 | 38.4 ± 5.4 |
| IAAT (cm2) | 48.3 ± 23.8 | 49.4 ± 26.1 | 55.8 ± 29.7 |
| Deep SAAT (cm2) | 80.4 ± 34.3 | 80.9 ± 36.5 | 111.3 ± 47.0 |
| Superficial SAAT (cm2) | 131.5 ± 41.9 | 133.7 ± 43.9 | 159.6 ± 54.5 |
| Fasting glucose (mg/dl) | 85 ± 6 | 84 ± 6 | 89 ± 9 |
| Fasting insulin (μIU/ml) | 8 ± 3 | 8 ± 3 | 9 ± 3 |
| Glucose effectiveness (min−1) | 0.0198 ± 0.0078 | 0.0195 ± 0.0080 | 0.0194 ± 0.0076 |
| Insulin sensitivity (× 10−4 min−1/(μIU/ml)) | 4.19 ± 1.97 | 4.13 ± 2.03 | 4.02 ± 1.98 |
| AIRg (μIU/ml × 10 min) | 625 ± 480 | 590 ± 402 | 702 ± 493 |
| Intravenous glucose tolerance (%/min) | 1.98 ± 1.07 | 1.89 ± 1.01 | 1.88 ± 0.96 |
| Glucose nadir (mg/dl) | 62 ± 12 | 63 ± 11 | 65 ± 13 |
| Glucose declinea (mg/dl) | 23 ± 12 | 22 ± 10 | 24 ± 12 |
AIRg, acute insulin response to glucose; DSAAT, deep subcutaneous abdominal adipose tissue; IAAT, intra-abdominal adipose tissue; SSAAT, superficial subcutaneous abdominal adipose tissue.
Fasting glucose minus glucose nadir during intravenous glucose tolerance test.
After 1 year, the average change in %fat was +5.3 ± 3.0% (range −2.6 to +14.8%). Associations between IVGTT-derived measures and change in %fat, adjusted for ethnicity, are shown in Table 2. Significant independent associations are shown in Table 3. Women with greater insulin sensitivity and higher fasting insulin gained significantly greater % fat.
Table 2.
Pearson partial correlation coefficients for change in % fat and fat distribution over 1 year vs. baseline measures of insulin secretion and action
| Fasting insulin | SI | AIRg | SG | KG | Glucose nadir | Glucose decline | |
|---|---|---|---|---|---|---|---|
| Δ%fat | 0.13 | 0.23* | −0.11 | 0.07 | 0.10 | −0.04 | 0.13 |
| ΔIAAT | −0.04 | −0.07 | −0.03 | −0.13 | −0.02 | 0.24* | −0.29* |
| ΔSSAAT | −0.02 | 0.02 | 0.02 | −0.09 | −0.07 | 0.04 | −0.09 |
| ΔDSAAT | −0.01 | −0.02 | −0.007 | −0.07 | −0.08 | 0.13 | −0.24* |
All data adjusted for ethnicity. IAAT and SAAT data adjusted for change in %fat.
AIRg, acute insulin response to glucose; IAAT, intra-abdominal adipose tissue; KG, glucose tolerance; SAAT, subcutaneous abdominal adipose tissue; SG, glucose effectiveness; SI, insulin sensitivity index.
P < 0.05.
Table 3.
Results from multiple linear regression analysis for gain in % fat
| Independent variable | Parameter estimate ± s.e.e. | P |
|---|---|---|
| Intercept | −1.72 ± 2.09 | 0.413 |
| Ethnicity | 1.46 ± 0.60 | 0.016 |
| Log insulin sensitivity | 4.52 ± 1.43 | 0.002 |
| Log fasting insulin | 4.06 ± 1.72 | 0.021 |
Model R2 = 0.12. Ethnicity coded 0 = European American, 1 = African American.
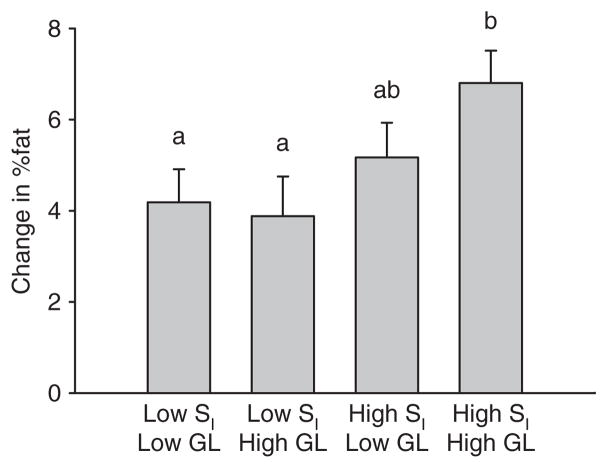
Women subsequently were divided by median SI and median dietary GL into “high” and “low” categories. Dietary data were available on 67 women. The mean GL in this subset was 95.0 ± 28.6 (mean ± s.d.). These women did not differ in any way from the group as a whole, being similar with respect to age, BMI, ethnic distribution, and insulin sensitivity (Table 1). Within the subgroup of women upon whom dietary data were available, relatively insulin sensitive women gained greater %fat (6.0 ± 0.4%) than did relatively insulin resistant women (4.5 ± 0.4%; P < 0.01; adjusted for ethnicity and fasting insulin; Figure 2). Women who were relatively insulin sensitive and who consumed a relatively high-GL diet gained greater %fat (mean ± s.e.m. 6.8 ± 0.71%) than did women who were less insulin sensitive, regardless of diet (P < 0.05), whereas women who were relatively insulin sensitive and who consumed a relatively low-GL diet did not differ from women who were less insulin sensitive. Results were similar when glycemic index was used in place of GL (data not shown).
Figure 2.
Change in %fat over 1 year by median GL and median SI (adjusted mean ± s.e.m.; data adjusted for ethnicity and fasting insulin). Different lower-case letters indicate significant differences (P < 0.05).
Change in IAAT was associated with change in %fat (r = 0.28; P < 0.01; adjusted for ethnicity). After adjusting for change in %fat and ethnicity, the associations of change in IAAT with glucose decline and glucose nadir were significant, as was the association of change in deep SAAT with glucose decline (Table 2). Multiple linear regression analysis indicated that women who showed a greater decline in glucose during the IVGTT gained less IAAT relative to adipose tissue in other regions (Table 4a). Results were similar if glucose decline was replaced with glucose nadir (model R2 = 0.09; P for glucose nadir = 0.09). Likewise, change in deep SAAT was independently associated with glucose decline (Table 4b). Change in superficial SAAT was not associated with any index of glucose disposal, either in partial correlation analysis (Table 2) or multivariate analysis (data not shown).
Table 4.
Results from multiple linear regression analysis for changes in IAAT and DSAAT
| Independent variable | Parameter estimate ± s.e.e | P |
|---|---|---|
| Change in IAAT; model R2 = 0.18 | ||
| Intercept | 6.78 ± 5.91 | 0.255 |
| Gain in %fat | 2.76 ± 0.75 | <0.001 |
| Glucose decline | −0.50 ± 0.18 | 0.008 |
| Change in DSAAT; model R2= 0.16 | ||
| Intercept | 20.75 ± 12.84 | 0.110 |
| Gain in %fat | 5.86 ± 1.62 | <0.001 |
| Glucose decline | −0.84 ± 0.40 | 0.038 |
DSAAT, deep subcutaneous abdominal adipose tissue; IAAT, intra-abdominal adipose tissue.
DISCUSSION
Inherent metabolic factors related to glucose metabolism may predispose to obesity, and this predisposition may be exacerbated by a high-GL diet. This study was designed to examine the independent and interactive effects of indexes of glucose metabolism and dietary GL on changes in body composition and fat distribution over 1 year among weight-reduced women. Results indicated that greater insulin sensitivity may increase risk for adiposity, but that this effect may be minimized by reducing dietary GL. Further, women with a robust ability to dispose of glucose may gain adipose tissue preferentially in subcutaneous depots that may be less detrimental to metabolic health.
Various aspects of glucose metabolism have been linked to individual differences in energy balance over time. The most widely studied are insulin sensitivity, and fasting and postchallenge insulin concentration, which may affect energy balance and partitioning via several potential mechanisms. The antilipolytic effect of insulin may prevent mobilization of fatty acids thereby facilitating accrual of triglyceride in adipose tissue (29). Insulin also may influence feeding behavior (30), an effect that may occur either at the level of the brain (31,32) or via glycogen storage and circulating glucose (15,33–37).
In our study, the strongest predictor of increasing adiposity was insulin sensitivity. Other studies have likewise reported a positive association between insulin sensitivity and weight gain in both children and adults (2–5,11), although one study in children indicated an inverse association (6). In agreement with the present results, in a longitudinal study involving Pima Indians, insulin sensitive subjects gained more weight over ~3.5 years than did insulin resistant subjects (2). Further, percent change in weight was statistically associated with insulin-stimulated glucose disposal during the euglycemic clamp.
Because insulin sensitivity is highly correlated with both fasting and postchallenge insulin concentrations (38), it is important to discern independent associations. In our population, fasting insulin was positively and independently associated with change in adiposity, suggesting that both fasting insulin and insulin sensitivity contribute uniquely to risk for adiposity. Existing literature indicates both positive (6–9,11) and inverse (10,39) associations of weight gain with fasting or postchallenge insulin concentration. We did not observe an association between postchallenge insulin (AIRg) and change in %fat in this study. Discrepancies among studies may be due to differences in subject population (e.g., age, gender, ethnic background) or a lack of control for confounding by related variables.
A novel finding of this study is the observation that dietary GL affected the association between insulin sensitivity status and adiposity. Weight-reduced women who were relatively insulin sensitive and who consumed a diet relatively high in GL showed a greater increase in %fat than other subgroups (Figure 1). The mechanism for this relationship is not clear. However, a high-GL diet would logically result in relatively high insulin secretion, which may promote a depression of blood glucose and thereby drive hunger. High insulin also may act to partition energy toward body fat at the expense of other tissues. It is possible that these effects are more pronounced among individuals who are also relatively insulin sensitive.
Although an interaction between indexes of glucose metabolism and diet regarding weight gain has been reported in rats (40), human data are sparse. In the Quebec Family study, 6-year weight gain in 276 healthy free-living adult men and women was associated with fasting insulin and with several insulin values obtained during an oral glucose tolerance test (11). The relationships were significant only among individuals consuming a higher carbohydrate (higher GL) diet. Thirty-minute insulin had the strongest relationship with weight gain, an observation interpreted to indicate that greater insulin secretion promoted weight gain. An inverse association of weight gain with 120-min insulin was interpreted as greater insulin sensitivity promoting weight gain. Glucose decline during the oral glucose tolerance test likewise predicted weight gain. In general, this pattern of relationships matches the results of the present study in that both studies suggest potential independent effects of insulin sensitivity and insulin concentration on weight gain.
We also examined the association between indexes of glucose metabolism and changes in fat distribution. We observed that subjects who showed a greater glucose decline during the IVGTT also showed lesser gain in deep abdominal adipose tissue (both IAAT and deep SAAT), depots that have been adversely associated with measures of metabolic health (21,22). In our population, glucose decline was related to both insulin sensitivity (r of 0.42), and glucose effectiveness (r of 0.54), and thus captures multiple processes that contribute to glucose tolerance. It remains to be determined whether a robust capacity for glucose disposal is causally related to fat distribution, or is simply a marker for a metabolic phenotype that includes a propensity to direct triglyceride storage to peripheral depots.
Strengths of the study were the comprehensive suite of measures of glucose metabolism collected using robust methodology, the direct measures of body fat distribution collected using computed tomography scan, and the longitudinal study design. Limitations were the observational nature of the study with associated risk of confounding and inability to determine the cause and effect nature of the observed relationships; the relatively small sample size; the incomplete diet data (67/103 women returned diet records); the inherent inaccuracy of self-reported dietary data; and the single dietary assessment at the 1-year follow-up evaluation.
In conclusion, among weight-reduced women, greater insulin sensitivity may increase risk for adiposity. However, this relationship was modulated by diet, suggesting that women who are more insulin sensitive may be more successful at weight loss maintenance if they consume a diet lower in GL. We also found that women with a robust ability to dispose of glucose may gain adipose tissue preferentially in subcutaneous depots that may not compromise metabolic health. These results in obesity-prone women should be verified in other populations. Development of a feasible, clinic-based means for “metabolic phenotyping,” including insulin sensitivity status, may prove useful for developing individualized approaches for weight loss and weight loss maintenance. Research is needed to determine if the mechanism through which insulin sensitivity increases risk for adiposity relates to energy partitioning toward adipose tissue, or increased food intake and positive energy balance.
Acknowledgments
This work was supported by R01DK51684, R01DK49779, M01-RR-00032, and P30-DK56336. Stouffer’s, Nestle Food Co., Weight Watchers, and HJ Heinz Foods provided food used prior to metabolic testing. David Bryan and Robert Petri provided technical assistance; Maryellen Williams and Cindy Zeng conducted laboratory analyses; Paul Zuckerman served as project coordinator.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
References
- 1.Elfhag K, Rössner S. Who succeeds in maintaining weight loss? A conceptual review of factors associated with weight loss maintenance and weight regain. Obes Rev. 2005;6:67–85. doi: 10.1111/j.1467-789X.2005.00170.x. [DOI] [PubMed] [Google Scholar]
- 2.Swinburn BA, Nyomba BL, Saad MF, et al. Insulin resistance associated with lower rates of weight gain in Pima Indians. J Clin Invest. 1991;88:168–173. doi: 10.1172/JCI115274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Travers SH, Jeffers BW, Eckel RH. Insulin resistance during puberty and future fat accumulation. J Clin Endocrinol Metab. 2002;87:3814–3818. doi: 10.1210/jcem.87.8.8765. [DOI] [PubMed] [Google Scholar]
- 4.Maffeis C, Moghetti P, Grezzani A, et al. Insulin resistance and the persistence of obesity from childhood into adulthood. J Clin Endocrinol Metab. 2002;87:71–76. doi: 10.1210/jcem.87.1.8130. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman RP, Stumbo PJ, Janz KF, Nielsen DH. Altered insulin resistance is associated with increased dietary weight loss in obese children. Horm Res. 1995;44:17–22. doi: 10.1159/000184584. [DOI] [PubMed] [Google Scholar]
- 6.Johnson MS, Figueroa-Colon R, Huang TT, Dwyer JH, Goran MI. Longitudinal changes in body fat in African American and Caucasian children: influence of fasting insulin and insulin sensitivity. J Clin Endocrinol Metab. 2001;86:3182–3187. doi: 10.1210/jcem.86.7.7665. [DOI] [PubMed] [Google Scholar]
- 7.Odeleye OE, de Courten M, Pettitt DJ, Ravussin E. Fasting hyperinsulinemia is a predictor of increased body weight gain and obesity in Pima Indian children. Diabetes. 1997;46:1341–1345. doi: 10.2337/diab.46.8.1341. [DOI] [PubMed] [Google Scholar]
- 8.Jernström H, Barrett-Connor E. Obesity, weight change, fasting insulin, proinsulin, C-peptide, and insulin-like growth factor-1 levels in women with and without breast cancer: the Rancho Bernardo Study. J Womens Health Gend Based Med. 1999;8:1265–1272. doi: 10.1089/jwh.1.1999.8.1265. [DOI] [PubMed] [Google Scholar]
- 9.Sigal RJ, El-Hashimy M, Martin BC, et al. Acute postchallenge hyperinsulinemia predicts weight gain: a prospective study. Diabetes. 1997;46:1025–1029. doi: 10.2337/diab.46.6.1025. [DOI] [PubMed] [Google Scholar]
- 10.Gould AJ, Williams DE, Byrne CD, Hales CN, Wareham NJ. Prospective cohort study of the relationship of markers of insulin resistance and secretion with weight gain and changes in regional adiposity. Int J Obes Relat Metab Disord. 1999;23:1256–1261. doi: 10.1038/sj.ijo.0801060. [DOI] [PubMed] [Google Scholar]
- 11.Chaput JP, Tremblay A, Rimm EB, Bouchard C, Ludwig DS. A novel interaction between dietary composition and insulin secretion: effects on weight gain in the Quebec Family Study. Am J Clin Nutr. 2008;87:303–309. doi: 10.1093/ajcn/87.2.303. [DOI] [PubMed] [Google Scholar]
- 12.Mosca CL, Marshall JA, Grunwald GK, Cornier MA, Baxter J. Insulin resistance as a modifier of the relationship between dietary fat intake and weight gain. Int J Obes Relat Metab Disord. 2004;28:803–812. doi: 10.1038/sj.ijo.0802621. [DOI] [PubMed] [Google Scholar]
- 13.Eckel RH, Hernandez TL, Bell ML, et al. Carbohydrate balance predicts weight and fat gain in adults. Am J Clin Nutr. 2006;83:803–808. doi: 10.1093/ajcn/83.4.803. [DOI] [PubMed] [Google Scholar]
- 14.Yost TJ, Jensen DR, Eckel RH. Weight regain following sustained weight reduction is predicted by relative insulin sensitivity. Obes Res. 1995;3:583–587. doi: 10.1002/j.1550-8528.1995.tb00193.x. [DOI] [PubMed] [Google Scholar]
- 15.Pannacciulli N, Ortega E, Koska J, et al. Glucose response to an oral glucose tolerance test predicts weight change in non-diabetic subjects. Obesity (Silver Spring) 2007;15:632–639. doi: 10.1038/oby.2007.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornier MA, Donahoo WT, Pereira R, et al. Insulin sensitivity determines the effectiveness of dietary macronutrient composition on weight loss in obese women. Obes Res. 2005;13:703–709. doi: 10.1038/oby.2005.79. [DOI] [PubMed] [Google Scholar]
- 17.Morrison JA, Glueck CJ, Horn PS, Schreiber GB, Wang P. Pre-teen insulin resistance predicts weight gain, impaired fasting glucose, and type 2 diabetes at age 18–19 y: a 10-y prospective study of black and white girls. Am J Clin Nutr. 2008;88:778–788. doi: 10.1093/ajcn/88.3.778. [DOI] [PubMed] [Google Scholar]
- 18.Ebbeling CB, Leidig MM, Feldman HA, Lovesky MM, Ludwig DS. Effects of a low-glycemic load vs low-fat diet in obese young adults: a randomized trial. JAMA. 2007;297:2092–2102. doi: 10.1001/jama.297.19.2092. [DOI] [PubMed] [Google Scholar]
- 19.Gower BA, Weinsier RL, Jordan JM, Hunter GR, Desmond R. Effects of weight loss on changes in insulin sensitivity and lipid concentrations in premenopausal African American and white women. Am J Clin Nutr. 2002;76:923–927. doi: 10.1093/ajcn/76.5.923. [DOI] [PubMed] [Google Scholar]
- 20.Shadid S, Jensen MD. Effects of pioglitazone versus diet and exercise on metabolic health and fat distribution in upper body obesity. Diabetes Care. 2003;26:3148–3152. doi: 10.2337/diacare.26.11.3148. [DOI] [PubMed] [Google Scholar]
- 21.Jensen MD. Role of body fat distribution and the metabolic complications of obesity. J Clin Endocrinol Metab. 2008;93:S57–S63. doi: 10.1210/jc.2008-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelley DE, Thaete FL, Troost F, Huwe T, Goodpaster BH. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am J Physiol Endocrinol Metab. 2000;278:E941–E948. doi: 10.1152/ajpendo.2000.278.5.E941. [DOI] [PubMed] [Google Scholar]
- 23.Salmerón J, Manson JE, Stampfer MJ, et al. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. JAMA. 1997;277:472–477. doi: 10.1001/jama.1997.03540300040031. [DOI] [PubMed] [Google Scholar]
- 24.Katsoulis K, Blaudeau TE, Roy JP, Hunter GR. Diet-induced Changes in Intra-abdominal Adipose Tissue and CVD Risk in American Women. Obesity (Silver Spring) 2009;17:2169–2175. doi: 10.1038/oby.2009.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kekes-Szabo T, Hunter GR, Nyikos I, et al. Development and validation of computed tomography derived anthropometric regression equations for estimating abdominal adipose tissue distribution. Obes Res. 1994;2:450–457. doi: 10.1002/j.1550-8528.1994.tb00092.x. [DOI] [PubMed] [Google Scholar]
- 26.Smith SR, Lovejoy JC, Greenway F, et al. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metab Clin Exp. 2001;50:425–435. doi: 10.1053/meta.2001.21693. [DOI] [PubMed] [Google Scholar]
- 27.Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest. 1981;68:1456–1467. doi: 10.1172/JCI110398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schakel SF. Maintaining a nutrient database in a changing marketplace: keeping pace with changing food products - A research perspective. J Food Comp and Anal. 2001;14:315–322. [Google Scholar]
- 29.Arner P. Control of lipolysis and its relevance to development of obesity in man. Diabetes Metab Rev. 1988;4:507–515. [PubMed] [Google Scholar]
- 30.Rodin J, Wack J, Ferrannini E, DeFronzo RA. Effect of insulin and glucose on feeding behavior. Metab Clin Exp. 1985;34:826–831. doi: 10.1016/0026-0495(85)90106-4. [DOI] [PubMed] [Google Scholar]
- 31.Woods SC, D’Alessio DA. Central control of body weight and appetite. J Clin Endocrinol Metab. 2008;93:S37–S50. doi: 10.1210/jc.2008-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lustig RH. Childhood obesity: behavioral aberration or biochemical drive? Reinterpreting the First Law of Thermodynamics. Nat Clin Pract Endocrinol Metab. 2006;2:447–458. doi: 10.1038/ncpendmet0220. [DOI] [PubMed] [Google Scholar]
- 33.Flatt JP. Carbohydrate balance and body-weight regulation. Proc Nutr Soc. 1996;55:449–465. doi: 10.1079/pns19960041. [DOI] [PubMed] [Google Scholar]
- 34.Shetty PS, Prentice AM, Goldberg GR, et al. Alterations in fuel selection and voluntary food intake in response to isoenergetic manipulation of glycogen stores in humans. Am J Clin Nutr. 1994;60:534–543. doi: 10.1093/ajcn/60.4.534. [DOI] [PubMed] [Google Scholar]
- 35.Sparti A, Milon H, Di Vetta V, et al. Effect of diets high or low in unavailable and slowly digestible carbohydrates on the pattern of 24-h substrate oxidation and feelings of hunger in humans. Am J Clin Nutr. 2000;72:1461–1468. doi: 10.1093/ajcn/72.6.1461. [DOI] [PubMed] [Google Scholar]
- 36.Snitker S, Larson DE, Tataranni PA, Ravussin E. Ad libitum food intake in humans after manipulation of glycogen stores. Am J Clin Nutr. 1997;65:941–946. doi: 10.1093/ajcn/65.4.941. [DOI] [PubMed] [Google Scholar]
- 37.Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA. 2002;287:2414–2423. doi: 10.1001/jama.287.18.2414. [DOI] [PubMed] [Google Scholar]
- 38.Phillips DI, Clark PM, Hales CN, Osmond C. Understanding oral glucose tolerance: comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion. Diabet Med. 1994;11:286–292. doi: 10.1111/j.1464-5491.1994.tb00273.x. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz MW, Boyko EJ, Kahn SE, Ravussin E, Bogardus C. Reduced insulin secretion: an independent predictor of body weight gain. J Clin Endocrinol Metab. 1995;80:1571–1576. doi: 10.1210/jcem.80.5.7745002. [DOI] [PubMed] [Google Scholar]
- 40.Pawlak DB, Kushner JA, Ludwig DS. Effects of dietary glycaemic index on adiposity, glucose homoeostasis, and plasma lipids in animals. Lancet. 2004;364:778–785. doi: 10.1016/S0140-6736(04)16937-7. [DOI] [PubMed] [Google Scholar]