Abstract
This study surveyed the distribution of tryptophan hydroxylase 2 (TPH2) mRNA, protein, and enzymatic activity throughout the male Sprague-Dawley rat brain. TPH2 is the genetic isoform of TPH that catalyzes the rate-limiting step in serotonin biosynthesis within the central nervous system. Although cell bodies of serotonergic neurons are located mainly in the raphe, serotonin-containing axons innervate many regions of the brain. In the present study, we assessed the levels of mRNA, protein expression, and enzyme activity of TPH2 in the rat raphe, ventral tegmental area (VTA), substantia nigra, hippocampus, cerebellum, dorsal striatum, nucleus accumbens, amygdala, and medial prefrontal cortex to more fully understand the distribution of this enzyme throughout the central nervous system. The pineal gland was used as a control tissue that expresses TPH1 (the peripheral enzyme), but not TPH2. As expected, the raphe showed the highest brain TPH2 activity and protein expression. In the contrast to other reports, however, the VTA followed the raphe as the region with the second-highest amount of TPH2 activity, mRNA and protein expression. There were significantly lower TPH activities and levels of TPH2 protein in the other regions. In addition, TPH2 immunocytochemistry demonstrated the presence of TPH-positive cell bodies within the VTA. The results of this study indicate that TPH2 and serotonergic signaling may play an important role in the mesolimbic/mesocortical reward pathway.
Keywords: Tryptophan hydroxylase 2 (TPH2), enzyme activity, raphe, ventral tegmental area
1. Introduction
Tryptophan hydroxylase (TPH) catalyzes the rate-limiting step in the biosynthesis of serotonin. A unique genetic enzyme isoform, TPH2, has been found to be specifically expressed in the central nervous system [30]. Serotonergic neuronal cell bodies are located primarily in the raphe nucleus [1] and these cells project throughout the brain where serotonin contributes to a variety of different behavioral and physiological effects [9]. Serotonin, while a low abundance neurotransmitter, is pivotal in health and disease. For instance, inhibition of serotonergic reuptake is a primary therapeutic target for treating depression.
The present study was designed to enhance the current understanding of TPH2 distribution and activity in the central nervous system. We assessed the levels of TPH activity in the raphe and compared it to other regions, including the VTA, substantia nigra, hippocampus, cerebellum, dorsal striatum, nucleus accumbens, amygdala and medial prefrontal cortex. Furthermore, using selective RT-PCR and a recently developed TPH2-specific antibody [23], we examined the levels of this particular protein (and its mRNA) within these same brain regions – an undertaking that has not previously been described.
Based on the previously reported presence of serotonergic cells in the VTA [12-13], and preliminary results from our own laboratory, attention was focused on this particular brain region. The VTA has been thought of as a catecholaminergic region that is part of the mesolimbic pathway that connects to the nucleus accumbens through the medial forebrain bundle [27, 31]. While the VTA contains dopaminergic, GABAergic and glutamatergic neurons, neuroanatomical experiments have documented the presence of a high density of serotonin immunoreactive fibers [12-13]. Moreover, serotonergic influences may be important modulators of brain reward mechanisms that reside in this region. For example, serotonin in the VTA has been found to play an important role in cocaine reinforcement [18]. The present studies suggest that the VTA has high levels of TPH2 and is a site of serotonergic neurons.
2. Materials and Methods
2.1. Animals
Fifteen adult (250–300 g), male Sprague–Dawley rats (Charles River Laboratories, Raleigh, NC) were used for this study. They were individually maintained in standard wire mesh cages with controlled temperature, humidity, and ventilation for 10 days. During the habituation and handling periods, rats were maintained on a 12 hour light/dark cycle (lights on at 0700 and lights off at 1900). Food and water were available ad libitum. All procedures involving animals were conducted in accordance with guidelines of the Institutional Animal Care and Use Committee of the Pennsylvania State University, as promulgated by the National Institutes of Health.
2.2. Tissue dissection
Rats were anesthetized with halothane (2ml halothane maintained in a sealed chamber) and then decapitated. The brain was removed from the skull, placed in pre-chilled phosphate buffered saline (PBS) and sectioned in a pre-chilled ASI brain slicer (ASI Instruments, Warren, MI). Brain regions were isolated using the coordinates of the rat brain stereotaxic atlas of Paxinos and Watson as we have previously described [11].
The following regions of the brain were dissected for this study: raphe (R), ventral tegmental area (VTA), substantia nigra (SN), hippocampus (H), cerebellum (C), dorsal striatum (DST), nucleus accumbens (NAc), amygdala (AMG), medial prefrontal cortex (mPFC), and pineal gland (PG). The entire pineal gland was removed with forceps. The raphe was collected from a coronal section (-6.6mm to -9.6mm; according to the coordinates of Paxinos and Watson[20]) and dissected using the ventricle as the dorsal landmark and the medial longitudinal fasciculus as the ventral landmark (including 1mm of tissue on each side of the midline). The VTA and SN were collected from a coronal section (-6.6 to -4.6mm). A horizontal cut was made at the dorsal tip of the substantia nigra and the pigmented nigra was collected leaving the medial aspect containing the VTA. The cerebellum was dissected by a single cut at the base. The dorsal striatum was collected from -2.8 to +2.2 by hand dissection using the external capsule as the landmark. The amygdala was dissected from -3.8 to -1.8 using the ventral tail of external capsule as the lateral landmark and the caudate putamen and internal capsule as ventral boundary. The hippocampus [11], medial prefrontal cortex and nucleus accumbens [10] were dissected as described previously. Following dissection, samples were placed in prechilled tubes, immediately frozen on dry ice, and then stored at −80°C.
2.3. TPH activity assay
All materials for activity assays were obtained from Sigma (St Louis, MO) with the exception of activated charcoal (Darco G-60; Fisher Scientific, Pittsburgh, PA).
TPH activity was assayed using a radio-enzymatic 3H2O release assay as previously described by Reinhard et al. [21] and modified by Vrana et al. [28]. Frozen tissue samples were pulverized in liquid nitrogen and homogenized in ten volumes (w/v) of buffer [25mM HEPES (pH 7), 250mM sucrose, 100μM EDTA, protease inhibitor cocktail, 1mM DTT, 10μM Fe(NH4)2(SO4)2·6H2O, 0.2% Triton-X-100]. Tissue samples (n=8; except for the pineal gland where n=7) were homogenized by sonication on ice (10 sec sonication, 20 sec on ice; repeated 4 times), centrifuged at 13000xg for 20 minutes at 4°C to pellet insoluble material, immediately subjected to protein concentration determination with subsequent enzyme activity assay and western blot analysis.
Activity values were expressed as nmol/h/mg after normalizing to the total protein present in the homogenized brain sample. Protein concentrations were determined by Bradford protein assay (Bio-Rad, Hercules, CA) prior to performance of activity assays and western blot analyses.
2.4. Western blot analysis
The presence of TPH2 in the VTA and in the raphe was determined by western blot analysis (n=8). NuPAGE (4-12% gradient) Bis-Tris gels from Invitrogen (Carlsbad, CA) were used to resolve protein samples at 200 V, 115 Amp for 50 minutes. Proteins were transferred to Immobilon P polyvinylidene fluoride membrane from Millipore Corporation (Billerica, MA) using a semi-dry electro-blot apparatus set at 30 V for 1.5h (Owl Scientific, Cambridge, MA). TPH2 was detected by probing with an anti-mouse TPH2 polyclonal antibody produced in rabbit (dilution: 1:5000; a generous gift from Dr. Donald Kuhn, Wayne State University). Kuhn and colleagues demonstrated that this antibody detects both mouse and rat TPH2 [23], and we confirmed its recognition of the rat enzyme in the present study using a commercially available TPH1/TPH2-specific antibody (data not shown). Mouse pan anti-TPH monoclonal antibody (Sigma, Catalog number: T0678, St Louis, MO) was used for TPH1 detection in the pineal gland (dilution: 1:5000). An HRP-conjugated donkey anti-rabbit (dilution: 1:3000) secondary antibody and an HRP-conjugated anti-mouse secondary antibody (dilution: 1:3000) (GE Healthcare, Lombard, Illinois) were used for detection. A monoclonal anti-β-actin-FITC primary antibody produced in mouse (Sigma, Catalog number: A3853, St Louis, MO) (dilution: 1:3000) and an HRP-conjugated anti-mouse (dilution: 1:3000) secondary antibody (GE Healthcare, Lombard, Illinois) were used to determine β-actin levels. Signals were detected by chemiluminescence (Immobilon Western; Millipore Corporation, Billerica, MA) and visualized following exposure to x-ray film. All film exposures were made in the linear range of response. The x-ray films were scanned and densities were quantified using ImageQuant TL v2005 semi-automated software from Molecular Dynamics Inc. (Sunnyvale, CA).
2.5. Immunocytochemistry
Rat brains (n=3) were dissected, sections containing the regions of interest were retrieved and embedded in OCT compound, and stored at -80°C. Tissues were cryosectioned at 7μm thickness by the Penn State College of Medicine Histology Core Facility. Tissue sections were subjected to two different staining techniques; DAB–horseradish peroxidase and fluorescence staining. For DAB–horseradish peroxidase staining, the endogenous peroxidase activity was quenched by incubating the sections with 1% H2O2 for 10 min and antigenic sites were retrieved by incubating slides with 0.01 M sodium citrate buffer (pH 6.0) for 10 min at 98°C and cooling to room temperature for 20 min. Nonspecific binding was blocked with 1% BSA in PBS. Sections were then covered with the rabbit-derived anti-mouse TPH2 specific polyclonal antibody (dilution: 1:1000 in 1% BSA in PBS as recommended by D.Kuhn) and incubated overnight at 4°C. Sections were then incubated with biotinylated anti-rabbit IgG secondary antibody (dilution: 1:200) for 30 min at RT. Following secondary antibody binding, sections were incubated with streptavidin-tagged peroxidase. TPH2 immunoreactivity was visualized following exposure to substrate-chromogen mixture (3,3-diaminobenzidine) for 2-5 min. Sections were counterstained with Meyer’s hematoxylin solution for 30 sec. Negative controls were performed by replacing the primary antibody with 1% BSA in PBS. Immunofluorescence staining was performed in a similar fashion except there was antigen retrieval without quenching. Antibody-antigen complexes were visualized using a fluorescent-conjugated secondary antibody (Alexa 488) and fluorescent microscope (Nikon EP600).
2.6. QRT-PCR analysis of gene expression
Total RNA was isolated from dissected brain samples (n=4 rats) as described previously [3] using TriReagent (Molecular Research Center Inc., Cincinnati, OH). RNA quantity and quality were assessed using the Agilent 2100 Bioanalyzer with the RNA 6000 Nano Assay (Agilent, Palo Alto, CA). cDNA synthesis was performed as previously described [16] on total RNA using Superscript III Reverse Transcriptase (Invitrogen, Carlsbad, CA). qPCR was performed on the 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA) in 384-well optical plates with Assay-On- Demand (Applied Biosystems) rat TPH2 specific primers. Relative quantities were calculated using ABI SDS 2.2.2 RQ software and the 2-ΔΔCt analysis method [3, 17] with GAPDH as the endogenous control. Relative quantity values were normalized with one of the raphe samples from control rats to aid in comparison across regions with varying basal abundance. These studies were conducted in the PSU Functional Genomics Core Facility.
2.7. Statistical analysis
All results are presented as mean ± SEM. The TPH activity determinations were analyzed using GraphPad Prism5 one-way ANOVA followed by a Newman-Keuls post-hoc test. Statistical significance was associated with values of p<0.05*, p<0.01**, p<0.001***.
3. Results
TPH activity was measured in nine different regions of the brain (presumably the activity of the brain specific TPH2 isoform) and the pineal gland (presumably TPH1) of eight male Sprague-Dawley rats (Fig. 1-A). We observed that tryptophan hydroxylase activity was highest in the raphe nucleus (0.67 nmol/hr/mg), followed by the ventral tegmental area (0.24 nmol/hr/mg). Significantly lower activities were observed in hippocampus (0.09 nmol/hr/mg), cerebellum (0.06 nmol/hr/mg), dorsal striatum (0.09 nmol/hr/mg), nucleus accumbens (0.14 nmol/hr/mg), amygdala (0.14 nmol/hr/mg), substantia nigra (0.13 nmol/hr/mg), and medial prefrontal cortex (0.13 nmol/hr/mg). Activity of TPH1 in pineal gland tissue (1.07 nmol/hr/mg) was higher than TPH2 activity in the raphe nucleus (0.67 nmol/hr/mg) (Fig. 1-A).
Figure 1.
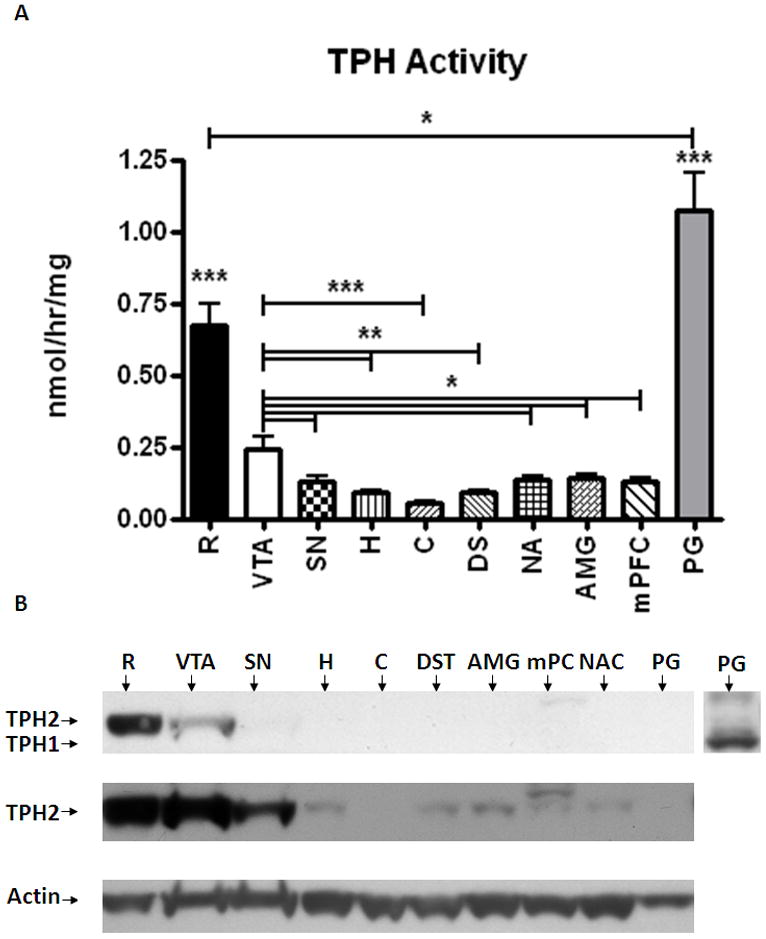
A- Relative TPH2 activity was measured with a radio-enzymatic assay in brain homogenates from raphe (R), ventral tegmental area (VTA), substantia nigra (SN), hippocampus (H), cerebellum (C), dorsal striatum (DST), amygdala (AMG), medial prefrontal cortex (mPFC), and nucleus accumbens (NAc). The pineal gland (PG) served as negative control, with no TPH2. Activity levels were normalized to the total protein levels in the homogenates. Data expressed as mean activity/total protein ±SEM. GraphPad Prism5 was used for one-way ANOVA with a Newman-Keuls post-hoc test. Statistical significance * p<0.05, ** p<0.01, *** p<0.001, n=8.
B- Determination of TPH2 expression levels in different brain regions used for the TPH activity assay. An aliquot of total protein (20μg) was loaded into each lane. The inset at the right represents the same pineal gland portion of the blot probed with an antibody that detects both TPH1 (peripheral) and TPH2 (central) isoforms of the enzyme. Top panel; TPH2-specific rabbit anti-TPH2 antibody (dilution: 1:5000). Middle panel; longer exposure of the blot presented above. Lower panel; β-actin bands were used as loading control to normalize TPH2 expression to protein levels.
Western blots were performed to correlate TPH activity with TPH2 immunoreactive protein. As with the TPH activity, TPH2 protein was found predominantly in the raphe and the VTA (Fig. 1-B; top panel); however, we were able detect TPH2 protein in other regions following prolonged exposures (Fig. 1-B middle panel). There was no TPH1 detected in the brain samples (data not shown). Following the quantification of western blot signals (and normalization to the actin control signals), the VTA displayed 60% less TPH2 immunoreactivity than the raphe [t(12)=2.4; P=0.033; Fig. 2]. QRT-PCR quantification agreed with the western blot analysis (i.e., TPH2 mRNA was highest in the raphe followed by VTA), albeit at greater magnitudes of expression. Specifically, the raphe expressed 8.5-fold more transcript than the VTA [t(5)=3.37, P=0.02] and 17-fold more message than the substantia nigra [t(5)=3.62, P=0.015] (Fig. 3). While very small levels of TPH2 mRNA were detected in the other regions, they were near the limits of detection and were orders of magnitude less than the midbrain nuclei (R, VTA, SN). In addition, levels of TPH2 mRNA were not different between VTA and SN.
Figure 2.

Relative TPH2 protein levels in raphe and VTA tissue homogenates were quantified by measuring the optical density of the TPH2 bands detected by western blot using TPH2-specific rabbit anti-TPH2 antibody (dilution: 1:5000) and normalized to the actin intensity. Results were analyzed by using GraphPad Prism5 one-way ANOVA with Newman-Keuls post-hoc test. The error bars represent standard error of the mean. Statistical significance * p<0.05, ** p<0.01, *** p<0.001, n=8.
Figure 3.
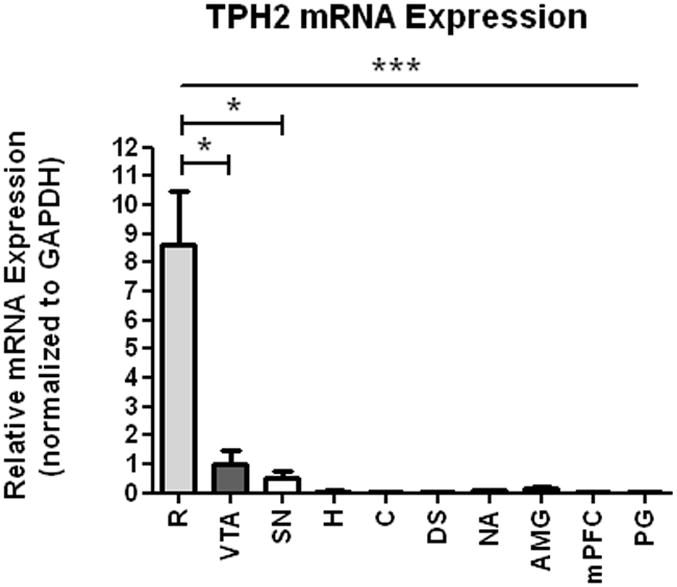
TPH2 mRNA expression in brain tissue from raphe (R), ventral tegmental area (VTA), substantia nigra (SN), hippocampus (H), cerebellum (C), dorsal striatum (DST), amygdala (AMG), medial prefrontal cortex (mPFC), nucleus accumbens (NAc), and pineal gland (PG). TPH2 mRNA expression levels were normalized to the expression levels of GAPDH in the samples. Results were analyzed using GraphPad Prism5 one-way ANOVA with Newman-Keuls post-hoc test. The error bars represent standard error of the mean. Statistical significance * p<0.05, ** p<0.01, *** p<0.001, n=2-4.
Based on the presence of TPH2 mRNA, the presence of TPH2-positive cells in the VTA was confirmed by immunocytochemistry. We found that the rat VTA region, but not the interfascicular (IF) nucleus or interpeduncular nucleus (IP), contained TPH2-positive cell bodies (Fig. 4). Cell bodies of TPH2-positive neurons were also found in the medial longitudinal fasciculus (MLF), which may represent neurons that are an extension of the dorsal raphe nucleus [2, 25].
Figure 4.
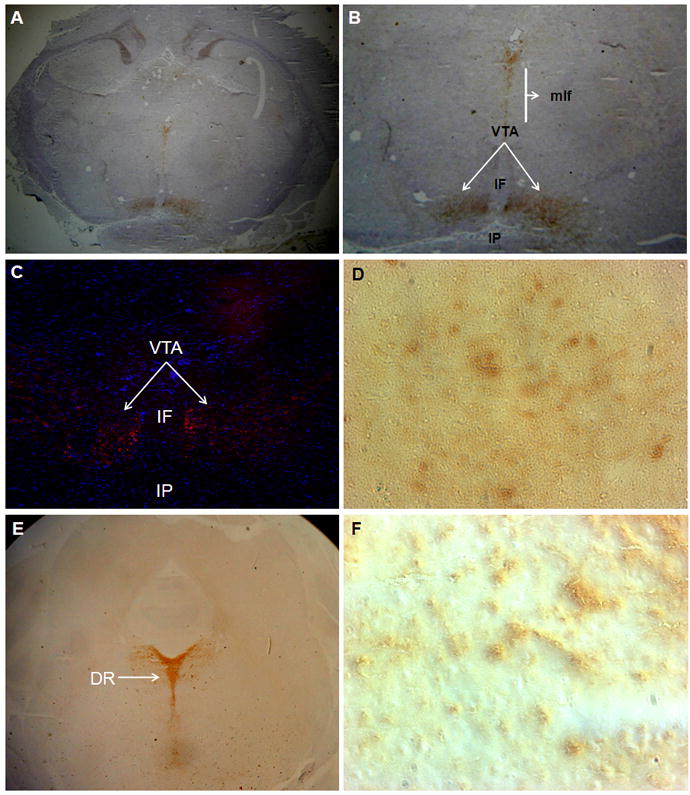
Immunocytochemistry assessment of TPH2 expression in the VTA region using rabbit anti-TPH2 specific antibody and HRP- (A,B,D,E and F) and immunofluorescent-conjugated secondary antibodies (C). A- TPH2 positive neuronal bodies are located within the VTA region (areas indicated by arrows), but not the surrounding areas such as the interfascicular nucleus (IF) and interpeduncular (IP) nucleus. In addition to the VTA, TPH2-positive neurons were found in the medial longitudinal fasciculus (MLF). B-Enlarged view of the VTA region. C- Immunofluorescent detection of TPH2 positive neurons (arrows) in the VTA region from an independent section using the same antibody. D- Enlarged view of the VTA region displays TPH2–positive neuronal bodies in the VTA. E- TPH2 positive neuronal bodies are located within the dorsal raphe (DR) region (areas indicated by arrows). F- Enlarged view of DR region displays TPH2–positive neuronal bodies in the DR.
4. Discussion
The aim of the present study was to investigate the enzymatic activities of TPH (1 and 2) and to assess TPH2 expression and localization in different brain regions of the adult male Sprague-Dawley rat. TPH2 is the rate-limiting enzyme in serotonin synthesis and hence serves as a molecular marker for serotonergic neurons. In general, serotonergic neurons have been thought to originate in the raphe nucleus and to project broadly and diffusely throughout the brain. In the present study, we confirmed the broad dissemination of TPH2, but also identified additional TPH2 neuronal cell body localization to the VTA.
This work demonstrates that enzymatic activity of TPH2 in the VTA was significantly higher than any other brain region we examined in the male rat, except the raphe nucleus. Immunocytochemistry findings confirmed the localization of TPH2 cell bodies within the VTA region. These results were supported by real time PCR findings that showed TPH2 mRNA levels in the VTA at 12% of the raphe nucleus levels (Fig. 3). Indeed, with the exception of the raphe and VTA, very low levels of TPH2 mRNA expression were detected throughout the brain. This may represent previously documented evidence of mRNA in terminal fields (for the related gene, tyrosine hydroxylase) [19]. It is important to note that, as these studies were confined to use of male subjects, the possibility of gender-based differences remains a topic for future studies.
Previous studies of Deguchi and co-workers investigated TPH activity in the rat brain and compared the raphe nucleus with the rest of the brain stem [6]. They reported that the raphe nucleus contained seven-fold higher TPH activity compared to the rest of the brain stem, and four-fold higher activity than the hypothalamus; a finding in contrast to their previous publications reporting higher activity in the hypothalamus [4-5]. Our findings show that VTA followed the raphe as the region with the second highest amount of TPH2 activity. Moreover, the immunoblot data on TPH2-specific protein levels support these activity findings (Fig. 1-B). Similarly, Koubi and coworkers [15] reported that the raphe nucleus expresses the highest levels of TPH activity (presumably TPH2), with lower but comparable levels in the VTA, hippocampus, frontal cortex and substantia nigra; a finding consistent with our own. In the present study, there are significant levels of TPH activity in the SN (Fig. 1-A), and lower, but detectable, levels of TPH2 protein (Fig. 1-B, middle panel). Indeed, there is TPH2 mRNA in this region (Fig. 3).
The observations of TPH2-positive neurons in the VTA may provide an area of further research involving interactions between serotonergic and dopaminergic systems within the VTA. Specially, this contributes to the growing understanding of the mesolimbic/mesocortical reward pathway and its role in addictive behaviors. There is evidence that the serotonin system in the VTA plays an inhibitory role on the mesolimbic dopaminergic system [7, 18]. Roberts and colleagues [22] showed that the reinforcing efficacy of cocaine can be improved by partial depletion of brain serotonin. Moreover, modulation of serotonin receptor activity will alter both cocaine and MDMA (ecstasy) behavioral effects [8, 14].
The presence of somata of neurons containing serotonin, within the VTA, could be an important biological factor for understanding the role of the VTA in depression [24] and addiction [26]. Koubi et al. [15] specifically characterized the VTA in animal models for the treatment of depression. In the rat, electroconvulsive shock (used to treat severe depression in humans) decreased TPH levels in the VTA within 72 hours of the treatment, whereas it increased TPH levels in hippocampus and frontal cortex. In addition, modulation of the serotonergic system in the VTA is a target of cocaine’s actions, as the serotonin transporter plays a very important role in the VTA for reward processes [18]. In this regard, we have previously reported a cocaine-mediated decrease in raphe TPH activity [29]. Recent preliminary findings from our laboratory suggest that VTA TPH activity significantly decreases following abstinence from heroin self-administration, while the TPH activity immediately following a period of self-administration remains unchanged (data not shown), suggesting that TPH2 and serotonin may play a role in drug abuse relapse behavior. Therefore, activity of serotonergic cells resident within the VTA, combined with serotonin inputs to the VTA, may play a role in several neuropsychiatric disorders. Characterizing the complexities of TPH2 expression within this brain region will contribute to enhanced understanding of behavioral phenomena controlled by the activity of neurons within the VTA.
Acknowledgments
This work was supported by United States Public Health Service Grant GM38931, DA13770 (to KEV), and DA021450 (to KLK). Dr. Donald Kuhn (Wayne State University) generously provided TPH2 specific antibodies. The authors thank Ms. Lauren Feinberg, Dr. Heather VanGuilder and Dr. John Hegarty for valuable editorial input.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Baker KG, Halliday GM, Hornung JP, Geffen LB, Cotton RG, Tork I. Distribution, morphology and number of monoamine-synthesizing and substance P-containing neurons in the human dorsal raphe nucleus. Neuroscience. 1991;42:757–775. doi: 10.1016/0306-4522(91)90043-n. [DOI] [PubMed] [Google Scholar]
- 2.Bendotti C, Servadio A, Samanin R. Distribution of GAP-43 mRNA in the brain stem of adult rats as evidenced by in situ hybridization: localization within monoaminergic neurons. J Neurosci. 1991;11:600–607. doi: 10.1523/JNEUROSCI.11-03-00600.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowyer JF, Pogge AR, Delongchamp RR, O’Callaghan JP, Patel KM, Vrana KE, Freeman WM. A threshold neurotoxic amphetamine exposure inhibits parietal cortex expression of synaptic plasticity-related genes. Neuroscience. 2007;144:66–76. doi: 10.1016/j.neuroscience.2006.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deguchi T, Barchas J. Effect of p-chlorophenylalanine on hydroxylation of tryptophan in pineal and brain of rats. Mol Pharmacol. 1972;8:770–779. [PubMed] [Google Scholar]
- 5.Deguchi T, Barchas J. Regional distribution and developmental change of tryptophan hydroxylase activity in rat brain. J Neurochem. 1972;19:927–929. doi: 10.1111/j.1471-4159.1972.tb01412.x. [DOI] [PubMed] [Google Scholar]
- 6.Deguchi T, Sinha AK, Barchas JD. Biosynthesis of serotonin in Raphe nuclei of rat brain: effect of p-chlorophenylalanine. J Neurochem. 1973;20:1329–1336. doi: 10.1111/j.1471-4159.1973.tb00244.x. [DOI] [PubMed] [Google Scholar]
- 7.Di Matteo V, Di Giovanni G, Di Mascio M, Esposito E. SB 242084, a selective serotonin2C receptor antagonist, increases dopaminergic transmission in the mesolimbic system. Neuropharmacology. 1999;38:1195–1205. doi: 10.1016/s0028-3908(99)00047-7. [DOI] [PubMed] [Google Scholar]
- 8.Fantegrossi WE, Ullrich T, Rice KC, Woods JH, Winger G. 3,4-Methylenedioxymethamphetamine (MDMA, “ecstasy”) and its stereoisomers as reinforcers in rhesus monkeys: serotonergic involvement. Psychopharmacology (Berl) 2002;161:356–364. doi: 10.1007/s00213-002-1021-6. [DOI] [PubMed] [Google Scholar]
- 9.Fox MA, Jensen CL, French HT, Stein AR, Huang SJ, Tolliver TJ, Murphy DL. Neurochemical, behavioral, and physiological effects of pharmacologically enhanced serotonin levels in serotonin transporter (SERT)-deficient mice. Psychopharmacology (Berl) 2008;201:203–218. doi: 10.1007/s00213-008-1268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freeman WM, Patel KM, Brucklacher RM, Lull ME, Erwin M, Morgan D, Roberts DC, Vrana KE. Persistent alterations in mesolimbic gene expression with abstinence from cocaine self-administration. Neuropsychopharmacology. 2008;33:1807–1817. doi: 10.1038/sj.npp.1301577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freeman WM, Vanguilder HD, Bennett C, Sonntag WE. Cognitive performance and age-related changes in the hippocampal proteome. Neuroscience. 2009;159:183–195. doi: 10.1016/j.neuroscience.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halliday GM, Tork I. Serotonin-like immunoreactive cells and fibres in the rat ventromedial mesencephalic tegmentum. Brain Res Bull. 1989;22:725–735. doi: 10.1016/0361-9230(89)90092-0. [DOI] [PubMed] [Google Scholar]
- 13.Herve D, Pickel VM, Joh TH, Beaudet A. Serotonin axon terminals in the ventral tegmental area of the rat: fine structure and synaptic input to dopaminergic neurons. Brain Res. 1987;435:71–83. doi: 10.1016/0006-8993(87)91588-5. [DOI] [PubMed] [Google Scholar]
- 14.Howell LL, Byrd LD. Serotonergic modulation of the behavioral effects of cocaine in the squirrel monkey. J Pharmacol Exp Ther. 1995;275:1551–1559. [PubMed] [Google Scholar]
- 15.Koubi D, Bezin L, Cottet-Emard JM, Gharib A, Bobillier P, Sarda N. Regulation of expression and enzymatic activities of tyrosine and tryptophan hydroxylases in rat brain after acute electroconvulsive shock. Brain Res. 2001;905:161–170. doi: 10.1016/s0006-8993(01)02524-0. [DOI] [PubMed] [Google Scholar]
- 16.Kuntz KL, Patel KM, Grigson PS, Freeman WM, Vrana KE. Heroin self-administration: II. CNS gene expression following withdrawal and cue-induced drug-seeking behavior. Pharmacol Biochem Behav. 2008;90:349–356. doi: 10.1016/j.pbb.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔΔC(T) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Mateo Y, Budygin EA, John CE, Jones SR. Role of serotonin in cocaine effects in mice with reduced dopamine transporter function. Proc Natl Acad Sci U S A. 2004;101:372–377. doi: 10.1073/pnas.0207805101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melia KR, Trembleau A, Oddi R, Sanna PP, Bloom FE. Detection and regulation of tyrosine hydroxylase mRNA in catecholaminergic terminal fields: possible axonal compartmentalization. Exp Neurol. 1994;130:394–406. doi: 10.1006/exnr.1994.1219. [DOI] [PubMed] [Google Scholar]
- 20.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1997. [Google Scholar]
- 21.Reinhard JF, Jr, Smith GK, Nichol CA. A rapid and sensitive assay for tyrosine-3-monooxygenase based upon the release of 3H2O and adsorption of [3H]-tyrosine by charcoal. Life Sci. 1986;39:2185–2189. doi: 10.1016/0024-3205(86)90395-4. [DOI] [PubMed] [Google Scholar]
- 22.Roberts DC, Loh EA, Baker GB, Vickers G. Lesions of central serotonin systems affect responding on a progressive ratio schedule reinforced either by intravenous cocaine or by food. Pharmacol Biochem Behav. 1994;49:177–182. doi: 10.1016/0091-3057(94)90473-1. [DOI] [PubMed] [Google Scholar]
- 23.Sakowski SA, Geddes TJ, Thomas DM, Levi E, Hatfield JS, Kuhn DM. Differential tissue distribution of tryptophan hydroxylase isoforms 1 and 2 as revealed with monospecific antibodies. Brain Res. 2006;1085:11–18. doi: 10.1016/j.brainres.2006.02.047. [DOI] [PubMed] [Google Scholar]
- 24.Schafer WR. How do antidepressants work? Prospects for genetic analysis of drug mechanisms. Cell. 1999;98:551–554. doi: 10.1016/s0092-8674(00)80042-2. [DOI] [PubMed] [Google Scholar]
- 25.Siegel G, Albers RW, Brady S, Price D. Basic Neurochemistry, Molecular, Cellular and Medical Aspects. 20006 [Google Scholar]
- 26.Sue D SDW, Sue S. Understanding abnormal behavior. Eight. Houghton Mifflin Company; Boston New York: 2006. [Google Scholar]
- 27.Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- 28.Vrana SL, Dworkin SI, Vrana KE. Radioenzymatic assay for tryptophan hydroxylase: [3H]H2O release assessed by charcoal adsorption. J Neurosci Methods. 1993;48:123–129. doi: 10.1016/s0165-0270(05)80014-7. [DOI] [PubMed] [Google Scholar]
- 29.Vrana SL, Vrana KE, Koves TR, Smith JE, Dworkin SI. Chronic cocaine administration increases CNS tyrosine hydroxylase enzyme activity and mRNA levels and tryptophan hydroxylase enzyme activity levels. J Neurochem. 1993;61:2262–2268. doi: 10.1111/j.1471-4159.1993.tb07468.x. [DOI] [PubMed] [Google Scholar]
- 30.Walther DJ, Peter JU, Bashammakh S, Hortnagl H, Voits M, Fink H, Bader M. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299:76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- 31.Yamaguchi T, Sheen W, Morales M. Glutamatergic neurons are present in the rat ventral tegmental area. Eur J Neurosci. 2007;25:106–118. doi: 10.1111/j.1460-9568.2006.05263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


