Abstract
Remifentanil is a relatively new opioid analgesic related to the fentanyl family of mu opioid receptor agonists and is used clinically for its unique property of having an ultra-short duration of action. However, there is little preclinical data on the analgesic (antinociceptive) effects of remifentanil and none obtained in non-mammalian animal models. The antinociceptive effects of remifentanil were assessed by using the acetic acid test in amphibians. Systemic and spinal administration of remifentanil was made by subcutaneous and intraspinal injections in the Northern grass frog, Rana pipiens. After administration, remifentanil produced dose-dependent and long-lasting antinociceptive effects which persisted for five hours after systemic administration but gave a shorter duration of action after spinal delivery. The antinociceptive effects of remifentanil were significantly blocked by pretreatment with systemic naltrexone. Systemic and spinal administration of remifentanil produced log dose–response curves which yielded ED50 values of 7.1 nmol/g and 3.2 nmol/animal respectively. The relative antinociceptive potency of remifentanil compared to other opioids administered to amphibians is similar to that found in mammalian models.
Keywords: Remifentanil, Analgesia, Opioid, Mu opioid receptor, Rana pipiens
1. Introduction
Remifentanil is a unique mu opioid analgesic with an ultra-short duration of action due to its inactivation by non-specific esterases in plasma and tissues (Michelsen and Hug, 1996). Marketed in Europe and in the United States as Ultiva®, remifentanil is used broadly in clinical anesthesiology; employed in obstetrics, pediatrics, cardiology and for a variety of surgical and outpatient procedures (Beers and Camporesi, 2004; Cohen and Royston, 2001; Van de Velde, 2005). Remifentanil is a potent opioid analgesic, as much as 60-times more potent than alfentanil but with an elimination half-life of about 10 min in humans (Dershwitz et al., 1995; Hoke et al., 1997).
Surprisingly, there is little data on the analgesic or antinociceptive effects of remifentanil in nonhuman animals. Early studies established the mu opioid receptor selectivity of remifentanil in tissue assays and the relative analgesic potency of systemically administered remifentanil in rodents (James et al., 1991). These studies showed that remifentanil was about equipotent to fentanyl and more potent than sufentanil or alfentanil. The analgesic potency of remifentanil and its short duration of action were confirmed after central administration by the spinal route using intrathecal catheters in rats (Buerkle and Yaksh, 1996). Remifentanil also inhibited nociceptive behavior following the hindpaw injection of formalin in rodents (Buerkle et al., 1998; Taylor et al., 1997) and the antinociceptive effects of remifentanil were studied in a rabbit model of anesthesia and analgesia (Hayashida et al., 2003a,b).
As part of an overall goal of testing novel mu opioid analgesics for use in a spinal opioid tolerance model in amphibians, we evaluated the nociceptive effects of systemic and spinal remifentanil in the Northern grass frog, Rana pipiens. This amphibian model is a well-developed alternative or adjunct model for the testing of opioid analgesics using non-mammalian vertebrates (Stevens, 1992, 2004). The algesiometric assay used in amphibians is called the acetic acid test and consists of monitoring the response of the animal to drops of log-spaced concentrations of acetic acid placed on the thigh of the unanesthetized animal. The nociceptive threshold obtained using the acetic acid test is robust and stable over a period of hours and days, and the wiping response used in the behavioral assay has not been observed in the absence of noxious stimuli. Thus, the acetic acid test appears to be specific for assessing a brief noxious event (Pezalla, 1983; Stevens et al., 1994). Previous work showed that systemic, supraspinal, and spinal administration of a series of opioid analgesics to amphibians produced antinociceptive effects on the acetic acid test with a rank order of relative potency correlated to that found in mammalian species and in humans (Stevens et al., 1994; Stevens, 1996; Stevens and Rothe, 1997). Given the lack of information on remifentanil’s action in non-mammalian vertebrates and the ongoing efforts to develop the amphibian model, the present study sought to obtain data on the time course, naltrexone antagonism, and dose–response characteristics of the antinociceptive effects of remifentanil after systemic and spinal administration in R. pipiens.
2. Materials and methods
All procedures were approved by the Institutional Animal Use Committee (IACUC) and adhere to the National Institutes of Health (U.S.A.) and the European Community guidelines on the use of animals.
2.1. Animals, drugs, and experimental procedures
Northern grass frogs, R. pipiens (mean weight, 28.0 g), were obtained from a commercial supplier (Sullivan Co., Inc, Nashville, TN, USA). Frogs were held in flow-through, stainless-steel aquaria in groups of 48 immediately after arrival and were fed live crickets 3 times a week.
Drugs used were remifentanil hydrochloride (3-[4-meth-oxycarbonyl-4-[(1-oxopropyl) phenylamino]-1-piperidine] propanoic acid methyl ester, Abbott Laboratories, North Chicago, IL, USA) and naltrexone hydrochloride (Sigma Chemical Co., St. Louis, MO. USA). Drugs were dissolved in sterile 0.9% saline to yield nanomole/gram body weight doses of the free base. Remifentanil was administered at doses of 1, 3, 10, and 30 nmol/g by the systemic route and given by intraspinal injection at doses of 3, 10, 30, 100 and 300 nmol/frog. Systemic administration was made by bolus injection into the dorsal lymph sac at a volume of 10 μl/g body weight (Stevens et al., 1994). Spinal administration was done using a Hamilton microsyringe and was made between the articulation of the lumbar vertebrate in a volume of 5 μl (Stevens, 1996). Saline-injected control animals were co-run with remifentanil and naltrexone antagonist experiments, and there were no significant changes from baseline values (data not shown). For opioid antagonism experiments, systemic naltrexone (100 nmol/g) or saline was administered 1 h before the systemic or spinal administration of remifentanil.
2.2. The acetic aid test and data analysis
At least two days before their use in any experiment, the frogs were transferred into the laboratory and placed in individual plastic cages with mesh tops and sufficient water. On experiment days, water was lowered to expose the dorsum of the thigh for acetic acid testing and 1 h later the baseline nociceptive threshold was obtained. The acetic acid test was used to assess the nociceptive threshold in frogs as fully described elsewhere (Pezalla, 1983). Briefly, 10 solutions of acetic acid were serially diluted (1:2, water:acid) from glacial acetic acid (17.5 M) to give log-spaced dilutions and labeled with the lowest number being equal to the lowest concentration of acetic acid. The nociceptive threshold was determined by placing a drop of acetic acid on the dorsal surface of the frog’s thigh. Testing began with the lowest concentration of acid and proceeded stepwise on alternative hind limbs until the nociceptive threshold was reached. The nociceptive threshold was defined as the code number of the lowest concentration of acid, which caused the frog to behave and wipe the treated leg with either hind limb. To prevent tissue damage, the drop of acetic acid was washed off immediately with a gentle stream of water once the animal responded or after 5 s if the animal failed to respond. In some instances, an nociceptive threshold of 11 (cutoff value) was recorded; this was only the case if the frog failed to respond to the highest concentration of acetic acid (10, glacial acetic acid).
To generate a time–response curve, baseline nociceptive threshold was obtained 1 h before and at post-treatment times of 15, 30, 60, 180 and 300 min after remifentanil administration. Peak maximum percent effect (MPE) values of individual animals from the time–response data were pooled for each dose to construct the dose–response curves. MPE was obtained from the raw nociceptive threshold (NT) values by the formula below:
MPE data were entered into the PHARM/PCS (v.4) program to calculate the dose producing a 50% effect (ED50) and the 95% confidence intervals. A Student’s t-test was used to assess the effects of naltrexone pretreatment vs. saline pretreatment on remifentanil administration. Treatment groups consisted of 6–12 animals and animals were used only once.
3. Results
At the doses reported, remifentanil did not produce any signs of adverse effects following systemic or spinal administration. Animals exhibited normal righting and corneal reflexes and remifentanil at times of testing and animals had NT values that returned to baseline levels 24 h after administration of remifentanil (data not shown).
3.1. Time course of the antinociceptive effects of systemic and spinal remifentanil
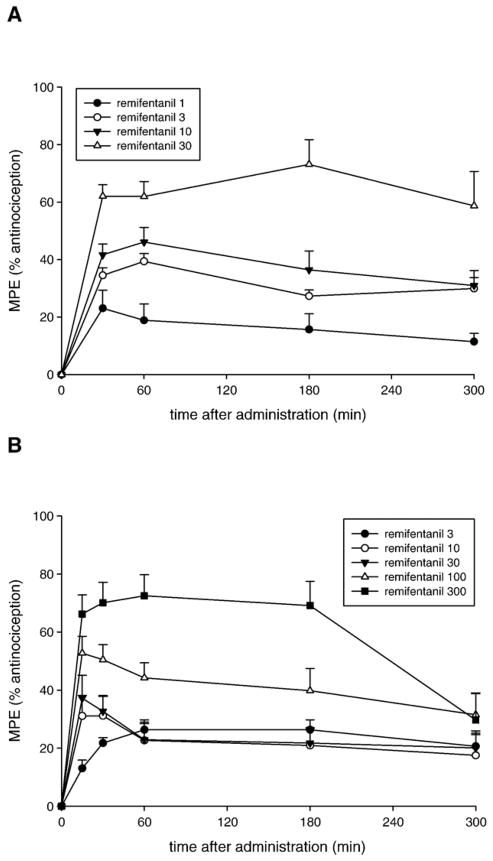
Fig. 1 shows the time course of the antinociceptive effects of remifentanil after systemic (A) and spinal administration (B). A dose-dependent increase in antinociceptive effects (MPE) of systemic remifentanil was observed at 30 min after 1, 3, 10, and 30 nmol/g remifentanil. The antinociceptive effects of these four doses of remifentanil remained constant over the 300 min (5 h) time course of the experiment. Following spinal administration of remifentanil, dose-dependent antinociceptive effects were noted at the first post-treatment test point (15 min). However, remifentanil at the highest doses of 100 or 300 nmol/frog showed a decrement of antinociceptive effects by 300 min after spinal administration.
Fig. 1.
Time course of the antinociceptive effect of remifentanil following systemic (A) or spinal (B) administration in amphibians. Doses used are given in boxes on the plots in nmol/g for systemic and nmol/frog for spinal administration. Data points are plotted as MPE+S.E.M. for N=6–12 animals per dose.
3.2. Effect of naltrexone pretreatment on the antinociceptive effects of remifentanil
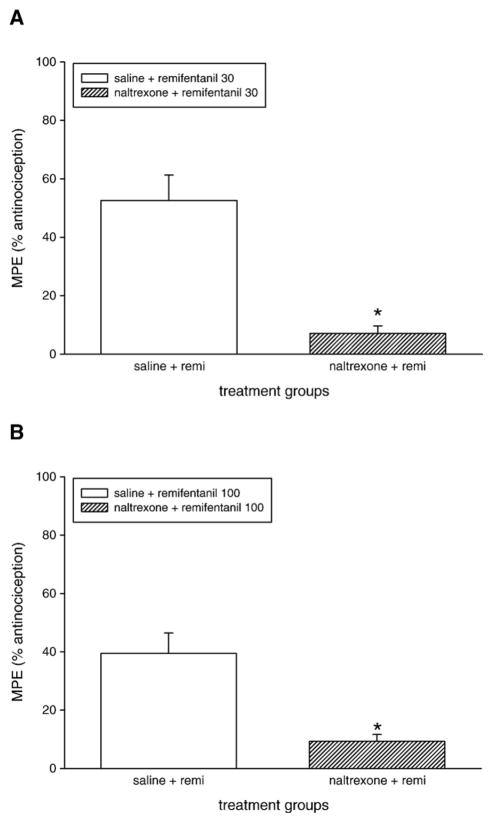
The opioid receptor antagonist naltrexone (100 nmol/g, s.c.) administered 1 h before the systemic administration of remifentanil (30 nmol/g) resulted in a significant reduction of the antinociceptive effect as shown by comparison of the saline-pretreated group and the naltrexone pretreated animals (Fig. 2A). The antinociception produced by spinal administration of remifentanil (100 nmol/frog) was also blocked by naltrexone pretreatment, as shown in Fig 2B.
Fig. 2.
Effect of naltrexone pretreatment on the antinociceptive effect of remifentanil following systemic (A) or spinal (B) administration in amphibians. Systemic administration of saline (filled bars) or naltrexone (100 nmol/g s.c., hatched bars) was given 60 min before systemic or spinal administration of remifentanil (doses given on plot). Data are plotted as the mean MPE+S.E.M. for six animals per treatment group. Asterisks (*) denote significant difference from saline-pretreated groups (P<0.05, Student’s t-test).
3.3. Relative potency of remifentanil in amphibians
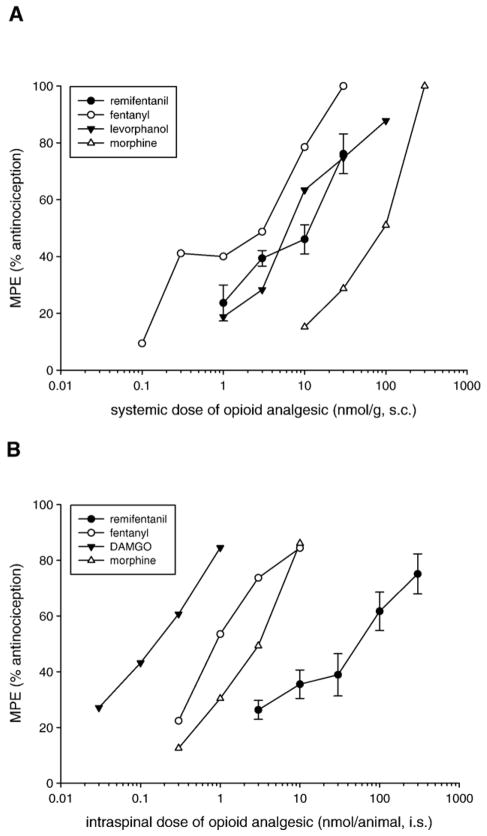
Log dose–response curves of the antinociceptive effect of remifentanil following systemic and spinal administration in amphibians are shown in Fig. 3. Systemic remifentanil gave an ED50 of 7.07 nmol/g (Fig. 3A), similar to that of levorphanol and fentanyl and more potent than morphine (from previous studies, Stevens et al., 1994). Spinal administration of remifentanil yielded an ED50 value of 3.21 nmol/frog (Fig. 3B), which was less potent than the mu opioid receptor agonists fentanyl, DAMGO ([D-Ala2,NMePhe4,Gly-ol]-enkephalin), and morphine (from previous studies, Stevens, 1996). As shown in Table 1, remifentanil was significantly more potent (12.2 times) than morphine, slightly less potent than fentanyl and equipotent to levorphanol after systemic administration. Following spinal administration, remifentanil was nearly equipotent to morphine and fentanyl, but significantly less potent than DAMGO.
Fig. 3.
Relative antinociceptive potency of remifentanil following systemic (A) or spinal (B) administration in amphibians. Data points were plotted from the maximum peak effect during the 5-h time course as the mean peak effect (MPE +S.E.M.) of individual animals grouped for each dose. N=6–12 animals per dose. Data from previous studies was not plotted with error bars for clarity (from Stevens et al., 1994; Stevens, 1996). ED50 values, 95% confidence intervals, and relative potency of remifentanil and other opioids compared to morphine are given in Table 1.
Table 1.
Antinociceptive potency of remifentanil after systemic and spinal administration in amphibians
| Agent | Systemic administration |
R.P.b | Spinal administration |
R.P. | ||
|---|---|---|---|---|---|---|
| ED50 (nmol/g) | (95% C.I.)a | ED50 (nmol/frog) | (95% C.I.) | |||
| Remifentanil | 7.1 | (4.7–10.7) | 12.2 | 3.2 | (1.2–9.0) | 0.7 |
| Morphine | 86.3 | (64.7–115) | 1.0 | 2.3 | (1.7–3.0) | 1.0 |
| Fentanyl | 1.4 | (0.9–2.3) | 61.6 | 0.9 | (0.5–1.9) | 2.4 |
| Levorphanol | 7.5 | (5.1–11.1) | 11.5 | – | ||
| DAMGOc | –d | 0.1 | (0.1–0.8) | 17.4 | ||
95% confidence interval, ED50 values with non-overlapping intervals are statistically different at P < 0.05;
relative potency compared to morphine (ED50 morphine/ED50 agent);
[D-Ala2,NMePhe4,Gly-ol]-enkephalin;
— (not tested). Morphine, fentanyl, levorphanol, DAMGO data from Stevens et al. (1994) and Stevens (1996).
4. Discussion
The present results demonstrate that remifentanil produces a potent and dose-dependent antinociceptive effect after systemic and spinal administration in an alternative pain research model using amphibians.
4.1. Time course of remifentanil antinociception in amphibians
Following systemic administration into the dorsal lymph sac, remifentanil produced a dose-dependent antinociceptive effect that was apparent at the time of the first post-treatment algesiometric test (Fig. 1A). The antinociceptive effect was persistent for at least 5 h following administration of the three highest doses of remifentanil, with animals returning to normal baseline thresholds the next day. In mammalian studies, systemic administration of remifentanil by intraperitoneal injection in rodents gave a short duration of action, in the range of 10–15 min (Buerkle and Yaksh, 1996). However, systemic administration of all opioid receptor agonists in amphibians produces a surprisingly long duration of action, which may be due to the depot-like injection into the dorsal lymph sac with slow release of analgesic into the systemic circulation, low body temperature and sluggish circulation, and decreased metabolic activity as discussed earlier (Stevens et al., 1994). There is no evidence of rapid metabolism of remifentanil following systemic administration in amphibians, although the pharmacokinetic differences just mentioned above may mask such an effect.
Following spinal administration of remifentanil in amphibians, peak antinociceptive effects are noted at 15 min after intraspinal administration for the four highest doses of remifentanil, with the lowest dose showing a slightly longer time to peak effect (Fig. 1B). However, the last time point of testing (5 h after administration) shows that three highest doses of remifentanil have decreased to 30–40% MPE. This is in contrast to previous studies of a number of mu, delta, and kappa-selective opioid receptor agonists administered via the spinal route in amphibians whereby all agents and doses maintained significant antinociceptive effects and flat effect curves throughout the time course of testing (Stevens, 1996). This may be evidence of a more rapid metabolism of remifentanil than other opioid analgesics after spinal administration in amphibians. The amphibian CNS and peripheral tissues are replete with esterase enzymes (Contestabile, 1976; Gabriel and Budai, 1992; Hardwick and Hebb, 1956) as is the case for all vertebrate animals tested (Chuiko et al., 2003; Fossi et al., 1992; Sanchez et al., 1997).
4.2. Naltrexone antagonism of remifentanil antinociception in amphibians
The putative action of remifentanil as an opioid receptor agonist was assessed in amphibians by systemic pretreatment of animals with the general opioid receptor antagonist, naltrexone. In previous studies, naltrexone at this dose of 100 nmol/g, s.c. given 1 h before systemic administration of ten different mu and kappa selective opioid analgesic agents (Stevens et al., 1994) or given before the spinal administration of twelve different mu, delta, or kappa selective opioid receptor agonists significantly blocked the antinociceptive effects of subsequent agonist administration (Stevens, 1996). As shown in Fig. 2A, pretreatment with naltrexone significantly blocked the antinociceptive effect of systemic remifentanil (30 nmol/g. s.c.). Remifentanil administration by the spinal route (100 nmol/frog, i.s.) was also blocked by naltrexone pretreatment (Fig. 2B). While these data demonstrate opioid receptor activation in amphibians mediates remifentanil antinociception, the use in amphibian studies of opioid receptor antagonists that are highly selective for each type of opioid receptor in mammals did not show convincing type-selectivity in behavioral and binding studies in amphibians (Newman et al., 2000a,b; Stevens and Newman, 1999). However, consistent findings from in vivo and in vitro studies in mammals (see Introduction) demonstrate that remifentanil is highly selective for the mu opioid receptor it is likely that remifentanil produces its effect predominantly by interaction of the species ortholog of the mu opioid receptor protein in amphibians (see below).
4.3. Relative antinociceptive potency of remifentanil in amphibians
The dose–response curves of the antinociceptive effects of remifentanil following systemic and spinal administration in amphibians are shown in Fig 3. For comparison, plotted also are the antinociceptive effects of morphine and related opioid receptor agonists in amphibians from previous larger studies (Stevens et al., 1994; Stevens, 1996). Systemic remifentanil gave a dose–response curve that did not differ in potency from fentanyl or levorphanol (overlapping 95% confidence intervals, see Table 1) but was significantly more potent than morphine. The ED50 of systemic remifentanil was 7.1 nmol/g compared to 86.3 nmol/g for morphine, making remifentanil about 12 times more potent than morphine. In rodent studies, remifentanil was also more potent than morphine and about equipotent to alfentanil following systemic administration (Buerkle and Yaksh, 1996). Following spinal administration, remifentanil had an ED50 value of 3.2 nmol/animal which was not significantly different than morphine (2.3 nmol/animal) or fentanyl (0.9 nmol/animal) but remifentanil was significantly less potent than the enkephalin analog, DAMGO (Table 1). Intrathecal administration of remifentanil in rodents found that remifentanil was significantly more potent than morphine (Buerkle and Yaksh, 1996). The overall relative potency between a number of opioid receptor agonists is well-correlated in rodents and amphibians following systemic, spinal, and intracerebroventricular administration (Stevens et al., 1994; Stevens, 1996; Stevens and Rothe, 1997). However, recent preliminary data following the cloning and characterization of amphibian mu, delta, and kappa opioid receptor proteins show that opioid receptor sequences are more similar among themselves in amphibians compared to sequences from mammals (Stevens, 2003, 2004). These findings suggest that opioid receptor type-selectivity may be developed less in earlier-evolved vertebrates and that, in general, greater type-selectivity among close members of the same receptor family is a driving force of molecular evolution.
In summary, remifentanil administration produced potent and dose-dependent antinociceptive effects following systemic and spinal administration in amphibians. The antinociceptive effects of remifentanil were significantly blocked by pretreatment of naltrexone and the relative potency of remifentanil compared to other opioids is similar to that found in mammalian models. The decrement of antinociceptive effects of remifentanil following spinal administration may reflect more rapid metabolism by this route compared to other opioid agents given in previous amphibian studies. Remifentanil may be a suitable choice as a mu opioid analgesic to continue studies of spinal opioid tolerance in the amphibian model.
Acknowledgments
We are grateful to the administration of OSU-Center for Health Sciences for their support and encouragement of our research program. Portions of this research were funded by the U.S. government by an NIH grant (NIDA 021148) to CWS.
References
- Beers R, Camporesi E. Remifentanil update: clinical science and utility. CNS Drugs. 2004;18:1085–1104. doi: 10.2165/00023210-200418150-00004. [DOI] [PubMed] [Google Scholar]
- Buerkle H, Yaksh TL. Continuous intrathecal administration of short-lasting mu opioids remifentanil and alfentanil in the rat. Anesthesiology. 1996;84:926–935. doi: 10.1097/00000542-199604000-00021. [DOI] [PubMed] [Google Scholar]
- Buerkle H, Marsala M, Yaksh TL. Effect of continuous spinal remifentanil infusion on behaviour and spinal glutamate release evoked by subcutaneous formalin in the rat. Br J Anaesth. 1998;80:348–353. doi: 10.1093/bja/80.3.348. [DOI] [PubMed] [Google Scholar]
- Chuiko GM, Podgornaya VA, Zhelnin YY. Acetylcholinesterase and butyrylcholinesterase activities in brain and plasma of freshwater teleosts: cross-species and cross-family differences. Comp Biochem Physiol, B Biochem Mol Biol. 2003;135:55–61. doi: 10.1016/s1096-4959(03)00048-4. [DOI] [PubMed] [Google Scholar]
- Cohen J, Royston D. Remifentanil. Curr Opin In Crit Care. 2001;7:227–231. doi: 10.1097/00075198-200108000-00003. [DOI] [PubMed] [Google Scholar]
- Contestabile A. Histochemical characterization of cholinesterase activity in the frog brain with special reference to its localization on the wall of blood vessels. Histochem J. 1976;8:513–521. doi: 10.1007/BF01003841. [DOI] [PubMed] [Google Scholar]
- Dershwitz M, Randel GI, Rosow CE, Fragen RJ, Connors PM, Librojo ES, Shaw DL, Peng AW, Jamerson BD. Initial clinical experience with remifentanil, a new opioid metabolized by esterases. Anesth Analg. 1995;81:619–623. doi: 10.1097/00000539-199509000-00035. [DOI] [PubMed] [Google Scholar]
- Fossi MC, Leonzio C, Massi A, Lari L, Casini S. Serum esterase inhibition in birds: a nondestructive biomarker to assess organophosphorus and carbamate contamination. Arch Environ Contam Toxicol. 1992;23:99–104. doi: 10.1007/BF00226001. [DOI] [PubMed] [Google Scholar]
- Gabriel R, Budai D. Seasonal variations in acetylcholine content and the levels of cholinergic enzymes in the alimentary tract and heart of Rana esculenta. J Auton Nerv Syst. 1992;40:223–227. doi: 10.1016/0165-1838(92)90204-t. [DOI] [PubMed] [Google Scholar]
- Hardwick DC, Hebb C. Pseudo-cholinesterase in the central nervous system of the frog. Nature. 1956;177:667. doi: 10.1038/177667a0. [DOI] [PubMed] [Google Scholar]
- Hayashida M, Fukunaga A, Hanaoka K. An animal model for surgical anesthesia and analgesia: characterization with isoflurane anesthesia and remifentanil analgesia. Anesth Analg. 2003a;97:1340–1346. doi: 10.1213/01.ANE.0000083369.63589.A5. [DOI] [PubMed] [Google Scholar]
- Hayashida M, Fukunaga A, Hanaoka K. Detection of acute tolerance to the analgesic and nonanalgesic effects of remifentanil infusion in a rabbit model. Anesth Analg. 2003b;97:1347–1352. doi: 10.1213/01.ANE.0000083370.80416.38. [DOI] [PubMed] [Google Scholar]
- Hoke JF, Shlugman D, Dershwitz M, Michalowski P, Malthouse-Dufore S, Connors PM, Martel D, Rosow CE, Muir KT, Rubin N, Glass PS. Pharmacokinetics and pharmacodynamics of remifentanil in persons with renal failure compared with healthy volunteers. Anesthesiology. 1997;87:533–541. doi: 10.1097/00000542-199709000-00012. [DOI] [PubMed] [Google Scholar]
- James MK, Feldman PL, Schuster SV, Bilotta JM, Brackeen MF, Leighton HJ. Opioid receptor activity of GI 87084B, a novel ultra-short acting analgesic, in isolated tissues. J Pharmacol Exp Ther. 1991;259:712–718. [PubMed] [Google Scholar]
- Michelsen LG, Hug CC. The pharmacokinetics of remifentanil. J Clin Anesth. 1996;8:679–682. doi: 10.1016/s0952-8180(96)00179-1. [DOI] [PubMed] [Google Scholar]
- Newman LC, Wallace DR, Stevens CW. Selective opioid agonist and antagonists displacement of [3H]-naloxone binding in amphibian brain. Eur J Pharmacol. 2000a;397:255–262. doi: 10.1016/s0014-2999(00)00265-x. [DOI] [PubMed] [Google Scholar]
- Newman LC, Wallace DR, Stevens CW. Selective opioid agonist and antagonists competition for [3H]-naloxone binding in amphibian spinal cord. Brain Res. 2000b;884:184–191. doi: 10.1016/s0006-8993(00)02967-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezalla PD. Morphine-induced analgesia and explosive motor behavior in amphibians. Brain Res. 1983;273:297–305. doi: 10.1016/0006-8993(83)90854-5. [DOI] [PubMed] [Google Scholar]
- Sanchez JC, Fossi MC, Focardi S. Serum “B” esterases as a nondestructive biomarker for monitoring the exposure of reptiles to organophosphorus insecticides. Ecotoxicol Environ Saf. 1997;38:45–52. doi: 10.1006/eesa.1997.1560. [DOI] [PubMed] [Google Scholar]
- Stevens CW. Alternatives to the use of mammals for pain research. Life Sci. 1992;50:901–912. doi: 10.1016/0024-3205(92)90167-n. [DOI] [PubMed] [Google Scholar]
- Stevens CW. Relative analgesic potency of mu, delta and kappa opioids after spinal administration in amphibians. J Pharmacol Exp Ther. 1996;276:440–448. [PubMed] [Google Scholar]
- Stevens CW. Opioid research in amphibians: a unique perspective on mechanisms of opioid analgesia and the evolution of opioid receptors. Rev Analgesia. 2003;7:69–82. [Google Scholar]
- Stevens CW. Opioid research in amphibians: an alternative pain model yielding insights on the evolution of opioid receptors. Brain Res Rev. 2004;46:204–215. doi: 10.1016/j.brainresrev.2004.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens CW, Newman LC. Spinal administration of selective opioid antagonists in amphibians: evidence for an opioid unireceptor. Life Sci. 1999;64:L125–L130. doi: 10.1016/s0024-3205(99)00013-2. [DOI] [PubMed] [Google Scholar]
- Stevens CW, Rothe KS. Supraspinal administration of opioids with selectivity for mu-, delta- and kappa-opioid receptors produces analgesia in amphibians. Eur J Pharmacol. 1997;331:15–21. doi: 10.1016/s0014-2999(97)01026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens CW, Klopp AJ, Facello JA. Analgesic potency of mu and kappa opioids after systemic administration in amphibians. J Pharmacol Exp Ther. 1994;269:1086–1093. [PubMed] [Google Scholar]
- Taylor BK, Peterson MA, Basbaum AI. Early nociceptive events influence the temporal profile, but not the magnitude, of the tonic response to subcutaneous formalin: effects with remifentanil. J Pharmacol Exp Ther. 1997;280:876–883. [PubMed] [Google Scholar]
- Van de Velde M. Remifentanil for obstetric analgesia and anesthesia: a review of the literature. Acta Anaesthesiol Belg. 2005;56:45–49. [PubMed] [Google Scholar]