Abstract
Atorvastatin has been associated with liver injury. We reported here two cases of aminotransferases elevation within 12 h of low-dose atorvastatin therapy. Liver functions were fully recovered to the baseline level 11 days after discontinuation of atorvastatin treatment. The possible relative risk factors included advanced age, chronic and systemic diseases, and co-administration of cytochrome P450 3A (CYP3A) enzyme-dependent metabolic drugs or its inhibitors such as clopidogrel and diltiazem. No significant transaminase elevation was observed after switching to pravastatin. Thus, pravastatin might be safer than atorvastain in patients with chronic or systemic diseases, or with co-administration of CYP3A enzyme-dependent drugs.
Keywords: atorvastatin, pravastatin, liver injury, cytochrome P450 3A enzyme
Introduction
The 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (also known as statins) are safe to the treatment of hyperlipidemia and prevention of coronary artery diseases. Few common side effects of statins include myopathy, gastrointestinal disturbance, depression, and liver injury. According to Motola et al. [1], the rate of statin-associated hepatic reactions is 10.9% in a total of 1,254 adverse drug reaction reports from January 1990 to May 2005. Statins are among those with the highest rate of hepatic reactions, higher than antiplatelet agents and non-steroidal anti-inflammatory drugs. Various hepatotoxic side effects related to atorvastatin therapy have been reported [2, 3], raising a potential concern of atorvastatin-associated liver injury in Chinese populations. Here we report two patients who demonstrated significant elevation of serum alanine amino-transferase (ALT) levels immediately after atorvastatin therapy, but without cross-toxicity to the livers after switching to pravastatin.
Case 1
A 58-year-old male was admitted to the hospital because of recurrent oral ulcers, Raynaud phenomenon, and leucocytopenia. Laboratory tests confirmed the diagnosis of systemic lupus erythematosus (SLE). He was subsequently treated with prednisone 20 mg daily and hydroxychloroquine 0.2 g twice a day. Past history included hypertension, diabetes and obsolete pulmonary tuberculosis. One week later, he developed chest pain and chest distress. ECG showed ST segment horizontally depression as well as T wave inversion on leads V1–V6. The serum myocardial injury biomarkers were normal. The patient was suspected of unstable angina pectoris, so therapies with atorvastatin (20 mg daily) and other medications were therefore started (Table 1). Subsequent coronary angiography confirmed the diagnosis of coronary artery disease, but abdominal ultrasound revealed no sign of liver disorder. Liver function tests (LFTs) were normal prior to atorvastatin therapy. 10 h after the atorvastatin, ALT began to rise, and persisted to more than 3 folds of the normal upper limit in 2 days. Myoglobin level also raised to 108 μg/l (compared with a normal range < 60 g/l) after 72 h (Table 1). Therefore, atorvastatin was withdrawn. ALT was recovered to the baseline level in 10 days. Then pravastatin was prescribed, which did not cause notable elevation of the ALT levels during the two-month follow up observation (Figure 1).
Table 1.
Atorvastatin-associated liver disorders: Clinical details of patients in this report.
| Case 1 | Case 2 | |
|---|---|---|
| Coexisting diseases | coronary heart disease, hypertension, systemic lupus erythematosus, old pulmonary tuberculosis | coronary heart disease, non-alcoholic fatty liver disease, mixed hyperlipidemia |
| Combined drugs | prednisone, hydroxychloroquine, bay-aspirin, clopidogrel, low molecular weight heparin, isosorbide dinitrate, metoprolol | bay-aspirin, clopidogrel, low molecular weight heparin, isosorbide dinitrate, metoprolol |
| HBsAg and HCV-Ab | negative | negative |
| Symptoms after ATV | obvious fatigue, anorexia | no symptoms |
| Peak ALT (n < 40 U/l) | 120 | 278 |
| Peak AST (n < 40 U/l) | 23 | 119 |
| Peak GGT (n < 67 U/l) | 40 | 124 |
| Peak ALP (n < 120 U/l) | 62 | 93 |
| Peak LDH (n < 270 U/l) | 148 | 171 |
| Peak direct bilirubin (n < 8.6 μmol/l) | 3.7 | 1.9 |
| Peak total bilirubin (n < 22 μmol/l) | 9.2 | 9.3 |
| RUCAM score | 7 | 7 |
HBsAg = hepatitis B surface antigen; HCV-Ab = hepatitis C virus antibody; ATV = atorvastatin; ALT = alanine aminotransferase; AST = aspartate aminotransferase; LDH = lactate dehydrogenase; GGT = gamma-glutamyl transpeptidase; ALP = alkaline phosphatase; RUCAM = Roussel-Uclaf causality assessment method; n = normal range.
Figure 1.
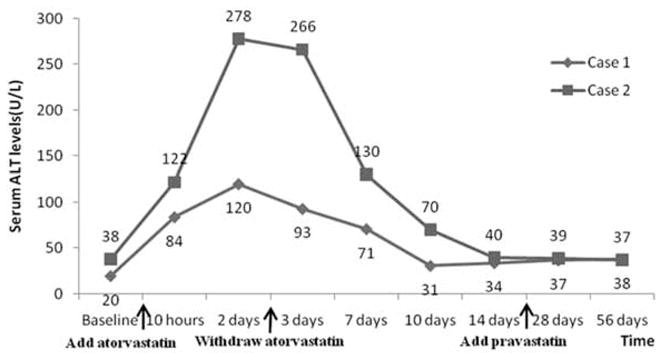
Serum alanine aminotransferase (ALT) levels vary with change of statins therapy in 2 cases.
Case 2
A 53-year-old male was referred to our department because of angina pectoris on exertion for 10 days. Past history included non-alcoholic fatty liver (NAFL), renal cyst and mixed hyperlipidemia. He has been a smoker for more than 30 years. The ECG showed ST segment depressed more than 0.5 mv, as well as flat T wave on leads I and aVL. His serum myocardial biomarkers were normal. Coronary angiography revealed a ≥ 90% luminal narrowing of left circumflex coronary artery and a drug-coating stent was implanted. Medications included atorvastatin 10 mg daily (Table 1). His baseline LFTs were normal. ALT significantly elevated immediately after the initiation of atorvastatin therapy, then atorvastatin was discontinued and ALT was gradually recovered to the baseline after 14 days. The other LFTs such as aspartate amino-transferase, lactate dehydrogenase, γ-glutamyl transpeptidase and alkaline phosphatase (ALP) were normal all the monitoring time (Table 1). He was also switched to pravastatin 20 mg daily, and his ALT elevated slightly but remained in the baseline ranges during the two-month follow-up observations (Figure 1).
Discussion
Atorvastatin and liver injury
Drug-induced liver injury can be divided into 3 types according to serum levels of ALT and ALP and the ratio (R) of ALT/ALP. Hepatocellular injury defined as ALT 2 times the upper limit of normal (ULN) and R > 5, cholestatic liver injury defined as ALP 2 times the ULN and R < 2, mixed liver injury defined as ALT 2 times the ULN and R > 2 but < 5 [4]. Our patients with ALT 3 folds higher than the ULN and normal serum levels of ALP and bilirubin can be attributed to hepatocellular injury group. Whether liver injury is caused by atorvastatin is evaluated by the Roussel-Uclaf causality assessment method (RUCAM). This scoring system is performed based on information about onset time of reaction, development of LFTs after cessation of atorvastatin, presence of risk factors, and known hepatotoxicity of the suspected drug and concomitant drugs. The results of assessment are defined as: 1 – 2 unlikely, 3 – 5 possible, 6 – 8 probable and > 8 highly probable [5, 6]. Our 2 patients were calculated to be probable cases (Table 1). However, as the second most commonly prescribed statin, atorvastatin-induced liver injury is not common based on the post-marketing experience. For example, the Anglo-Scandinavian Cardiac Outcomes Trial –Lipid Lowering Arm reported no difference in hepatic adverse events in 5,168 hypertensive patients who were treated with atorvastatin 10 mg/day over 3.3 years when compared with 5,137 controls receiving the placebo [7]. In fact, no idiosyncratic drug-induced liver injury (DILI) with atorvastatin was reported according to the investigation on relationship between daily dose of several statins and DILI, while 3 patients with simvastatin and 2 patients with cerivastatin were reported to develop liver failure leading to liver transplantation or death eventually [8]. Asymptomatic hepatic enzyme elevations are the most common forms of hepatic side effects associated with atorvastatin, which do not necessarily indicate hepatic damage. In retrospective analyses, the incidence of persistent elevations of serum transaminases is 0.5% in the atorvastatin-treated population [9]. However, Bhardwaj et al. summarized several forms of side effects attributed to the use of atorvastatin, including hepatocellular injury, cholestatic injury, mixed pattern of atorvastatin-associated hepatocellular and cholestatic injury, autoimmune-type reaction and fulminant liver failure [2]. The duration from atorvastatin exposure to the onset of hepatic toxicity can be variable, with a mean period of 9.4 weeks, range from 1 to 52 weeks, and the majority of transaminase elevations occurred in the first 16 weeks of statin therapy, and was considered to be dose-dependent [3]. There has been no case of significant ALT elevations immediately after taking statins. From both cases we reported in this study, however, serum transaminases began to rise within 12 h of a low dose atorvastatin. Elevation of serum transaminases is often self-limiting and may be related to the alteration of the hepatocyte cellular membrane with enzyme leakage rather than direct injury to the liver cells [3]. LFTs are often performed before and 6 and 12 weeks after the statin therapy to evaluate liver functions. However, both of our cases showed more frequent LFT value changes, suggesting more frequent liver function evaluations are required.
High risks and mechanism
It is still uncertain to which group of population is more prone to develop liver injury and what other factors may be associated with atorvastatin-initiated liver damages. But it seems that most warning drugs due to hepatotoxicity were prescribed greater than 50 mg daily [8]. However, our present cases were both given low doses of atorvastatin (10 – 20 mg per day), in accordance with the generally speaking that DILI is unpredictable and not dose-dependent [8].
Clarke et al. [3] reported 14 patients from U.K. suffered from serious hepatotoxicity associated with atorvastatin. These patients had average ages > 60 years with a female/male ratio of 2: 1, and 6 out of them had a marked hyperbilirubinemia (> 100 μmol/l). In other 2 cases, one 72-year-old male from Spain and a 71 years old female from USA in the reports of de Castro and Gershovich [10, 11], atorvastatin-induced cholestatic injury was proven by rapid improvement with cessation of the drug and recurrence with re-initiation of atorvastatin. Besides advanced age, hepatic injury appeared associated with systemic diseases and medications. Our patient in Case 1 had SLE and underwent combined therapies of prednisolone and immunosuppressant. Jiménez-Alonso et al. also reported a young woman with SLE suffered from atorvastatin-induced cholestatic hepatitis. After withdrawal of atorvastatin, they found that this patient’s LFTs were also normalized [12]. Clarke and Mills reported 7 cases of hepatic reactions from atorvastatin treatment [3]. The majority of them had underlying diseases and co-administration of drugs: 1 taking prednisolone for polymyalgia rheumatica, 3 taking coproxamol for osteoarthritis, 1 with chronic renal failure and 1 with coeliac disease and taking warfarin because of paroxysmal atrial fibrillation. In another case report from Nakad et al. [13], a 70-year-old female from Belgium developed acute hepatitis due to atorvastatin. She had taken phenobarbital (100 mg/day) for idiopathic seizure for years. According to these previous reports and our own experience, we conclude that atorvastatin-associated liver injury might occur more commonly in patients with chronic diseases, especially connective tissue diseases such as SLE, and in patients with multiple medications. Such adverse effect of atorvastatin seems race-independent.
The exact mechanism of atorvastatin-associated liver injury remains unclear. One possibility is drug-drug interactions [14], based on the observations that patients with multiple underlying disorders and treatments showed more liver injury cases [3]. Alterations of the cytochrome P450 (CYP450) system can be a key. All statins, except for pravastatin, are metabolized by CYP450 [15]. For example, atorvastatin is metabolized by CYP 3A4 and atorvastatin plasma concentration may increase after CYP3A4 inhibitor treatments, such as ritonavir, antifungals, macrolides, calcium channel antagonists (verapamil and diltiazem), and amiodarone [15, 16], or decrease by CYP3A4 inducers such as corticosteroids [17]. Prednisone dosage reduction is often required when using statins. In our report, Case 1 patient took diltiazem and prednisone, both may affect CYP3A4 metabolism. Both our cases took clopidogrel because of acute coronary syndrome and implantation of drug-eluting stent. Clopidogrel was also CYP3A-dependent [18]. Previous studies suggested that atorvastatin, but not pravastatin, decreased the inhibitory effect of clopidogrel on platelet aggregation [19]. As an inactive prodrug, clopidogrel is metabolized mainly by CYP3A4 and CYP3A5 isozymes, whereas atorvastatin could reduce the effects of clopidogrel by inhibiting the CYP3A-dependent formation of its active metabolites [15]. A population-based cohort study investigated the interactions between CYP3A4-metabolized statins and clopidogrel [18]. Compared to the control group (non-CYP3A4-metabolized statins), co-prescription of CYP3A4-metabolized statins associated with an increase of adverse outcomes (hazard ratio 1.16, 95% confidence interval 0.91 – 1.47), although not statistically significant. Thus we conclude that combination of atorvastatin and clopidogrel may induce hepatic injury via competitive activation and excessive consumption of CYP3A4.
Pharmacokinetics of pravastatin is clearly different from atorvastatin. Pravastatin plasma concentration is not affected by CYP inhibition [15] and is less hepatotoxic than other statins [18]. There are concerns of atorvastatin hepatotoxicity in patients with NAFL and those with abnormal LFT baselines, but pravastatin has been proven safe in patients with NAFL. In a recent multicenter, randomized, double-blind, placebo-controlled parallel-group trial, pravastatin is well tolerated and effective in 326 hypercholesterolemic subjects with well-compensated chronic liver diseases (NAFL was present in 64%), when compared with the placebo group [20]. Based on previous research results, and the non-CYP-based metabolism, as well as the hydrophilic nature of pravastatin, pravastatin might be safer than atorvastatin in patients with chronic liver disease such as NAFL.
In conclusion, our report emphasized the importance of careful monitoring of LFTs to avoid severe atorvastatin-associated hepatotoxicity. Atorvastatin therapy combined with CYP3A-dependent metabolic drugs such as clopidogrel, or CYP3A inhibitors such as diltiazem, might increase the risk of hepatic injury attributing to drug-drug interactions. Our observations also suggested that pravastatin was safer than atorvastatin in patients with advanced age, liver diseases (such as NAFL) or systemic disorders (such as SLE).
References
- 1.Motola D, Vargiu A, Leone R, et al. Hepatic adverse drug reactions: a case/non-case study in Italy. Eur J Clin Pharmaco. 2007;63:73–79. doi: 10.1007/s00228-006-0222-z. [DOI] [PubMed] [Google Scholar]
- 2.Bhardwaj SS, Chalasani N. Lipid lowering agents that cause drug-induced hepatotoxicity. Clin Liver Dis. 2007;11:597–613. vii. doi: 10.1016/j.cld.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clarke AT, Mills PR. Atorvastatin associated liver disease. Dig Liver Dis. 2006;38:772–777. doi: 10.1016/j.dld.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 4.Benichou C. Criteria of drug-induced liver disorders. Report of an international consensus meeting. J Hepatol. 1990;11:272–276. doi: 10.1016/0168-8278(90)90124-a. [DOI] [PubMed] [Google Scholar]
- 5.Danan G, Benichou C. Causality assessment of adverse reactions to drugs – I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46:1323–1330. doi: 10.1016/0895-4356(93)90101-6. [DOI] [PubMed] [Google Scholar]
- 6.Benichou C, Danan G, Flahault A. Causality assessment of adverse reactions to drugs – II. An original model for validation of drug causality assessment methods: case reports with positive rechallenge. J Clin Epidemiol. 1993;46:1331–1336. doi: 10.1016/0895-4356(93)90102-7. [DOI] [PubMed] [Google Scholar]
- 7.The ASCOT investigators. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial – Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361:1149–1158. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- 8.Lammert C, Einarsson S, Saha C, et al. Relationship between daily dose of oral medications and idiosyncratic drug-induced liver injury: search for signals. Hepatology. 2008;47:2003–2009. doi: 10.1002/hep.22272. [DOI] [PubMed] [Google Scholar]
- 9.Newman CB, Palmer G, Silbershatz H, et al. Safety of atorvastatin derived from analysis of 44 completed trials in 9,416 patients. Am J Cardiol. 2003;92:670–676. doi: 10.1016/s0002-9149(03)00820-8. [DOI] [PubMed] [Google Scholar]
- 10.de Castro ML, Hermo JA, Baz A, et al. Acute cholestatic hepatitis after atorvastatin reintroduction. Gastroenterol Hepatol. 2006;29:21–24. doi: 10.1157/13083248. [DOI] [PubMed] [Google Scholar]
- 11.Gershovich OE, Lyman AE. Liver function test abnormalities and pruritis in a patient treated with atorvastatin: case report and review of the literature. Pharmacotherapy. 2004;24:150–154. doi: 10.1592/phco.24.1.150.34803. [DOI] [PubMed] [Google Scholar]
- 12.Jiménez-Alonso J, Osorio JM, Gutiérrez-Cabello F, et al. Atorvastatin-induced cholestatic hepatitis in a young woman with systemic lupus erythematosus. Arch Intern Med. 1999;159:1811–1812. doi: 10.1001/archinte.159.15.1811-a. [DOI] [PubMed] [Google Scholar]
- 13.Nakad A, Bataille L, Hamoir V, et al. Atorvastatin-induced acute hepatitis with absence of cross-toxicity with simvastatin. Lancet. 1999;353:1763–1764. doi: 10.1016/S0140-6736(99)00569-3. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson TA. Comparative pharmacokinetic interaction profiles of pravastatin, simvastatin, and atorvastatin when coadministered with cytochrome P450 inhibitors. Am J Cardiol. 2004;94:1140–1146. doi: 10.1016/j.amjcard.2004.07.080. [DOI] [PubMed] [Google Scholar]
- 15.Neuvonen PJ, Niemi M, Backman JT. Drug interactions with lipid-lowering drugs: mechanisms and clinical relevance. Clin Pharmacol Ther. 2006;80:565–581. doi: 10.1016/j.clpt.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Borders-Hemphill V. Concurrent use of statins and amiodarone. Consult Pharm. 2009;24:372–379. doi: 10.4140/tcp.n.2009.372. [DOI] [PubMed] [Google Scholar]
- 17.Lam S, Partovi N, Ting LS, et al. Corticosteroid interactions with cyclosporine, tacrolimus, myco-phenolate, and sirolimus: fact or fiction? Ann Pharmacother. 2008;42:1037–1047. doi: 10.1345/aph.1k628. [DOI] [PubMed] [Google Scholar]
- 18.Blagojevic A, Delaney JA, Lévesque LE, et al. Investigation of an interaction between statins and clopidogrel after percutaneous coronary intervention: a cohort study. Pharmacoepidemiol Drug Saf. 2009;18:362–369. doi: 10.1002/pds.1716. [DOI] [PubMed] [Google Scholar]
- 19.Lau WC, Waskell LA, Watkins PB, et al. Atorvastatin reduces the ability of clopidogrel to inhibit platelet aggregation: a new drugdrug interaction. Circulation. 2003;107:32–37. doi: 10.1161/01.cir.0000047060.60595.cc. [DOI] [PubMed] [Google Scholar]
- 20.Lewis JH, Mortensen ME, Zweig S, et al. Pravastatin in chronic liver disease study investigators. Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: Results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology. 2007;46:1453–1463. doi: 10.1002/hep.21848. [DOI] [PubMed] [Google Scholar]


