Abstract
Adoptive transfer of autologous tumor-reactive T cells holds promise as a cancer immunotherapy. In this approach, T cells are harvested from a tumor-bearing host, expanded in vitro and infused back to the same host. Conditioning of the recipient host with a lymphodepletion regimen of chemotherapy or radiotherapy before adoptive T cell transfer has been shown to substantially improve survival and anti-tumor responses of the transferred cells. These effects are further enhanced when the adoptive T cell transfer is followed by vaccination with tumor antigens in combination with a potent immune adjuvant. Although significant progress has been made toward an understanding of the reasons underlying the beneficial effects of lymphodepletion to T cell adoptive therapy, the precise mechanisms remain poorly understood. Recent studies, including ours, would indicate a more central role for antigen presenting cells, in particular dendritic cells. Unraveling the exact role of these important cells in mediation of the beneficial effects of lymphodepletion could provide novel pathways toward the rational design of more effective anti-cancer immunotherapy. This article focuses on how the frequency, phenotype, and functions of dendritic cells are altered during the lymphopenic and recovery phases post-induction of lymphodepletion, and how they affect the anti-tumor responses of adoptively transferred T cells.
Keywords: Adoptive T cell transfer, Cancer, Chemotherapy, Dendritic cells, Lymphodepletion, Tumor, Vaccination
Introduction
The success of anti-tumor immunity depends on generation of effector T cells that can differentiate into functional long-lived memory cells [62, 86, 102]. The current inability to fully elucidate the critical factors involved in the generation of effective T cell responses has hindered the successful development of effective cancer vaccine therapy. Defining approaches that can accentuate anti-tumor T cell responses would help developing potential immunotherapeutic protocols in the clinical setting. Due to the limited numbers and prevalent dysfunction of the tumor-reactive T cells found in a tumor-bearing host, studies have been focusing on how to correct the functions of these cells while effectively increasing their numbers. Adoptive T cell transfer is a potential approach in which several intrinsic factors affecting T cells harvested from tumor bearing host can be improved in vitro [34]. Extrinsic factors induced by preconditioning of the recipient host with cytoreductive regimens, such as chemotherapy (e.g. cyclophosphamide; CTX) or sublethal total body irradiation (TBI) can also significantly improve the anti-tumor efficacy of adoptive T cell therapy in particular when the latter is followed by active vaccination [5, 42, 51, 115, 118, 139]. In the following sections, we will discuss how the application of these cytoreductive regimens mediate the beneficial effects of the adoptive T cell therapy by focusing on the potential roles of dendritic cells (DCs) in the vaccination setting.
Adoptive T cell transfer system in cancer setting
Adoptive transfer of autologous tumor-reactive T cells in the clinical stetting consists of harvesting T cells from the patient’s own peripheral blood, draining lymph nodes, or tumor bed (tumor infiltrating lymphocytes, TIL) followed by their expansion in vitro using polyclonal stimulation with anti-CD3 and anti-CD28 mAbs in the presence of IL-2 as a growth factor. The cells are then infused back to the same patient blood with administration of IL-2 to improve cell engraftment and survival [62]. Most of the earlier clinical studies of adoptive T cell therapy are based on the use of T cells expanded in vitro for several cycles before their adoptive transfer [26, 73, 77, 109, 111]. Although the use of these cells for adoptive transfer can mediate melanoma regression with an objective response rate of about 34%, the response rates in these studies were not better than treatment with cytokines or cancer vaccines [110]. Interestingly, recent studies clearly demonstrated that adoptively transferred T cells that had expanded in vitro for several cycles of expansion are considered terminally differentiated (exhausted), expressing the phenotype of effector memory T cells (TEM; CD62LlowCCR7lowCD127RlowCD44high). Although these TEM cells can express rapid effector functions in vitro, they show poor survival, trafficking, and persistence in vivo [62, 86, 102]. The limited in vivo anti-tumor responses of terminally differentiated TEM cells were attributed to their acquisition of the phenotype of senescent cells and the loss of lymph node homing receptors, in particular CD62L and CCR7. These exhausted phenotypes limit their responses to vaccination with tumor antigens and accordingly their anti-tumor efficacy [62]. By contrast, antigen stimulation of T cells in vitro for a short period can generate early effector cells with central memory phenotype (TCM; CD62LlowCCR7lowCD127RlowCD44high) with better survival, persistence, and anti-tumor efficacy than their TEM counterparts upon their adoptive transfer into a lymphodepleted host [26, 73, 77, 109, 111]. The advantage of generation of TCM cells over TEM cells in vitro has been attributed to the higher expression levels of CD62L homing molecule in the early effector cells with TCM phenotype than in the late effector cells with TEM phenotype [61, 62]. This homing receptor facilitates T cells to traffic to lymph nodes and their crosstalk with antigen presenting cells [57, 108], resulting in the differentiation of T cells into effector cytolytic cells capable of combating cancer. Importantly, the type of cytokine added to T cells during their antigen stimulation in vitro can shape their TCM versus TEM phenotypes. For instance, IL-12 [24] and IL-15 favor T cells, which sustain the expression of CD62L and differentiate into a TCM phenotype, while IL-2 favors differentiation of cells into a TEM phenotype [61]. It would seem that these cytokines, besides their ability to modulate the magnitude of CD62L expression, can induce distinct intrinsic mechanisms in T cells that affect the antigen-specific responses in vivo. These intrinsic mechanisms of T cells have been reviewed before [34, 62, 63, 83, 95, 112, 120, 135] and are beyond the scope of this article.
Improving adoptive T cell therapy with lymphodepletion
Accumulating evidence now supports that induction of immune lymphodepletion in the recipient host by treatment with sublethal TBI or anti-cancer chemotherapeutic drugs, such as CTX and doxorubicin before adoptive transfer of in vitro-activated T cells can markedly improve the survival, persistence, and anti-tumor efficacy of the transferred T cells [33, 34]. These studies reported a long-term engraftment of the infused T cells, which comprised a larger fraction of the patient repertoire. Although recent studies have explored some of the cellular and molecular mechanisms underlying the beneficial effects of lymphodepletion regimen to the homeostatic- and antigen-driven responses of the adoptively transferred T cells, the precise mechanisms behind these effects are poorly understood [105]. What has been defined includes the following.
Enhanced engraftment and survival of the transferred T cells by creation of a space “niche” [63].
A rapid induction of homeostatic cytokines [17, 115, 119, 134].
Elimination of regulatory CD4+CD25+ T (Treg), NKT cells, and myeloid-derived suppressor cells [6, 9, 46, 53, 95].
Depletion of endogenous cells that compete with the transferred T cells for cytokines “cytokine sink” [17, 33, 63].
Recent studies would suggest, however, that these mechanisms might not be the principal means by which lymphodepletion regimens augment adoptive immunotherapy, in particular in the presence of active vaccination [74, 80, 101, 118, 134]. Moreover, these mechanisms have been investigated during the lymphopenic phase, and few studies addressed the role of the cellular components that might be altered at the recovery phase, in which the host recovers from the induced lymphopenia; days 5–18. It could be postulated that some mechanisms related to the recognition of tumor antigen by the host cells, in particular dendritic cells (DCs), might play a critical role. Indeed, recent preclinical studies including ours have pointed to the active roles of DCs in mediation of the beneficial effects of lymphodepletion to adoptive T cell therapy [96, 115]. In the following sections, we will discuss how the numbers and activation phenotype of DCs are altered during the lymphopenic and recovery phases after induction of lymphodepletion and how this alteration affects responses of adoptively transferred T cells.
Alteration in the frequency and phenotype of DCs after lymphodepletion
Rapid activation of DCs during the lymphopenic phase
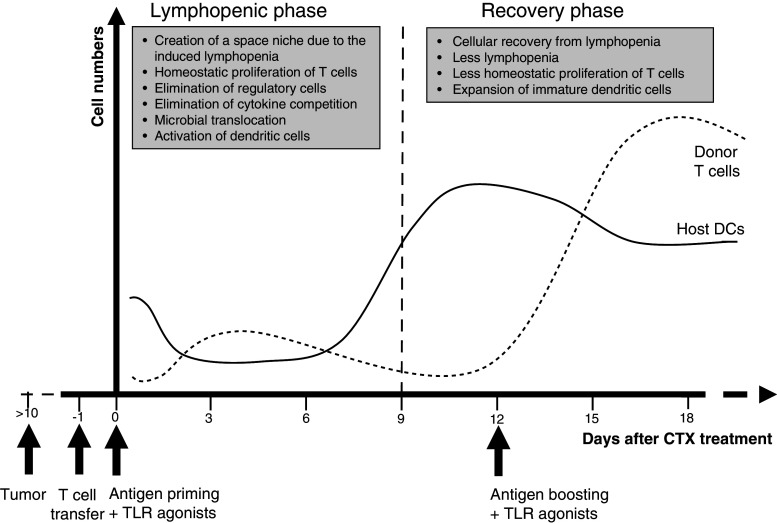
DCs are the most potent professional antigen presenting cells and as such have been a promising target for the development of new cancer treatments [106]. The presence of physiological numbers of DCs is considered a crucial factor for both homeostatic and antigen-driven expansion of the peripheral pool size of memory TCM and TEM subsets [36, 55, 100]. Given this central role of DCs in shaping the quantity and quality of immune responses, we have been focusing our recent studies on understanding their roles in T cell responses in the context of lymphodepletion. Using the OT-1 and pmel-1 TCR transgenic mouse models, in which CD8+ T cells are engineered to recognize MHC class-I OVA (SIINFEKL) [23] and melanoma (gp10025–33) [92] peptides, respectively, we have been addressing the role of DCs at the lymphopenic and recovery phases after CTX treatment. These models are based on the adoptive transfer of a few (1 million) trackable CD8+ T cells from a TCR transgenic mouse into a recipient mouse followed by vaccination. These models allow for visualization of the responses (expansion, contraction; activation, trafficking, function) of the antigen-specific CD8+ T cells (the donor cells) within a large population of CD8+ T cells (host cells). This also allows for a side-by-side testing of the responses of the host innate immune cells, including DCs. Using these TCR CD8+ T cells with B16 melanoma, we have established that adoptive transfer of OT-1 CD8+ T cells at the lymphopenic phase after CTX treatment results in marked increases in post-vaccination T cell responses, including enhanced expansion, function, and delayed contraction of adoptively transferred CD8+ T cells; these effects were further augmented when peptide vaccination was combined with the TLR3 agonist poly(I:C) [118].
Consistent with other studies that showed space-independent enhanced immune responses of adoptively transferred CD4+ T cells after CTX treatment [80], the enhanced effects of CTX preconditioning to CD8+ T cells were not felt to be mediated by creation of a “space niche” since infusion of up to 200 million wild type naïve cells immediately after CTX treatment did not block its beneficial effects [118]. Interestingly, however, the beneficial effect of CTX was associated with a rapid activation of DCs in the liver and spleen during the early phase of lymphopenia (days 1–4) and were dependent on both the presence of CD11b-expressing cells and intact IFN-α/β signaling pathways. Given that, DCs express CD11b and are capable of producing IFN-α/β, these results suggested a possible role for DCs during the lymphopenic phase in mediation of the adjuvant effects of CTX preconditioning regimen. Indeed, earlier studies have reported that mouse interdigitating DCs isolated 2 days after CTX treatment showed an enhanced accessory function compared with the control DCs [70], and that follicular DCs harvested from lymph nodes during the lymphopenic phase post-CTX treatment retained exogenous antigen for long time and were capable of inducing a better antibody responses [98]. Similar to CTX, TBI also induces a rapid activation of DCs coincided with augmented T cell responses [18, 28, 69, 141]. Human studies also showed a rapid activation of DCs after myeloablative allogenic hematopoietic stem cell transplantation [47, 69]. These studies would indicate to the rapid activation of DCs as a potential mechanism contributing to the enhanced anti-tumor responses to vaccination with different antigen formulations during the lymphopenic phase [16, 37, 67, 104, 115, 118, 125, 131].
How activation of DCs is induced early after lymphodepletion
DCs have been reported to be required for the lymphopenia-driven homeostatic (i.e. in absence of antigen priming) proliferation of naïve and memory CD8+ T cells [36, 138]. Furthermore, the antigen-specific responses of adoptively transferred T cells into a lymphodepleted host was found to be likely due to an enhanced MHC Class I-restricted antigen presentation by elements of the transplanted bone marrow [134]. Taken together, these studies would suggest that DCs post-induction of lymphodepletion would play an important role in the enhanced anti-tumor immune responses. In the following sections, we will discuss studies that would suggest this role of DCs at the lymphopenic and recovery phases post-lymphodepletion. Although it is not clear how lymphodepletion regimens induces the rapid DC activation and how DCs are possibly contributing to lymphodepletion-induced enhanced effects on T cell responses, several mechanisms are possible:
A massive apoptosis of the host cells as well as tumor cells occurs rapidly after induction of lymphodepletion [11, 118]. In this setting, cells undergoing apoptosis are rapidly and specifically recognized by phagocytic cells, in particular DCs. After recognition, apoptotic bodies are silently removed by phagocytosis which can result in activation of DCs [49, 59, 126].
The induction of tumor cell apoptosis and the release of endogenous TLR agonists such as heat-shock proteins and uric acid can act through TLRs and thus induce DC maturation [31, 113]. Uric acid, at least in its crystal form can activate DCs and augment tumor rejection [48, 122, 123]. Dying tumor cells induced by local irradiation of tumor can also release the high-mobility-group box 1 alarmin protein, which can bind to TLR4 expressed by DCs and increase the efficiency of tumor antigen processing and presentation [7].
The rapid induction of inflammatory cytokines such as IFN-α [38, 45, 54, 88] can act as danger signals [79, 81, 113, 119]. These signals would result in activation of DCs, their maturation, and migration to lymph nodes, a critical step required for optimal antigen-specific T cell responses [10]. We and others have reported induction of several inflammatory cytokines, in particular type I IFNs, post-CTX treatment [17, 107, 117, 128, 136, 141], and that the absence of type I IFNs in vivo abrogated the beneficial effects of CTX to the anti-tumor responses of adoptively transferred immune cells [101, 118].
The release of LPS (a TLR4 ligand) due to the TBI- or chemotherapy-induced damage in the integrity of mucosal barriers in the intestinal tract and the translocation of microbial products [1, 85]. This microbial translocation would lead to induction of inflammatory cytokines [17, 117, 119] and a rapid activation of DCs and the associated enhanced effector immune responses [69, 141]. In line with this notion, exogenous LPS can substitute for the endogenous TBI-induced LPS for augmentation of the anti-tumor responses of CD8+ T cells to active vaccination when they were adoptively transferred to immune cell (NK and CD4+ T) cell-ablated recipient mice [96]. Similarly, we have found that addition of the TLR3 agonist poly(I:C) to OVA vaccination during the lymphopenic phase after CTX preconditioning markedly augmented CD8+ T cell expansion, coinciding with significant activation of DCs in the spleen and liver [118].
Lymphodepletion removes the endogenous lymphocytes that compete with the donor T cells for the access to the host DCs, allowing the transferred T cell to crosstalk with a relatively larger pool of antigen-bearing DCs [58].
Killing of significant numbers of tumor cells and the release of tumor antigens from the dead cells, which can be cross-presented to T cells by DCs [43]. The enhanced effects on immune-mediated tumor regression of radiotherapy and CTX therapy have been found to be due to the disruption of the stroma-tumor network within the tumor bed cells [52, 140], coinciding with induction of the expression of adhesion molecules, such as P-selectin, enhancing infiltration of immune effectors into the tumor stroma [39, 40, 113]. The disruption of the stroma-tumor network would result in the release of tumor antigen that can be picked up and presented by the stromal cells themselves of the localized DCs for cytolytic effector T cells. The possibility of the cross presentation of the antigens released from the dead tumor cells would explain the enhanced anti-tumor effects of adoptively transferred T cells into a CTX-preconditioned host in the absence of active vaccination [9, 15, 17, 129] or even in absence of adoptive T cell transfer [8, 12–14, 16, 37, 131]. It would also explain the anti-tumor efficacy of vaccination with naïve (i.e. with no antigen loading) DCs [20], where the antigens released from the dead tumor cells can be further cross presented by the exogenous DCs.
Therefore, it appears that the rapid induction of an inflammatory and apoptotic milieu by lymphodepletion can provide an environment, which is conducive toward the induction of activated DCs. The inflammatory milieu induced by lymphodepletion, however, is transient and diminishes during the recovery phase. Therefore, the suggested role of DCs would be transient and can be effective only when vaccination is performed during lymphopenia. In general, the optimization of increases in the numbers, activation, survival, and the anti-tumor responses of the adoptively transferred T cells into a lymphodepleted host have been found to require antigen boosting [74, 89], which is often performed during the recovery from lymphopenia. Since this time frame would be after the normalization of the activation state of the DCs logic would indicate the presence of additional mechanism(s) that would be in effect during the rebound phase. As discussed in the following section, there is increasing evidence that DCs develop an augmented presence during the rebound phase and could, at least in part, be one factor contributing to the augmented T cell responses to antigen boosting at the recovery from lymphodepletion.
Expansion of DCs during the recovery phase
While we were investigating the role of DCs in mediation of the beneficial effects of CTX-induced lymphopenia to adoptive T cell therapy, we observed substantial increases in both the relative and absolute numbers of DCs with myeloid (CD11chighCD11bhighLy6GlowB220low) and immature (CD40lowCD80lowCD86low) phenotype during the recovery phase after CTX-induced lymphodepletion [115]. Despite their immature phenotype, these CTX-expanded DCs were functional based on the phagocytosis and in vivo and in vitro antigen presenting function assays. Recent studies in human showed also increases in the numbers of DCs during the recovery phase in the peripheral blood of cancer patients receiving a combinatorial treatment of CTX and the growth factors G-CSF or GM-CSF [35, 103, 130]. Although it was not clear in these studies whether the increase in the frequency of DCs is solely due to the effects induced by CTX or the growth factors, our results demonstrate that CTX per se is capable of inducing DC expansion in the peripheral blood in a murine model. This notion is consistent with the capability of CTX to induce mobilization of hematopoietic stem cells (CD34+) from bone marrow to circulation [71, 82, 87, 133]; these CD34+ showed higher differentiation rate into DCs when cultured in vitro with growth factors [35]. Our in vitro studies also demonstrated higher tendency of BM from CTX-treated mice to generate DCs in vitro [116]. Furthermore, the anti-tumor T cell response and protective immunity in mice that received TBI and immune reconstitution was found to be associated with significant increases in the number of lymph node DCs, which were further enhanced after vaccination [75]. Additionally, one recent study showed that the beneficial effects of CTX to the anti-tumor effects of T cells associate with DC turnover in spleen, liver, and tumor site. These newly recruited DCs were suggested to be originated from proliferating early DC progenitors and secreted more IL-12 and less IL-10 compared to those from untreated tumor-bearing animals. They were also fully capable of priming T cell responses and ineffective in inducing expansion of Treg cells [104]. In the same vein, we also found that post-CTX expansion of DCs was associated with proliferation of DCs in bone marrow during the lymphopenic phase and in the blood and spleen during the recovery phase [114]. Interestingly, CTX induced a dynamic surge in the expression of growth factors and chemokines in bone marrow, where CCR2 and Flt3 signaling pathways were critical for DC expansion [114]. Taken together, it could be suggested that the beneficial effects of lymphodepletion, such as those induced by CTX, to enhance the antitumor potency of T cells extend beyond the well-documented cytotoxicity and lymphodepletion and include the augmented presence of DCs during the recovery phase. This observation of DC rebounding at certain time points after induction of lymphodepletion indicate that in addition to the rapid activation of DCs following the induction of lymphopenia, increases in the numbers of these cells also occur at the recovery phase. This would form a foundation for a new rationale design for immunotherapeutic strategies post-lymphodepletion.
Besides its induction of myeloid DC expansion, CTX can also expand other myeloid cells. Several previous studies, including ours, have reported increases in the numbers of cells expressing the phenotype (Gr-1+CD11b+) of the myeloid-derived suppressor cells (MDSC) in the peripheral blood and spleen [3, 4, 97, 118]. Our most recent studies showed that this MDSC expansion start to gradually decrease prior the peak of DC expansion (Fig. 1). Consistent with the previous studies, we have observed recently that cancer patients treated with chemotherapy containing CTX harbor high number of MDSC, which are capable of suppressing T cell responses in vitro [25]. Previous clinical studies also showed that CTX-induced MDSC expansion could limit T cell responses [3, 4, 97]. We found in our preclinical studies, however, that MDSC expansion by CTX does not abrogate its beneficial effects to adoptively transferred T cell responses to peptide vaccination [115, 118]. Because vaccination, in particular, in the presence of a potent adjuvant such TLR agonists, can induce post-vaccination inflammatory mediators, it can be suggested that the presence of an inflammatory microenvironment may induce activation of MDSC and drive their differentiation into beneficial T cell activators or at least in part block their suppressor function. A previous study also described a role of MDSC in mediating the antitumor effects of CTX by a nitric oxide-dependent mechanism [97]. This notion is consistent with recent data in the literature showing that treatment of Gr-1+CD11b+ cells, isolated from immunocompromised animals or patients, with stimuli such as GM-CSF/IL-4, and all-trans-retinoic acid, can block the suppressive activity of these cells and drive their differentiation to mature DCs [2, 32, 93, 94]. Moreover, Gr-1+CD11b+ cells from tumor-bearing mice have been found to acquire the phenotype characteristic to DCs (CD11chigh) upon their adoptive transfer into naïve, but not tumor-bearing, recipient mice [64, 76]. Therefore, it might be feasible that application of strategies that can induce simultaneous activation of both DCs and MDSC expanded post-chemotherapy favor the creation of an overall stimulatory rather than inhibitory host microenvironment. Alternatively, the immunosuppressive activities of the expanded MDSC by CTX and the tumor can be blocked by targeting their regulatory pathways, including increased production of reactive oxygen species, the metabolism of the amino acid l-arginine by arginase I and nitric oxide synthetase 2, and the high levels of indoleamine-2,3-dioxygenase (IDO). Drugs such as the IDO inhibitor 1-methyl-d-tryptophan (d-1mT) and the multi-targeted receptor tyrosine kinase inhibitors sunitinib have been found to block the suppressor function of MDSC [22, 30, 64, 65, 84, 137].
Fig. 1.
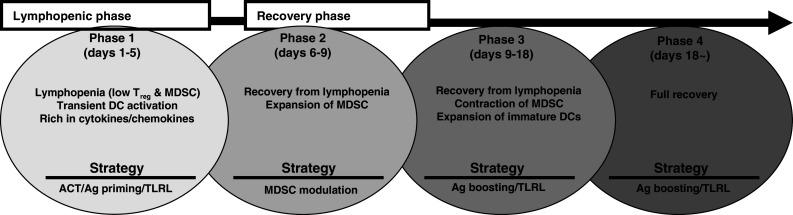
Suggested phases post-CTX therapy and proposed approaches for their manipulation in vivo to benefit adoptive T cell therapy (ACT). CTX treatment induces a rapid lymphopenia for about 5 days followed by a gradual cellular recovery with a full recovery after 18–20 days of treatment. The cellular recovery is characterized by an expansion of cells with immature myeloid-derived suppressor cell (MDSC) phenotype (Gr-1+CD11b+) followed by an expansion of DCs with CD11c+CD11b+ phenotype in the peripheral blood, spleen, and liver. The expansion of DCs occurs when the expanded MDSC start to contract. Vaccination with a tumor antigen (Ag) and a potent adjuvant, such as a TLR ligand (TLRL) can activate the expanded DCs and MDSC, resulting in beneficial host microenvironment to T cell responses
How expansion of DCs at recovery phase benefits adoptive T cell therapy
If DCs at the lymphopenic phase are important because of their activation state, how could an augmented presence of DCs during the recovery phase benefit T cell responses if they express immature phenotype? It could be postulated that inflammatory cytokines produced by effector cells after antigen priming at the lymphopenic phase induce the activation of DCs during their expansion and thus augment the immune response to antigen boosting. The beneficial effect of the inflammatory cytokine milieu on DC activation, however, is expected to be weak since its peak (days 5–7) of induction precedes the peak (days 10–14) of DC expansion. Therefore, either a second vaccination or provision of exogenous inflammatory adjuvant such as a TLR agonist at the recovery phase of CTX would be required to activate rebounding DCs (Fig. 2). This notion is consistent with our studies showing the minimal increases in the post-vaccination responses of OT-1 CD8+ cells when the antigen priming was performed at the time of DCs expansion in absence of exogenous adjuvant poly(I:C) administration in contrast to the marked increase in the post-vaccination CD8+ T cell responses when poly(I:C) was co-administered with peptide vaccination at the time of DC expansion [118]. The advantage of the provision of poly(I:C) could be attributed to its rapid induction of the inflammatory cytokines TNF-α, MCP-1, IFN-α, and IL-6 [114, 115, 118]. Indeed, induction of these cytokines was associated with the appearance of activated CCR7highCD40highCD80high DCs in lymph nodes, coinciding with a significant decrease in the numbers of DCs in the peripheral blood. Given that CCR7 is essential for migration of activated DCs to lymph nodes [19], its up-regulation would suggest that the appearance of DCs in lymph nodes of CTX-treated mice after poly(I:C) treatment is, at least in part, due to their recruitment from the peripheral blood upon their maturation.
Fig. 2.
Proposed paradigm for DC activation post-lymphodepletion and its impact on antigen-specific responses of adoptive T cell therapy. Treatment with a lymphodepleting dose of CTX or TBI induces a rapid lymphopenia likely associated with microbial translocation due to the damage of the intestinal tract. Lymphopenia can result in elimination or an inbalance of regulatory elements, including Treg cells and myeloid-derived suppressor cells. Microbial translocation can also result in the release of microbial products such as LPS, which leads to activation of antigen presenting cells, in particular DCs. The intensity of these events, however, decreases gradually during the recovery phase from lymphopenia, in which DCs are expanded in circulation due to mobilization of DC precursors (solid line). Adoptively transferred antigen-specific T cells (dotted line) during the lymphopenic phase can benefit from the space “niche” and the more available survival cytokines and thus show homeostatic proliferation. The proliferation of these cells is further increased if the host is also primed with a specific antigen, particularly in the presence of endogenous activated DCs. The inflammatory microenvironment created during the peak of the antigen-specific responses of donor T cells to antigen priming might result in activation of the expanded DCs during the recovery phase, and thus could slightly augment the T cell responses when the antigen boosting occurs at this time point. Addition of an inflammatory adjuvant (e.g. TLR agonist) at the time of DC surge, however, would accentuate the inflammatory microenvironment that can induce the full activation and maturation of the expanded DCs and their migration to LNs, resulting in robust antigen-specific responses of donor cells (dotted line)
Using the pmel-1 mouse model, in which CD8+ T cells can recognize gp10025–33 peptide only in the presence of a potent adjuvant [92], we found that priming and boosting with gp100 peptide plus poly(I:C) treatment at the lymphopenic and recovery phases induced dramatic increases in CD8+ T cell responses and regression of B16 tumor growth than those obtained after vaccination at either phase or at both phases but in absence of poly(I:C) treatment [115]. Importantly, depletion of DCs before boosting resulted in a significant abrogation of CD8+ T cell responses, indicating to the importance of post-CTX expanded DCs. Similar requirement of DCs to antigen recall responses has been reported in the viral setting [19]. Taken together, it appears that expansion of a large pool of DCs per se can slightly benefit the CD8+ T cell responses, but can dramatically augment the responses if an inflammatory signal capable of maturing DCs is concomitantly induced. This would explain why prime-boost vaccination with peptide post-chemotherapy or irradiation is only effective when exogenous adjuvant is co-administered during vaccination [16, 41, 60, 67, 74, 90, 91, 99, 113, 115]. It would also explain the enhanced T cell responses after induction of expansion of endogenous DCs with mobilizing factors, in particular Flt3L, followed by vaccination in combination of the TLR7 agonist imiquimod [121] and TLR9 agonist CpG ODN [29, 56].
DC rebounding post-lymphodepletion may benefit ex vivo DC vaccines
The expansion of endogenous DCs during recovery from lymphodepletion could also benefit ex vivo DC-based vaccination. Recent studies have shown that single vaccination with antigen-loaded DCs immediately after TBI- or CTX-induced lymphopenia is not sufficient to induce significant tumor-specific T cell responses, unless a second DC vaccination is performed after 7–10 days [21, 74, 89]. Because DCs are already expanded during the second DC vaccination, it could be postulated that endogenous DCs provide a help to the exogenous DCs. Exogenously administrated DCs migrate to draining lymph nodes, leading to a strong anti-tumor response. Migration of DCs to draining lymph nodes, however, is limited when the draining lymph nodes are saturated with the migrated cells [27, 50, 68, 89]. Conditioning lymph nodes, for example with pre-injection of naïve unpulsed DCs or inflammatory cytokines, such as TNF-α, can significantly enhance the migration and retention of exogenous DCs, resulting in a robust anti-tumor immunity [78]. Therefore, several scenarios could be postulated to explain how expansion of endogenous DCs post-lymphodepletion augments vaccination with exogenous DC vaccine.
Induction of lymphopenia per se creates an immune niche in the lymph nodes, enhancing their capacity to recruit higher numbers of endogenous DCs upon creation of the inflammatory microenvironment and their activation. This would explain the enhanced survival and migration of exogenous DCs administered after chemotherapy [20].
Migration of endogenous DCs into lymph nodes would condition the latter to further increase their saturation (ceiling) capability for the exogenous DCs, resulting in arrival of higher numbers of antigen-bearing DCs. Since the magnitude of the antigen-specific T cell responses correlates with the numbers of DCs in the lymphoid tissues, in particular lymph nodes [27], arrival of more numbers of antigen-bearing DCs along with the arrival of adoptively transferred T cells would limit the antigen competition for the antigen recognition and results in higher frequency of the antigen-specific T cells. This would explain our recent observation of the appearance of high numbers of activated DCs mainly in lymph nodes after injection of poly(I:C) at the peak of DC expansion even in the absence of vaccination [27, 115]. It would also explain the enhanced ex vivo DC-based vaccination in combination with CTX treatment without adjuvant [44, 72] or with poly(I:C) [37].
It is also possible that some of the antigen loaded in the exogenous DCs might be transferred into the endogenous DCs when both arrive to lymph nodes, resulting in antigen presentation by both of these DCs and better antigen display to T cells. This would explain the enhanced anti-tumor responses after the administration of DCs with no antigen [20, 124, 127].
Advantages of the sequential activation of DC at lymphopenic and recovery phases
Recent studies have reported the capability of CD8+ T cells to induce regression of established B16 tumor. The anti-tumor effects in these studies, however, required aggressive treatment protocols consisting of: (1) TBI-induced lymphodepletion or myelodepletion followed by hematopoietic stem cell transplant; (2) adoptive transfer of in vitro cytokine-conditioned antigen-stimulated T cells; (3) vaccination with 2 × 107 plaque forming units of a recombinant fowlpox virus encoding gp10025–33 or with repeated ex vivo vaccination with peptide-pulsed DCs; and (4) exogenous administration of high doses of IL-2 [61, 132, 134]. The efficacy of these anti-tumor treatment protocols could be explained by the hierarchy of activation of DCs during the lymphopenic phase and their expansion during the restoration phase (Fig. 2). This hierarchy of DC responses would suggest a harmonized multiple mechanisms involved in the beneficial effect of lymphodepletion to adoptive T cell therapy and warrants the reconsideration of the timing between adoptive T cell transfer into a lymphodepleted host and subsequent vaccinations. As shown in Fig. 2, antigen priming along with a TLR agonist could be delivered at the lymphopenic phase, when T cells could benefit from the activated DCs, and then at the recovery phase again with a TLR agonist when T cells can benefit from the DC surge. This prime-boost vaccination regimen at these certain time points post-induction of lymphodepletion would obviate the need for more complicated and potentially toxic treatment regimens such as in vivo IL-2 therapy. In line with this notion, a complete regression of a large B16 melanoma was achieved when adoptive T cell transfer and vaccination with adenoviral vector expressing gp100 antigen post-CTX preconditioning was boosted by persistent stimulation of innate immunity through adjuvant peritumoral injections of the TLR9 agonist CpG and the TLR3 agonist poly(I:C) [66]. Interestingly, the administration of CpG and poly(I:C) in this study was performed about 12 days after CTX treatment, the time point when DC rebounding is maximal [104, 114, 115], indicating that the requirement of CPG/poly(I:C) injection to the success of this protocol is due to the activation of the rebounding DCs. Given the robust anti-tumor effect of our prime-boost vaccination with gp100 peptide in combination with poly(I:C), we suggest that not only IL-2 can be replaced by TLR agonist but also vaccination with adenoviral expressing the target antigen can be replaced with antigenic peptide mixed with a TLR agonist. This strategy would lead to a significant improvement in the application of chemo-immunotherapy, opening a new avenue for a simple but effective anti-cancer therapy.
Conclusion and prospective
There is growing evidence that DCs are altered in numbers and phenotypes at precise time points after the induction of lymphodepletion, resulting in a biphasic effect on DCs: a rapid induction of DC activation during the lymphopenic phase, and increasing their frequency without affecting their activation during the recovery phase. Exogenous administration of inflammatory adjuvants such as TLR agonists can induce stimulation and maturation of this expanded DCs and their migration to LNs. These fine-tuned responses of DCs are of a paramount significance since a precise prime-boost vaccination along with a TLR-mediated targeting of DCs at both the lymphopenic and recovery phases post-lymphodepletion could profoundly improve the anti-tumor responses of adoptive T cell therapy. These roles of DCs would provide a useful foundation for the design of immunotherapy regimens combining tumor vaccines, primed T cells, and lymphodepletion. Future studies are required to focus on maximizing the role of DCs during both the lymphopenic and recovery phases post-lymphodepletion. For instance, DC rebounding can be augmented by agents such as Flt3L, and DC survival and trafficking to lymph nodes can be enhanced by chemokines such as CCL21 (CCR7 ligand), and DC activation can be augmented by combination of multiple TLR agonists. Further studies are also required to unveil the molecular mechanisms underlying the expansion of DCs post-induction of lymphodepletion. Progress in the research in this area can advance our understanding of the application of lymphodepletion for the maximal benefit of immunotherapy in the different clinical settings.
Acknowledgments
This work was supported by the National Institutes of Health Grant 1 R01 CA94856-01.
References
- 1.Abad JD, Wrzensinski C, Overwijk W, De Witte MA, Jorritsma A, Hsu C, Gattinoni L, Cohen CJ, Paulos CM, Palmer DC, Haanen JB, Schumacher TN, Rosenberg SA, Restifo NP, Morgan RA. T-cell receptor gene therapy of established tumors in a murine melanoma model. J Immunother. 2008;31:1–6. doi: 10.1097/CJI.0b013e31815c193f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 3.Angulo I, de las Heras FG, Garcia-Bustos JF, Gargallo D, Munoz-Fernandez MA, Fresno M. Nitric oxide-producing CD11b(+)Ly-6G(Gr-1)(+)CD31(ER-MP12)(+) cells in the spleen of cyclophosphamide-treated mice: implications for T-cell responses in immunosuppressed mice. Blood. 2000;95:212–220. [PubMed] [Google Scholar]
- 4.Angulo I, Jimenez-Diaz MB, Garcia-Bustos JF, Gargallo D, de las Heras FG, Munoz-Fernandez MA, Fresno M. Candida albicans infection enhances immunosuppression induced by cyclophosphamide by selective priming of suppressive myeloid progenitors for NO production. Cell Immunol. 2002;218:46–58. doi: 10.1016/s0008-8749(02)00521-x. [DOI] [PubMed] [Google Scholar]
- 5.Antony PA, Paulos CM, Ahmadzadeh M, Akpinarli A, Palmer DC, Sato N, Kaiser A, Hinrichs CS, Klebanoff CA, Tagaya Y, Restifo NP. Interleukin-2-dependent mechanisms of tolerance and immunity in vivo. J Immunol. 2006;176:5255–5266. doi: 10.4049/jimmunol.176.9.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antony PA, Piccirillo CA, Akpinarli A, Finkelstein SE, Speiss PJ, Surman DR, Palmer DC, Chan CC, Klebanoff CA, Overwijk WW, Rosenberg SA, Restifo NP. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, Yang H, Amigorena S, Ryffel B, Barrat FJ, Saftig P, Levi F, Lidereau R, Nogues C, Mira JP, Chompret A, Joulin V, Clavel-Chapelon F, Bourhis J, Andre F, Delaloge S, Tursz T, Kroemer G, Zitvogel L. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 8.Apostolopoulos V, Popovski V, McKenzie IF. Cyclophosphamide enhances the CTL precursor frequency in mice immunized with MUC1-mannan fusion protein (M-FP) J Immunother. 1998;21:109–113. doi: 10.1097/00002371-199803000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Awwad M, North RJ. Cyclophosphamide (Cy)-facilitated adoptive immunotherapy of a Cy- resistant tumour. Evidence that Cy permits the expression of adoptive T- cell mediated immunity by removing suppressor T cells rather than by reducing tumour burden. Immunology. 1988;65:87–92. [PMC free article] [PubMed] [Google Scholar]
- 10.Belardelli F, Ferrantini M. Cytokines as a link between innate and adaptive antitumor immunity. Trends Immunol. 2002;23:201–208. doi: 10.1016/s1471-4906(02)02195-6. [DOI] [PubMed] [Google Scholar]
- 11.Bellone M. Apoptosis, cross-presentation, and the fate of the antigen specific immune response. Apoptosis. 2000;5:307–314. doi: 10.1023/a:1009671105696. [DOI] [PubMed] [Google Scholar]
- 12.Ben-Hur H, Kossoy G, Kossoy N, Zusman I. Response of the immune system of mammary tumor-bearing rats to cyclophosphamide and soluble low-molecular-mass tumor-associated antigens: the bone marrow and thymus. Int J Mol Med. 2002;10:517–521. [PubMed] [Google Scholar]
- 13.Ben-Hur H, Kossoy G, Tendler Y, Kossoy N, Zusman I. Effects of cyclophosphamide and soluble tumor-associated antigens on lymphoid infiltration, proliferative activity and rate of apoptosis in chemically-induced rat mammary tumors. In Vivo. 2002;16:287–292. [PubMed] [Google Scholar]
- 14.Ben-Hur H, Kossoy G, Zandbank J, Zusman I. Response of the immune system of mammary tumor-bearing rats to cyclophosphamide and soluble low-molecular-mass tumor-associated antigens: rate of lymphoid infiltration and distribution of T lymphocytes in tumors. Int J Mol Med. 2002;9:425–430. [PubMed] [Google Scholar]
- 15.Berenson JR, Einstein AB, Jr, Fefer A. Syngeneic adoptive immunotherapy and chemoimmunotherapy of a Friend leukemia: requirement for T cells. J Immunol. 1975;115:234–238. [PubMed] [Google Scholar]
- 16.Berraondo P, Nouze C, Preville X, Ladant D, Leclerc C. Eradication of large tumors in mice by a tritherapy targeting the innate, adaptive, and regulatory components of the immune system. Cancer Res. 2007;67:8847–8855. doi: 10.1158/0008-5472.CAN-07-0321. [DOI] [PubMed] [Google Scholar]
- 17.Bracci L, Moschella F, Sestili P, La Sorsa V, Valentini M, Canini I, Baccarini S, Maccari S, Ramoni C, Belardelli F, Proietti E. Cyclophosphamide enhances the antitumor efficacy of adoptively transferred immune cells through the induction of cytokine expression, B-cell and T-cell homeostatic proliferation, and specific tumor infiltration. Clin Cancer Res. 2007;13:644–653. doi: 10.1158/1078-0432.CCR-06-1209. [DOI] [PubMed] [Google Scholar]
- 18.Brode S, Cooke A. Immune-potentiating effects of the chemotherapeutic drug cyclophosphamide. Crit Rev Immunol. 2008;28:109–126. doi: 10.1615/critrevimmunol.v28.i2.20. [DOI] [PubMed] [Google Scholar]
- 19.Carbone FR, Belz GT, Heath WR. Transfer of antigen between migrating and lymph node-resident DCs in peripheral T-cell tolerance and immunity. Trends Immunol. 2004;25:655–658. doi: 10.1016/j.it.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Cavanagh WA, Tjoa BA, Ragde H. Chemotherapy followed by syngeneic dendritic cell injection in the mouse: findings and implications for human treatment. Urology. 2007;70:36–41. doi: 10.1016/j.urology.2007.06.1127. [DOI] [PubMed] [Google Scholar]
- 21.Cohen S, Haimovich J, Hollander N. Dendritic cell-based therapeutic vaccination against myeloma: vaccine formulation determines efficacy against light chain myeloma. J Immunol. 2009;182:1667–1673. doi: 10.4049/jimmunol.182.3.1667. [DOI] [PubMed] [Google Scholar]
- 22.Corzo CA, Cotter MJ, Cheng P, Cheng F, Kusmartsev S, Sotomayor E, Padhya T, McCaffrey TV, McCaffrey JC, Gabrilovich DI. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J Immunol. 2009;182:5693–5701. doi: 10.4049/jimmunol.0900092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curtsinger JM, Lins DC, Mescher MF. CD8+ memory T cells (CD44high, Ly-6C+) are more sensitive than naive cells to (CD44low, Ly-6C-) to TCR/CD8 signaling in response to antigen. J Immunol. 1998;160:3236–3243. [PubMed] [Google Scholar]
- 24.Diaz-Montero CM, El Naggar S, Al Khami A, El Naggar R, Montero AJ, Cole DJ, Salem ML. Priming of naive CD8+ T cells in the presence of IL-12 selectively enhances the survival of CD8+CD62Lhi cells and results in superior anti-tumor activity in a tolerogenic murine model. Cancer Immunol Immunother. 2008;57:563–572. doi: 10.1007/s00262-007-0394-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eberlein TJ, Rosenstein M, Rosenberg SA. Regression of a disseminated syngeneic solid tumor by systemic transfer of lymphoid cells expanded in interleukin 2. J Exp Med. 1982;156:385–397. doi: 10.1084/jem.156.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eggert AA, Schreurs MW, Boerman OC, Oyen WJ, de Boer AJ, Punt CJ, Figdor CG, Adema GJ. Biodistribution and vaccine efficiency of murine dendritic cells are dependent on the route of administration. Cancer Res. 1999;59:3340–3345. [PubMed] [Google Scholar]
- 28.Eyrich M, Burger G, Marquardt K, Budach W, Schilbach K, Niethammer D, Schlegel PG. Sequential expression of adhesion and costimulatory molecules in graft-versus-host disease target organs after murine bone marrow transplantation across minor histocompatibility antigen barriers. Biol Blood Marrow Transplant. 2005;11:371–382. doi: 10.1016/j.bbmt.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Fong L, Hou Y, Rivas A, Benike C, Yuen A, Fisher GA, Davis MM, Engleman EG. Altered peptide ligand vaccination with Flt3 ligand expanded dendritic cells for tumor immunotherapy. Proc Natl Acad Sci USA. 2001;98:8809–8814. doi: 10.1073/pnas.141226398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 32.Garrity T, Pandit R, Wright MA, Benefield J, Keni S, Young MR. Increased presence of CD34+ cells in the peripheral blood of head and neck cancer patients and their differentiation into dendritic cells. Int J Cancer. 1997;73:663–669. doi: 10.1002/(sici)1097-0215(19971127)73:5<663::aid-ijc9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 33.Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, Hwang LN, Yu Z, Wrzesinski C, Heimann DM, Surh CD, Rosenberg SA, Restifo NP. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gattinoni L, Powell DJ, Jr, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6:383–393. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gazitt Y, Akay C, Thomas C., 3rd No polarization of type 1 or type 2 precursor dendritic cells in peripheral blood stem cell collections of non-hodgkin’s lymphoma patients mobilized with cyclophosphamide plus G-CSF, GM-CSF, or GM-CSF followed by G-CSF. Stem Cells Dev. 2006;15:269–277. doi: 10.1089/scd.2006.15.269. [DOI] [PubMed] [Google Scholar]
- 36.Gruber A, Brocker T. MHC class I-positive dendritic cells (DC) control CD8 T cell homeostasis in vivo: T cell lymphopenia as a prerequisite for DC-mediated homeostatic proliferation of naive CD8 T cells. J Immunol. 2005;175:201–206. doi: 10.4049/jimmunol.175.1.201. [DOI] [PubMed] [Google Scholar]
- 37.Guo F, Chang CK, Fan HH, Nie XX, Ren YN, Liu YY, Zhao LH. Anti-tumour effects of exosomes in combination with cyclophosphamide and polyinosinic–polycytidylic acid. J Int Med Res. 2008;36:1342–1353. doi: 10.1177/147323000803600623. [DOI] [PubMed] [Google Scholar]
- 38.Hallahan DE, Spriggs DR, Beckett MA, Kufe DW, Weichselbaum RR. Increased tumor necrosis factor alpha mRNA after cellular exposure to ionizing radiation. Proc Natl Acad Sci USA. 1989;86:10104–10107. doi: 10.1073/pnas.86.24.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hallahan DE, Staba-Hogan MJ, Virudachalam S, Kolchinsky A. X-ray-induced P-selectin localization to the lumen of tumor blood vessels. Cancer Res. 1998;58:5216–5220. [PubMed] [Google Scholar]
- 40.Hallahan DE, Virudachalam S. Accumulation of P-selectin in the lumen of irradiated blood vessels. Radiat Res. 1999;152:6–13. [PubMed] [Google Scholar]
- 41.He H, Wisner P, Yang G, Hu HM, Haley D, Miller W, O’Hara A, Alvord WG, Clegg CH, Fox BA, Urba WJ, Walker EB. Combined IL-21 and Low-Dose IL-2 therapy induces anti-tumor immunity and long-term curative effects in a murine melanoma tumor model. J Transl Med. 2006;4:24. doi: 10.1186/1479-5876-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hinrichs CS, Spolski R, Paulos CM, Gattinoni L, Kerstann KW, Palmer DC, Klebanoff CA, Rosenberg SA, Leonard WJ, Restifo NP. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood. 2008;111:5326–5333. doi: 10.1182/blood-2007-09-113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hirschhorn-Cymerman D, Rizzuto GA, Merghoub T, Cohen AD, Avogadri F, Lesokhin AM, Weinberg AD, Wolchok JD, Houghton AN. OX40 engagement and chemotherapy combination provides potent antitumor immunity with concomitant regulatory T cell apoptosis. J Exp Med. 2009;206:1103–1116. doi: 10.1084/jem.20082205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holtl L, Ramoner R, Zelle-Rieser C, Gander H, Putz T, Papesh C, Nussbaumer W, Falkensammer C, Bartsch G, Thurnher M. Allogeneic dendritic cell vaccination against metastatic renal cell carcinoma with or without cyclophosphamide. Cancer Immunol Immunother. 2005;54:663–670. doi: 10.1007/s00262-004-0629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hong JH, Chiang CS, Tsao CY, Lin PY, McBride WH, Wu CJ. Rapid induction of cytokine gene expression in the lung after single and fractionated doses of radiation. Int J Radiat Biol. 1999;75:1421–1427. doi: 10.1080/095530099139287. [DOI] [PubMed] [Google Scholar]
- 46.Hoover SK, Barrett SK, Turk TM, Lee TC, Bear HD. Cyclophosphamide and abrogation of tumor-induced suppressor T cell activity. Cancer Immunol Immunother. 1990;31:121–127. doi: 10.1007/BF01742376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Horvath R, Budinsky V, Kayserova J, Kalina T, Formankova R, Stary J, Bartunkova J, Sedlacek P, Spisek R. Kinetics of dendritic cells reconstitution and costimulatory molecules expression after myeloablative allogeneic haematopoetic stem cell transplantation: implications for the development of acute graft-versus host disease. Clin Immunol. 2009;131:60–69. doi: 10.1016/j.clim.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 48.Hu DE, Moore AM, Thomsen LL, Brindle KM. Uric acid promotes tumor immune rejection. Cancer Res. 2004;64:5059–5062. doi: 10.1158/0008-5472.CAN-04-1586. [DOI] [PubMed] [Google Scholar]
- 49.Huang J, Wang Y, Guo J, Lu H, Lin X, Ma L, Teitz-Tennenbaum S, Chang AE, Li Q. Radiation-induced apoptosis along with local and systemic cytokine elaboration is associated with DC plus radiotherapy-mediated renal cell tumor regression. Clin Immunol. 2007;123:298–310. doi: 10.1016/j.clim.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 50.Huck SP, Tang SC, Andrew KA, Yang J, Harper JL, Ronchese F. Activation and route of administration both determine the ability of bone marrow-derived dendritic cells to accumulate in secondary lymphoid organs and prime CD8+ T cells against tumors. Cancer Immunol Immunother. 2008;57:63–71. doi: 10.1007/s00262-007-0350-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hwang LN, Yu Z, Palmer DC, Restifo NP. The in vivo expansion rate of properly stimulated transferred CD8+ T cells exceeds that of an aggressively growing mouse tumor. Cancer Res. 2006;66:1132–1138. doi: 10.1158/0008-5472.CAN-05-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ibe S, Qin Z, Schuler T, Preiss S, Blankenstein T. Tumor rejection by disturbing tumor stroma cell interactions. J Exp Med. 2001;194:1549–1559. doi: 10.1084/jem.194.11.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ikezawa Y, Nakazawa M, Tamura C, Takahashi K, Minami M, Ikezawa Z (2005) Cyclophosphamide decreases the number, percentage and the function of CD25(+) CD4(+) regulatory T cells, which suppress induction of contact hypersensitivity. J Dermatol Sci [DOI] [PubMed]
- 54.Ishihara H, Tanaka I, Nemoto K, Tsuneoka K, Cheeramakara C, Yoshida K, Ohtsu H. Immediate-early, transient induction of the interleukin-1 beta gene in mouse spleen macrophages by ionizing radiation. J Radiat Res (Tokyo) 1995;36:112–124. doi: 10.1269/jrr.36.112. [DOI] [PubMed] [Google Scholar]
- 55.Jabbari A, Harty JT. Cutting edge: differential self-peptide/MHC requirement for maintaining CD8 T cell function versus homeostatic proliferation. J Immunol. 2005;175:4829–4833. doi: 10.4049/jimmunol.175.8.4829. [DOI] [PubMed] [Google Scholar]
- 56.Jahrsdorfer B, Weiner GJ. CpG oligodeoxynucleotides as immunotherapy in cancer. Update Cancer Ther. 2008;3:27–32. doi: 10.1016/j.uct.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kawashima H. Roles of sulfated glycans in lymphocyte homing. Biol Pharm Bull. 2006;29:2343–2349. doi: 10.1248/bpb.29.2343. [DOI] [PubMed] [Google Scholar]
- 58.Kedl RM, Rees WA, Hildeman DA, Schaefer B, Mitchell T, Kappler J, Marrack P. T cells compete for access to antigen-bearing antigen-presenting cells. J Exp Med. 2000;192:1105–1113. doi: 10.1084/jem.192.8.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kepp O, Tesniere A, Schlemmer F, Michaud M, Senovilla L, Zitvogel L, Kroemer G. Immunogenic cell death modalities and their impact on cancer treatment. Apoptosis. 2009;14:364–375. doi: 10.1007/s10495-008-0303-9. [DOI] [PubMed] [Google Scholar]
- 60.Klebanoff CA, Finkelstein SE, Surman DR, Lichtman MK, Gattinoni L, Theoret MR, Grewal N, Spiess PJ, Antony PA, Palmer DC, Tagaya Y, Rosenberg SA, Waldmann TA, Restifo NP. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci USA. 2004;101:1969–1974. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, Palmer DC, Antony PA, Hwang ST, Rosenberg SA, Waldmann TA, Restifo NP. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci USA. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klebanoff CA, Gattioni L, Restifo NP. CD8+ T-cell memory in tumot immunology and immunotherapy. Immunol Rev. 2006;211:214–224. doi: 10.1111/j.0105-2896.2006.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Klebanoff CA, Khong HT, Antony PA, Palmer DC, Restifo NP. Sinks, suppressors and antigen presenters: how lymphodepletion enhances T cell-mediated tumor immunotherapy. Trends Immunol. 2005;26:111–117. doi: 10.1016/j.it.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ko HJ, Lee JM, Kim YJ, Kim YS, Lee KA, Kang CY. Immunosuppressive myeloid-derived suppressor cells can be converted into immunogenic APCs with the help of activated NKT cells: an alternative cell-based antitumor vaccine. J Immunol. 2009;182:1818–1828. doi: 10.4049/jimmunol.0802430. [DOI] [PubMed] [Google Scholar]
- 65.Ko JS, Bukowski RM, Fincke JH. Myeloid-derived suppressor cells: a novel therapeutic target. Curr Oncol Rep. 2009;11:87–93. doi: 10.1007/s11912-009-0014-6. [DOI] [PubMed] [Google Scholar]
- 66.Kohlmeyer J, Cron M, Landsberg J, Bald T, Renn M, Mikus S, Bondong S, Wikasari D, Gaffal E, Hartmann G, Tuting T. Complete regression of advanced primary and metastatic mouse melanomas following combination chemoimmunotherapy. Cancer Res. 2009;69:6265–6274. doi: 10.1158/0008-5472.CAN-09-0579. [DOI] [PubMed] [Google Scholar]
- 67.Koike N, Pilon-Thomas S, Mule JJ. Nonmyeloablative chemotherapy followed by T-cell adoptive transfer and dendritic cell-based vaccination results in rejection of established melanoma. J Immunother. 2008;31:402–412. doi: 10.1097/CJI.0b013e31816cabbb. [DOI] [PubMed] [Google Scholar]
- 68.Lappin MB, Weiss JM, Delattre V, Mai B, Dittmar H, Maier C, Manke K, Grabbe S, Martin S, Simon JC. Analysis of mouse dendritic cell migration in vivo upon subcutaneous and intravenous injection. Immunology. 1999;98:181–188. doi: 10.1046/j.1365-2567.1999.00850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lau J, Sartor M, Bradstock KF, Vuckovic S, Munster DJ, Hart DN. Activated circulating dendritic cells after hematopoietic stem cell transplantation predict acute graft-versus-host disease. Transplantation. 2007;83:839–846. doi: 10.1097/01.tp.0000258731.38149.61. [DOI] [PubMed] [Google Scholar]
- 70.Limpens J, Van Meijer M, Van Santen HM, Germeraad WT, Hoeben-Schornagel K, Breel M, Scheper RJ, Kraal G. Alterations in dendritic cell phenotype and function associated with immunoenhancing effects of a subcutaneously administered cyclophosphamide derivative. Immunology. 1991;73:255–263. [PMC free article] [PubMed] [Google Scholar]
- 71.Liu F, Poursine-Laurent J, Link DC. The granulocyte colony-stimulating factor receptor is required for the mobilization of murine hematopoietic progenitors into peripheral blood by cyclophosphamide or interleukin-8 but not flt-3 ligand. Blood. 1997;90:2522–2528. [PubMed] [Google Scholar]
- 72.Liu JY, Wu Y, Zhang XS, Yang JL, Li HL, Mao YQ, Wang Y, Cheng X, Li YQ, Xia JC, Masucci M, Zeng YX. Single administration of low dose cyclophosphamide augments the antitumor effect of dendritic cell vaccine. Cancer Immunol Immunother. 2007;56:1597–1604. doi: 10.1007/s00262-007-0305-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lotze MT, Rosenberg SA. Results of clinical trials with the administration of interleukin 2 and adoptive immunotherapy with activated cells in patients with cancer. Immunobiology. 1986;172:420–437. doi: 10.1016/S0171-2985(86)80122-X. [DOI] [PubMed] [Google Scholar]
- 74.Lou Y, Wang G, Lizee G, Kim GJ, Finkelstein SE, Feng C, Restifo NP, Hwu P. Dendritic cells strongly boost the antitumor activity of adoptively transferred T cells in vivo. Cancer Res. 2004;64:6783–6790. doi: 10.1158/0008-5472.CAN-04-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ma J, Urba WJ, Si L, Wang Y, Fox BA, Hu HM. Anti-tumor T cell response and protective immunity in mice that received sublethal irradiation and immune reconstitution. Eur J Immunol. 2003;33:2123–2132. doi: 10.1002/eji.200324034. [DOI] [PubMed] [Google Scholar]
- 76.Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev. 2008;222:162–179. doi: 10.1111/j.1600-065X.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 77.Marincola FM, Ettinghausen S, Cohen PA, Cheshire LB, Restifo NP, Mule JJ, Rosenberg SA. Treatment of established lung metastases with tumor-infiltrating lymphocytes derived from a poorly immunogenic tumor engineered to secrete human TNF-alpha. J Immunol. 1994;152:3500–3513. [PMC free article] [PubMed] [Google Scholar]
- 78.MartIn-Fontecha A, Sebastiani S, Hopken UE, Uguccioni M, Lipp M, Lanzavecchia A, Sallusto F. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J Exp Med. 2003;198:615–621. doi: 10.1084/jem.20030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McBride WH, Chiang CS, Olson JL, Wang CC, Hong JH, Pajonk F, Dougherty GJ, Iwamoto KS, Pervan M, Liao YP. A sense of danger from radiation. Radiat Res. 2004;162:1–19. doi: 10.1667/rr3196. [DOI] [PubMed] [Google Scholar]
- 80.Mihalyo MA, Doody AD, McAleer JP, Nowak EC, Long M, Yang Y, Adler AJ. In vivo cyclophosphamide and IL-2 treatment impedes self-antigen-induced effector CD4 cell tolerization: implications for adoptive immunotherapy. J Immunol. 2004;172:5338–5345. doi: 10.4049/jimmunol.172.9.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mokyr MB, Place AT, Artwohl JE, Valli VE. Importance of signaling via the IFN-alpha/beta receptor on host cells for the realization of the therapeutic benefits of cyclophosphamide for mice bearing a large MOPC-315 tumor. Cancer Immunol Immunother. 2006;55:459–468. doi: 10.1007/s00262-005-0029-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Morrison SJ, Wright DE, Weissman IL. Cyclophosphamide/granulocyte colony-stimulating factor induces hematopoietic stem cells to proliferate prior to mobilization. Proc Natl Acad Sci USA. 1997;94:1908–1913. doi: 10.1073/pnas.94.5.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Muranski P, Boni A, Wrzesinski C, Citrin DE, Rosenberg SA, Childs R, Restifo NP. Increased intensity lymphodepletion and adoptive immunotherapy–how far can we go? Nat Clin Pract Oncol. 2006;3:668–681. doi: 10.1038/ncponc0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nagaraj S, Collazo M, Corzo CA, Youn JI, Ortiz M, Quiceno D, Gabrilovich DI. Regulatory myeloid suppressor cells in health and disease. Cancer Res. 2009;69:7503–7506. doi: 10.1158/0008-5472.CAN-09-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nakayama M, Itoh K, Takahashi E. Cyclophosphamide-induced bacterial translocation in Escherichia coli C25-monoassociated specific pathogen-free mice. Microbiol Immunol. 1997;41:587–593. doi: 10.1111/j.1348-0421.1997.tb01896.x. [DOI] [PubMed] [Google Scholar]
- 86.Ndejembi MP, Tang AL, Farber DL. Reshaping the past: Strategies for modulating T-cell memory immune responses. Clin Immunol. 2007;122:1–12. doi: 10.1016/j.clim.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 87.Neben S, Marcus K, Mauch P. Mobilization of hematopoietic stem and progenitor cell subpopulations from the marrow to the blood of mice following cyclophosphamide and/or granulocyte colony-stimulating factor. Blood. 1993;81:1960–1967. [PubMed] [Google Scholar]
- 88.Nemoto K, Ishihara H, Tanaka I, Suzuki G, Tsuneoka K, Yoshida K, Ohtsu H. Expression of IL-1 beta mRNA in mice after whole body X-irradiation. J Radiat Res (Tokyo) 1995;36:125–133. doi: 10.1269/jrr.36.125. [DOI] [PubMed] [Google Scholar]
- 89.Okada N, Tsujino M, Hagiwara Y, Tada A, Tamura Y, Mori K, Saito T, Nakagawa S, Mayumi T, Fujita T, Yamamoto A. Administration route-dependent vaccine efficiency of murine dendritic cells pulsed with antigens. Br J Cancer. 2001;84:1564–1570. doi: 10.1054/bjoc.2001.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Overwijk WW, de Visser KE, Tirion FH, de Jong LA, Pols TW, van der Velden YU, van den Boorn JG, Keller AM, Buurman WA, Theoret MR, Blom B, Restifo NP, Kruisbeek AM, Kastelein RA, Haanen JB. Immunological and antitumor effects of IL-23 as a cancer vaccine adjuvant. J Immunol. 2006;176:5213–5222. doi: 10.4049/jimmunol.176.9.5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA, Dellemijn TA, Antony PA, Spiess PJ, Palmer DC, Heimann DM, Klebanoff CA, Yu Z, Hwang LN, Feigenbaum L, Kruisbeek AM, Rosenberg SA, Restifo NP. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Overwijk WW, Tsung A, Irvine KR, Parkhurst MR, Goletz TJ, Tsung K, Carroll MW, Liu C, Moss B, Rosenberg SA, Restifo NP. gp100/pmel 17 is a murine tumor rejection antigen: induction of “self”-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J Exp Med. 1998;188:277–286. doi: 10.1084/jem.188.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pak AS, Wright MA, Matthews JP, Collins SL, Petruzzelli GJ, Young MR. Mechanisms of immune suppression in patients with head and neck cancer: presence of CD34(+) cells which suppress immune functions within cancers that secrete granulocyte-macrophage colony-stimulating factor. Clin Cancer Res. 1995;1:95–103. [PubMed] [Google Scholar]
- 94.Pandit R, Lathers DM, Beal NM, Garrity T, Young MR. CD34+ immune suppressive cells in the peripheral blood of patients with head and neck cancer. Ann Otol Rhinol Laryngol. 2000;109:749–754. doi: 10.1177/000348940010900809. [DOI] [PubMed] [Google Scholar]
- 95.Paulos CM, Kaiser A, Wrzesinski C, Hinrichs CS, Cassard L, Boni A, Muranski P, Sanchez-Perez L, Palmer DC, Yu Z, Antony PA, Gattinoni L, Rosenberg SA, Restifo NP. Toll-like receptors in tumor immunotherapy. Clin Cancer Res. 2007;13:5280–5289. doi: 10.1158/1078-0432.CCR-07-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Paulos CM, Wrzesinski C, Kaiser A, Hinrichs CS, Chieppa M, Cassard L, Palmer DC, Boni A, Muranski P, Yu Z, Gattinoni L, Antony PA, Rosenberg SA, Restifo NP. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J Clin Invest. 2007;117:2197–2204. doi: 10.1172/JCI32205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pelaez B, Campillo JA, Lopez-Asenjo JA, Subiza JL. Cyclophosphamide induces the development of early myeloid cells suppressing tumor cell growth by a nitric oxide-dependent mechanism. J Immunol. 2001;166:6608–6615. doi: 10.4049/jimmunol.166.11.6608. [DOI] [PubMed] [Google Scholar]
- 98.Phipps RP, Mandel TE, Schnizlein CT, Tew JG. Anamnestic responses induced by antigen persisting on follicular dendritic cells from cyclophosphamide-treated mice. Immunology. 1984;51:387–397. [PMC free article] [PubMed] [Google Scholar]
- 99.Prins RM, Shu CJ, Radu CG, Vo DD, Khan-Farooqi H, Soto H, Yang MY, Lin MS, Shelly S, Witte ON, Ribas A, Liau LM. Anti-tumor activity and trafficking of self, tumor-specific T cells against tumors located in the brain. Cancer Immunol Immunother. 2008;57:1279–1289. doi: 10.1007/s00262-008-0461-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Probst HC, van den Broek M. Priming of CTLs by lymphocytic choriomeningitis virus depends on dendritic cells. J Immunol. 2005;174:3920–3924. doi: 10.4049/jimmunol.174.7.3920. [DOI] [PubMed] [Google Scholar]
- 101.Proietti E, Greco G, Garrone B, Baccarini S, Mauri C, Venditti M, Carlei D, Belardelli F. Importance of cyclophosphamide-induced bystander effect on T cells for a successful tumor eradication in response to adoptive immunotherapy in mice. J Clin Invest. 1998;101:429–441. doi: 10.1172/JCI1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pulendran B, Ahmed R. Translating innate immunity into immunological memory: implications for vaccine development. Cell. 2006;124:849–863. doi: 10.1016/j.cell.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 103.Radcliff FJ, Caruso DA, Koina C, Riordan MJ, Roberts AW, Tang ML, Baum CM, Woulfe SL, Ashley DM. Mobilization of dendritic cells in cancer patients treated with granulocyte colony-stimulating factor and chemotherapy. Br J Haematol. 2002;119:204–211. doi: 10.1046/j.1365-2141.2002.03717.x. [DOI] [PubMed] [Google Scholar]
- 104.Radojcic V, Bezak KB, Skarica M, Pletneva MA, Yoshimura K, Schulick RD, Luznik L. Cyclophosphamide resets dendritic cell homeostasis and enhances antitumor immunity through effects that extend beyond regulatory T cell elimination. Cancer Immunol Immunother. 2009;59(1):137–148. doi: 10.1007/s00262-009-0734-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ramakrishnan R, Antonia S, Gabrilovich DI. Combined modality immunotherapy and chemotherapy: a new perspective. Cancer Immunol Immunother. 2008;57:1523–1529. doi: 10.1007/s00262-008-0531-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Reis e Sousa C. Dendritic cells in a mature age. Nat Rev Immunol. 2006;6:476–483. doi: 10.1038/nri1845. [DOI] [PubMed] [Google Scholar]
- 107.Rigby SM, Rouse T, Field EH. Total lymphoid irradiation nonmyeloablative preconditioning enriches for IL-4-producing CD4+-TNK cells and skews differentiation of immunocompetent donor CD4+ cells. Blood. 2003;101:2024–2032. doi: 10.1182/blood-2002-05-1513. [DOI] [PubMed] [Google Scholar]
- 108.Rosen SD. Ligands for l-selectin: homing, inflammation, and beyond. Annu Rev Immunol. 2004;22:129–156. doi: 10.1146/annurev.immunol.21.090501.080131. [DOI] [PubMed] [Google Scholar]
- 109.Rosenberg SA. Immunotherapy of cancer by systemic administration of lymphoid cells plus interleukin-2. J Biol Response Mod. 1984;3:501–511. [PubMed] [Google Scholar]
- 110.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380–384. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 111.Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, Simon P, Lotze MT, Yang JC, Seipp CA, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988;319:1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 112.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Roses RE, Xu M, Koski GK, Czerniecki BJ. Radiation therapy and Toll-like receptor signaling: implications for the treatment of cancer. Oncogene. 2008;27:200–207. doi: 10.1038/sj.onc.1210909. [DOI] [PubMed] [Google Scholar]
- 114.Salem ML, AL-Khami AA, EL-Naggar SA, Díaz-Montero CM, Chen Y, Cole DJ (2009) Cyclophosphamide induces dynamic alterations in the host microenvironments resulting in a FLT3L-dependent expansion of dendritic cells. J Immunol (under revision) [DOI] [PMC free article] [PubMed]
- 115.Salem ML, Diaz-Montero CM, Al-Khami AA, El-Naggar SA, Naga O, Montero AJ, Khafagy A, Cole DJ. Recovery from cyclophosphamide-induced lymphopenia results in expansion of immature dendritic cells which can mediate enhanced prime-boost vaccination antitumor responses in vivo when stimulated with the TLR3 agonist poly(I:C) J Immunol. 2009;182:2030–2040. doi: 10.4049/jimmunol.0801829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Salem ML, El-Naggar SA, Cole DJ (2009) Cyclophosphamide induces bone marrow to yield higher numbers of precursor dendritic cells in vitro capable of functional antigen presentation to T cells in vivo. Cell Immunol (submitted) [DOI] [PMC free article] [PubMed]
- 117.Salem ML, El-Naggar SA, Kadima A, Gillanders WE, Cole DJ. The adjuvant effects of the toll-like receptor 3 ligand polyinosinic-cytidylic acid poly (I:C) on antigen-specific CD8+ T cell responses are partially dependent on NK cells with the induction of a beneficial cytokine milieu. Vaccine. 2006;24:5119–5132. doi: 10.1016/j.vaccine.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 118.Salem ML, Kadima AN, El-Naggar SA, Rubinstein MP, Chen Y, Gillanders WE, Cole DJ. Defining the ability of cyclophosphamide preconditioning to enhance the antigen-specific CD8+ T-cell response to peptide vaccination: creation of a beneficial host microenvironment involving type I IFNs and myeloid cells. J Immunother. 2007;30:40–53. doi: 10.1097/01.cji.0000211311.28739.e3. [DOI] [PubMed] [Google Scholar]
- 119.Schiavoni G, Mattei F, Di Pucchio T, Santini SM, Bracci L, Belardelli F, Proietti E. Cyclophosphamide induces type I interferon and augments the number of CD44(hi) T lymphocytes in mice: implications for strategies of chemoimmunotherapy of cancer. Blood. 2000;95:2024–2030. [PubMed] [Google Scholar]
- 120.Schumacher TN, Restifo NP. Adoptive T cell therapy of cancer. Curr Opin Immunol. 2009;21:187–189. doi: 10.1016/j.coi.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shackleton M, Davis ID, Hopkins W, Jackson H, Dimopoulos N, Tai T, Chen Q, Parente P, Jefford M, Masterman KA, Caron D, Chen W, Maraskovsky E, Cebon J. The impact of imiquimod, a Toll-like receptor-7 ligand (TLR7L), on the immunogenicity of melanoma peptide vaccination with adjuvant Flt3 ligand. Cancer Immun. 2004;4:9. [PubMed] [Google Scholar]
- 122.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–521. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 123.Shi Y, Galusha SA, Rock KL. Cutting edge: elimination of an endogenous adjuvant reduces the activation of CD8 T lymphocytes to transplanted cells and in an autoimmune diabetes model. J Immunol. 2006;176:3905–3908. doi: 10.4049/jimmunol.176.7.3905. [DOI] [PubMed] [Google Scholar]
- 124.Song W, Levy R. Therapeutic vaccination against murine lymphoma by intratumoral injection of naive dendritic cells. Cancer Res. 2005;65:5958–5964. doi: 10.1158/0008-5472.CAN-05-0406. [DOI] [PubMed] [Google Scholar]
- 125.Taieb J, Chaput N, Schartz N, Roux S, Novault S, Menard C, Ghiringhelli F, Terme M, Carpentier AF, Darrasse-Jeze G, Lemonnier F, Zitvogel L. Chemoimmunotherapy of tumors: cyclophosphamide synergizes with exosome based vaccines. J Immunol. 2006;176:2722–2729. doi: 10.4049/jimmunol.176.5.2722. [DOI] [PubMed] [Google Scholar]
- 126.Tesniere A, Panaretakis T, Kepp O, Apetoh L, Ghiringhelli F, Zitvogel L, Kroemer G. Molecular characteristics of immunogenic cancer cell death. Cell Death Differ. 2008;15:3–12. doi: 10.1038/sj.cdd.4402269. [DOI] [PubMed] [Google Scholar]
- 127.Tong Y, Song W, Crystal RG. Combined intratumoral injection of bone marrow-derived dendritic cells and systemic chemotherapy to treat pre-existing murine tumors. Cancer Res. 2001;61:7530–7535. [PubMed] [Google Scholar]
- 128.Torihata H, Ishikawa F, Okada Y, Tanaka Y, Uchida T, Suguro T, Kakiuchi T. Irradiation up-regulates CD80 expression through two different mechanisms in spleen B cells, B lymphoma cells, and dendritic cells. Immunology. 2004;112:219–227. doi: 10.1111/j.1365-2567.2004.01872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vierboom MP, Bos GM, Ooms M, Offringa R, Melief CJ. Cyclophosphamide enhances anti-tumor effect of wild-type p53-specific CTL. Int J Cancer. 2000;87:253–260. doi: 10.1002/1097-0215(20000715)87:2<253::aid-ijc17>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 130.Vuckovic S, Kim M, Khalil D, Turtle CJ, Crosbie GV, Williams N, Brown L, Williams K, Kelly C, Stravos P, Rodwell R, Hill GR, Wright S, Taylor K, Gill D, Marlton P, Bradstock K, Hart DN. Granulocyte-colony stimulating factor increases CD123hi blood dendritic cells with altered CD62L and CCR7 expression. Blood. 2003;101:2314–2317. doi: 10.1182/blood-2002-03-0973. [DOI] [PubMed] [Google Scholar]
- 131.Wada S, Yoshimura K, Hipkiss EL, Harris TJ, Yen HR, Goldberg MV, Grosso JF, Getnet D, Demarzo AM, Netto GJ, Anders R, Pardoll DM, Drake CG. Cyclophosphamide augments antitumor immunity: studies in an autochthonous prostate cancer model. Cancer Res. 2009;69:4309–4318. doi: 10.1158/0008-5472.CAN-08-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang LX, Li R, Yang G, Lim M, O’Hara A, Chu Y, Fox BA, Restifo NP, Urba WJ, Hu HM. Interleukin-7-dependent expansion and persistence of melanoma-specific T cells in lymphodepleted mice lead to tumor regression and editing. Cancer Res. 2005;65:10569–10577. doi: 10.1158/0008-5472.CAN-05-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wright DE, Cheshier SH, Wagers AJ, Randall TD, Christensen JL, Weissman IL. Cyclophosphamide/granulocyte colony-stimulating factor causes selective mobilization of bone marrow hematopoietic stem cells into the blood after M phase of the cell cycle. Blood. 2001;97:2278–2285. doi: 10.1182/blood.v97.8.2278. [DOI] [PubMed] [Google Scholar]
- 134.Wrzesinski C, Paulos CM, Gattinoni L, Palmer DC, Kaiser A, Yu Z, Rosenberg SA, Restifo NP. Hematopoietic stem cells promote the expansion and function of adoptively transferred antitumor CD8 T cells. J Clin Invest. 2007;117:492–501. doi: 10.1172/JCI30414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wrzesinski C, Restifo NP. Less is more: lymphodepletion followed by hematopoietic stem cell transplant augments adoptive T-cell-based anti-tumor immunotherapy. Curr Opin Immunol. 2005;17:195–201. doi: 10.1016/j.coi.2005.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xun CQ, Thompson JS, Jennings CD, Brown SA, Widmer MB. Effect of total body irradiation, busulfan-cyclophosphamide, or cyclophosphamide conditioning on inflammatory cytokine release and development of acute and chronic graft-versus-host disease in H-2-incompatible transplanted SCID mice. Blood. 1994;83:2360–2367. [PubMed] [Google Scholar]
- 137.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zaft T, Sapoznikov A, Krauthgamer R, Littman DR, Jung S. CD11chigh dendritic cell ablation impairs lymphopenia-driven proliferation of naive and memory CD8+ T cells. J Immunol. 2005;175:6428–6435. doi: 10.4049/jimmunol.175.10.6428. [DOI] [PubMed] [Google Scholar]
- 139.Zeng R, Spolski R, Finkelstein SE, Oh S, Kovanen PE, Hinrichs CS, Pise-Masison CA, Radonovich MF, Brady JN, Restifo NP, Berzofsky JA, Leonard WJ. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201:139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang B, Bowerman NA, Salama JK, Schmidt H, Spiotto MT, Schietinger A, Yu P, Fu YX, Weichselbaum RR, Rowley DA, Kranz DM, Schreiber H. Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J Exp Med. 2007;204:49–55. doi: 10.1084/jem.20062056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang Y, Louboutin JP, Zhu J, Rivera AJ, Emerson SG. Preterminal host dendritic cells in irradiated mice prime CD8+ T cell-mediated acute graft-versus-host disease. J Clin Invest. 2002;109:1335–1344. doi: 10.1172/JCI14989. [DOI] [PMC free article] [PubMed] [Google Scholar]