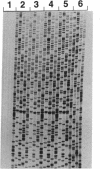
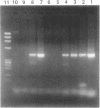
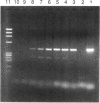
Abstract
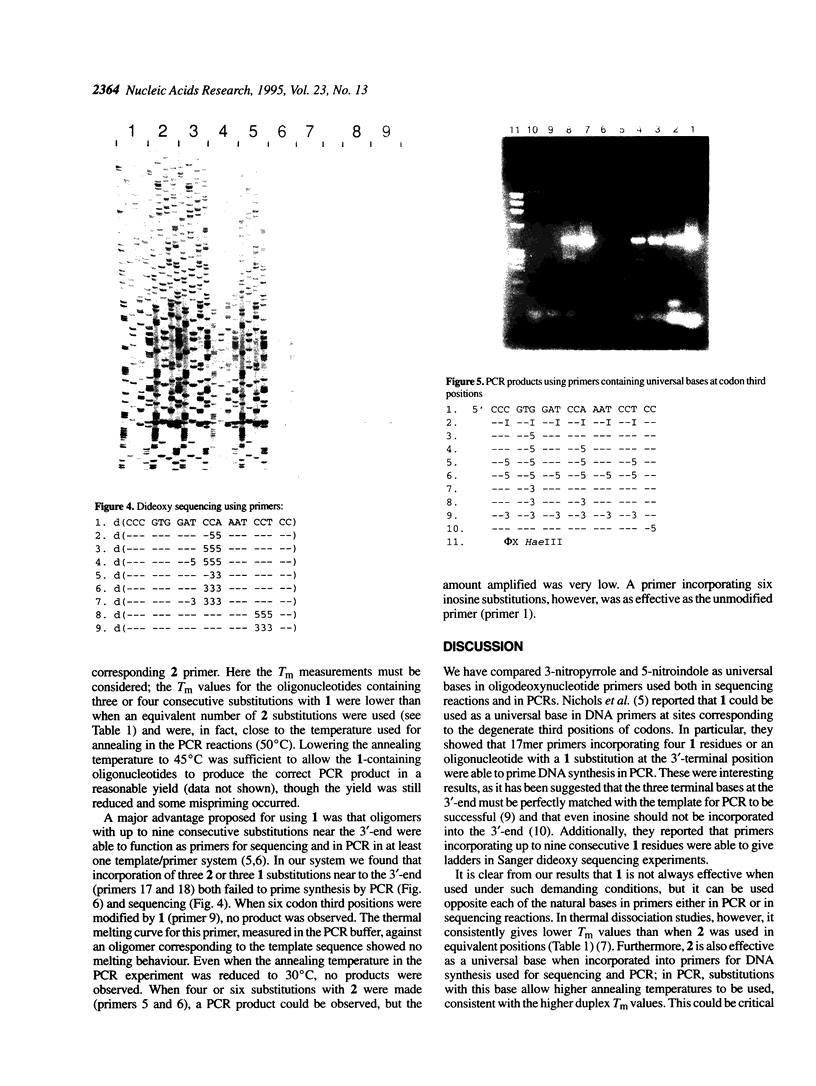
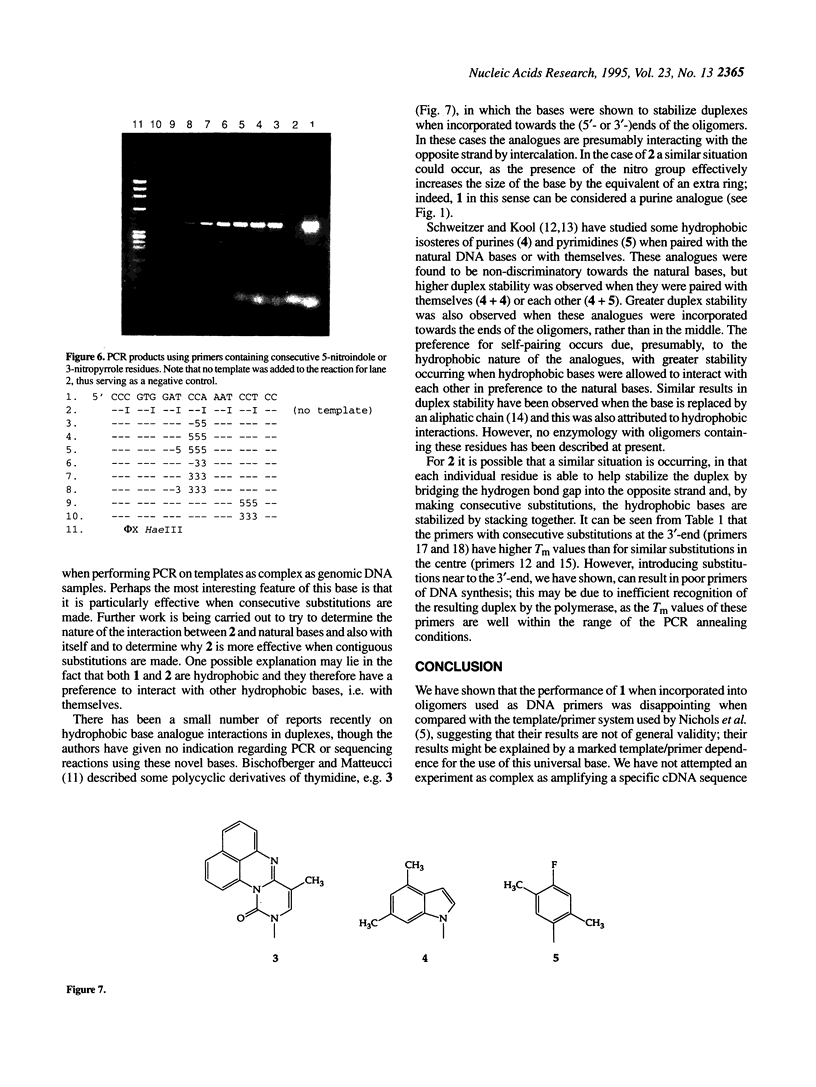
3-Nitropyrrole and 5-nitroindole have been assessed as universal bases in primers for dideoxy DNA sequencing and in the polymerase chain reaction (PCR). In contrast to a previous report, we have found that the introduction of more than one 3-nitropyrrole residue at dispersed positions into primers significantly reduced their efficiency in PCR and sequencing reactions. Primers containing 5-nitroindole at multiple dispersed positions were similarly affected; for both bases only a small number of substitutions were tolerated. In PCR experiments neither base, when incorporated into primers in codon third positions, was as effective as hypoxanthine, which was incorporated in six codon third positions in a 20mer oligomer. However, primers containing up to four consecutive 5-nitroindole substitutions performed well in both PCR and sequencing reactions. Consecutive 3-nitropyrrole substitutions were tolerated, but less well in comparable reactions.
Full text
PDF
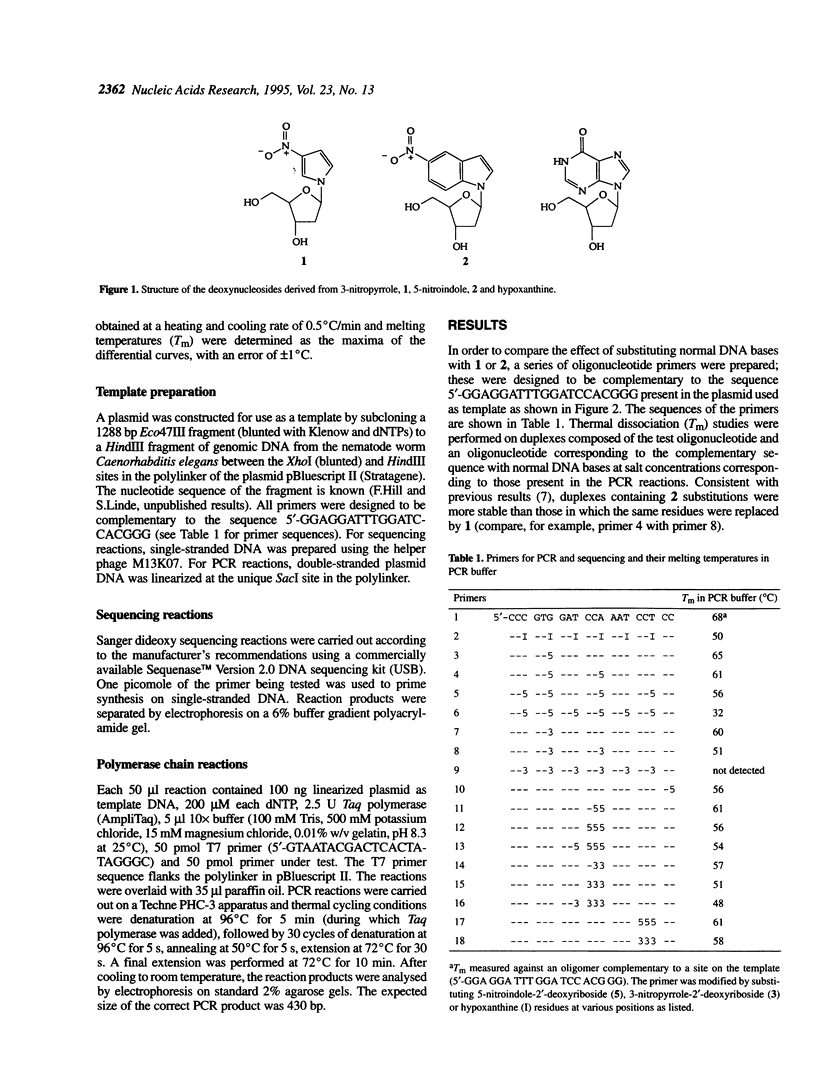


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Kamiya H., Sakaguchi T., Murata N., Fujimuro M., Miura H., Ishikawa H., Shimizu M., Inoue H., Nishimura S., Matsukage A. In vitro replication study of modified bases in ras sequences. Chem Pharm Bull (Tokyo) 1992 Oct;40(10):2792–2795. doi: 10.1248/cpb.40.2792. [DOI] [PubMed] [Google Scholar]
- Liu H., Nichols R. PCR amplification using deoxyinosine to replace an entire codon and at ambiguous positions. Biotechniques. 1994 Jan;16(1):24–26. [PubMed] [Google Scholar]
- Loakes D., Brown D. M. 5-Nitroindole as an universal base analogue. Nucleic Acids Res. 1994 Oct 11;22(20):4039–4043. doi: 10.1093/nar/22.20.4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millican T. A., Mock G. A., Chauncey M. A., Patel T. P., Eaton M. A., Gunning J., Cutbush S. D., Neidle S., Mann J. Synthesis and biophysical studies of short oligodeoxynucleotides with novel modifications: a possible approach to the problem of mixed base oligodeoxynucleotide synthesis. Nucleic Acids Res. 1984 Oct 11;12(19):7435–7453. doi: 10.1093/nar/12.19.7435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols R., Andrews P. C., Zhang P., Bergstrom D. E. A universal nucleoside for use at ambiguous sites in DNA primers. Nature. 1994 Jun 9;369(6480):492–493. doi: 10.1038/369492a0. [DOI] [PubMed] [Google Scholar]