Abstract
Background
Resolvin E1 (RvE1), an endogenous lipid mediator derived from eicosapentaenoic acid (EPA), has been identified in local inflammation during the healing stage. RvE1 reduces inflammation in several types of animal models including peritonitis and retinopathy, and blocks human neutrophil transendothelial cell migration. The RvE1 receptor ChemR23 is expressed on myeloid cells such as macrophages and dendritic cells. The aim of this study was to determine whether RvE1 regulates colonic inflammation when the innate immune response of macrophages plays a key role in the pathogenesis and tissue damage.
Methods/Results
RvE1 receptor, ChemR23, was expressed in mouse peritoneal macrophages as defined by flow cytometry. Peritoneal macrophages were pretreated with RvE1, followed by lipopolysaccharide (LPS) stimulation whereupon of the transcriptional levels of proinflammatory cytokines were analyzed. RvE1 treatment led to the inhibition of proinflammatory cytokines including TNF-α and IL-12p40. In HEK293 cells, pretreatment with RvE1 inhibited TNF-α-induced nuclear translocation of NF-κB in a ChemR23 dependent manner. These results suggested that RvE1 could regulate pro-inflammatory responses of macrophages expressing ChemR23. Therefore, we investigated the beneficial effects of RvE1 in dextran sulfate sodium (DSS) induced colitis. RvE1 treatment led to amelioration of colonic inflammation.
Conclusions
These results indicate that RvE1 suppresses pro-inflammatory responses of macrophages. RvE1 and its receptor may therefore be useful as therapeutic targets in the treatment of human inflammatory bowel disease (IBD) and other inflammatory disorders.
Keywords: Resolvin E1, macrophage, NF-κB, DSS-induced colitis
Introduction
ω3 Polyunsaturated fatty acids (PUFAs) such as EPA and docosahexaenoic acid, which are enriched in fish oils, are held to be beneficial in a wide range of human inflammatory disorders, including cardiovascular diseases, rheumatoid arthritis, psoriasis, and inflammatory bowel disease (IBD) (1–5). However, the molecular basis of ω3 fatty acid action remains unknown. It is now appreciated that endogenous lipid mediators play an important role in the promotion and inhibition of inflammation. During the active inflammatory phase, arachidonic acid is converted to proinflammatory eicosanoids (such as prostaglandin E2 and leukotriene B4) which recruit neutrophils to the site of injury and elicit an inflammatory response. In contrast, during the resolution of inflammation, pro-resolution lipid mediators such as lipoxins and resolvins including Resolvin E1 (RvE1), derived from EPA, reduce neutrophil infiltration, increase the removal of apoptotic cells and microorganisms by macrophages in inflamed sites and promote the clearance of neutrophils from the inflammatory site (6).
RvE1 was originally identified in murine inflammatory exudates in vivo. RvE1 is generated in vitro from EPA through aspirin-acetylated cyclooxygenase-2 (COX2) in vascular endothelial cells, and 5-lipoxygenase in leukocytes (7). Moreover microbial cytochrome P450 enzymes can also contribute to RvE1 biosynthesis (8). RvE1 directly interacts with at least two G-protein-coupled receptors (GPCRs) in a cell-type-specific manner. RvE1 activates ChemR23 expressed on macrophages and dendritic cells and inhibits the leukotriene B4 receptor (BLT1) that is expressed by human neutrophils (7, 9, 10).
The pathogenesis of IBD such as ulcerative colitis and Crohn’s disease involves macrophages. However, the mechanisms involved are incompletely understood but certainly involve excessive production of pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), and IL-6 (11, 12).
We therefore examined the effects of RvE1 in regulating macrophages in vitro, and the effects of RvE1 in vivo using DSS induced colitis. DSS is a widely used in animal IBD model that is primarily initiated by innate immune mechanisms (13), allowing for an evaluation of the effects of RvE1 on this cell type.
Materials and Methods
Flow cytometry analysis
Cells were incubated with Rat anti-mouse ChemR23 mAb (BZ194) or Rat anti-human ChemR23 (BZ322; gifts from Dr. Brian A. Zabel (14)) followed by reaction with PE labeled goat anti-rat IgG, or FITC labeled rat anti-mouse CD11b (BD Pharmingen, Franklin Lakes, NJ). The stained cells were subjected to flow cytometer (BD Biosciences, San Jose, CA) and analyzed.
Nuclear translocation of NF-κB
HEK293 (human embryonic kidney) cells transfected with ChemR23 (9) were plated on Glass Bottom Culture Dishes (35mm, poly-d-lysine coated MatTek Corporation, Ashland, CA) for 24 h before RvE1 treatment, and were pretreated with 100 ng/ml RvE1 for 1 hour followed by treatment with 4 ng/ml TNF-α for HEK293 cells. After 2 hours, cells were fixed by methanol, blocked with 10% goat serum for 1 h, and incubated with rabbit anti-NF-κB p65 Antibody (Ab) (sc-372; Santa Cruz Biotechnology, Santa Cruz, CA). Bound Ab was detected with Alexa 488-labeled goat anti-rabbit IgG Ab (Invitrogen Life Technologies and Molecular Probes, Carlsbad, CA). Cells were then analyzed by confocal laser scanning microscopy (Zeiss LSM510 META; Carl Zeiss, Thornwood, NY).
Western Blot analysis
HEK293 cells were pretreated with 100 nM RvE1 for 2 hours followed by 4 ng/ml TNF-α stimulation. After 1 hour, nuclear proteins were isolated from HEK293 cells as previously described (15). The protein concentration of nuclear proteins was measured by a BCA assay. Nuclear proteins (30–50 µg proteins) were heated at 95°C in sample buffer (1 M Tris-HCl, pH 7.5, 640 mM 2-mercaptoethanol, 0.2% bromphenol blue, 4% SDS, and 20% glycerol), and then separated on 10% SDS-polyacrylamide gels. Proteins on the gels were transferred to a PVDF membrane. The membrane was blocked with 1% skim milk in TBS-T (10 mM Tris-HCl, 100 mM NaCl, and 0.5% Tween-20), and probed with an anti-NF-κB p65 Ab (1:1000) followed by a horseradish peroxidase-conjugated secondary antibody. The protein-antibody complex was detected by using ChemiLumiONE (nacalai tesque, Kyoto, Japan) and Image Reader (LAS-4000mini Imaging System; Fuji Film Corporation, Tokyo, Japan). Western blot images were quantified by Multi Gauge v3.2 software, and quantitative data were exported into Microsoft Excel software for further analysis.
Quantitative Real-Time PCR
Total RNA from colon or macrophages was prepared using Sepazol (Nakarai Tesuque, Kyoto, Japan) following the manufacturer’s instructions. RNA purity was determined spectrophotometrically by absorbance at 260 nm in relation to absorbance at 280 nm. Reverse transcription of mRNA was performed by using random hexamer primers. Real-time PCR was carried out by using SYBR Green in a 7500 Real Time PCR system (Applied Biosystems, Foster City, CA), according to the manufacturer’s instructions. The PCR primers for TNF-α, IL-12p40, IL-1β, and β-actin were designed as described (16). Primers for mChemR23 were designed as described (17). The relative expression of each gene was normalized against the housekeeping gene β-actin.
Mice
All animal treatments in this study were approved by the Institutional Animal Care and Use Committee and carried out according to the Kobe University Animal Experimentation Regulations. Six-to 8 week old female C57BL/6 mice were obtained from Clea Japan (Tokyo, Japan) and maintained for 1–2 weeks in the same environment before starting DSS treatment. All mice were housed and bred in the Animal Unit of the Kobe University School of Medicine in a specific-pathogen-free facility and had ad libitum access to standard diet and water until reaching the desired age (8–10 weeks).
Induction of Colitis by DSS
Colitis was induced in WT 8–10 weeks old female mice by addition of 3.5% (wt/vol) DSS (molecular weight 36,000–50,000; MP Biomedicals, Solon, OH) to the sterile drinking water. On day 5, DSS supplementation was discontinued, and mice were sacrificed on day 8 (3 days after cessation of DSS administration). Clinical assessment of all DSS-treated animals for body weight, stool consistency, rectal bleeding, and general appearance was performed daily. Colons were excised, and their length and thickening documented. Histological examination was performed in a blinded manner. The degree of inflammation and epithelial damage was determined by microscopic examination of hematoxylin and eosin stained sections of the colon. The inflammation score was a combined score of (i) severity of inflammation (0; no inflammation, 1; mild, 2; moderate, and 3; severe) and (ii) thickness of inflammatory involvement (0; no inflammation, 1; mucosa, 2; mucosa plus submucosa, and 3; transmural); epithelial damage score consisting of character (0; intact epithelium, 1; disruption of architectural structure, 2; erosion, and 3; ulceration), and; extent of lesions (0; no lesions, 1; punctuate, 2; multifocal, and 3; diffuse) as previously described (18).
Immunohistochemistry
Frozen sections of distal colon were cut at a thickness of 5 µm, and fixed in acetone for 10 min. After the frozen sections were washed with PBS, residual endogenous peroxidase activity was quenched by incubation with 3% H2O2 for 10min. Slides were then incubated with 10% normal goat serum in PBS for 30 min. Next, sections were incubated with a primary antibody against Ser276-phosphorylated NF-κB p65 Ab (Cell signaling Technology, Danvers, MA) in PBS at a dilution of 1/50 overnight at 4°C. After washing with PBS three times, slides were incubated with HRP-labeled polymer anti-rabbit IgG Ab for 30 min, and then 3, 3’-diaminibenzidine (Dako North America. Inc., Carpinteria, CA) following the manufacturer’s instructions. Slides were stained with hematoxylin-eosin. Control sections were treated in the same way except that they were incubated with the secondary antibody alone. Staining was scored by counting the number of Ser276-phosphorylated- NF-κB p65 positive cells per high-power field (×400 magnification). A total of five fields in each section were analyzed as described previously (19).
Statistical Analysis
All results are expressed as mean ± SE of n mice per group. Statistical significance was determined by Student’s t test. P < 0.05 was considered significant.
Results
ChemR23 is expressed by macrophages and regulates proinflammatory cytokines
Mouse peritoneal exudate cells were isolated and stained with anti-CD11b, a well-defined macrophage marker, and anti-ChemR23 antibodies. Flow cytometry analysis revealed that the majority of CD11b+ macrophages were positive for ChemR23 (Fig. 1A). Mouse peritoneal macrophages were treated with RvE1 for 4 h prior to LPS stimulation. After 2 h of incubation with LPS, the expression of TNF-α and IL-12p40 mRNA was quantified by real-time PCR. The LPS-induced expression of TNF-α and IL-12p40 mRNA was significantly inhibited in RvE1 treatment group compared to the control group treated with LPS alone (Fig. 1B).
Figure 1. ChemR23 expresses on macrophages, and regulates proinflammatory cytokines.
(A) Anti-mChemR23 mAb and FITC labeled anti-mCD11b mAb staining of primary mouse peritoneal macrophages. (B) Primary peritoneal macrophages were pretreated with RvE1 (100 ng/ml) for 4 hours followed by stimulation with LPS (100 ng/ml), and cytokine mRNA was analyzed by quantitative RT-PCR. Data are shown as the mean ± S.E. (n=3).
RvE1 inhibits nuclear translocation of NF-κB via ChemR23
In order to determine the mechanisms of the immunomodulatory effects of RvE1 induced through ChemR23 stimulation, HEK293 cells were stably transfected with ChemR23. Flow cytometry using an anti-ChemR23 antibody showed that ChemR23 was expressed on ChemR23-transfected HEK293 cells (Fig. 2A). In addition, immunohistochemistry indicated that nuclear translocation of NF-κB was observed in the ChemR23 transfected cells after TNF-α stimulation (Fig. 2B). However, RvE1 treatment inhibited the TNF-α stimulated nuclear translocation of NF-κB in the transfected cells. In contrast, NF-κB translocation induced by TNF-α stimulation was not inhibited in non-transfected, wild type HEK293 cells regardless of RvE1 treatment (Fig. 2B). In addition, Western blot analysis also showed that RvE1 could suppress TNF-α-stimulated nuclear translocation of NF-κB in the ChemR23-transfected HEK293 cells, but not in nontransfected HEK293 cells (Fig. 2C). These results indicate that RvE1 can suppress TNF-α-induced NF-κB activation in the context of ChemR23.
Figure 2. RvE1 inhibits the nuclear translocation of NF-κB via ChemR23.
(A) Staining of Human ChemR23 stably-expressed HEK293 cells by anti-human ChemR23 mAb. (B) HEK293 wild type cells or ChemR23 transfected cells were incubated with 100 ng/ml RvE1 for 2 hours followed by treatment with 4 ng/ml TNF-α for 1 hour, and then subjected to immunocytochemistry. (C) HEK293 wild type cells or ChemR23 transfected cells were incubated with 100 ng/ml RvE1 for 2 hours followed by treatment with 4 ng/ml TNF-α for 1 hour. The nuclear proteins were prepared and analyzed for NF-κB p65 by Western blotting analysis. The band density of NF-κB was quantified, and the values relative to a vehicle control are shown. Data are shown as the mean ± S.E. (n=3).
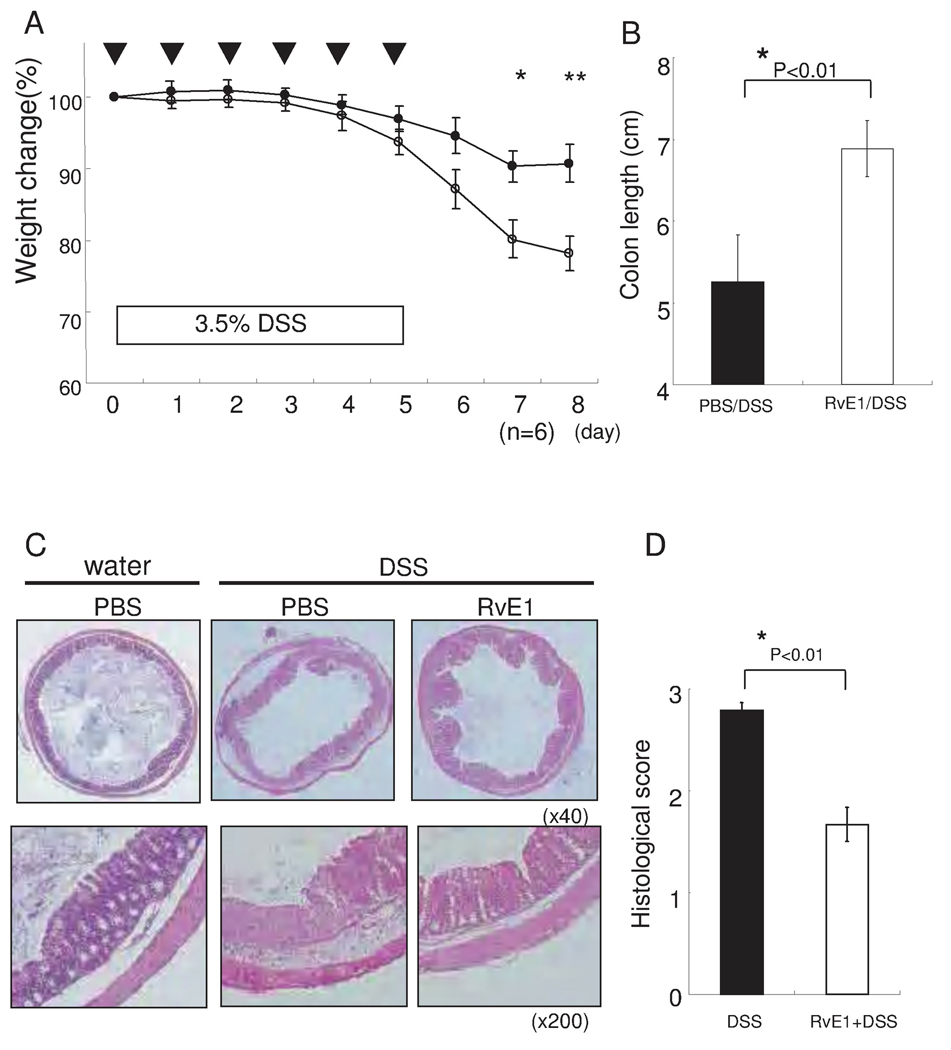
RvE1 protects mice from DSS induced colitis
We next assessed the effects of RvE1 in the DSS induced colitis model. Mice were exposed to 3.5% DSS in the drinking water for 5 days together with daily intraperitoneal injections of PBS or RvE1 for a total of 5 days. After 5 days of DSS administration, severe illness that was characterized by bloody diarrhea and severe wasting disease was observed. However, whereas the relative body weight was 78.1 ± 2.6 % of that observed at baseline at 8 days after DSS treatment in the PBS treated control group, treatment of mice with RvE1 (1 µg i.p. per mouse) reduced the body weight loss (90.7 ± 2.4 % relative to baseline at 8 days (Fig. 3A). These differences were directly reflected in the degree of macroscopic injury observed. Without RvE1 treatment, significant shortening and thickening of the colon were observed (Fig. 3B). Consistent with these macroscopic changes, mice treated with PBS exhibited marked transmural infiltration with inflammatory cells such as neutrophils and mononuclear cells in association with ulceration (Fig. 3C middle panels). In contrast, mice treated with RvE1 exhibited less severe histologic features of colitis (Fig. 3C right panels). Quantification of these histological differences were highly significant (Fig. 3D).
Figure 3. RvE1 dramatically attenuates DSS-induced colitis.
(A) Wasting disease as measured by body weight loss in mice with DSS-induced colitis is improved by RvE1 treatment. Open circles, DSS alone; filled circles, DSS treated with RvE1 (1 µg per mouse) daily for five days (day0–5). *, P< 0.05, **, P< 0.01 (n=6). (B) Length of the inflamed colons of mice treated with either DSS alone or DSS plus RvE1. *P< 0.01, compared with DSS alone. (C) Colon sections of mice exposed to drinking water only (Left), day 8 after DSS-induced colitis (Center), and RvE1-treated mice (Right).(D) Histological scores of the colon of mice receiving DSS alone or DSS plus RvE1. *P<0.05, compared with DSS alone. Data are shown as the mean ± S.E. (n=6).
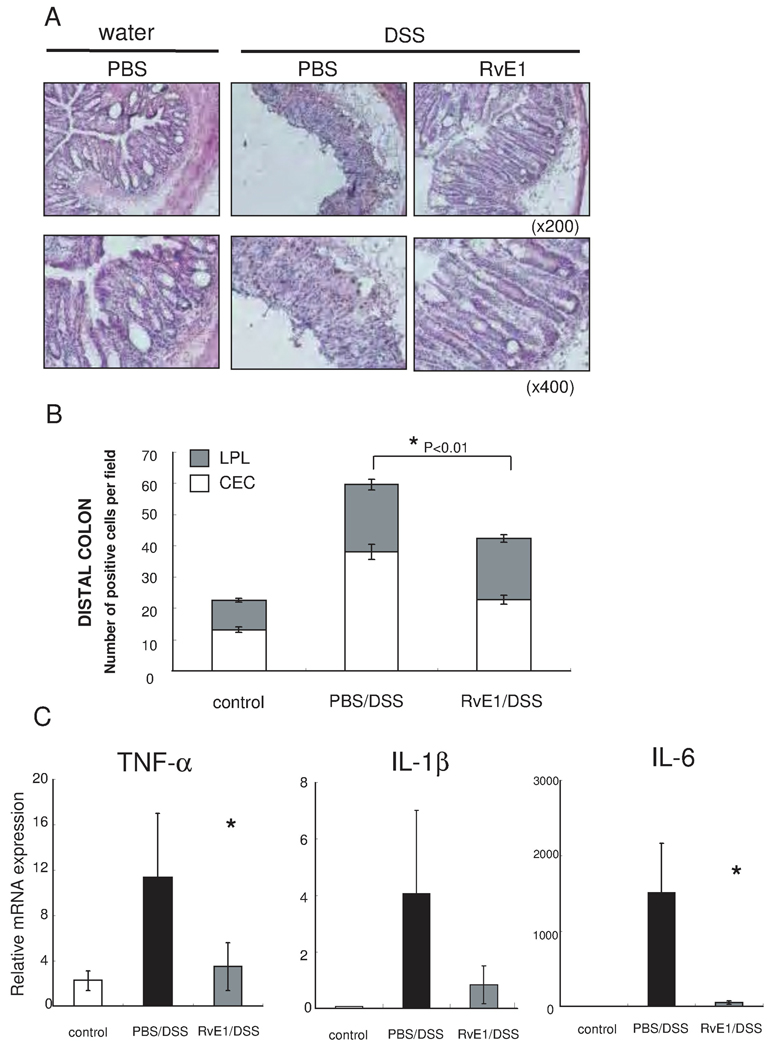
To determine whether the RvE1-mediated protection from colitis was associated with alterations in NF-κB activation and additionally NF-κB induced proinflammatory cytokine gene expression, we investigated the influence of RvE1 on NF-κB p65 phosphorylation at Ser276 by immunohistochemistry. NF-κB p65 phosphorylation at Ser276 was significantly increased in the distal colon of DSS induced colitis; a model which is known to induce more severe inflammation of the distal colon than the proximal colon. Furthermore, RvE1 treatment reduced the Ser276 phosphorylation of NF-κB p65 in the distal colon (Fig. 4A). In addition, the number of epithelial Ser276 phosphorylation positive cells was significantly decreased in the RvE1 treatment group in comparison to the PBS treatment, although the number of such positive cells in lamina propria lymphocytes was not significantly reduced (Fig. 4B). Finally, we assessed the NF-κB induced proinflammatory cytokine mRNA levels in colon tissues by using quantitative real-time PCR (Fig. 4C). The expression levels of TNF-α, IL-1β, and IL-6 mRNA were significantly reduced in the RvE1 treatment group compared to the PBS control group (Fig. 4C).
Figure 4. RvE1 reduces NF-κB phosphorylation and NF-κB-dependent proinflammatory mediators in DSS-induced colitis.
(A) Immunohistchemical analysis of the effects of RvE1 on NF-κB activation (NF-κB p65 Phosphorylation at Ser276) in distal colon of mice receiving water only, DSS only, and DSS plus RvE1. Brown staining indicates cells positive for Ser276 phosphorylation of NF-κB p65. (magnification ×200: upper side, and ×400: lower side) (B) Staining was scored by counting the number of Ser276-phosphorylated NF-κB p65-positive cells (colonic epithelial cells; CEC: open bars, and lamina propria lymphocytes; LPL: shaded bars) per visualized high-power field (magnification × 400) in distal colon of mice receiving water only, DSS only, and DSS plus RvE1. (C) Quantitative real-time PCR analysis of mRNA expression of inflammatory mediators in colons obtained on day 8 from control mice (white column), mice treated with DSS alone (black column) or mice receiving DSS plus RvE1 (gray column). *, P<0.05, compared with DSS alone. Data are shown as the mean ± S.E. (n=6).
ChemR23 is upregulated by inflammation
To determine whether ChemR23 expression levels are altered during inflammation, we examined ChemR23 expression on peritoneal macrophages which are known to express this receptor. As shown in Fig. 5A, LPS stimulation significantly unregulated the levels of ChemR23 mRNA expression in comparison to unstimulated peritoneal macrophages (Fig.5A). Moreover, murine ChemR23 mRNA was significantly increased in mouse colon after DSS treatment in comparison to nontreated wild type animals (Fig. 5B). These results indicate that ChemR23 is increased by inflammation consistent with a role in inhibiting inflammation.
Figure 5. ChemR23 mRNA increases during LPS-stimulation and DSS induced colitis.
(A) ChemR23 expression on peritoneal macrophages after 100 ng/ml LPS stimulation for 24 hours (black column) in comparison to no stimulation (white column). (B) ChemR23 expression in colon tissues of DSS induced colitis (black column) in comparison to control mice (white column). Data are shown as the mean ± S.E. (n=6).
Discussion
IBD represents a dysregulated immune response to the commensal microbiota in a genetically susceptible host. One important pathway that is abnormally activated in IBD which is an important pathological relevance is associated with macrophages which produce high levels of pro-inflammatory cytokines such as TNF-α, IL-6 and IL-1β (20). Little is however known about regulatory pathways which function to inhibit macrophage activation during intestinal inflammation. In this report, we show that resolvins may provide such an important regulatory function. Specifically, we examined the effects of RvE1 generated from EPA on macrophage activation. We showed that RvE1 receptor, ChemR23, was highly expressed in mouse resident peritoneal macrophages and upregulated during LPS-stimulation and DSS colitis. Such observations are consistent with a previous report that ChemR23 is highly expressed in the monocyte lineage in humans (9). Additionally we observed that LPS-induced expression of TNF-α and IL-12p40 mRNA was inhibited by RvE1 pretreatment. These results indicate that RvE1 is able to down-regulate the pro-inflammatory properties of macrophages after activation.
We further examined the mechanisms of the resolvin mediated regulation of macrophages and focused on the role of NF-κB. NF-κB is a key regulator of inflammation in IBD (21). NF-κB activity is significantly upregulated in IBD and promotes the expression of various proinflammatory genes, resulting in mucosal inflammation (22). In this study, we demonstrated that RvE1 inhibits TNF-α-induced NF-κB nuclear translocation in HEK293 cells stably expressing recombinant human ChemR23, and NF-κB activation in colon tissues of DSS induced colitis. This suggests that RvE1 may attenuate inflammatory immune responses associated with macrophages through inhibition of NF-κB signaling pathways via ChemR23 which is prominently expressed on cells of the monocyte lineage (9). RvE1 also interacts with BLT1 and attenuates LTB4-induced leukocyte migration and activation as a partial agonist/antagonist suggesting that RvE1 may play a broad role in inhibiting inflammation (23).
In a previous report, RvE1 protected against 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis (23). TNBS induced colitis is known to be characterized by massive infiltration of neutrophils and macrophages producing high levels of proinflammatory cytokines such as TNF-α, IL-1β, and IL-6 in the early stage followed by CD4+ T-cell infiltration which produce high levels of IFN-γ. The inflammatory reaction in this model is associated with a Th1 response (24). Therefore, we examined the effects of RvE1 in DSS-induced colitis, which involves macrophage activation as an important component of its pathogenesis (25–27). In this study, RvE1 pretreatment significantly improved the histological damage and clinical score and inhibited the expression of proinflammatory cytokine mRNAs mainly produced by macrophages including TNF-α, IL-1β, and IL-6, which are associated with DSS-induced colitis. In a previous report, Schwab et al. showed that the phagocytic activity of macrophages was increased by RvE1 treatment during mouse zymosan-induced peritonitis. It was also observed that proinflammatory cytokine production from macrophages was reduced by RvE1 pretreatment. Taken together, these studies suggest that RvE1 attenuates inflammation through reduction of proinflammatory cytokine production from macrophages, increased phagocytic activity of macrophages and reduced tissue infiltration with neutrophils (7, 28). It is predicted that resolvin mediated mechanisms likely function at later points of tissue inflammation when ChemR23 expression is significantly elevated as shown here, and increased anti-inflammatory lipid mediators such as RvE1 and Lipoxin A4 and known to be synthesized.
It is known that unresolved inflammation is a major mechanism of disease pathogenesis in many chronic disorders (29). RvE1 and other anti-inflammatory lipid mediators are derived from PUFAs during the resolution phase of inflammation whereupon they actively promote the resolution of inflammation. In this study, we show that a significant mechanism of anti-inflammation imposed by RvE1 is through ChemR23 mediated inhibition of macrophage function.
Acknowledgement
T.I. was supported by the grant from Initiatives for Attractive Education in Graduate Schools: “Program of Raising Young Research Leaders in Biomedical Sciences” from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. M.Y. was supported by grants from the Japanese Society of Gastroenterology, Hyogo Science and Technology Association, Grant-in-aid for Scientific Research (C), Suzuken Memorial Foundation, and the Global COE Program "Global Center of Excellence for Education and Research on Signal Transduction Medicine in the Coming Generation" from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. R.S.B. was supported by grants from the National Institutes of Health (RO1 DK53056, DK51362, and DK44319).
References
- 1.Yokoyama M, Origasa H, Matsuzaki M, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomized open-label, blinded endpoint analysis. Lancet. 2007;369:1090–1098. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 2.Belluzzi A, Brignola C, Campieri M, et al. Effect of an enteric-coated fish-oil preparation on relapses in Crohn's disease. N Engl J Med. 1996;334:1557–1560. doi: 10.1056/NEJM199606133342401. [DOI] [PubMed] [Google Scholar]
- 3.Teitelbaum JE, Allan Walker W. Review: the role of omega 3 fatty acids in intestinal inflammation. J Nutr Biochem. 2001;12:21–32. doi: 10.1016/s0955-2863(00)00141-8. [DOI] [PubMed] [Google Scholar]
- 4.Mayser P, Grimm H, Grimminger F. n-3 fatty acids in psoriasis. Reprod Nutr Dev. 2005;45:1–28. doi: 10.1079/bjn2001459. [DOI] [PubMed] [Google Scholar]
- 5.Kremer JM. n-3 fatty acid supplements in rheumatoid arthritis. Am J Clin Nutr. 2000;71:349–351. doi: 10.1093/ajcn/71.1.349s. [DOI] [PubMed] [Google Scholar]
- 6.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serhan CN, Clish CB, Brannon J, et al. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haas-Stapleton EJ, Lu Y, Hong S, et al. Candida albicans modulates host defense by biosynthesizing the pro-resolving mediator resolvin E1. PLoS ONE. 2007;2:e1316. doi: 10.1371/journal.pone.0001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arita M, Bianchini F, Aliberti J, et al. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arita M, Ohira T, Sun YP, et al. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol. 2007;178:3912–3917. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- 11.Reimund JM, Wittersheim C, Dumont S, et al. Increased production of tumour necrosis factor-alpha interleukin-1 beta, and interleukin-6 by morphologically normal intestinal biopsies from patients with Crohn's disease. Gut. 1996;39:684–689. doi: 10.1136/gut.39.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reimund JM, Wittersheim C, Dumont S, et al. Mucosal inflammatory cytokine production by intestinal biopsies in patients with ulcerative colitis and Crohn's disease. J Clin Immunol. 1996;16:144–150. doi: 10.1007/BF01540912. [DOI] [PubMed] [Google Scholar]
- 13.Okayasu I, Hatakeyama S, Yamada M, et al. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 14.Zabel BA, Ohyama T, Zuniga L, et al. Chemokine-like receptor 1 expression by macrophages in vivo: regulation by TGF-beta and TLR ligands. Exp Hematol. 2006;34:1106–1114. doi: 10.1016/j.exphem.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 15.Nishiumi S, Yamamoto N, Kodoi R, et al. Antagonistic and agonistic effects of indigoids on the transformation of an aryl hydrocarbon receptor. Arch Biochem Biophys. 2008;470:187–199. doi: 10.1016/j.abb.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 16.Overbergh L, Valckx D, Waer M, et al. Quantification of murine cytokine mRNAs using real time quantitative reverse transcriptase PCR. Cytokine. 1999;11:305–312. doi: 10.1006/cyto.1998.0426. [DOI] [PubMed] [Google Scholar]
- 17.Mårtensson UE, Bristulf J, Owman C, et al. The mouse chemerin receptor gene, mcmklr1, utilizes alternative promoters for transcription and is regulated by all-trans retinoic acid. Gene. 2005;350:65–77. doi: 10.1016/j.gene.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Hudert CA, Weylandt KH, Lu Y, et al. Transgenic mice rich in endogenous omega-3 fatty acids are protected from colitis. Proc Natl Acad Sci U S A. 2006;103:11276–11281. doi: 10.1073/pnas.0601280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larmonier CB, Uno JK, Lee KM, et al. Limited effects of dietary curcumin on Th-1 driven colitis in IL-10 deficient mice suggest an IL-10-dependent mechanism of protection. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1079–G1091. doi: 10.1152/ajpgi.90365.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis. 2006;12:S3–S9. doi: 10.1097/01.mib.0000195385.19268.68. [DOI] [PubMed] [Google Scholar]
- 21.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 22.Neurath MF, Pettersson S, Meyer zum Büschenfelde KH, et al. Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF-kappa B abrogates established experimental colitis in mice. Nat Med. 1996;2:998–1004. doi: 10.1038/nm0996-998. [DOI] [PubMed] [Google Scholar]
- 23.Arita M, Yoshida M, Hong S, et al. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc Natl Acad Sci U S A. 2005;102:7671–7676. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abad C, Martinez C, Juarranz MG, et al. Therapeutic effects of vasoactive intestinal peptide in the trinitrobenzene sulfonic acid mice model of Crohn's disease. Gastroenterology. 2003;124:961–971. doi: 10.1053/gast.2003.50141. [DOI] [PubMed] [Google Scholar]
- 25.Hasturk H, Kantarci A, Goguet-Surmenian E. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J Immunol. 2007;179:7021–7029. doi: 10.4049/jimmunol.179.10.7021. [DOI] [PubMed] [Google Scholar]
- 26.Ohkawara T, Nishihira J, Takeda H, et al. Amelioration of dextran sulfate sodium-induced colitis by anti-macrophage migration inhibitory factor antibody in mice. Gastroenterology. 2002;123:256–270. doi: 10.1053/gast.2002.34236. [DOI] [PubMed] [Google Scholar]
- 27.Tessner TG, Cohn SM, Schloemann S, et al. Prostaglandins prevent decreased epithelial cell proliferation associated with dextran sodium sulfate injury in mice. Gastroenterology. 1998;115:874–882. doi: 10.1016/s0016-5085(98)70259-8. [DOI] [PubMed] [Google Scholar]
- 28.Schwab JM, Chiang N, Arita M, et al. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;14:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]