Abstract
Background
MRP-8 (S100A8) and MRP-14 (S100A9) are members of the S100-family of calcium-modulated proteins that regulate myeloid cell function and control inflammation, in part, through activation of toll-like receptor-4 and RAGE. A transcriptional profiling approach in patients with acute coronary syndromes identified MRP-14 as a novel predictor of myocardial infarction. Further studies demonstrated that elevated plasma levels of MRP-8/14 heterodimer predict increased risk of first and recurrent cardiovascular events. Beyond its serving as a risk marker, whether MRP-8/14 participates directly in arterial inflammation and disease remains unclear.
Methods and Results
We evaluated vascular inflammation in wild-type (WT) and MRP-14-deficient (MRP-14−/−) mice that lack MRP-8/14 complexes with experimental arterial injury, vasculitis, or atherosclerosis. After femoral artery wire injury, MRP-14−/− mice had significant reductions in leukocyte accumulation, cellular proliferation, and neointima formation compared to WT mice. In a cytokine-induced local Schwartzman-like reaction that produces thrombohemorrhagic vasculitis, MRP-14−/− mice had significant reductions in neutrophils accumulation, lesion severity and hemorrhagic area. In response to high-fat feeding, mice doubly deficient in ApoE and MRP-8/14 complexes had attenuation in atherosclerotic lesion area and in macrophage accumulation in plaques compared to mice deficient in ApoE alone.
Conclusions
This study demonstrates that MRP-8/14 broadly regulates vascular inflammation and contributes to the biological response to vascular injury by promoting leukocyte recruitment.
Keywords: MRP-8/14, S100A-9, atherosclerosis, vasculitis, inflammation
INTRODUCTION
A wealth of experimental and clinical research supports the premise that inflammation plays an important role in hyperplastic arterial diseases (e.g., neointimal hyperplasia and restenosis after coronary intervention and cardiac allograft vasculopathy), in vasculitis, and in the initiation, progression, and complications of atherosclerosis 1. The pathobiology of arterial disease begins with an inciting injury that activates inflammatory responses from well-characterized cellular and molecular regulatory pathways. Vascular inflammation involves complex heterotypic interactions between endothelial cells, platelets, and inflammatory cells including neutrophils, monocytes, lymphocytes, and mast cells 2. Under normal circumstances, the cellular and molecular processes that control vascular injury responses direct repair and vascular healing. In pathological conditions however, dysregulation of inflammatory responses results in persistent vascular inflammation and adverse arterial remodeling and contributes to the development of clinical vascular diseases.
Our previous study used a transcriptional profiling strategy to identify novel regulators of vascular inflammation and atherothrombosis by examining platelet mRNA transcripts that are differentially expressed in patients with ST-segment elevation myocardial infarction (STEMI) compared to stable coronary artery disease 3. Myeloid-related protein-14 (MRP-14, also referred to as S100A9) was one of the strongest candidate proteins that arose from the transcriptional profiling analysis. MRP-14 complexes with MRP-8 (S100A8), another member of the S100-family of calcium-modulated proteins, and together MRP-8 and MRP-14 regulate myeloid cell function by binding to Toll-like receptor-4 (TLR-4) 4 and RAGE 5 and by modulating calcium signaling 6 and cytoskeletal reorganization 7. In 2 prospective nested case-control studies, one in apparently healthy post-menopausal women 3 and the other in patients presenting with acute coronary syndromes 8, elevated plasma levels of MRP-8/14 predict the risk of future cardiovascular events, independent of traditional cardiovascular risk factors and C-reactive protein. Elevated plasma levels of MRP-8/14 also serve as an early and sensitive marker of myocardial necrosis in the setting of chest pain9.
Emerging evidence from studies using isolated cells from MRP-14-deficient (MRP-14−/−) mice strongly suggests that MRP-8 and MRP-14 regulate leukocyte migration 10. Despite the presence of MRP-8 mRNA transcripts, MRP-14−/− mice lack both MRP-8 and MRP-14 protein due to the instability of MRP-8 in the absence of MRP-14 6, 11. In vitro studies with MRP-14−/− neutrophils show markedly diminished migration through endothelial monolayers and attenuated chemokinesis in a three dimensional collagen matrix 11. An essential role for MRP-14 in leukocyte recruitment in vivo is uncertain since MRP-14−/− mice have normal neutrophil migration during chemical peritonitis 6, 11 and interleukin-8 (IL-8)-induced skin inflammation, but have diminished granulocyte recruitment during tissue wound healing 7 and during acute pancreatitis 12. Recent studies utilizing MRP-14−/− mice have demonstrated that MRP-8 and MRP-14 play a regulatory role in endotoxin-induced phagocyte function. MRP-8 and MRP-14 are endogenous regulators of TLR-4, affecting myeloid MyD88-dependent activation of nuclear factor-κB (NF-κB) and expression of tumor necrosis factor-α (TNF-α)4.
Although MRP-8 and MRP-14 serve as risk biomarkers and can modulate inflammatory cell function, whether these proteins participate directly in vascular inflammation and in cardiovascular disease remains uncertain. The current study used MRP-14−/− mice to test the hypotheses that MRP-8/14 complexes contribute to neutrophil-dependent and monocyte/macrophage-dependent vascular inflammatory responses in experimental arterial injury, vasculitis, and atherosclerosis.
MATERIALS AND METHODS
MRP-14−/− mice were generated in the laboratory of Dr. Nancy Hogg 6. For atherosclerosis experiments, MRP-14−/− mice were crossbred with apolipoprotein E-deficient (ApoE−/−) mice (Jackson Laboratories) to generate compound mutant mice doubly-deficient in MRP-14 and ApoE (MRP-14−/−ApoE−/−). Single mutant ApoE−/− mice were used as controls for atherogenesis experiments. All mice had a congenic C57BL/6 background and were maintained in animal facilities at Harvard Medical School and Case Western Reserve University School of Medicine. Animal care and procedures were reviewed and approved by the Institutional Animal Care and Use Committees and performed in accordance with the guidelines of the American Association for Accreditation of Laboratory Animal Care and the National Institutes of Health.
Femoral Artery Wire Injury
Bilateral wire injury (0.010 in) of the femoral artery was performed as described previously 13, 14 and as detailed in Supplemental Data and Methods. Six hours, 7 days or 28 days after vascular injury, the right and left femoral arteries were excised, paraffin embedded, stained with hematoxylin and eosin (H&E) and Verhoeff tissue elastin stain. A histologist blinded to genotype measured the luminal, intimal, and medial areas of each cross-sectional plane using a microscope equipped with a CCD camera (Zeiss AxioCam MRc5) interfaced to a computer running NIH Image. For immunohistochemistry, standard avidin-biotin procedures were used. Percent Mac-3-positive area, percent 7/4-positive area, and percent glycoprotein IIb (GPIIb) positive area was defined as the fraction of immunopositive staining to total area measured. Methods for in human aortic smooth muscle cell culture (SMC), mouse aortic SMC culture, and SMC MTT proliferation assays are detailed in Supplemental Data and Methods.
Vasculitis Experiments: Local Shwartzman Reaction
Induction of vasculitis (the local Shwartzman reaction) was performed as described previously 15 and as detailed in Supplemental Data and Methods. Briefly, 200 μg of lipopolysaccharide (LPS) was injected subcutaneously into the skin and 24 hours after the LPS injection, 300ng TNF-α was injected in the same site to enhance vasculitic lesion formation. On day 4, mice were euthanized and skin tissue was harvested for semi-quantitative macroscopic grading of skin lesions and histological analysis. For immunohistochemistry, standard avidin-biotin procedures were used. The extravascular red blood cell signal in H&E-stained sections indicated hemorrhage while the red signal inside arteries and veins was excluded. Percent hemorrhage area was defined as the fraction of extravascular red signal area to total area measured. Neutrophil accumulation was assessed by determining mAb 7/4-positive area.
Atherosclerosis Experiments: ApoE Model of High-Fat Diet-induced Atherosclerosis
To induce atherosclerosis, 8-week-old male ApoE−/− (n=22) or doubly deficient ApoE−/−MRP-14−/− (n=20) mice consumed a high-fat diet (Clinton/Cybulsky Rodent Diet D12108 with 1.25% cholesterol, Research Diets New Brunswick, NJ) for 20 weeks. As detailed in Supplemental Data and Methods, Sudan IV staining was used to quantify atherosclerotic lesion area. Macrophage (Mac-3-positive cells) accumulation in atherosclerotic lesions was assessed in the descending aorta and the aortic arch (lesser curvature), and percent macrophage area was calculated by determining Mac-3-positive area using computer-assisted imaging analysis. Methods for in vitro cytokine production and transwell assays are detailed in Supplemental Data and Methods.
Statistical Analysis
Unless otherwise noted, data are represented as the mean value ± standard deviation, and statistical analysis was done using a Student T-test. P-values < 0.05 were considered significant. Correlation analyses for the atherosclerosis experiments (open vs. closed lesion area, and macrophage staining of the descending aorta vs. aortic arch) were done using the correlation function in Microsoft Excel.
RESULTS
MRP-8/14 Complexes Regulate Neutrophil and Monocyte Recruitment and Neointimal Thickening After Femoral Artery Injury
To determine whether MRP-8/14 complexes influence arterial injury responses and neointima formation, we performed femoral artery wire injury in wild type (WT) and MRP-14−/− mice. Wire injury causes endothelial denudation, medial injury, platelet and fibrin deposition, and yields prominent inflammation of all arterial layers 13. Inflammation following wire injury involves both neutrophils and monocytes. We and others have demonstrated the critical participation of inflammatory cell recruitment in neointima formation in wire injured mouse arteries13, 14.
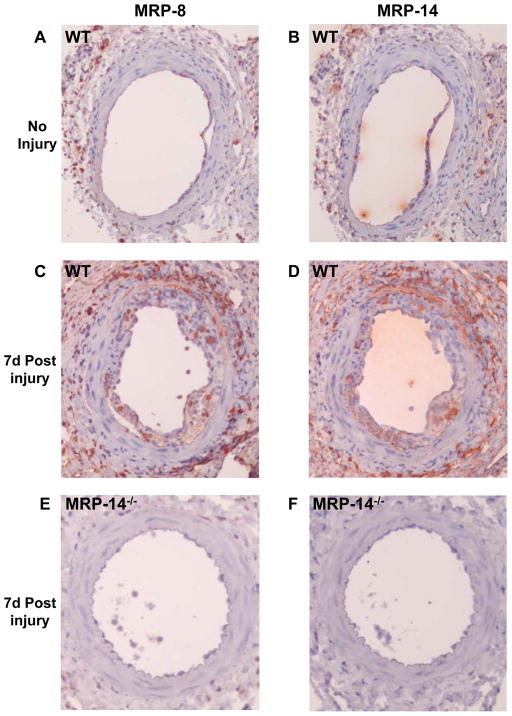
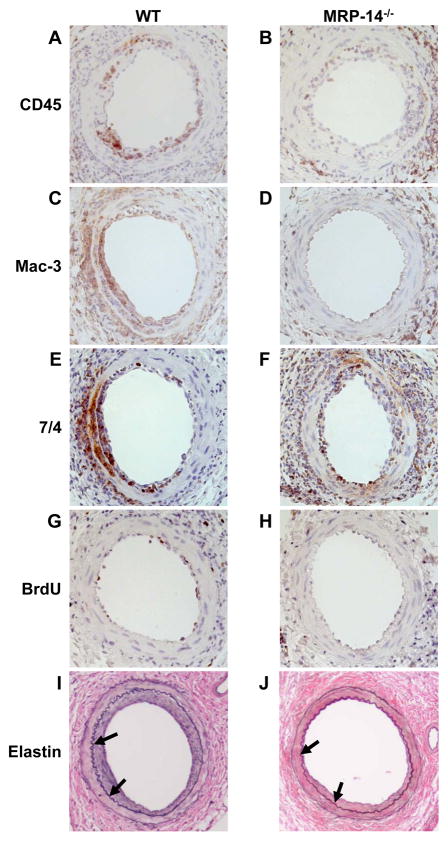
Wire injury resulted in significant increase in MRP-8 and MRP-14 expression in the vessel wall (No injury: Figure 1A–B, 7d post injury: Figure 1C–D). As expected, neither MRP-8 nor MRP-14 expression were detected in the injured arteries of MRP-14−/− mice (Figure 1E–F). We next examined leukocyte recruitment 7 days after wire injury, the time corresponding to peak inflammatory cell infiltration in this model 13. Accumulation of leukocytes in the developing neointima (CD45 staining) was reduced significantly by 45% (% CD45-positive area WT: 13.1 ± 7.2% vs. MRP-14−/−: 7.2 ± 5.4%; P=0.028) in MRP-14−/− compared to WT mice (Figure 2A–B, Table 1). We expanded the CD45 analysis by immunostaining for the macrophage-specific marker Mac-3 and the neutrophil-specific marker 7/4 (Figure 2C-F, Table 1). In the absence of MRP-14, macrophages accumulating in the intima (% Mac-3-positive area WT: 27.8 ± 7.5% vs. MRP-14−/−: 16.1 ± 9.6%; P=0.013) and invading the media (WT: 6.9±5.2% vs. MRP-14−/−: 1.6 ± 1.3%; P=0.023) at 7d fell significantly by 42% and 76%, respectively, while neutrophils invading the intima fell significantly by 58% (% 7/4-positive area WT: 20.4 ± 9.4% vs. MRP-14−/−: 8.4 ± 5.5%; P=0.046).
Figure 1. Photomicrographs of MRP-8 and MRP-14 immunostaining of normal and injured femoral arteries from WT and MRP-14−/− mice.
A-B, MRP-8 or MRP-14 immunostaining of an uninjured femoral artery from a WT mouse. C–D, MRP-8 or MRP-14 immunostaining of a femoral artery from a WT mouse 7d post injury. E–F, MRP-8 or MRP-14 immunostaining of a femoral artery from a MRP-14−/− mouse 7d post injury (original magnification 10X).
Figure 2. Photomicrographs of injured femoral arteries from WT and MRP-14−/− mice after wire injury.
A–B, CD45 immunostaining 7d after injury. C–D, Mac-3 immunostaining 7d after injury. E–F, 7/4 immunostaining 7d after injury. G–H, BrdU immunostaining 7d after injury. I–J, VerHoeff elastin stain 28d after injury. Arrows delineate the internal elastic lamina (original magnification 10X).
Table 1.
Quantitative Morphometry and Immunohistochemistry
| Wild Type | MRP-14−/− | P-value | |
|---|---|---|---|
| % CD45-positive area 7d | |||
| Intima | 13.1±7.2 | 7.2±5.4 | 0.028 |
| Media | 4.8±8.0 | 1.4±2.3 | 0.17 |
| % Mac-3-positive area 7d | |||
| Intima | 27.8±7.5 | 16.1±9.6 | 0.013 |
| Media | 6.9±5.2 | 1.6±1.3 | 0.023 |
| % 7/4-positive area 7d | |||
| Intima | 20.4±9.4 | 8.4±5.5 | 0.046 |
| Media | 13.6±7.8 | 5.0±6.5 | 0.093 |
| % BrdU-positive cells 7d | |||
| Intima | 15.2±5.4 | 4.2±3.5 | 0.001 |
| Media | 5.3±3.6 | 1.1±1.2 | 0.019 |
| Intimal area 28d, μm2 | 17,561±4,984 | 12,252±4610 | 0.031 |
| Medial area 28d, μm2 | 11,060±1,573 | 12,862±1,743 | 0.031 |
| Intimal-to-medial ratio 28 d | 1.81±0.63 | 1.00±0.34 | 0.004 |
| External elastic lamina area 28d, μm2 | 47,585±6,719 | 48,885±9,752 | 0.73 |
Morphometry: WT n=9 mice, MRP-14−/−n=7 mice. Immunohistochemistry: analysis of n=9–14 sections per data point. Data are presented as mean ± standard deviation
Since increasing evidence suggests that leukocytes play an important role in regulating cellular proliferation, we assessed cellular proliferation by quantifying incorporation of BrdU. Substantial proliferation was observed 7d after injury in WT vessels (15.2% of intimal cells, 5.3% of medial cells). Cellular proliferation at 7d fell significantly by 72% in the intima (WT: 15.2 ± 5.4% vs. MRP-14−/−: 4.2 ± 3.5%, P=0.001) and 80% in the media (% BrdU-positive cells WT: 5.3 ± 3.6% vs. MRP-14−/−: 1.1 ± 1.2%, P=0.019) in MRP-14−/− mice compared to WT mice (Figure 2G–H, Table 1).
MRP-14 deficient mice had less neointima formation assessed 28d after femoral artery injury compared to WT mice (neointimal area WT: 17,561 ± 4,984 μm2 vs. MRP-14−/−: 12,862 ± 1,743 μm2; P=0.031) (Figure 2I–J, Table 1). Medial area increased slightly in the absence of MRP-14 (WT: 11,060 ± 1,573 μm2 vs. MRP-14−/−: 12,862 ± 1,743 μm2, P=0.031). MRP-14−/−mice had a 45% lower intima-to-media area ratio at 28 days compared with WT mice (WT: 1.81 ± 0.63 vs. MRP-14−/−: 1.00 ± 0.34 P=0.004) (Table 1). Taken together, these observations indicate that MRP-8/14 complexes modulate neutrophil- and monocyte-dependent vascular inflammation and neointimal thickening after experimental mechanical arterial injury, and suggest that MRP-8/14 complexes modulate cellular proliferation that is stimulated by platelets and/or leukocytes in this setting. Importantly, differences in neointimal formation between WT and MRP-14−/− mice did not appear to be related to altered platelet deposition, as WT and MRP-14−/−mice showed similar staining for the platelet marker GPIIb 6 hours following injury (P=0.96)(Supplemental Figure 1). In addition, altered hemodynamics did not appear to influence inflammatory responses in the MRP-14−/− mice because WT and MRP-14−/− mice had similar systolic, diastolic, and mean arterial pressures in the awake and conscious state as measured by tail cuff method (Table 2).
Table 2.
Blood Pressure Measurements
| WT (n=8) | MRP-14−/− (n=8) | P | |
|---|---|---|---|
| Systolic, mmHg | 99.6 ± 2.3 | 98.5 ± 4.7 | 0.53 |
| Diastolic, mmHg | 71.9 ± 7.1 | 72.5 ± 8.1 | 0.87 |
| Mean arterial pressure, mmHg | 81.1 ± 4.8 | 81.2 ± 6.1 | 0.99 |
Data are presented as mean ± standard deviation
Measurements were made on age and sex matched mice that were 12 weeks of age
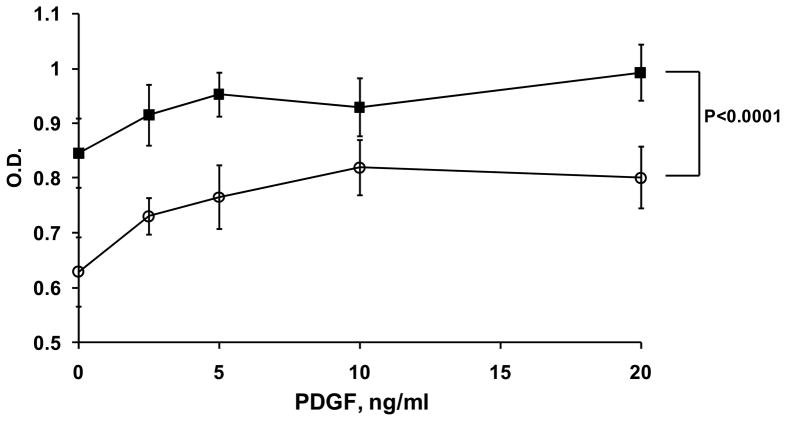
Vascular smooth muscle cells (SMC) are known to express MRP-14 16. To examine the role of MRP-8/14 in SMC proliferation relevant to vascular injury and restenosis, we isolated SMC from the aortas of WT and MRP-14−/− mice (muAoSMC) and examined basal and PDGF-stimulated proliferation in vitro by MTT assay (Figure 3). MuAoSMC from MRP-14−/− mice had significantly reduced basal and PDGF-stimulated proliferation in vitro (P<0.0001 by ANOVA). In contrast, the addition of purified human MRP-8 and MRP-14 had no effect on the proliferation of human aortic smooth muscle cells (huAoSMC)(Supplemental Figure 2). Taken together, these data likely indicate a role for intracellular MRP-8/14 action (calcium transients, cytoskeletal reorganization, arachidonic acid metabolism) in the regulation of SMC proliferation.
Figure 3. Deficiency of MRP-14 attenuates smooth muscle cell proliferation in vitro.
Basal and PDGF-stimulated proliferative activity of cultured mouse aortic smooth muscle cells derived from WT or MRP-14−/− mice. Proliferation was measured 18 hours following addition of PDGF. Representative experiment, mean ± standard deviation, n= 3 (■ WT, ○ MRP-14−/−)(P<0.0001 by ANOVA).
MRP-8/14 Complexes Regulate Neutrophil-dependent Thrombohemorrhagic Vasculopathy
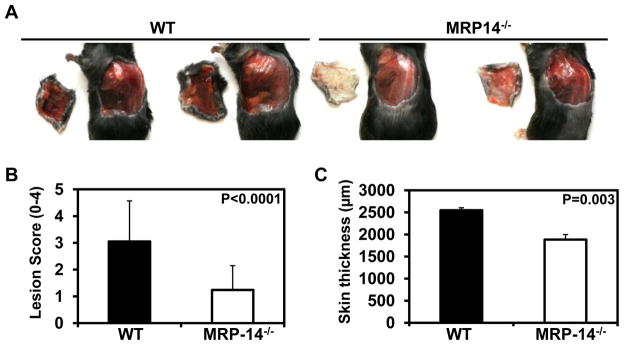
Next, we examined the role of MRP-8/14 complexes in cytokine- and neutrophil-dependent inflammation. MRP-14 regulates neutrophil functions in vitro and neutrophil-derived cytotoxic products play an important role in cytokine-induced inflammation of the vessel wall. The local Shwartzman-like reaction (LSR), induced by successive LPS and cytokine injection in the skin, produces a thrombohemorrhagic vasculitis 15, 17. Consistent with prior reports4, flow cytometry demonstrated equivalent expression of the LPS receptor TLR-4 on bone marrow derived and thioglycolate-elicited peritoneal macrophages harvested from WT and MRP-14 mice (data not shown). The LSR depends on neutrophils and their interaction with activated platelets and endothelial cells 15, 17, 18. Neutrophil-derived microparticles promote the accumulation of fibrin, generation of occlusive thrombi, and eventual hemorrhage from inflamed blood vessels 17. The LSR produces hemorrhage in the intact skin 24 hr after the TNF-α injection. MRP-14−/− mice subjected to LSR exhibited significant reduction in the development of hemorrhagic lesions (n=13 mice per group)(Figure 4A). In analysis blinded to genotype, MRP-14−/− mice had a 59% reduction in lesion severity (lesion hemorrhage score WT: 3.1 ± 1.52 vs. MRP-14−/−: 1.24 ± 0.91, P<0.0001) (Figure 4A-B).
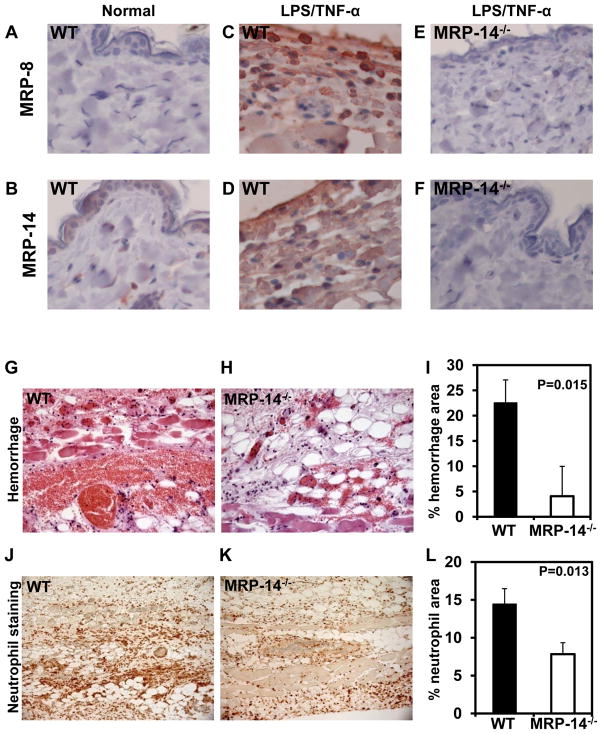
Figure 4. Deficiency of MRP-14 attenuates lesion severity during cytokine-induced thrombohemorrhagic vasculitis.
A, Necropsy images showing representative vasculitis lesions in WT and MRP-14−/− mice. B, Scoring of vasculitic lesions from WT and MRP-14−/− mice. C, Quantification of skin thickness (dermis plus epidermis) from histologic section of vasculitic lesions. Data represent mean ± standard deviation, n=13 mice per group.
Histopathology of vasculitic lesions demonstrated that the reduction in vasculitis severity was associated with less edema in the skin quantified by a 26% reduction in skin thickness in MRP-14−/− compare to WT mice (WT: 2,548 ± 57 μm vs. MRP-14−/−: 1,883 ± 115 μm, P=0.003)(Figure 4C). Histologic analysis of skin tissue affected by the LSR revealed significant increase in MRP-8 and MRP-14 expression compared to normal skin (Figure 5A–D). As expected, neither MRP-8 nor MRP-14 was detected in vasculitic lesions from MRP-14−/− mice (Figure 5E–F). The vasculitic lesions had substantial erythrocyte extravasation, edema, neutrophil accumulation, and occlusive thrombi containing neutrophils (Figure 5G–L). MRP-14−/− mice had an 82% reduction in hemorrhagic area, as assessed by computer-assisted imaging analysis (WT: 22.5 ± 4.5% vs. MRP-14−/−: 4.1 ± 5.9%, P<0.015)(Figure 5G–I). Neutrophil (mAb7/4-positive cells) accumulation in the vascular wall and skin also fell by 45% in the MRP-14−/− mice (% neutrophil area; WT: 14.4 ± 2.0% vs. MRP-14−/−: 7.8 ± 1.5%, P=0.013)(Figure 5J–L). These data indicate that in the LSR MRP-8/14 complexes participate in neutrophil-dependent tissue injury leading to the development of thrombohemorrhagic vasculitis.
Figure 5. Photomicrographs of skin lesions from WT and MRP-14−/− mice during cytokine-induced thrombohemorrhagic vasculitis.
A–B, MRP-8 or MRP-14 immunostaining of normal skin from a WT mouse. C–D, MRP-8 or MRP-14 immunostaining of vasculitic lesion from a WT mouse. E–F, MRP-8 or MRP-14 immunostaining of vasculitic lesion from a MRP-14−/− mouse. G–H, Hematoxylin and eosin staining of representative vasculitic lesions demonstrating hemorrhage (original magnification 40X). I, Quantification of % hemorrhage area. J–K, Immunohistochemical staining for neutrophils in vasculitic lesions (mAb7/4-positive cells)(20X). L, Quantification of % neutrophil-positive area. Data represent mean ± SD, n=13 mice per group.
MRP-8/14 Complexes Modulate Monocyte/Macrophage-dependent Atherosclerotic Lesion Formation
Examination of the role of MRP-8/14 in monocyte/macrophage-dependent vascular inflammation used mice with experimental atherosclerosis, a process driven by monocyte recruitment and function 19–21. Because we have shown that plasma levels of MRP-8/14 predict cardiovascular events related to atherosclerotic plaque rupture and thrombosis 3, 8, we examined whether plasma levels of MRP-14 increase during the development of atherosclerosis in mice. WT and atherosclerosis-prone apolipoprotein E-deficient (ApoE−/−) mice commenced an atherogenic diet at 8 weeks of age and 12 weeks later plasma levels of MRP-14 were assessed by immunoblotting. After 12 weeks of consumption of the atherogenic diet, ApoE−/− mice developed atherosclerotic lesions in the aorta (data not shown). Immunoblot analysis of MRP-14 expression demonstrated more MRP-14 in the plasma of ApoE−/− mice fed a normal chow diet compared to WT controls (Supplemental Figure 3). In addition, ApoE−/− mice fed the atherogenic diet had further increases in MRP-14 levels compared to ApoE−/− mice that were maintained on normal chow (Supplemental Figure 3).
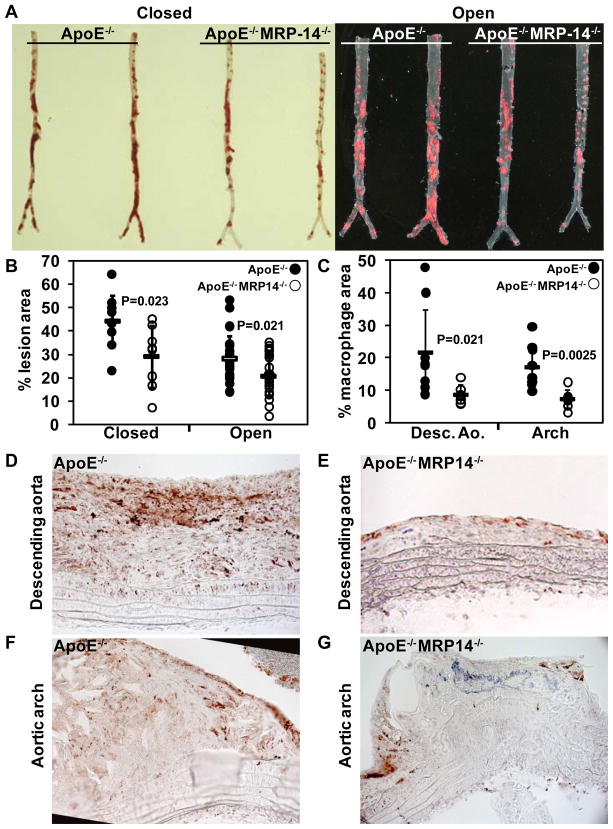
We generated compound mutant mice deficient in both ApoE and MRP-14 (ApoE−/−MRP-14−/−), and examined the development of atherosclerotic lesions in doubly deficient ApoE−/−MRP-14−/− compared to singly deficient ApoE−/− mice. Mice consumed an atherogenic diet from 8 weeks of age, and the thoracic and abdominal aortas were harvested at 20 weeks for atherosclerotic lesion analysis. Importantly, plasma lipid profiles did not differ significantly between ApoE−/− and ApoE−/−MRP-14−/− mice on atherogenic diet (Table 3). ApoE−/−MRP-14−/− mice had a 34% reduction in lesion area in en face (closed) analysis of Sudan IV stained thoraco-abdominal aortas at 20 weeks (ApoE−/−: 44.0 ± 11.0% vs. ApoE−/−MRP-14−/−: 29.0 ± 13.2%, n=9 per group, P=0.023), and a 25% reduction in lesion area when the aortas were opened longitudinally, pinned, and analyzed (ApoE−/−: 28.1 ± 9.8% vs. ApoE−/−MRP-14−/−:21.2 ± 9.0%, n=22 per group, P=0.021) (Figure 6A–B).
Table 3.
Plasma Lipid Levels
| ApoE−/− | ApoE−/−MRP-14−/− | P | |
|---|---|---|---|
| Total Cholesterol, mg/dL | 1,168 ± 200 | 1,325 ± 183 | 0.12 |
| LDL Cholesterol | 506 ± 80.0 | 527 ± 101 | 0.46 |
| HDL Cholesterol | 24.2 ± 7.9 | 18.5 ± 7.9 | 0.07 |
| Triglycerides, mg/dL | 145 ± 54 | 117 ± 37 | 0.12 |
Data are presented as mean ± standard deviation
Measurements were made on mice that were fed high-fat for 9–12 weeks (n=10–15 mice)
Figure 6. Deficiency of MRP-14 attenuates atherosclerotic lesion formation and plaque inflammation.
A, Sudan IV staining of intact closed and longitudinally opened and pinned thoracoabdominal aortas harvested from ApoE−/− and ApoE−/−MRP-14−/− mice after 20 weeks of high-fat feeding. B, Quantification of % lesion area as assessed by Sudan IV staining and computer-assisted imaging analysis. C, Quantification of plaque macrophage content (% Mac-3-positive area) in longitudinal sections from the descending aorta and the lesser curvature of the aortic arch. D–G, Representative photomicrographs staining for macrophages (Mac-3-positive cells) within atherosclerotic lesions in the descending aorta (D, E) and aortic arch (F, G) (original magnification 20X). For paired samples in the atherosclerotic lesion area analysis, comparison of the % lesion area in closed vs. open analysis resulted in a correlation value of 0.63. For macrophage staining, comparison of the percent macrophage area in the descending aorta vs. the aortic arch resulted in a correlation value of 0.41.
We examined plaque inflammation by staining atherosclerotic lesions for the macrophage-specific marker Mac-3. Plaque inflammation fell significantly in ApoE−/− MRP-14−/− mice in both the descending aorta atheroma (% macrophage area: ApoE−/− 21.6 ± 13.3% vs. ApoE−/−MRP-14−/−: 8.7 ± 2.9%, n= 9 per group, P=0.021) and in the aortic arch atheroma (ApoE−/−: 17.1 ± 6.6% vs. ApoE−/−MRP-14−/−: 7.3 ± 2.8%, n=9 per group, P=0.0025) (Figure 6C–G). Additional analyses demonstrated no significant differences in T cell accumulation (CD3-positive cells), collagen content, or elastin content in atherosclerotic lesions from ApoE−/− and ApoE−/− MRP-14−/− mice (Supplemental Figure 4). These observations indicate that MRP-8/14 complexes promote monocyte/macrophage recruitment, plaque inflammation and the development of atherosclerotic lesions in ApoE−/− mice.
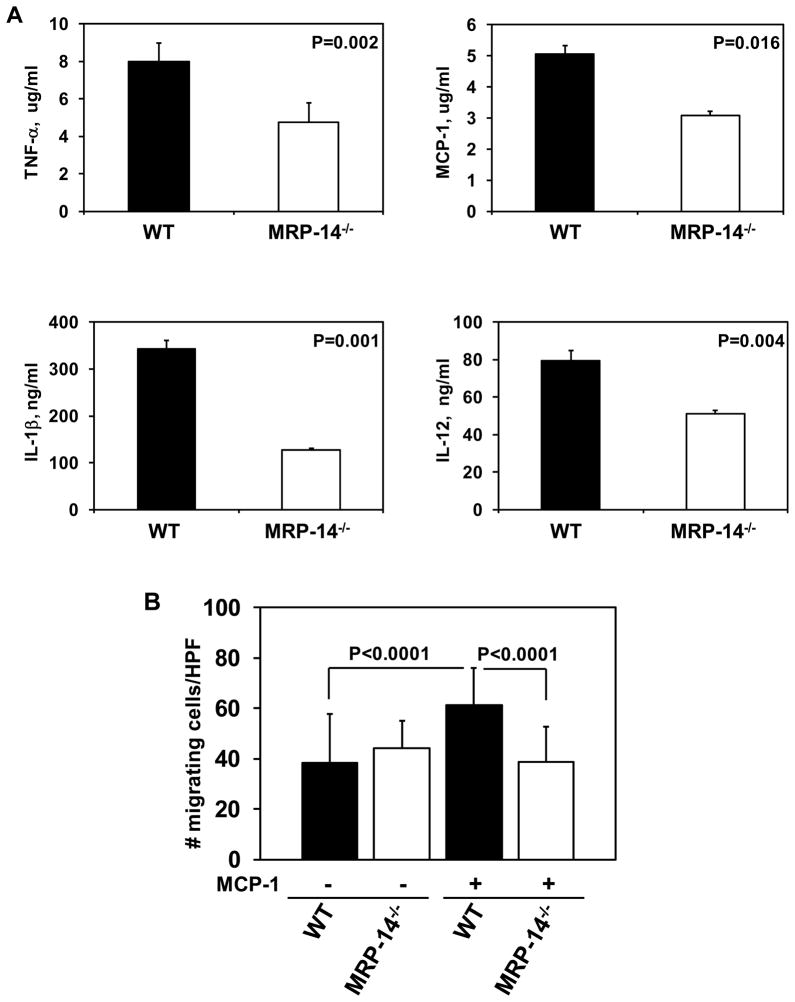
To specifically examine the role of MRP-8/14 in the regulation of macrophage function relevant to atherosclerosis, we evaluated the production of atherogenic cytokines by cultured macrophages derived from WT or MRP-14−/− mice (Figure 7A–B). Compared to WT, in response to LPS challenge, MRP-14−/− macrophages demonstrated a 40% reduction in TNF-α production (WT: 7.9 ± 0.2 μg/ml vs. MRP-14−/−: 4.8 ± 0.01 μg/ml, P=0.002), a 39% reduction in MCP-1 production (WT: 5.0 ± 0.3 μg/ml vs. MRP-14−/−: 3.1 ± 0.14 μg/ml, P=0.016), a 62% reduction in Il-1β production (WT: 342.5 ± 17.5 ng/ml vs. MRP-14−/−: 127.5 ± 2.5 ng/ml, P=0.001), and a 36% reduction in Il-12 production (WT: 79.5 ± 5.0 ng/ml vs. MRP-14−/−: 51 ± 2.0 ng/ml, P=0.004). In addition to reduction in atherogenic cytokine production, macrophages derived from MRP-14−/− mice had a 37% reduction in MCP-1-stimulated migration in transwell margination assays (WT: 61.2 ± 14.9 cells/high power field (HPF) vs. MRP-14−/−: 38.7 ± 14.0 cells/HPF, P<0.0001). Analysis of macrophage lipid uptake and foam cell formation in vitro by thioglycolate-elicited macrophages from WT and MRP-14−/− mice demonstrated equivalent uptake of fluorescently labeled Acetyl LDL (WT: 4460 ± 248 relative fluorescence units (RFU) vs. MRP-14−/−:4587 ± 728 RFU at 48h, P=0.87) and similar uptake of oxidized LDL (oxLDL) measured by oil red O (WT: 0.317 ±0.041 optical density (O.D.) vs. MRP-14−/−: 0.294 ± 0.009 O.D. at 48h, P=0.44). Taken together, these data demonstrate that MRP-8/14 influences the production of key cytokines involved in the pathobiology of restenosis, vasculitis and atherosclerosis, and that MRP-8/14 regulates cell migratory function in response to MCP-1, which is a critical mediator of vascular inflammation.
Figure 7. MRP-8/14 regulates macrophage cytokine production and migration.
ELISA analysis of LPS-induced (5μg/ml) cytokine production by cultured macrophages derived from WT and MRP-14−/− mice. Twelve hour stimulation, representative experiment of n=3 per cytokine. A, TNF-α, MCP-1, Il-1β, IL-12 production. B, Transwell assay of MCP-1-stimulated peripheral blood mononuclear cell migration.
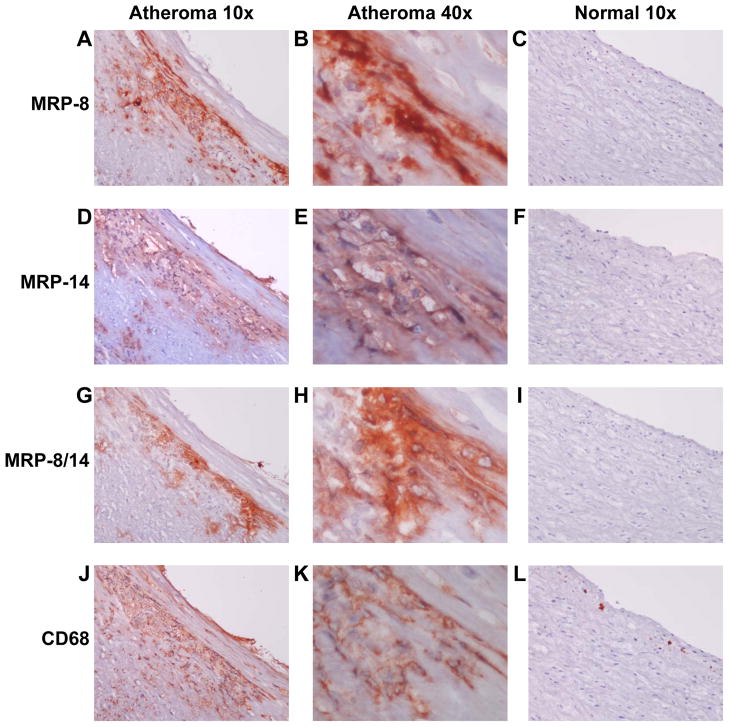
Human Carotid Atheroma Contain MRP-8/14
To begin to investigate the relevance of MRP-8/14 in human disease, we examined the expression of MRP-8, MRP-14, and MRP-8/14 in normal human carotid arteries and in carotid atheroma. Carotid atheroma, but not normal arteries (n=5), contain abundant MRP-8, MRP-14 and heterodimeric MRP-8/14 (identified using a heterodimer complex-specific antibody 27E10)(Figure 8A–L). Analysis of adjacent tissue sections demonstrated that MRP-8, MRP-14, and MRP-8/14 staining is largely associated with lesional CD68-positive macrophages (Figure 8 40x serial sections B,E,H,K). MRP-8, MRP-14, and MRP-8/14 were expressed robustly in all types of atherosclerotic lesions including fatty streaks (n=6 samples), smooth muscle cell-rich atheroma with stable features (n=6), and inflammatory cell-rich atheroma with thin caps and features of plaque instability (n=4) (Supplemental Figure 5).
Figure 8. MRP-8/14 is expressed in human carotid atheroma.
A–C, MRP-8 staining of carotid atheroma or normal carotid artery. D–F, MRP-14 staining of carotid atheroma or normal carotid artery. G–I, MRP-8/14 complex-specific staining of carotid atheroma or normal carotid artery. J–K, CD68 staining of carotid atheroma or normal carotid artery. B,E,H,K, Adjacent tissue sections demonstrating MRP-8, MRP-14, and MRP-8/14 association with lesional CD68-positive macrophages (original magnification 10x or 40x as indicated).
DISCUSSION
This study shows that deficiency of MRP-8 and MRP-14 reduces neutrophil- and monocyte-dependent vascular inflammation and attenuates the severity of diverse vascular injury responses in vivo. These observations establish that MRP-8 and MRP-14 participate importantly in the biological response to vascular injury.
Analyses of mice that lack MRP-8 and MRP-14 have provided important insights into the function of these proteins. Although the homozygous deletion of MRP-8 results in embryonic lethality 22, deletion of the MRP-14 gene does not affect viability and results in the additional loss of MRP-8 protein. Failure to produce mature MRP-8 protein in the presence of normal MRP-8 mRNA production likely results from instability of MRP-8 in the absence of MRP-14 6, 11. Thus the MRP-14−/− mice used in this and previous studies lack MRP-8 and MRP-14 protein, and MRP-8/14 complexes.
Recent observations in MRP-14−/− mice indicate that MRP-8/14 is essential for LPS-induced septic shock4, neutrophil-dependent S. aureus killing 23, and neutrophil infiltration in acute pancreatitis 12, however a direct role for MRP-8 and MRP-14 in vascular inflammation and in the pathophysiology of vascular disease was heretofore unrecognized. Prior studies examining MRP-8/14 in vascular inflammation found high MRP-8/14 levels in neutrophils in large-vessel vasculitis 24 and in macrophages and foam cells in human 25 and mouse 26 atherosclerotic lesions. The 3 experimental forms of vascular inflammation utilized in our study depend variably upon neutrophils (vascultis), monocyte/macrophages (atherosclerosis), or their combination (arterial wire injury). This study shows that MRP-8 and MRP-14 promote the recruitment of inflammatory cells and tissue injury, as assessed by neointimal formation (wire injury), hemorrhage area (vasculitis), and atherosclerotic plaque area (atherosclerosis).
Our data strongly suggest that MRP-8/14 influences vascular inflammation and the development of vascular disease through multiple mechanisms that include regulation of vascular smooth muscle cell proliferation, regulation of MCP-1-stimulated monocyte migration, and regulation of the production of key cytokines (TNF-α, MCP-1, IL-1β, and IL-12). A particularly notable feature of the phenotype of MRP-14−/− mice in all three disease models (wire injury, vasculitis, atherosclerosis), is the significant reduction in the accumulation of neutrophils and macrophages in the inflamed tissue. This phenotype of reduced leukocyte accumulation is supported by several studies demonstrating MRP-8/14 regulation of myeloid adhesion and transmigration across the blood vessel wall 11, 27–30. In vitro studies with MRP-14−/− neutrophils show markedly diminished migration through endothelial monolayers and attenuated chemokinesis in collagen gels 11, and although MRP-14−/− mice have normal neutrophil migration during chemical peritonitis, MRP-14 promotes the recruitment of neutrophils into granulation tissue during wound healing in vivo 7, into pancreatic tissue during acute pancreatitis 12, and into the skin during IL-8-stimulated inflammation 6, 11. Recently, MRP-8/14 was linked to leukocyte activation in lethal endotoxin-mediated shock, however, this report did not identify the in vivo source of MRP-8/14 and did not study the effects of MRP-8/14-deficiency on leukocyte recruitment during sepsis 4.
It is possible that MRP-8/14 regulates leukocyte adhesion during vascular inflammation through control of the expression and function of the leukocyte integrin adhesion molecule Mac-1 (CD11b/CD18, αMβ2)11, 30, 31. MRP-14−/− neutrophils have reduced Mac-1 expression at baseline and in response to cell activation 11, and MRP-14 binds to a distinct neutrophil receptor resulting in inside-out signaling events that promote Mac-1 activation and increase cell adhesiveness 31. MRP-8/14 induces Mac-1 expression on monocytes and enhances endothelial transmigration via intracellular adhesion molecule-1 (ICAM-1)-dependent pathways 32. We have shown previously that Mac-1 directs leukocyte recruitment and is critical for the biological response to vascular injury and neointimal formation 14, 33. These studies linking MRP-8/14 function to leukocyte adhesion and transmigration are consistent with our data that MRP-14-deficiency reduces MCP-1-stimulated monocyte transmigration. It is also possible that extracellular MRP-8/14 regulates leukocyte recruitment during the development of vascular disease by functioning as a chemoattractant or by binding to cell receptors that activate leukocytes. MRP-8/14 interacts with RAGE 5 and TLR-44 receptors which both modulate vascular inflammation and have important roles in the pathobiology of atherosclerosis34, 35. Other putative receptors for MRP-8/14 on target cells include CD36 36, special carboxylated N-glycans 37, and heparin-like glycoaminoglycans 38.
In the vascular injury model, MRP-8/14 appears to control the inflammatory response at several levels including regulation of macrophage and neutrophil accumulation, and regulation of vascular smooth muscle cell proliferation. Our finding that MRP-14 deficiency attenuates mouse vascular SMC proliferation is consistent with a recent report linking MRP-8/14 function to bacterial-induced proliferative responses in human vascular SMCs 16.
Despite equivalent TLR-4 receptor expression in WT and MRP-14−/− mice, mechanistic interpretation of the LSR response in MRP-14−/− mice is complicated by the fact that LPS is used to stimulate the LSR. Since MRP-8/14 itself modulates LPS signaling via TLR-4 4, attenuated LSR response could be secondary to dampened TLR-4 signaling in MRP-14−/− mice. However, the LSR requires subcutaneous injection of TNF-α 24h after LPS, making it unlikely that reduced TLR-4 signaling (i.e., production of TNF-α) could solely account for the effect in MRP-14−/− mice. Rather, we believe that a combination of the intracellular actions of MRP-14 (regulation of calcium-dependent signaling 5, 6, integration of MAP kinase activity 7, regulation of microtubule reorganization 7, regulation of arachidonic acid metabolism and trafficking 27, 39) and extracellular actions of MRP-14 (receptor-dependent signaling through TLR-4 4, RAGE 5, CD36 36 and possibly other receptors 38) together account for the attenuated thrombohemorrhagic vasculitic response in vivo. Furthermore, reduced leukocyte Mac-1 expression and ligand binding is well-documented in MRP-14−/− mice, and reduced MRP-8/14-dependent Mac-1 adhesion/activation 11, 30, 31 could also account for attenuation of the LSR response because this model is dependent on Mac-1-mediated neutrophil function 15.
With regard to the role of MRP-8/14 in atherosclerosis, recent studies have demonstrated that free cholesterol accumulation in macrophage membranes activates TLRs and p38 Mitogen-activated protein kinase, and induces production and secretion of MRP-8 40. It is possible that hyperlipidemia-induced macrophage expression of MRP-8/14 could promote leukocyte recruitment and atherogenic cytokine production, a hypothesis that is supported by our demonstration of MRP-8/14 expression in fatty streaks and advanced atheroma. Furthermore, the demonstration of reduced cytokine production by MRP-14−/− macrophages, and the demonstration of reduced atherosclerotic lesion size and plaque macrophage accumulation in ApoE−/−MRP-14−/− mice, suggest a regulatory for MRP-8/14 in leukocyte recruitment and cytokine production during atherogenesis. A regulatory role for MRP-8/14 in atherogenesis is further supported by the recent discovery that MRP-8/14 expression levels are increased in microarray analysis of gene expression in normal vs. atherosclerotic human arteries where statistical analysis of gene expression patterns suggested a role for MRP-8/14 in the development of atherosclerotic vascular disease 41.
Limitations
The present study does not clarify whether neutrophil-, monocyte-, or platelet-derived MRP-8 and/or MRP-14 account for the action of these proteins in the pathophysiology of inflammatory vascular disease. Although platelet deposition following wire injury was similar in WT and MRP-14−/− mice, we have not excluded a regulatory role for MRP-14 in platelet functions that influence vascular injury responses. Tissue-specific knockdown of MRP-14 will be required to establish the relative contributions of MRP-8 and MRP-14 action in specific hematopoietic cell types, and investigations into the role of MRP-14 in platelet function will be the focus of future research. The relevance of these observations to human disease awaits expanded studies using human tissues, although the present observations document high levels of MRP-8/14 in human carotid artery atheroma (Figure 8 and Supplemental Figure 5), and elevated plasma levels of MRP-8/14 are associated with adverse cardiovascular outcomes in patients 3, 8.
Conclusions
This study demonstrates that MRP-8/14 broadly regulates vascular inflammation and contributes to biological responses to various vascular insults by controlling neutrophil and macrophage accumulation, macrophage cytokine production, and smooth muscle cell proliferation. These findings implicate MRP-8/14 in the development of diverse forms of vascular disease including hyperplastic responses to arterial intervention, vasculitis and atherosclerosis.
Supplementary Material
Acknowledgments
FUNDING SOURCES
This work was supported in part by grants from the National Heart, Lung, and Blood Institute to D.S. (HL85816, HL57506 MERIT Award, HL73852), to K.C. (1K08HL086672), and to PL (HL34636), the Donald W. Reynolds Foundation to P.L., a Future Leaders in Cardiovascular Medicine Fellowship Grant from Schering Plough to K.C., and an award from the Michael Lerner Foundation to K.C.
Footnotes
DISCLOSURE
The authors report no significant conflicts of interest.
Author contributions: K.C. designed research, performed research, analyzed data, and wrote paper; H.G. designed research, performed research, and analyzed data; Y.W. designed research, performed research, analyzed data, and wrote paper; M.S. performed research and analyzed data; T.M. performed research and analyzed data; C.S. performed research; G.S. performed research and analyzed data; R.P. performed research; N.H. contributed vital reagent and wrote paper; P.L. provided experimental input, and helped with data analysis and writing; D.I.S. designed research, analyzed data, and wrote paper.
References
- 1.Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 2.Hansson GK, Libby P, Schonbeck U, Yan ZQ. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circulation research. 2002;91(4):281–291. doi: 10.1161/01.res.0000029784.15893.10. [DOI] [PubMed] [Google Scholar]
- 3.Healy AM, Pickard MD, Pradhan AD, Wang Y, Chen Z, Croce K, Sakuma M, Shi C, Zago AC, Garasic J, Damokosh AI, Dowie TL, Poisson L, Lillie J, Libby P, Ridker PM, Simon DI. Platelet expression profiling and clinical validation of myeloid-related protein-14 as a novel determinant of cardiovascular events. Circulation. 2006;113(19):2278–2284. doi: 10.1161/CIRCULATIONAHA.105.607333. [DOI] [PubMed] [Google Scholar]
- 4.Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MA, Nacken W, Foell D, van der Poll T, Sorg C, Roth J. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med. 2007;13(9):1042–1049. doi: 10.1038/nm1638. [DOI] [PubMed] [Google Scholar]
- 5.Boyd JH, Kan B, Roberts H, Wang Y, Walley KR. S100A8 and S100A9 Mediate Endotoxin-Induced Cardiomyocyte Dysfunction via the Receptor for Advanced Glycation End Products. Circulation research. 2008 doi: 10.1161/CIRCRESAHA.107.167544. [DOI] [PubMed] [Google Scholar]
- 6.Hobbs JA, May R, Tanousis K, McNeill E, Mathies M, Gebhardt C, Henderson R, Robinson MJ, Hogg N. Myeloid cell function in MRP-14 (S100A9) null mice. Mol Cell Biol. 2003;23(7):2564–2576. doi: 10.1128/MCB.23.7.2564-2576.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vogl T, Ludwig S, Goebeler M, Strey A, Thorey IS, Reichelt R, Foell D, Gerke V, Manitz MP, Nacken W, Werner S, Sorg C, Roth J. MRP8 and MRP14 control microtubule reorganization during transendothelial migration of phagocytes. Blood. 2004;104(13):4260–4268. doi: 10.1182/blood-2004-02-0446. [DOI] [PubMed] [Google Scholar]
- 8.Morrow D, Wang Y, Croce K, Sakuma M, Sabatine M, Gao H, Pradhan A, Healy A, Buros J, McCabe C, Libby P, Cannon C, Braunwald E, Simon DI. Myeloid-Related Protein-8/14 and the Risk of Cardiovascular Death or Myocardial Infarction after an Acute Coronary Syndrome in the PROVE IT-TIMI 22 Trial. American Heart Journal. 2007 doi: 10.1016/j.ahj.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Altwegg LA, Neidhart M, Hersberger M, Muller S, Eberli FR, Corti R, Roffi M, Sutsch G, Gay S, von Eckardstein A, Wischnewsky MB, Luscher TF, Maier W. Myeloid-related protein 8/14 complex is released by monocytes and granulocytes at the site of coronary occlusion: a novel, early, and sensitive marker of acute coronary syndromes. Eur Heart J. 2007;28(8):941–948. doi: 10.1093/eurheartj/ehm078. [DOI] [PubMed] [Google Scholar]
- 10.Nacken W, Roth J, Sorg C, Kerkhoff C. S100A9/S100A8: Myeloid representatives of the S100 protein family as prominent players in innate immunity. Microsc Res Tech. 2003;60(6):569–580. doi: 10.1002/jemt.10299. [DOI] [PubMed] [Google Scholar]
- 11.Manitz MP, Horst B, Seeliger S, Strey A, Skryabin BV, Gunzer M, Frings W, Schonlau F, Roth J, Sorg C, Nacken W. Loss of S100A9 (MRP14) results in reduced interleukin-8-induced CD11b surface expression, a polarized microfilament system, and diminished responsiveness to chemoattractants in vitro. Mol Cell Biol. 2003;23(3):1034–1043. doi: 10.1128/MCB.23.3.1034-1043.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schnekenburger J, Schick V, Kruger B, Manitz MP, Sorg C, Nacken W, Kerkhoff C, Kahlert A, Mayerle J, Domschke W, Lerch MM. The calcium binding protein S100A9 is essential for pancreatic leukocyte infiltration and induces disruption of cell-cell contacts. J Cell Physiol. 2008;216(2):558–567. doi: 10.1002/jcp.21433. [DOI] [PubMed] [Google Scholar]
- 13.Roque M, Fallon JT, Badimon JJ, Zhang WX, Taubman MB, Reis ED. Mouse model of femoral artery denudation injury associated with the rapid accumulation of adhesion molecules on the luminal surface and recruitment of neutrophils. Arterioscler Thromb Vasc Biol. 2000;20(2):335–342. doi: 10.1161/01.atv.20.2.335. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Sakuma M, Chen Z, Ustinov V, Shi C, Croce K, Zago AC, Lopez J, Andre P, Plow E, Simon DI. Leukocyte engagement of platelet glycoprotein Ibalpha via the integrin Mac-1 is critical for the biological response to vascular injury. Circulation. 2005;112(19):2993–3000. doi: 10.1161/CIRCULATIONAHA.105.571315. [DOI] [PubMed] [Google Scholar]
- 15.Hirahashi J, Mekala D, Van Ziffle J, Xiao L, Saffaripour S, Wagner DD, Shapiro SD, Lowell C, Mayadas TN. Mac-1 signaling via Src-family and Syk kinases results in elastase-dependent thrombohemorrhagic vasculopathy. Immunity. 2006;25(2):271–283. doi: 10.1016/j.immuni.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 16.Inaba H, Hokamura K, Nakano K, Nomura R, Katayama K, Nakajima A, Yoshioka H, Taniguchi K, Kamisaki Y, Ooshima T, Umemura K, Murad F, Wada K, Amano A. Upregulation of S100 calcium-binding protein A9 is required for induction of smooth muscle cell proliferation by a periodontal pathogen. FEBS letters. 2009;583(1):128–134. doi: 10.1016/j.febslet.2008.11.036. [DOI] [PubMed] [Google Scholar]
- 17.Brozna JP. Shwartzman reaction. Semin Thromb Hemost. 1990;16(4):326–332. doi: 10.1055/s-2007-1002685. [DOI] [PubMed] [Google Scholar]
- 18.Argenbright LW, Barton RW. Interactions of leukocyte integrins with intercellular adhesion molecule 1 in the production of inflammatory vascular injury in vivo. The Shwartzman reaction revisited. J Clin Invest. 1992;89(1):259–272. doi: 10.1172/JCI115570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boring L, Gosling J, Cleary M, Charo IF. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394(6696):894–897. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- 20.Gosling J, Slaymaker S, Gu L, Tseng S, Zlot CH, Young SG, Rollins BJ, Charo IF. MCP-1 deficiency reduces susceptibility to atherosclerosis in mice that overexpress human apolipoprotein B. J Clin Invest. 1999;103(6):773–778. doi: 10.1172/JCI5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan ZQ, Hansson GK. Innate immunity, macrophage activation, and atherosclerosis. Immunol Rev. 2007;219:187–203. doi: 10.1111/j.1600-065X.2007.00554.x. [DOI] [PubMed] [Google Scholar]
- 22.Passey RJ, Williams E, Lichanska AM, Wells C, Hu S, Geczy CL, Little MH, Hume DA. A null mutation in the inflammation-associated S100 protein S100A8 causes early resorption of the mouse embryo. J Immunol. 1999;163(4):2209–2216. [PubMed] [Google Scholar]
- 23.Corbin BD, Seeley EH, Raab A, Feldmann J, Miller MR, Torres VJ, Anderson KL, Dattilo BM, Dunman PM, Gerads R, Caprioli RM, Nacken W, Chazin WJ, Skaar EP. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science. 2008;319(5865):962–965. doi: 10.1126/science.1152449. [DOI] [PubMed] [Google Scholar]
- 24.Hirono K, Foell D, Xing Y, Miyagawa-Tomita S, Ye F, Ahlmann M, Vogl T, Futatani T, Rui C, Yu X, Watanabe K, Wanatabe S, Tsubata S, Uese K, Hashimoto I, Ichida F, Nakazawa M, Roth J, Miyawaki T. Expression of myeloid-related protein-8 and -14 in patients with acute Kawasaki disease. J Am Coll Cardiol. 2006;48(6):1257–1264. doi: 10.1016/j.jacc.2006.02.077. [DOI] [PubMed] [Google Scholar]
- 25.McCormick MM, Rahimi F, Bobryshev YV, Gaus K, Zreiqat H, Cai H, Lord RS, Geczy CL. S100A8 and S100A9 in human arterial wall. Implications for atherogenesis. J Biol Chem. 2005;280(50):41521–41529. doi: 10.1074/jbc.M509442200. [DOI] [PubMed] [Google Scholar]
- 26.Eue I, Langer C, Eckardstein A, Sorg C. Myeloid related protein (MRP) 14 expressing monocytes infiltrate atherosclerotic lesions of ApoE null mice. Atherosclerosis. 2000;151(2):593–597. doi: 10.1016/s0021-9150(00)00476-7. [DOI] [PubMed] [Google Scholar]
- 27.Kerkhoff C, Eue I, Sorg C. The regulatory role of MRP8 (S100A8) and MRP14 (S100A9) in the transendothelial migration of human leukocytes. Pathobiology. 1999;67(5–6):230–232. doi: 10.1159/000028098. [DOI] [PubMed] [Google Scholar]
- 28.Vandal K, Rouleau P, Boivin A, Ryckman C, Talbot M, Tessier PA. Blockade of S100A8 and S100A9 suppresses neutrophil migration in response to lipopolysaccharide. J Immunol. 2003;171(5):2602–2609. doi: 10.4049/jimmunol.171.5.2602. [DOI] [PubMed] [Google Scholar]
- 29.Ryckman C, McColl SR, Vandal K, de Medicis R, Lussier A, Poubelle PE, Tessier PA. Role of S100A8 and S100A9 in neutrophil recruitment in response to monosodium urate monohydrate crystals in the air-pouch model of acute gouty arthritis. Arthritis Rheum. 2003;48(8):2310–2320. doi: 10.1002/art.11079. [DOI] [PubMed] [Google Scholar]
- 30.Ryckman C, Vandal K, Rouleau P, Talbot M, Tessier PA. Proinflammatory activities of S100: proteins S100A8, S100A9, and S100A8/A9 induce neutrophil chemotaxis and adhesion. J Immunol. 2003;170(6):3233–3242. doi: 10.4049/jimmunol.170.6.3233. [DOI] [PubMed] [Google Scholar]
- 31.Newton RA, Hogg N. The human S100 protein MRP-14 is a novel activator of the beta 2 integrin Mac-1 on neutrophils. J Immunol. 1998;160(3):1427–1435. [PubMed] [Google Scholar]
- 32.Eue I, Pietz B, Storck J, Klempt M, Sorg C. Transendothelial migration of 27E10+ human monocytes. Int Immunol. 2000;12(11):1593–1604. doi: 10.1093/intimm/12.11.1593. [DOI] [PubMed] [Google Scholar]
- 33.Simon DI, Chen Z, Seifert P, Edelman ER, Ballantyne CM, Rogers C. Decreased neointimal formation in Mac-1(−/−) mice reveals a role for inflammation in vascular repair after angioplasty. J Clin Invest. 2000;105(3):293–300. doi: 10.1172/JCI7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Basta G. Receptor for advanced glycation endproducts and atherosclerosis: From basic mechanisms to clinical implications. Atherosclerosis. 2008;196(1):9–21. doi: 10.1016/j.atherosclerosis.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 35.Michelsen KS, Doherty TM, Shah PK, Arditi M. TLR signaling: an emerging bridge from innate immunity to atherogenesis. J Immunol. 2004;173(10):5901–5907. doi: 10.4049/jimmunol.173.10.5901. [DOI] [PubMed] [Google Scholar]
- 36.Kerkhoff C, Sorg C, Tandon NN, Nacken W. Interaction of S100A8/S100A9-arachidonic acid complexes with the scavenger receptor CD36 may facilitate fatty acid uptake by endothelial cells. Biochemistry. 2001;40(1):241–248. doi: 10.1021/bi001791k. [DOI] [PubMed] [Google Scholar]
- 37.Srikrishna G, Panneerselvam K, Westphal V, Abraham V, Varki A, Freeze HH. Two proteins modulating transendothelial migration of leukocytes recognize novel carboxylated glycans on endothelial cells. J Immunol. 2001;166(7):4678–4688. doi: 10.4049/jimmunol.166.7.4678. [DOI] [PubMed] [Google Scholar]
- 38.Robinson MJ, Tessier P, Poulsom R, Hogg N. The S100 family heterodimer, MRP-8/14, binds with high affinity to heparin and heparan sulfate glycosaminoglycans on endothelial cells. J Biol Chem. 2002;277(5):3658–3665. doi: 10.1074/jbc.M102950200. [DOI] [PubMed] [Google Scholar]
- 39.Kerkhoff C, Klempt M, Kaever V, Sorg C. The two calcium-binding proteins, S100A8 and S100A9, are involved in the metabolism of arachidonic acid in human neutrophils. J Biol Chem. 1999;274(46):32672–32679. doi: 10.1074/jbc.274.46.32672. [DOI] [PubMed] [Google Scholar]
- 40.Sun Y, Ishibashi M, Seimon T, Lee M, Sharma SM, Fitzgerald KA, Samokhin AO, Wang Y, Sayers S, Aikawa M, Jerome WG, Ostrowski MC, Bromme D, Libby P, Tabas IA, Welch CL, Tall AR. Free Cholesterol Accumulation in Macrophage Membranes Activates Toll-Like Receptors and p38 Mitogen-Activated Protein Kinase and Induces Cathepsin K. Circulation research. 2009 doi: 10.1161/CIRCRESAHA.108.182568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cagnin S, Biscuola M, Patuzzo C, Trabetti E, Pasquali A, Laveder P, Faggian G, Iafrancesco M, Mazzucco A, Pignatti PF, Lanfranchi G. Reconstruction and functional analysis of altered molecular pathways in human atherosclerotic arteries. BMC genomics. 2009;10(1):13. doi: 10.1186/1471-2164-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei J, Gorman TE, Liu X, Ith B, Tseng A, Chen Z, Simon DI, Layne MD, Yet SF. Increased neointima formation in cysteine-rich protein 2-deficient mice in response to vascular injury. Circulation research. 2005;97(12):1323–1331. doi: 10.1161/01.RES.0000194331.76925.5c. [DOI] [PubMed] [Google Scholar]
- 43.Fukumoto Y, Deguchi JO, Libby P, Rabkin-Aikawa E, Sakata Y, Chin MT, Hill CC, Lawler PR, Varo N, Schoen FJ, Krane SM, Aikawa M. Genetically determined resistance to collagenase action augments interstitial collagen accumulation in atherosclerotic plaques. Circulation. 2004;110(14):1953–1959. doi: 10.1161/01.CIR.0000143174.41810.10. [DOI] [PubMed] [Google Scholar]
- 44.Shi C, Sakuma M, Mooroka T, Liscoe A, Gao H, Croce KJ, Sharma A, Kaplan D, Greaves DR, Wang Y, Simon DI. Down-regulation of the forkhead transcription factor Foxp1 is required for monocyte differentiation and macrophage function. Blood. 2008;112(12):4699–4711. doi: 10.1182/blood-2008-01-137018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Packard RR, Maganto-Garcia E, Gotsman I, Tabas I, Libby P, Lichtman AH. CD11c(+) dendritic cells maintain antigen processing, presentation capabilities, and CD4(+) T-cell priming efficacy under hypercholesterolemic conditions associated with atherosclerosis. Circulation research. 2008;103(9):965–973. doi: 10.1161/CIRCRESAHA.108.185793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sukhova GK, Schonbeck U, Rabkin E, Schoen FJ, Poole AR, Billinghurst RC, Libby P. Evidence for increased collagenolysis by interstitial collagenases-1 and -3 in vulnerable human atheromatous plaques. Circulation. 1999;99(19):2503–2509. doi: 10.1161/01.cir.99.19.2503. [DOI] [PubMed] [Google Scholar]
- 47.Falk E, Shah P, Fuster V. Coronary plaque disruption. Circulation. 1995;92:657–671. doi: 10.1161/01.cir.92.3.657. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.