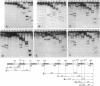
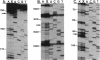
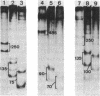
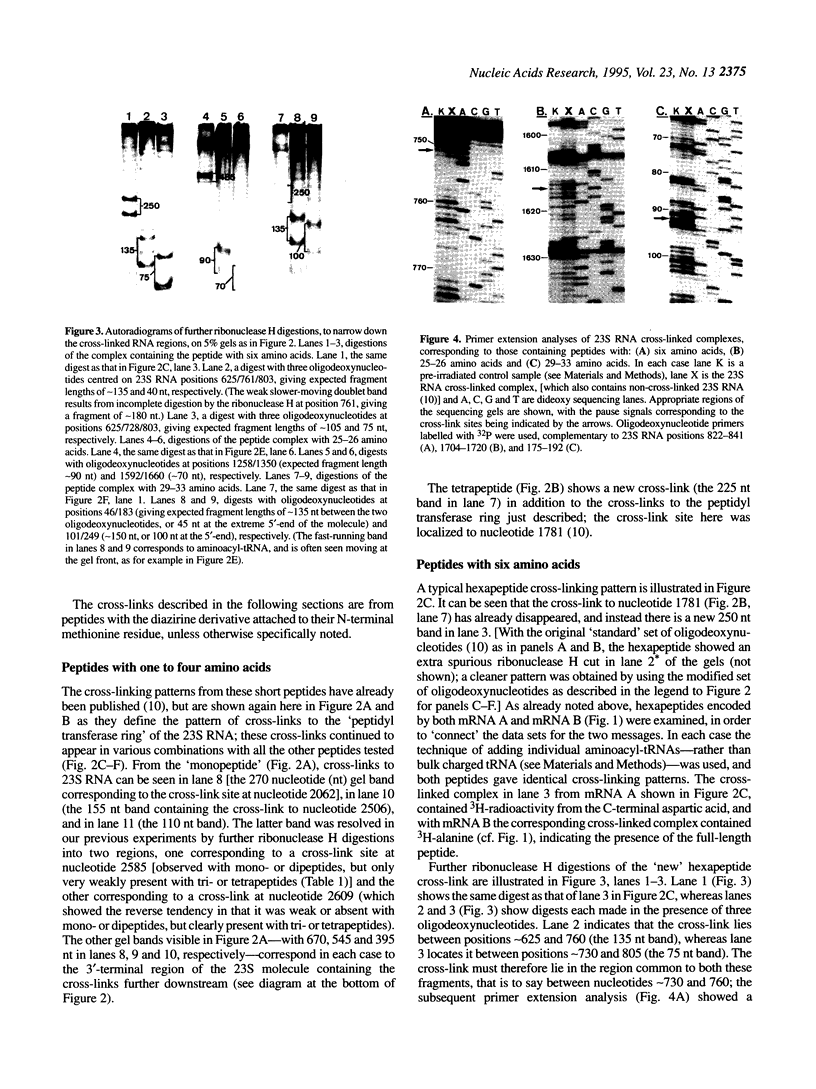
Abstract
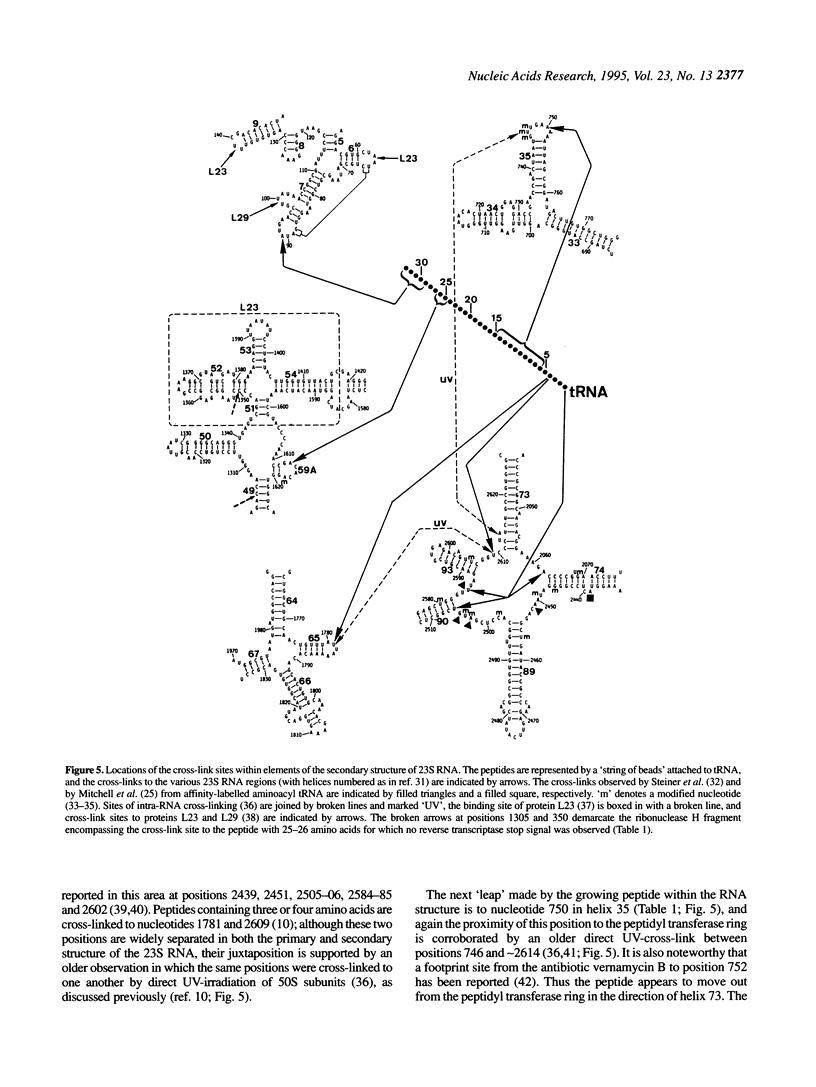
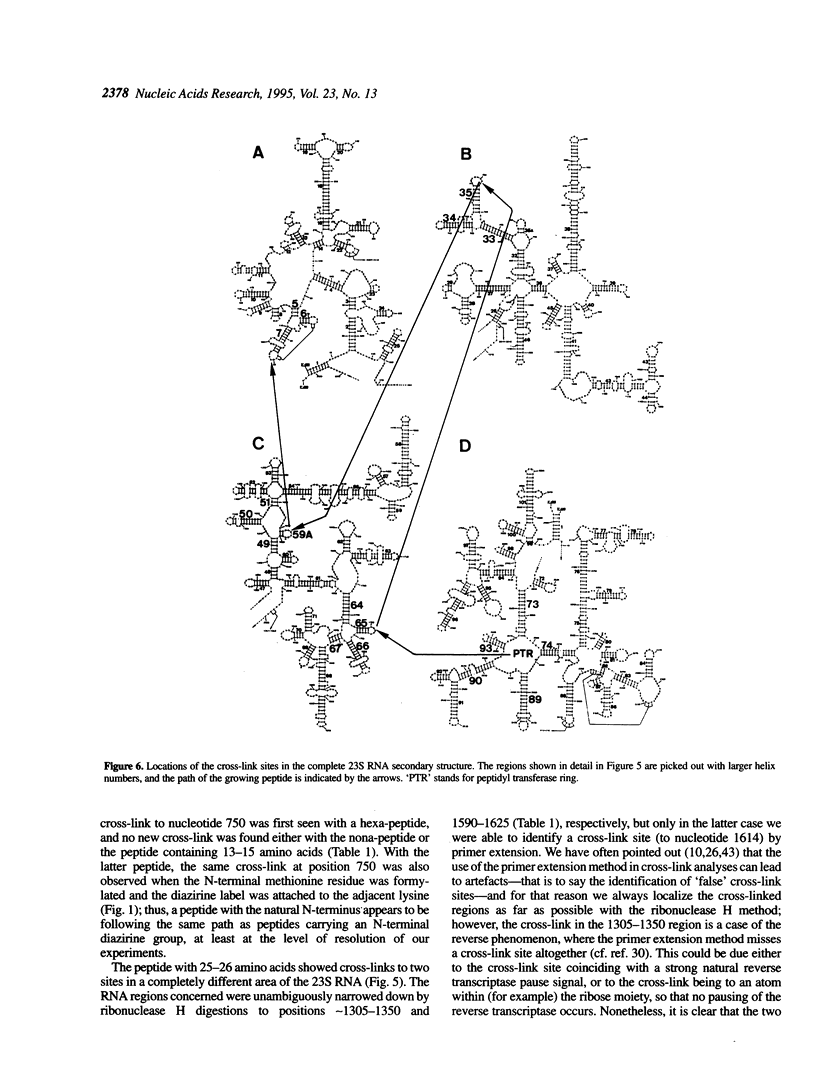
Peptides of different lengths encoded by suitable mRNA fragments were biosynthesized in situ on Escherichia coli ribosomes. The peptides carried a diazirine derivative bound to their N-terminal methionine residue, which was photoactivated whilst the peptides were still attached to the ribosome. Subsequently, the sites of photo-cross-linking to 23S RNA were analyzed by our standard procedures. The N-termini of peptides of increasing length became progressively cross-linked to nucleotide 750 (peptides of 6, 9 or 13-15 amino acids), to nucleotide 1614 and concomitantly to a second site between nucleotides 1305 and 1350 (a peptide of 25-26 amino acids), and to nucleotide 91 (a peptide of 29-33 amino acids). Previously we had shown that peptides of 1 or 2 amino acids were cross-linked to nucleotides 2062, 2506 and 2585 within the peptidyl transferase ring, whereas tri-and tetrapeptides were additionally cross-linked to nucleotides 2609 and 1781. Taken together, the data demonstrate that the path of the nascent peptide chain moves from the peptidyl transferase ring in domain V of the 23S RNA to domain IV, then to domain II, then to domain III, and finally to domain I. These cross-linking results are correlated with other types of topographical data relating to the 50S subunit.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakin A., Ofengand J. Four newly located pseudouridylate residues in Escherichia coli 23S ribosomal RNA are all at the peptidyltransferase center: analysis by the application of a new sequencing technique. Biochemistry. 1993 Sep 21;32(37):9754–9762. doi: 10.1021/bi00088a030. [DOI] [PubMed] [Google Scholar]
- Bernabeu C., Lake J. A. Nascent polypeptide chains emerge from the exit domain of the large ribosomal subunit: immune mapping of the nascent chain. Proc Natl Acad Sci U S A. 1982 May;79(10):3111–3115. doi: 10.1073/pnas.79.10.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Sabatini D. D. Controlled proteolysis of nascent polypeptides in rat liver cell fractions. I. Location of the polypeptides within ribosomes. J Cell Biol. 1970 Apr;45(1):130–145. doi: 10.1083/jcb.45.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochkariov D. E., Kogon A. A. Application of 3-[3-(3-(trifluoromethyl)diazirin-3-yl)phenyl]-2,3- dihydroxypropionic acid, carbene-generating, cleavable cross-linking reagent for photoaffinity labeling. Anal Biochem. 1992 Jul;204(1):90–95. doi: 10.1016/0003-2697(92)90144-v. [DOI] [PubMed] [Google Scholar]
- Branlant C., Krol A., Machatt M. A., Pouyet J., Ebel J. P., Edwards K., Kössel H. Primary and secondary structures of Escherichia coli MRE 600 23S ribosomal RNA. Comparison with models of secondary structure for maize chloroplast 23S rRNA and for large portions of mouse and human 16S mitochondrial rRNAs. Nucleic Acids Res. 1981 Sep 11;9(17):4303–4324. doi: 10.1093/nar/9.17.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimacombe R., Mitchell P., Osswald M., Stade K., Bochkariov D. Clustering of modified nucleotides at the functional center of bacterial ribosomal RNA. FASEB J. 1993 Jan;7(1):161–167. doi: 10.1096/fasebj.7.1.8422963. [DOI] [PubMed] [Google Scholar]
- Brimacombe R. The structure of ribosomal RNA: a three-dimensional jigsaw puzzle. Eur J Biochem. 1995 Jun 1;230(2):365–383. [PubMed] [Google Scholar]
- Crowley K. S., Reinhart G. D., Johnson A. E. The signal sequence moves through a ribosomal tunnel into a noncytoplasmic aqueous environment at the ER membrane early in translocation. Cell. 1993 Jun 18;73(6):1101–1115. doi: 10.1016/0092-8674(93)90640-c. [DOI] [PubMed] [Google Scholar]
- Dontsova O., Dokudovskaya S., Kopylov A., Bogdanov A., Rinke-Appel J., Jünke N., Brimacombe R. Three widely separated positions in the 16S RNA lie in or close to the ribosomal decoding region; a site-directed cross-linking study with mRNA analogues. EMBO J. 1992 Aug;11(8):3105–3116. doi: 10.1002/j.1460-2075.1992.tb05383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döring T., Mitchell P., Osswald M., Bochkariov D., Brimacombe R. The decoding region of 16S RNA; a cross-linking study of the ribosomal A, P and E sites using tRNA derivatized at position 32 in the anticodon loop. EMBO J. 1994 Jun 1;13(11):2677–2685. doi: 10.1002/j.1460-2075.1994.tb06558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görlich D., Kurzchalia T. V., Wiedmann M., Rapoport T. A. Probing the molecular environment of translocating polypeptide chains by cross-linking. Methods Cell Biol. 1991;34:241–262. doi: 10.1016/s0091-679x(08)61684-2. [DOI] [PubMed] [Google Scholar]
- Hartl F. U., Wiedmann M. Prokaryotic secretion: a signal recognition particle in Escherichia coli? Curr Biol. 1993 Feb;3(2):86–89. doi: 10.1016/0960-9822(93)90161-g. [DOI] [PubMed] [Google Scholar]
- Krieg U. C., Johnson A. E., Walter P. Protein translocation across the endoplasmic reticulum membrane: identification by photocross-linking of a 39-kD integral membrane glycoprotein as part of a putative translocation tunnel. J Cell Biol. 1989 Nov;109(5):2033–2043. doi: 10.1083/jcb.109.5.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudlicki W., Odom O. W., Kramer G., Hardesty B. Chaperone-dependent folding and activation of ribosome-bound nascent rhodanese. Analysis by fluorescence. J Mol Biol. 1994 Dec 2;244(3):319–331. doi: 10.1006/jmbi.1994.1732. [DOI] [PubMed] [Google Scholar]
- Leffers H., Kjems J., Ostergaard L., Larsen N., Garrett R. A. Evolutionary relationships amongst archaebacteria. A comparative study of 23 S ribosomal RNAs of a sulphur-dependent extreme thermophile, an extreme halophile and a thermophilic methanogen. J Mol Biol. 1987 May 5;195(1):43–61. doi: 10.1016/0022-2836(87)90326-3. [DOI] [PubMed] [Google Scholar]
- Lim V. I., Spirin A. S. Stereochemical analysis of ribosomal transpeptidation. Conformation of nascent peptide. J Mol Biol. 1986 Apr 20;188(4):565–574. doi: 10.1016/s0022-2836(86)80006-7. [DOI] [PubMed] [Google Scholar]
- Malkin L. I., Rich A. Partial resistance of nascent polypeptide chains to proteolytic digestion due to ribosomal shielding. J Mol Biol. 1967 Jun 14;26(2):329–346. doi: 10.1016/0022-2836(67)90301-4. [DOI] [PubMed] [Google Scholar]
- Milligan R. A., Unwin P. N. Location of exit channel for nascent protein in 80S ribosome. Nature. 1986 Feb 20;319(6055):693–695. doi: 10.1038/319693a0. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Osswald M., Schueler D., Brimacombe R. Selective isolation and detailed analysis of intra-RNA cross-links induced in the large ribosomal subunit of E. coli: a model for the tertiary structure of the tRNA binding domain in 23S RNA. Nucleic Acids Res. 1990 Aug 11;18(15):4325–4333. doi: 10.1093/nar/18.15.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P., Stade K., Osswald M., Brimacombe R. Site-directed cross-linking studies on the E. coli tRNA-ribosome complex: determination of sites labelled with an aromatic azide attached to the variable loop or aminoacyl group of tRNA. Nucleic Acids Res. 1993 Feb 25;21(4):887–896. doi: 10.1093/nar/21.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moazed D., Noller H. F. Chloramphenicol, erythromycin, carbomycin and vernamycin B protect overlapping sites in the peptidyl transferase region of 23S ribosomal RNA. Biochimie. 1987 Aug;69(8):879–884. doi: 10.1016/0300-9084(87)90215-x. [DOI] [PubMed] [Google Scholar]
- Moazed D., Noller H. F. Interaction of tRNA with 23S rRNA in the ribosomal A, P, and E sites. Cell. 1989 May 19;57(4):585–597. doi: 10.1016/0092-8674(89)90128-1. [DOI] [PubMed] [Google Scholar]
- Moazed D., Noller H. F. Sites of interaction of the CCA end of peptidyl-tRNA with 23S rRNA. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3725–3728. doi: 10.1073/pnas.88.9.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moazed D., Stern S., Noller H. F. Rapid chemical probing of conformation in 16 S ribosomal RNA and 30 S ribosomal subunits using primer extension. J Mol Biol. 1986 Feb 5;187(3):399–416. doi: 10.1016/0022-2836(86)90441-9. [DOI] [PubMed] [Google Scholar]
- Osswald M., Greuer B., Brimacombe R. Localization of a series of RNA-protein cross-link sites in the 23S and 5S ribosomal RNA from Escherichia coli, induced by treatment of 50S subunits with three different bifunctional reagents. Nucleic Acids Res. 1990 Dec 11;18(23):6755–6760. doi: 10.1093/nar/18.23.6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden K. W. Revised sequence of the tetracycline-resistance gene of pBR322. Gene. 1983 May-Jun;22(2-3):277–280. doi: 10.1016/0378-1119(83)90112-9. [DOI] [PubMed] [Google Scholar]
- Picking W. D., Odom O. W., Hardesty B. Evidence for RNA in the peptidyl transferase center of Escherichia coli ribosomes as indicated by fluorescence. Biochemistry. 1992 Dec 22;31(50):12565–12570. doi: 10.1021/bi00165a004. [DOI] [PubMed] [Google Scholar]
- Picking W. D., Picking W. L., Odom O. W., Hardesty B. Fluorescence characterization of the environment encountered by nascent polyalanine and polyserine as they exit Escherichia coli ribosomes during translation. Biochemistry. 1992 Mar 3;31(8):2368–2375. doi: 10.1021/bi00123a023. [DOI] [PubMed] [Google Scholar]
- Rapoport T. A. Transport of proteins across the endoplasmic reticulum membrane. Science. 1992 Nov 6;258(5084):931–936. doi: 10.1126/science.1332192. [DOI] [PubMed] [Google Scholar]
- Rheinberger H. J., Geigenmüller U., Wedde M., Nierhaus K. H. Parameters for the preparation of Escherichia coli ribosomes and ribosomal subunits active in tRNA binding. Methods Enzymol. 1988;164:658–670. doi: 10.1016/s0076-6879(88)64076-6. [DOI] [PubMed] [Google Scholar]
- Rinke-Appel J., Jünke N., Brimacombe R., Dukudovskaya S., Dontsova O., Bogdanov A. Site-directed cross-linking of mRNA analogues to 16S ribosomal RNA; a complete scan of cross-links from all positions between '+1' and '+16' on the mRNA, downstream from the decoding site. Nucleic Acids Res. 1993 Jun 25;21(12):2853–2859. doi: 10.1093/nar/21.12.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryabova L. A., Selivanova O. M., Baranov V. I., Vasiliev V. D., Spirin A. S. Does the channel for nascent peptide exist inside the ribosome? Immune electron microscopy study. FEBS Lett. 1988 Jan 4;226(2):255–260. doi: 10.1016/0014-5793(88)81434-0. [DOI] [PubMed] [Google Scholar]
- Smith J. E., Cooperman B. S., Mitchell P. Methylation sites in Escherichia coli ribosomal RNA: localization and identification of four new sites of methylation in 23S rRNA. Biochemistry. 1992 Nov 10;31(44):10825–10834. doi: 10.1021/bi00159a025. [DOI] [PubMed] [Google Scholar]
- Stade K., Riens S., Bochkariov D., Brimacombe R. Contacts between the growing peptide chain and the 23S RNA in the 50S ribosomal subunit. Nucleic Acids Res. 1994 Apr 25;22(8):1394–1399. doi: 10.1093/nar/22.8.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stade K., Rinke-Appel J., Brimacombe R. Site-directed cross-linking of mRNA analogues to the Escherichia coli ribosome; identification of 30S ribosomal components that can be cross-linked to the mRNA at various points 5' with respect to the decoding site. Nucleic Acids Res. 1989 Dec 11;17(23):9889–9908. doi: 10.1093/nar/17.23.9889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner G., Kuechler E., Barta A. Photo-affinity labelling at the peptidyl transferase centre reveals two different positions for the A- and P-sites in domain V of 23S rRNA. EMBO J. 1988 Dec 1;7(12):3949–3955. doi: 10.1002/j.1460-2075.1988.tb03281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiege W., Glotz C., Brimacombe R. Localisation of a series of intra-RNA cross-links in the secondary and tertiary structure of 23S RNA, induced by ultraviolet irradiation of Escherichia coli 50S ribosomal subunits. Nucleic Acids Res. 1983 Mar 25;11(6):1687–1706. doi: 10.1093/nar/11.6.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Vester B., Garrett R. A. Structure of a protein L23-RNA complex located at the A-site domain of the ribosomal peptidyl transferase centre. J Mol Biol. 1984 Nov 5;179(3):431–452. doi: 10.1016/0022-2836(84)90074-3. [DOI] [PubMed] [Google Scholar]
- Wagenknecht T., Carazo J. M., Radermacher M., Frank J. Three-dimensional reconstruction of the ribosome from Escherichia coli. Biophys J. 1989 Mar;55(3):455–464. doi: 10.1016/S0006-3495(89)82839-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzmann C., Cooperman B. S. On the structural specificity of puromycin binding to Escherichia coli ribosomes. Biochemistry. 1985 Apr 23;24(9):2268–2274. doi: 10.1021/bi00330a022. [DOI] [PubMed] [Google Scholar]
- Yonath A., Leonard K. R., Wittmann H. G. A tunnel in the large ribosomal subunit revealed by three-dimensional image reconstruction. Science. 1987 May 15;236(4803):813–816. doi: 10.1126/science.3576200. [DOI] [PubMed] [Google Scholar]