Abstract
Elevating body temperature or just the temperature of the dorsal medulla by approximately 2 °C prolongs the laryngeal chemoreflex (LCR) in decerebrate neonatal piglets. We tested the hypothesis that transient receptor potential vanilloid 1 (TRPV1) receptors in the nucleus of the solitary tract (NTS) mediate thermal prolongation of the LCR. We studied the effect of a selective TRPV1 receptor antagonist on thermal prolongation of the LCR, and we tested the effect of a TRPV1 agonist on the duration of the LCR under normothermic conditions. We studied 37 decerebrate neonatal piglets between the ages of post-natal days 4 and 7. The TRPV1 receptor antagonist, 5−iodoresiniferatoxin (65 microM/L in 100 nL), blocked thermal prolongation of the LCR when injected bilaterally into the region of the NTS. The TRPV1 agonist, resiniferatoxin (0.65-1.0 mM/L in 100 nL), prolonged the LCR after bilateral injection into the NTS even when the body temperature of each piglet was normal. The effect of the TRPV1 agonists could be blocked by treatment with the GABAA receptor antagonist, bicuculline, whether given intravenously (0.3 mg/kg) or focally injected bilaterally into the NTS (10 mM in 100nL). We conclude that TRPV1 receptors in the NTS mediate thermal prolongation of the LCR.
Keywords: SIDS, laryngeal chemoreflex, hyperthermia, GABA, TRPV1 channels, nucleus of the solitary tract
1. Introduction
The laryngeal chemoreflex (LCR) is elicited when fluid enters the larynx, particularly fluid with a low chloride content or low pH (Boggs and Bartlett, 1982; Downing and Lee, 1975; Lee et al., 1977). The LCR is made up of behaviors that prevent aspiration of fluids in the upper airway, clear the fluids from the airway and preserve oxygen delivery to vital organs. To prevent aspiration, respiration is inhibited - this ranges from prolonged apnea to short-lived respiratory disruptions (Downing and Lee, 1975), and the glottis is closed (Haraguchi et al., 1983; Sasaki, 1979). To clear the airway, coughing and swallowing are activated (Thach, 2001; van der Velde et al., 2003). To preserve oxygen delivery, bradycardia occurs and blood flow may be redistributed to vital organs (Grogaard et al., 1982). These behaviors are elicited to varying degrees depending on the strength of the reflex response. Weak reflex responses may consist of brief respiratory disruption, coughing and swallowing; whereas the entire range of behaviors may be elicited when the LCR is strongly activated (Thach, 2001; van der Velde et al., 2003). The LCR is particularly prominent in neonatal animals and is frequently elicited in the normal course of neonatal life. Many investigators believe that the LCR initiates processes that may lead to Sudden Infant Death Syndrome (SIDS) (Leiter and Böhm, 2007; Thach, 2001; Thach, 2005). Elevated body temperature increases the strength of the LCR, but the thermal enhancement of the LCR diminishes as animals mature (Haraguchi et al., 1983; Xia et al., 2008b; Xia et al., 2010). The effect of elevated body temperature on the LCR seems to originate in the central nervous system (CNS). Focal warming of the brain in the region of the nucleus of the solitary tract (NTS) by ~2.0 °C reversibly prolongs the LCR in decerebrate piglets, even while body temperature is held constant at ~38 °C (Xia et al., 2006). These findings imply that the thermal prolongation of the LCR originates from elevated temperatures in or near the NTS.
What thermal sensor is responsible for thermal prolongation of the LCR has not been identified. There are temperature sensitive neurons in and around the NTS in rabbits (Inoue and Murakami, 1976), but the mechanism of temperature sensation in these neurons has not been studied so far as we know. Transient receptor potential vanilloid-1 (TRPV1) receptors are thermally sensitive, ligand-gated ion channels, and TRPV1 receptors are found in the NTS (Guo et al., 1999; Mezey et al., 2000). The usual temperature range over which TRPV1 channels are activated is > 42 °C (Caterina, 2007). However, activity-dependent activation of central TRPV1 channels has a temperature dependence that encompasses the range of normal body temperatures (Peters et al., 2010). Moreover, the function of TRPV1 channels is modified by at least four factors that may be relevant to SIDS, and these TRPV1 modulators allow activation of TRPV1 channels at temperatures < 42 °C. Reduced pH - for example during hypercapnia, bradykinin - perhaps related to coexisting infection or inflammation, and nicotine appear to enhance TRPV1 sensitivity (Venkatachalam and Montell, 2007). Interestingly, the thermal prolongation of the LCR is enhanced in neonatal rats born to mothers exposed to tobacco smoke or nicotine during pregnancy (Xia et al., 2009; Xia et al., 2010). Finally, serotonergic activation of neurons may enhance TRPV1-dependent neurotransmitter release (Ohta et al., 2006; Suguira et al., 2004); a finding of interest in view of the evidence that brainstem serotonergic function may be altered in many infants who died of SIDS (Paterson et al., 2006) For all these reasons, we were interested in testing the hypothesis that TRPV1 receptors mediate the thermal prolongation of the LCR originating in the NTS.
2. Methods
Experiments were performed on 37 piglets (18 male and 19 female) ranging in age from 4 to 7 days (5.1±0.1 days; mean±SEM) with an average weight of 2.3±0.1 kg. The Institutional Animal Care and Use Committee of Dartmouth College approved all surgery and experimental protocols.
2.1 Surgical preparation
Animals were anesthetized with 2% isoflurane (2-chloro-2-(difluoromethoxy)-1,1,1-trifluoro-ethane; Halocarbon Laboratories, NJ) in O2. A rectal probe was inserted, and rectal temperature (‘body temperature’) was maintained between 38 and 39 °C using a servo-controlled heating pad. Femoral arterial and venous catheters were inserted to measure blood pressure and administer drugs, respectively. Each animal was tracheostomized and artificially ventilated (Harvard Apparatus Dual Phase Respirator, South Natick, MA) to maintain the end-tidal CO2 concentration at approximately 5%, which was also servo-controlled so that end-tidal CO2 did not vary as a function of any of the experimental treatments. After exposing the carotid sinus regions bilaterally, the internal and external carotid arteries were ligated to facilitate decerebration. The vagus nerves were sectioned bilaterally to prevent entrainment of the phrenic rhythm to the mechanical ventilator (Graves et al., 1986; Petrillo et al., 1983). Each animal was placed prone with its head in a stereotaxic apparatus (Kopf Instruments, Tujunga, CA). The skull was opened, the animal was decerebrated at the level of the superior colliculi and all brain tissue rostral to the section was removed by suction. Following decerebration, isoflurane anesthesia was discontinued. Each animal was paralyzed using pancuronium bromide (1 mg/kg, iv; Elkins-Sinn Inc., Cherry Hill, NJ), and supplemental doses of pancuronium were given as required, usually at a rate of 0.5 mg/kg/hr. A phrenic nerve was exposed and sectioned, and the central cut end was placed on a bipolar recording electrode to monitor respiratory output. Phrenic activity was amplified (Gould Universal Amplifier, Cleveland, OH), and the moving time average (“integrated activity”) was calculated electronically (100 ms time constant; CWE, Ardmore, PA). Integrated phrenic nerve activity, body temperature, end-tidal CO2, and blood pressure were recorded on a computer (PowerLab, ADI, Australia) for later analysis.
Intravenous drugs were delivered through the femoral venous catheter. We made microinjections of drugs into the dorsal area of the brainstem using a 0.5 μl syringe (SGE Analytical Sciences, Austin, TX) with a 0.47 mm O.D. The needle of the injection syringe was flexible, and to control the location of the injections more effectively, the needle was passed through a rigid guide tube that ended just above the surface of the dorsal brainstem. The needle was advanced into the dorsal medulla perpendicular to the dorsal surface using visual landmarks - the obex primarily (Niblock et al., 2005). The needle for microinjections was placed in the medulla approximately 5-10 minutes before any tests of the LCR were performed. Respiratory activity was stable at the time of each injection. All drugs injected into the medulla were combined with 0.5 micron diameter fluorescent microbeads (Fluoresbrite® YG Microspheres, Polysciences, Inc., Warrington, PA). The beads were added to the drugs dissolved in the solvent vehicle (5 μl stock solution of microbeads, which contained 3.64 × 1011 particles/ml, were added to 100 μl vehicle) and distributed within the solvent by shaking before being aspirated into the injection syringe.
We placed a pharyngeal catheter (PE-90) through a nostril and positioned the tip just above the larynx. The catheter was filled with water, and 0.1 ml of water was injected into the larynx using a computer controlled syringe pump each time that we elicited the LCR. Water remained in the catheter between tests, and as a consequence, the temperature of the water injected was near body temperature. The larynx was suctioned periodically as needed. At least 5 minutes elapsed between tests of the LCR, and the LCR was not tested unless phrenic respiratory activity was stable.
2.2 Neuroanatomy
At the conclusion of each experiment, each piglet was killed with an injection of 500 mg/kg pentobarbital sodium followed by 5-10 ml of saturated potassium chloride administered I.V. The brainstem was removed from the animal, placed in cryoembedding medium (Tissue-Tek O.C.T. 458, Sakura Finetek, Torrance, CA) and frozen in isopentane at −70 °C. Brainstems were sectioned (50 μm) in a cryostat at −18 °C, and sections were mounted on gelatinized glass slides, fixed for 15 min in 4% paraformaldehyde in phosphate buffered saline (pH 7.0) and stained with cresyl violet (Bandroft and Cook, 1994; Luna, 1992). The location of the microinjection was identified using a combination of tissue disruption caused by the microinjection syringe tip and the location of fluorescent microbeads. The distribution of fluorescent microbeads was examined under fluorescent light using a TRITC filter (excitation 540 ± 25 nm; dichroic mirror 565 nm) on a Nikon Eclipse E800 microscope (Nikon Instruments Inc., Melville, NY). We recorded the location of the highest concentration of beads as the center of the injection, but we also noted the rostro-caudal extent of spread of the beads.
2.3 Experimental protocols: TRPV1 antagonist study
To examine the effect of the TRPV1 antagonist, studies began with a control period during which the body temperature was held at 38-39°C, and the LCR was elicited three times. Next, the body temperature was elevated approximately 2.5°C by warming each animal with a heating pad. Once the body temperature reached a stable elevated temperature, the LCR was stimulated three more times. This series of studies established that each animal did, or did not, have demonstrable thermal prolongation of the LCR. After these control tests, the body temperature of each animal was maintained at an elevated temperature, and individual injections of 100 nL of 65 μM of 5′iodoresiniferitoxin were made bilaterally in the caudal NTS. The LCR was studied three times after these injections. In the final test condition, each animal was cooled by swabbing it with isopropyl alcohol until body temperature was reduced to the control value, after which the LCR was tested three times in a second, normothermic period following the 5′iodoresiniferatoxin injections.
2.4 TRPV1 agonist studies
In the next set of studies, we examined the effect of administering a TRPV1 agonist on the LCR during normothermic conditions. Initial normothermic and hyperthermic studies were performed as described above. In the third treatment condition, each animal was cooled after hyperthermic studies by swabbing it with isopropyl alcohol until body temperature was reduced to the control value. At this point the LCR was studied three times to re-establish the baseline normothermic LCR response. In the fourth condition of this study, injections of 100 nL of 0.65 to 1.0 mM of resiniferitoxin, aTRPV1 agonist, were given bilaterally in the caudal NTS and the LCR was tested three final times.
2.5 Bicuculline effects on TRPV1 agonist responses
In the final set of studies, we determined whether TRPV1 channel activation affected the LCR through a GABAergic mechanism. We measured the control response of the LCR at normal body temperature and during hyperthermia as described above. In the third condition, the body temperature was returned to the control level and the LCR was assessed three times. In the fourth condition, either bicuculline (0.3 mg/kg I.V.) was given or bilateral 100 nl injections of 1.0 mM of resiniferatoxin were made in the NTS. In the fifth condition, each animal received either a bicuculline treatment or the resiniferatoxin injections, whichever treatment it had not previously received, so that each animal received each treatment once, but the order of bicuculline and resiniferatoxin treatments was randomized. In a final set of studies, we followed the design of the bicuculline/resiniferatoxin study except that we gave the bicuculline (10 mM) as 100 nL microinjections bilaterally into the NTS before each animal received the resiniferatoxin injections in the same locations to determine whether the bicuculline effect was mediated centrally within the NTS.
2.6 Data analysis and statistics
We defined the duration of the LCR as the period of respiratory instability (defined as variability of phrenic amplitude and/or respiratory timing) from the beginning of the breath during which the water stimulus was delivered to the onset of at least five regular breaths. These five breaths did not need to have the same frequency or amplitude as the control breaths; we simply required that they be regular (Curran et al., 2005; van der Velde et al., 2003; Xia et al., 2006). The respiratory disruption measured in this way included both periods of unstable respiratory activity and apneas. We kept the definition of the LCR duration simple and applied it consistently across all animals, but it can be difficult to decide what constitutes five regular breaths to define the end of the LCR. Therefore, we also measured the longest apnea duration of each reflex trial, which is less subject to interpretation. Apnea was defined as a cessation of phrenic activity greater than the duration of the two cycles preceding the cycle during which the stimulus was delivered. However, apnea did not occur in all tests of the LCR. Measuring both the LCR duration and apnea duration, when present, provided a more complete analysis of the response since apnea duration alone does not capture the behavioral complexity of the LCR, and measuring the LCR duration alone can require subjective judgments. Stimulation of the LCR may induce bradycardia as well as apnea in intact animals. However, we did not analyze the heart rate responses because the animals were vagotomized.
Even though we studied two factors, body temperature and drug treatment, the studies were not balanced. Within each study, each animal was exposed to all treatments, so we used a repeated-measures one-way ANOVA to analyze these studies (SYSTAT 9.0, SPSS, Inc, Chicago, IL). When the ANOVA indicated that significant differences existed among the treatments, specific pre-planned comparisons were made using orthogonal contrasts and P-values adjusted by the Bonferroni method. We first compared the two normothermic conditions (the first and third treatments when these conditions were present in any particular study). In none of the studies did the variables differ in these conditions, so we made orthogonal contrasts between the means of these normothermic control conditions and each of the other treatment conditions. The apnea and LCR durations were not normally distributed, and these data did not conform to the assumption of sphericity (one of the assumptions upon which the ANOVA is predicated). Therefore, these data were log transformed for the statistical analysis, as we have done previously (Curran et al., 2005; Duy et al., 2010), and the ANOVA was performed on the log transformed data for these two variables. Data are presented as the mean ± the standard error of the mean.
3. Results
3.1 Effects of 5′-iodoresiniferatoxin a TRPV1 antagonist, on hyperthermic prolongation of the LCR
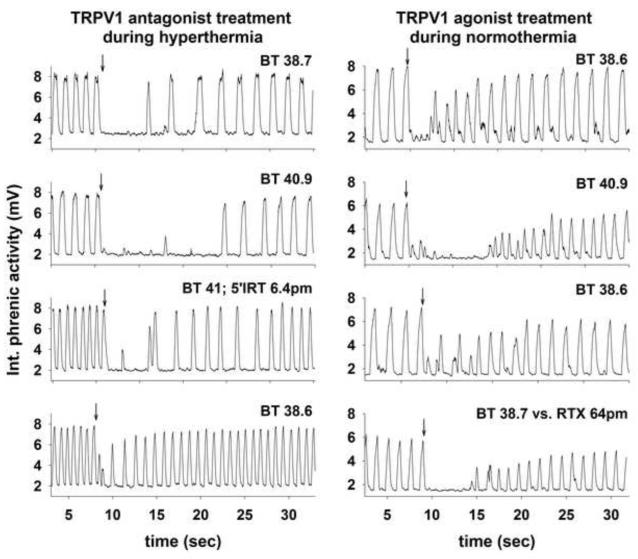
Two examples of the responses of integrated phrenic nerve activity during different treatment conditions are shown in Fig. 1. The results on the left in Fig. 1 show the effect of bilateral 100 nL microinjections of 65 μM 5′-iodoresiniferatoxin, a TRPV1 antagonist, into the caudal NTS on the LCR. The injection site in this piglet was caudal to the obex (the yoked symbols marked by an asterisk in Fig. 2). In the initial control condition (uppermost panel on the left), body temperature was 38.7 °C, and introducing 0.1 ml of water into the larynx (arrow) caused apnea and a slightly longer period of respiratory instability. After elevating the animal’s body temperature to 40.9 °C, 0.1 ml of water injected into the larynx caused a more prolonged period of respiratory disruption and apnea. We maintained the hyperthermic body temperature and made bilateral injections of 5′iodoresiniferatoxin into the caudal NTS. After these injections, the apnea and respiratory disruption associated with the LCR were relatively short lived, and similar to the normothermic control condition even though the animal remained hyperthermic. The animal was cooled to restore the initial, normothermic body temperature in the final condition, and the manifestations of the LCR following stimulation of the larynx with water were quite brief; there was no measurable apnea and only a limited period of respiratory irregularity. Thus, focal injection of a TRPV1 antagonist in the NTS prevented thermal prolongation of the LCR in this animal.
Figure 1.
In the left set of tracings, integrated phrenic activity is shown during four tests of the LCR in a male piglet (post-natal age 6 days). The control response to laryngeal injection of 0.1 ml of water (at the downward arrow) at a normal body temperature is shown in the top panel. Elevating the body temperature (second panel) prolonged the duration of the LCR. The hyperthermic prolongation of the apnea and LCR duration was blocked after bilateral 100 nL microinjections of 6.5 μM 5′-idodresiniferatoxin into the caudal NTS (third panel). The LCR response during a final normothermic trial after the 5′-idodresiniferatoxin treatment (fourth panel) was quite short ‘BT’ indicates body temperature. The locations of the injections in this animal are marked by an asterisk in the schematic anatomical drawings in Fig. 2.
In the right set of records, integrated phrenic activity is shown during four tests of the LCR in a male piglet (post-natal age 5 days). The control response to laryngeal injection of 0.1 ml of water (at the downward arrow) at a normal body temperature is shown in the top panel. Elevating the body temperature (second panel) prolonged the duration of the LCR. The hyperthermic prolongation of the apnea and LCR duration was reversed when the animal was cooled back to normal body temperature (third panel). The LCR response during a final normothermic trial after bilateral 100 nL microinjections of 65 mM resiniferatoxin into the caudal NTS (fourth panel) was prolonged even though the animal was normothermic. The locations of the injections in this animal are marked by an asterisk in the schematic anatomical drawings in Fig. 3.
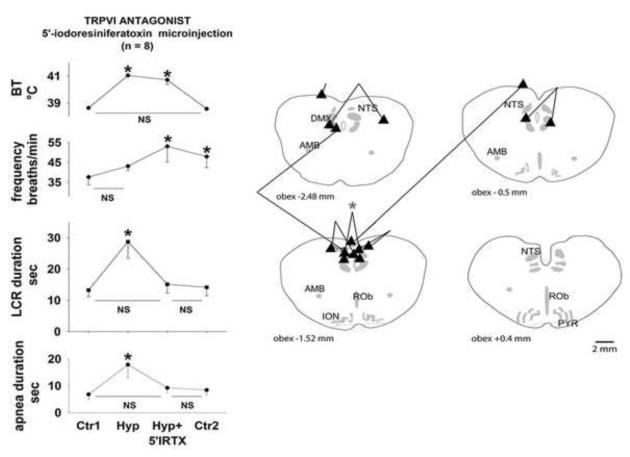
Figure 2.
Body temperature (BT), respiratory frequency, the duration of the LCR and the duration of apnea have been plotted as functions of experimental conditions (‘Ctr1,’ control normothermia condition; ‘Hyp,’ hyperthermic condition; ‘Hyp+5′IRTX,’ hyperthermia after bilateral 100 nL microinjections of 65 μM 5′iodoresiniferatoxin; and ‘Ctr2,’ normothermic conditions after bilateral 100 nL microinjections of 65 μM 5′iodoresiniferatoxin. ‘*’ indicates P < 0.05 compared to mean of the initial control condition, and ‘NS’ indicates that no significant difference existed between the treatments indicated. Schematic cross-sections starting caudal to the obex of the neonatal piglet medulla are shown on the right side of the figure. The pair of injections marked by the asterisk marks the site of the injections in the animal from the left side of Fig. 1. Anatomical abbreviations: 7, facial nucleus; ROb, raphé obscurus; RP, raphé pallidus; DMX, dorsal vagal motor nucleus; ION, inferior olivary nucleus; NTS, nucleus of the solitary tract; AMB, nucleus ambiguus.
The average values of the LCR duration, the longest apnea duration, respiratory frequency and body temperature during each of these four treatment conditions (normothermic control, hyperthermia, hyperthermia plus 5′-iodoresiniferatoxin microinjections in the NTS and normothermia following the 5′-iodoresiniferatoxin microinjections) are shown in Fig. 2. The sites of injection of 5′-iodoresiniferatoxin are shown in schematic cross-sections of the piglet brainstem in the same figure. When the temperature was increased from a control value of 38.6 ± 0.1 °C to 41.0 ± 0.1 °C, the apnea duration increased by approximately 3-fold (P < 0.05), and the LCR duration increased approximately two-fold, also a significant change (P < 0.05). The average respiratory frequency rose slightly, but this was not significant. These changes in apnea and LCR duration were reversed after bilateral injection of 5′iodoresiniferatoxin into the NTS even though body temperature remained elevated at 40.7 ± 0.3 °C. The respiratory frequency rose significantly after the drug treatment regardless of the body temperature (there was a significant main effect of drug treatment, P < 0.05). After each animal was cooled to the control body temperature (38.6 ± 0.1 °C), apnea and LCR duration were not significantly different from the initial normothermic control period. As noted above, the respiratory frequency remained elevated after 5′iodoresiniferatoxin microinjection into the NTS even after the animal was made normothermic. There were no changes in end-tidal CO2 or mean arterial blood pressure during these treatment conditions that might have contributed to these changes in apnea and LCR duration and respiratory frequency.
The sites of microinjections were concentrated in the dorsal medulla in close proximity to the caudal NTS. As we have conducted more of these studies, we have gotten better at ‘hitting’ the NTS. The beads tend to be concentrated in any cross-section, but they spread rostro-caudally between 500 and 1000 μm. The 5′-iodoresiniferatoxin almost certainly diffuses beyond the volume delineated by the beads, and so the potential sites of the TRPV1 channels responding to 5′-iodoresiniferatoxin may extend beyond the margins of the caudal NTS. So far as we can tell, 5′-iodoresiniferatoxin blunted the thermal prolongation in 7 of the 8 piglets; the microinjections in the 7 ‘responders’ were all close to the NTS. In the one non-responder, one injection site was on the very dorsal surface of the brainstem well above the NTS, and the other was extremely caudal (−4.48 mm caudal to the obex; so far caudal that we did not include a cross-section of this part of the piglet brainstem). Therefore, 5′-iodoresiniferatoxin seems to be having its effect within or closely adjacent to the NTS.
3.2 Effects of resiniferatoxin, a TRPV1 agonist, on normothermic prolongation of the LCR
An example of the effect of microinjection of a TRPV1 agonist into the NTS on the LCR is shown in the right sided panels of Fig. 1. Resiniferatoxin was less potent than 5′-iodoresiniferatoxin, and in preliminary studies, we determined that an approximately 10-fold increase in drug concentration compared to 5′-iodoresiniferatoxin was required to see an effect of the TRPV1 agonist on the LCR. In the example shown in Fig. 1, we studied the effect of bilateral 100 nL microinjections of 0.85 mM resiniferatoxin into the caudal NTS. The injection site in this piglet was caudal to the obex (the yoked symbols marked by an asterisk in Fig. 3). In the initial control condition (uppermost panel on the right), body temperature was 38.6 °C, and introducing 0.1 ml of water into the larynx (arrow) caused a brief apnea, and a slightly longer period of respiratory instability. After elevating the animal’s body temperature to 40.9 °C, the LCR and accompanying apnea were prolonged. The animal was cooled to 38.6 °C, and the LCR was tested again. The thermal prolongation of the LCR was reversible, and the LCR was short and similar to the initial normothermic control condition. While the animal remained normothermic, bilateral injections of resiniferatoxin were made into the NTS, and the LCR was retested. Even though the animal was normothermic, the LCR duration and particularly the apnea duration were prolonged after the resiniferatoxin injections. Thus, focal injection of a TRPV1 agonist in the NTS prolonged the LCR in this animal while body temperature was in the normal range.
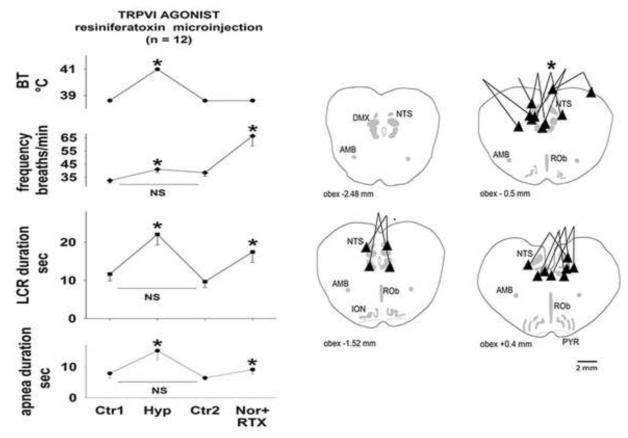
Figure 3.
The symbol conventions are the same as in Fig. 2 except that the third and fourth treatments were ‘Ctr2,’ a second normothermic control and ‘Nor+RTX,’ normothermia following injection of resiniferatoxin bilaterally into the region of the NTS.
The average values of the LCR duration, the longest apnea duration, respiratory frequency and body temperature during each of four experimental conditions (normothermic control, hyperthermia, a second normothermic control period and after resiniferatoxin microinjection in the NTS plus normothermia) are shown in Fig. 3. The sites of injection of resiniferatoxin are shown in schematic cross-sections of the piglet brainstem in the same figure. Although we used concentrations of resiniferatoxin ranging from 0.65 to 1 mM in each 100 nL microinjection, the responses to these different doses were similar, and for this reason, the data from the entire dose range were pooled in the following analysis. We studied 14 animals in this group, but two of the animals did not demonstrate any thermal prolongation of the LCR. This presents two difficulties: we cannot compare the drug response to the thermal response (since there was no thermal response), and perhaps more interestingly, animals that lack a thermal response have consistently not responded to any of the drug treatments that we have studied (Duy et al., 2010). For these reasons, results from these animals were excluded from analysis.
When the temperature was increased from a control value of 38.6 ± 0.1 °C to 40.9 ± 0.1 °C, the apnea duration increased significantly (P < 0.05), and the LCR duration approximately doubled (P < 0.05). The respiratory frequency also increased significantly during hyperthermia (P < 0.05). These changes in apnea and LCR duration were reversed when body temperature was reduced to 38.6 ± 0.1 °C. Apnea and LCR durations and the respiratory frequency all fell during the second normothermic control period, and each variable was indistinguishable statistically from the initial control values. After each animal received bilateral 100 nL microinjections of 0.65 – 1.0 mM/L resiniferatoxin in the caudal NTS, the LCR durations and respiratory frequency increased significantly (P < 0.05 for all three variables). There were no changes in end-tidal CO2 or mean arterial blood pressure that might have contributed to these changes in apnea and LCR duration and respiratory frequency.
The sites of the microinjections overlapped those in the first study, and there were no injections outside the general region of the NTS. All 12 of these animals had some response to the TRPV1 agonist treatment: the magnitude of the response varied, but none of them could be considered a ‘non-responder.’
3.3 Effect of bicuculline on the LCR response after microinjection of a TRPV1 agonist
In previous studies, we found that thermal prolongation of the LCR could be blocked by GABAA receptor antagonists (Böhm et al., 2007; Duy et al., 2010; Xia et al., 2007). Therefore, we tested the hypothesis that bicuculline, a GABAA receptor antagonist, could block prolongation of the LCR following microinjection of a TRPV1 agonist, resiniferatoxin, into the NTS. We studied the LCR during five conditions: an initial normothermic control, hyperthermia, a second normothermic control, normothermia plus 0.3 mg/kg bicuculline given I.V. and normothermia plus bilateral microinjection of 100 nL of 1.0 mM resiniferatoxin. The order of the last two conditions was alternated to avoid any confounding effects of treatment order. Four animals received bicuculline followed by resiniferatoxin, and four animals received microinjections of resiniferatoxin first, followed by intravenous bicuculline.
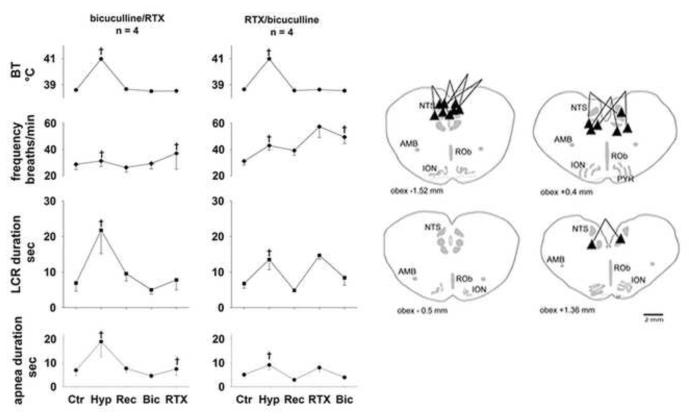
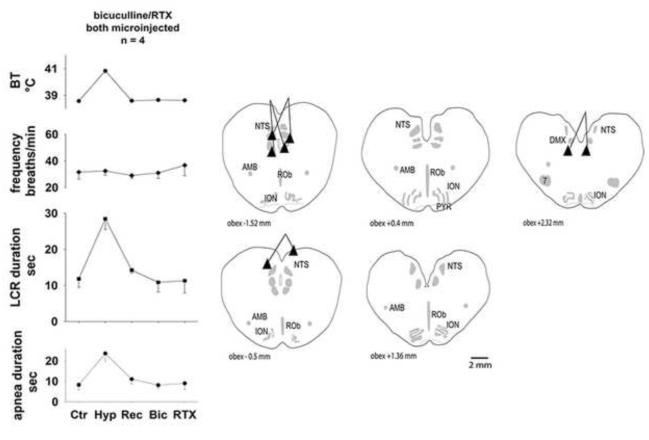
The average values of body temperature, respiratory frequency and LCR and apnea durations and the sites of injection are shown in Fig. 4. In all eight of the animals in this study, the LCR and apnea durations were prolonged during the hyperthermic condition (P < 0.05 for both variables), and this effect was reversible (the variables measured in the first and third normothermic conditions were not different from each other). When bicuculline was given first (left set of panels in Fig. 4), there were no changes in respiratory frequency or LCR or apnea durations. Respiratory frequency increased significantly when resiniferatoxin was microinjected into the NTS following bicuculline treatment, but microinjection of resiniferatoxin did not prolong the LCR. On the other hand, when resiniferatoxin was given before bicuculline (right set of panels in Fig. 4), respiratory frequency and LCR and apnea durations were increased, as shown for a larger number of animals in Fig. 3. No statistical comparisons were made for the data shown in Fig. 4 because small numbers of animals received each different treatment. In the final treatment condition, all animals had received I.V bicuculline and microinjections of resiniferatoxin into the region of the NTS. Despite receiving resiniferatoxin microinjections, apnea and LCR durations were not significantly different from the control conditions: in the bicuculline first group, resiniferatoxin failed to prolong apnea and LCR durations, and in the resiniferatoxin first group, the extended durations of apnea and the LCR that had been present after resiniferatoxin treatment were reversed following bicuculline treatment. Respiratory frequency, however, was significantly increased following resiniferatoxin microinjection regardless of the order of bicuculline treatment (P < 0.05). There were no significant treatment effects on end-tidal CO2 or blood pressure that might have contributed to the treatment related changes that we observed. All of the injections were in or near to the NTS although the injections tended to be concentrated at the caudal extreme of the region that we have been studying. In summary, bicuculline treatment, whether given before or after resiniferatoxin, seemed to block the effect of TRPV1 channel activation on the duration of the LCR. The sites of injections were no different from the previous studies described above.
Figure 4.
The symbol conventions are the same as in Figures 2 and 3. The ‘*’ indicates that these conditions were significantly different from the combined normothermic control values when data from both treatment orders (left and right panels) were combined. Conditions without any symbols indicating statistical differences were not significantly different from the combined normothermic control values.
GABAA receptors are widely distributed, and intravenous treatment with bicuculline is not anatomically specific. We believe, however, that GABAA receptors within the dorsal medulla in or near the NTS mediate thermal prolongation of the LCR (Xia et al., 2007). To test the hypothesis that GABAA receptors within or near the NTS mediate the TRPV1 channel mediated prolongation of the LCR described above, we followed the same protocol as described above, but we gave the bicuculline by microinjection into the NTS rather than intravenously. We studied four additional animals using this protocol, and bicuculline microinjections were made consistently before the resiniferatoxin injections. The average responses of body temperature, respiratory frequency and LCR and apnea durations are shown in Fig. 5 along with schematic anatomical cross-sections showing the sites of injection. As in previous studies, hyperthermia prolonged the LCR, and this effect was reversed when each animal’s body temperature was reduced to the control level. Microinjections of bicuculline into the NTS tended to reduce the duration of apneas and the LCR, but we studied only a small number of animals in this protocol. Resiniferatoxin microinjections into the NTS did not prolong the LCR or apnea duration in any of these animals that had received bicuculline microinjections into the same region. As before, the respiratory frequency tended to be higher after the resiniferatoxin injections, and so far as we can tell with only four animals, bicuculline injected into the NTS did not block this tendency. The locations of these injections were at the caudal and rostral ends of the region of the NTS that we have been testing. Thus, blocking GABAA receptors in the NTS seemed to prevent the effect on the LCR of TRPV1 receptor activation by resiniferatoxin.
Figure 5.
The symbol conventions are the same as in foregoing figures.
4. Discussion
The main finding in this study is that hyperthermic prolongation of the LCR seems to depend on activation of TRPV1 channels in the region of the caudal NTS. As in previous studies in decerebrate piglets, elevating body temperature from ~38 °C to ~ 40.5 °C was associated with increased LCR duration (Böhm et al., 2007; Curran et al., 2005; Duy et al., 2010; Xia et al., 2008a; Xia et al., 2007; Xia et al., 2006). The effect of hyperthermia was reversible: when normothermic body temperature was restored, the LCR duration was no longer significantly different from the initial normothermic condition. An antagonist of the TRPV1 receptor, 5′- iodoresiniferatoxin, microinjected into the NTS blocked prolongation of the LCR while the animal was hyperthermic, and a TRPV1 agonist, resiniferatoxin, microinjected into the region of the NTS prolonged the LCR even while the animals were normothermic. Finally, bicuculline, whether administered systemically or focally within the NTS, blocked the LCR prolongation that followed administration of the TRPV1 agonist in the NTS. Thus, TRPV1 channels within or close to the NTS seem to mediate thermal prolongation of the LCR, and once activated, the effect of TRPV1 channel activity seems to modify the duration of the LCR through GABAergic mechanisms in the same region.
4.1 Role of TRPV1 channels in the LCR
The LCR is mediated by ‘water’ receptors within the mucosa of the larynx, and the water receptors do not seem to express any temperature sensitivity (Xia et al., 2005). Afferent C-fibers in the internal branch of the superior laryngeal nerve, which is a branch of the vagus, transmit sensory information that mediates the LCR from the larynx to the brainstem (Thach, 2007). Laryngeal afferents pass through the nodose ganglion before entering the brainstem, where they innervate second order neurons within the caudal NTS (Hayakawa et al., 2001; Patrickson et al., 1991). TRPV1 channels are present in the laryngeal mucosa (Hamamoto et al., 2009), and in the nodose ganglion (Helliwell et al., 1998) and also expressed presynaptically on visceral afferent fibers within the NTS (Mezey et al., 2000; Patterson et al., 2003; Sun et al., 2009). Moreover, the pattern of TRPV1 receptor expression differs among types of visceral afferent fibers. Visceral afferent C-fibers express pre-synaptic TRPV1 receptors and synapse on second order neurons that express a specific set of potassium channels. A-fibers express presynaptic purinergic receptors and synapse onto a different set of second order neurons (Jin et al., 2004). Visceral afferents are glutamatergic, and the second order neurons in the NTS express non-NMDA glutamate receptors. Thus, when visceral afferents are stimulated, glutamatergic excitatory post-synaptic currents (EPSCs) are elicited in the second order neurons (Doyle et al., 2002; Jin et al., 2004; Peters et al., 2010). Activation of TRPV1 channels within the pre-syanptic membrane on unmyelinated C-fibers seems to amplify glutamate release. TRPV1 channels are usually thought to have a thermal threshold of greater than 42 °C (Julius and Basbaum, 2001), but the opening probability of TRPV1 channels on visceral afferent terminals within the NTS was affected by temperatures well within the range of normal body temperatures. TRPV1 channel activation in the NTS, which enhances asynchronous release of glutamate following visceral afferent stimulation, was thermally modulated by temperatures between 25-38 °C (Peters et al., 2010). Moreover, spontaneous glutamatergic excitatory post-synaptic currents (currents occurring in the absence of visceral afferent activation) were strongly influenced by TRPV1 channel activity, and TRPV1 activity in this setting had a thermal range of 30-42 °C (Shoudai et al., 2010). It is our hypothesis that pre-synaptic TRPV1 receptors on laryngeal C-fiber afferents within the NTS probably amplify glutamate release at second order neurons in the NTS when the body temperature is increased and thereby amplify the potency of the afferent information that leads to respiratory inhibition. although TRPV1 receptors are present in the laryngeal mucosa, we doubt that water receptors mediating the LCR express TRPV1 receptors in the periphery in the larynx since water receptors demonstrate no appreciable temperature sensitivity (Xia et al., 2005). Such an hypothesis is also consistent with our previous observation that focal heating of the NTS prolonged the LCR even while the rest of the animal was normothermic – the temperature sensor for thermal prolongation of the LCR seems to reside within the NTS (Xia et al., 2008a) and seems to be active at temperatures only slightly above normal body temperature. We have never cooled the NTS, but it is possible that thermal modulation of TRPV1 channels, and thereby thermal modulation of the LCR, persists even into the hypothermic temperature range (32-34 °C) – a range of body temperatures experienced by many small neonatal mammals.
The effect of 5′iodoresiniferatoxin on TRPV1 receptor during hyperthermia and the TRPV1 agonist during normothermia support the hypothesis that TRPV1 channels mediate thermal prolongation of the LCR, but there are some limitations in our data. The affinity of resiniferatoxin for the TRPV1 receptor was greater than the affinity of 5′iodoresiniferatoxin for the rat TRPV1 receptor expressed in HEK293 cells (Wahl et al., 2001). Nonetheless, we found that it was necessary to use a dose of resiniferatoxin approximately 10-fold larger than the dose of 5′iodoresiniferatoxin to elicit comparable, though opposite physiological effects. The sites of injections in the NTS and the volumes of drug injected in both studies of these drugs were quite similar (and therefore an unlikely explanation for this asymmetry of dose effects). We do not have a satisfactory explanation for the requirement of different agonist and antagonist doses, but there are several possible explanations. First, the TRPV1 receptors are susceptible to a variety of modifications, such as phosphorylation and interactions with bradykinnin, endocannabinoids and other ligands, which may modify the binding characteristics of the TRPV1 receptors (Julius and Basbaum, 2001; Venkatachalam and Montell, 2007), and the channels expressed in cell culture systems may not represent the affinity characteristics of the native channels in vivo since post-translational modifications, alternate splicing and heteromultimerization may alter the biophysical and pharmacological properties of the receptors (Caterina, 2007). Second, the network properties of the second order neurons and communicating inter-neurons in the NTS may create differential sensitivity to agonists and antagonists of apparently equal pharmacological strength. For example, bilateral excitotoxic lesions in central chemoreceptor regions in the ventral medulla completely disrupt respiratory activity for up to 90 minutes in anesthetized or decerebrate animals (Nattie and Li, 1990). On the other hand, focal stimulation with the agonist, hypercapnic low pH solutions, only modestly alters ventilation (Li and Nattie, 1997). Thus, network interactions within the interneurons of the NTS may make the LCR more sensitive to inhibitory influences than stimulatory effects, and the asymmetry of agonist/antagonist effects may not reflect anything about relative receptor affinities.
One further limitation is that virtually all of the microinjections of TRPV1 agonists and antagonists were in or very close to the NTS. We have demonstrated that activation of TRPV1 receptors in the NTS is sufficient to prolong the LCR, but since we have so few injections outside the NTS, we cannot state that TRPV1 receptors outside the NTS would not have similar effects, though we do know that focal heating is effective only in the NTS region (Xia et al., 2006).
4.2 Origin of thermal sensitivity of the LCRs
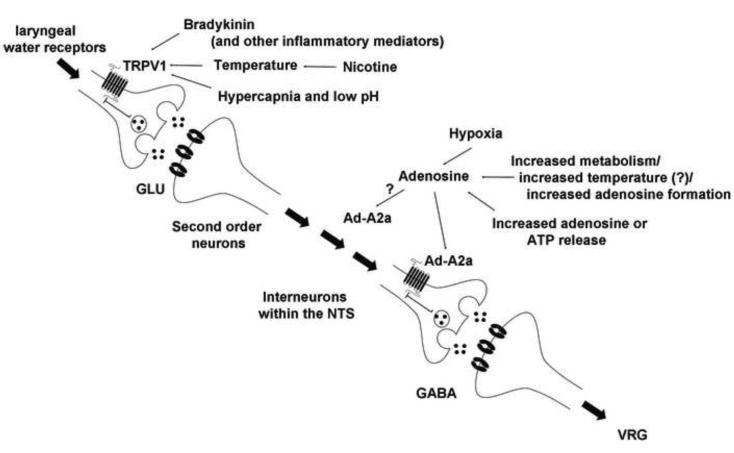
We have used the foregoing results to create a working model of the circuitry of the LCR in the NTS (Fig. 6). As noted above, the LCR is elicited when water receptors within the larynx are stimulated. This information is transmitted by C-fiber visceral afferents, which relay the information through a glutamatergic mechanism to second order neurons in the NTS. There are a variety of interneuruons in the NTS, but ultimately there appear to be GABAergic synapses within the NTS, which relays inhibitory information to the ventral medullary respiratory neurons. There appear to be two amplification processes in this chain of command. First, TRPV1 activity modulates glutamate release at the synapse between the visceral afferent nerve terminals and the second order neurons within the NTS (Peters et al., 2010; Shoudai et al., 2010). Second, adenosine A2a (Ad-A2a) receptors, which may be presynaptic on GABAergic terminals within the NTS, seem to amplify GABA release (Duy et al., 2010). Presynaptic Ad-A2a receptors on GABAergic neurons have been described in the hypothalamus (Hong et al., 2005), the globus pallidus (Ochi et al., 2000) and the cortex (Phillis, 1998). Thus, TRPV1 seems to provide excitatory amplification, and adenosine, acting through Ad-A2a receptors, seems to provide amplification of GABA-mediated inhibition. Both the TRPV1 and the adenosinergic processes may be thermally sensitive since both are enhanced when body temperature is elevated. The thermal sensitivity of TRPV1 receptors originates from the intrinsic properties of the ion channel. The thermal sensitivity of the adenosinergic process may originate from thermal modulation of the rate of formation of adenosine or from direct neuronal or glial release of ATP or adenosine (Erlichman et al., 2010). It is our hypothesis that adenosinergic modulation of the LCR is thermally sensitive (Duy et al., 2010), but if this were so, we might have expected some residual thermal prolongation of the LCR after inhibiting TRPV1 channels. And we did not see this (Fig. 2). So it may be that the intensity of adenosinergic activation is not intrinsically thermally sensitive, but rather related through some other mechanism to the intensity of TRPV1 activation. In the final analysis, both of these amplifying processes serve to enhance GABAergic inhibitory activity leaving the NTS to interact with respiratory neurons controlling expiration in the VRG. The dominant role of GABA within the NTS is supported by the observation that systemic administration of bicuculline (Böhm et al., 2007) and focal administration of gabazine in the NTS (Xia et al., 2007) completely blocked the hyperthermic prolongation of the LCR and block the effect of adenosine agonists (Duy et al., 2010) and TRPV1 agonists (Figs. 4 and 5). In summary, thermal prolongation of the LCR may result from modulation of a GABAergic process by TRPV1-mediated thermal enhancement of glutamate release at second order neurons in the NTS and may also be influenced by enhancement of adenosine release at the site of GABA release in the final inhibitory process within the NTS before this information is transmitted to the respiratory control element(s) of the reflex circuit in the respiratory neurons in the ventral medulla. The apnea induced by laryngeal stimulation originates from a centrally mediated prolongation of post-inspiratory neuronal activity, so that the normal sequential activation of expiratory neurons that would lead to the next breath is prevented and/or delayed (Remmers et al., 1986). Hyperthermic prolongation of the LCR seems to extend the duration of this ‘post-inspiratory apneusis.’
Figure 6.
Our working model of thermal modulation of the LCR is shown schematically in this figure. Thermal amplification of the LCR occurs within the NTS, and it seems to occur by virtue of activating TRPV1 receptors and increasing adenosinergic activation of adenosine A2a (Ad-A2a) receptors. TRPV1 receptor activation, whether by thermal or other mechanisms, increases glutamate release (GLU) at second order neurons within the NTS. Adenosine formation may be enhanced either by elevated brain temperature, release from neurons or glia or through some process proportional to the intensity of TRPV1 activation, and adenosine, operating through Ad-A2a receptors enhance activation of GABAA receptors. We believe that Ad-A2a receptors are presynaptic on GABAergic neurons and enhance GABA release, but we have not investigated the exact cellular location of the Ad-A2a receptors in the NTS. The Ad-A2a receptors may be on other interneurons neurons (indicated by the ‘?’). In any event, enhanced activation of GABAA receptors seems to be the final pathway within the NTS leading to prolonged activation of post-inspiratory and expiratory decrementing neurons in the ventral respiratory group (VRG), which maintain the prolonged expiratory apnea during thermal prolongation of the LCR. The modulators of TRPV1 activation and adenosine formation and/or adenosine release relevant to SIDS are also shown.
4.3 Respiratory effects of hyperthermia and TRPV1 agonist and antagonists
The most frequent thermal effect on respiration in decerebrate piglets was an increase in respiratory frequency, but the effect has been inconsistent among animals within each study and among studies (Böhm et al., 2007; Curran et al., 2005; Xia et al., 2008a). Even in the current study, the increase in respiratory frequency was statistically significant in the TRPV1 agonist studies with and without bicuculline (Figs. 3 and 4), but not significant in the initial study of the TRPV1 receptor antagonist (Fig. 2). Many elements of the thermoregulatory system, which interact with the respiratory system to regulate body temperature, were removed by decerebration. The level of decerebration has an impact on the frequency response to a variety of treatments (St. John, 1979), and subtle differences in the level of decerebration may explain the divergent frequency responses whether the differences in decerebration altered the contribution of ponto-medullary neurons to respiratory or thermal control of frequency.
There is a similar problem explaining the frequency responses to injections of resiniferatoxin and 5′idoresiniferatoxin. Following injections of the TRPV1 receptor agonist and antagonist, respiratory frequency increased significantly. The changes in respiratory frequency were tightly correlated with the drug injections and cannot be attributed to some non-specific effect of the injections. In previous studies, we injected solutions containing saline and DMSO (Duy et al., 2010; Xia et al., 2008a), and we have never observed an injection-related change in respiratory frequency in these control studies. One might have expected respiratory frequency to change in opposite directions when comparing the TRPV1 receptor agonist and antagonist responses. 5′iodoresiniferatoxin is, however, a partial agonist in some studies (Shimizu et al., 2005), and perhaps the agonist properties of the drug contribute to the increased respiratory frequency that we observed. Whatever the explanation for the similarity of frequency responses to TRPV1 agonists and antagonists, the frequency response is not dependent on a GABAergic mechanism (the frequency response to resiniferatoxin and 5′iodoresiniferatoxin treatments persisted after systemic bicuculline treatment). These results are similar to the effect of Ad-A2a agonists, which also increase respiratory frequency when administered in the NTS, an effect that also persisted after systemic bicuculline administration (Duy et al., 2010).
4.4 Limitations of the methods
We have discussed the limitations of studying the LCR in decerebrate neonatal piglets in previous studies (Duy et al., 2010; Xia et al., 2008a). We pointed out in these discussions that thermal stress has been identified as a risk factor for SIDS, but this does not mean necessarily that infants who died of SIDS had an elevated body temperature. In this respect, our studies of decerebrate piglets may not accurately mimic the thermal risk factors for SIDS. In light of the current work, this limitation may not be as serious as we had thought. If TRPV1 channels express thermal sensitivity in the normal body temperature range, then frank hyperthermia need not be present for TRPV1 channel activity to modulate the strength and duration of the LCR. Our studies of hyperthermic piglets may be relevant to normothermic conditions in sleeping infants, particularly if other factors that modify TRPV1 activity and thermal sensitivity are present (Julius and Basbaum, 2001; Venkatachalam and Montell, 2007).
4.5 Thermal stresses, TRPV1 channels, adenosine, the LCR and SIDS
SIDS is often associated with heat stress.(Blair et al., 2008; Guntheroth and Spiers, 2001; Kleemann et al., 1998; Williams et al., 1996). Although there is not universal agreement (Sheers-Masters et al., 2004), many reports indicate that death frequently occurs in overheated rooms, with excessive bundling or wrapping of the infant (Fleming et al., 1990; Ponsonby et al., 1998; Williams et al., 1996), who occasionally is found covered with sweat (Kleemann et al., 1996). As emphasized by Guntheroth and Spiers (2001), the danger of the prone sleeping position may relate more to heat stress than to asphyxia. Prone infants—particularly those who are over blanketed and/or have their faces in the bedclothes—lose heat more slowly than supine infants do, both from the body surface and via the respiratory tract (Bolton et al., 1996).We hypothesize, therefore, that thermal stress, by enhancing the LCR, may increase the likelihood of prolonged apneas that result ultimately in sudden death in neonates in whom the LCR and the thermal effects on the LCR are particularly strong. TRPV1 receptor activity may play an important role in strengthening the potency of sensory information that elicits the LCR. Adenosinergic mechanisms also seem to increase the inhibitory power of the LCR (Duy et al., 2010). Thus, when the LCR is elicited, any factors that promote activation of TRPV1 receptors and promote the formation of adenosine in the NTS may be expected to favor the pathogenesis of SIDS.
The demonstration that TRPV1 channels provide a thermal sensor that enhances the LCR provides a molecular mechanism through which heat stress and other epidemiologically identified risk factors for SIDS may operate to increase the risk of prolonged apnea and sudden infant death. Recent upper respiratory tract infections are also a risk factor for SIDS (Dalveit et al., 2003), and recent infection, particularly with Respiratory Syncytial Virus, seems to increase the potency of the LCR (Lindgren et al., 1992). The infection-related increase in potency of the LCR seems to be mediated by inflammatory mediators, either transmitted retrograde from the larynx into the central nervous system through the superior laryngeal nerves or made locally within the central nervous system (Lindgren and Grogaard, 1996; Lindgren et al., 1992; Thach, 2003). To the extent that inflammatory mediators sensitize TRPV1 channels (Julius and Basbaum, 2001; Venkatachalam and Montell, 2007) and increase the activity of the channels even at normal body temperatures, TRPV1 amplification of the LCR may increase the likelihood of prolonged apneas and increase the risk of SIDS following infections. Thus, TRPV1 channel activity may provide a common molecular mechanism for the epidemiological association of SIDS with thermal stress and recent infections.
Acknowledgments
This work was supported by grants 36379 and 42707 from the NICHD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Bandroft JD, Cook HC. The central and peripheral nervous system. In: Bandroft JD, editor. Manual of Histological Techniques and Their Diagnostic Application. Churchill Livingstone; New York: 1994. pp. 350–351. [Google Scholar]
- Blair PS, Mitchell EA, Heckstall-Smith EMA, Fleming PJ. Head covering - A major modifiable risk factor for Sudden Infant Death Syndrome: A systematic review. Arch. Dis. Child. 2008;93:778–783. doi: 10.1136/adc.2007.136366. [DOI] [PubMed] [Google Scholar]
- Boggs DF, Bartlett D., Jr. Chemical specificity of a laryngeal apneic reflex in puppies. J. Appl. Physiol. 1982;53:455–462. doi: 10.1152/jappl.1982.53.2.455. [DOI] [PubMed] [Google Scholar]
- Böhm I, Xia L, Leiter JC, Bartlett D., Jr. GABAergic processes mediate thermal prolongation of laryngeal reflex apnea in decerebrate piglets. Respir. Physiol. Neurobiol. 2007;156:229–233. doi: 10.1016/j.resp.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Bolton DPG, Nelson EAS, Taylor BJ, Weatherall IL. Thermal balance in infants. J. Appl. Physiol. 1996;80:2234–2242. doi: 10.1152/jappl.1996.80.6.2234. [DOI] [PubMed] [Google Scholar]
- Caterina MJ. Transient receptor potential ion channels as participants in thermosensation and thermoregulation. Am. J. Physiol. 2007;292:R64–R76. doi: 10.1152/ajpregu.00446.2006. [DOI] [PubMed] [Google Scholar]
- Curran AK, Xia L, Leiter JC, Bartlett D., Jr. Elevated body temperature enhances the laryngeal chemoreflex in decerebrate piglets. J. Appl. Physiol. 2005;98:780–786. doi: 10.1152/japplphysiol.00906.2004. [DOI] [PubMed] [Google Scholar]
- Dalveit AK, Irgens LM, Øyen N, Skjaerven R, Markstad T, Wennergren G. Circadian variations in sudden infant death syndrome: associations with maternal smoking, sleeping position and infections. The Nordic Epidemiological SIDS Study. Acta Paediatr. 2003;92:1007–1013. [PubMed] [Google Scholar]
- Downing SE, Lee JC. Laryngeal chemosensitivity: A possible mechanism of sudden infant death. Pediatrics. 1975;55:640–649. [PubMed] [Google Scholar]
- Doyle MW, Bailey TW, Jin Y-H, Andresen MC. Vanilloid receptors presynaptically modulate cranial visceral afferent synaptic transmission in nucleus tractus solitarius. J. Neurosci. 2002;22:8222–8229. doi: 10.1523/JNEUROSCI.22-18-08222.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duy MP, Xia L, Bartlett D, Jr., Leiter JC. An adenosine A2A agonist injected in the NTS prolongs the LCR by a GABAergic mechanism in decerebrate piglets. Exp. Physiol. 2010 doi: 10.1113/expphysiol.2010.052647. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlichman JS, Leiter JC, Gourine AV. ATP, glia and central respiratory control. Respir. Physiol. Neurobiol. 2010;173:305–311. doi: 10.1016/j.resp.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming PJ, Gilbert R, Azaz Y, Berry PJ, Rudd PT, Stewart A, Hall E. Interaction between bedding and sleeping position in the SIDS: a population based case-control study. BMJ. 1990;301:85–89. doi: 10.1136/bmj.301.6743.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves C, Glass L, Laporta D, Meloche R, Grassino A. Respiratory phase locking during mechanical ventilation in anesthetized subjects. Am. J. Physiol. 1986;250:R902–R909. doi: 10.1152/ajpregu.1986.250.5.R902. [DOI] [PubMed] [Google Scholar]
- Grogaard J, Lindstrom DP, Stahlman MT, Marchal F, Sundell H. The cardiovascular response to laryngeal water administration in young lambs. J. Dev. Physiol. 1982;4:353–370. [PubMed] [Google Scholar]
- Guntheroth WG, Spiers PS. Thermal stress in Sudden Infant Death: Is there an ambiguity with the rebreathing hypothesis? Pediatrics. 2001;107:693–698. doi: 10.1542/peds.107.4.693. [DOI] [PubMed] [Google Scholar]
- Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur. J. Neurosci. 1999;11:946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- Hamamoto T, Takumida M, Kirakawa K, Tatsukawa T, Ishibashi T. Localization of transient receptor potential vanilloid (TRPV) in the human larynx. Acta Otolaryngol. 2009;129:560–568. doi: 10.1080/00016480802273108. [DOI] [PubMed] [Google Scholar]
- Haraguchi S, Fung RQ, Sasaki R. Effect of hyperthermia on the laryngeal closure reflex. Implications in the sudden infant death syndrome. Ann. Otol. Rhinol. Laryngol. 1983;92:24–28. doi: 10.1177/000348948309200106. [DOI] [PubMed] [Google Scholar]
- Hayakawa T, Takanaga A, Maeda S, Seki M, Yajima Y. Subnuclear distribution of afferents from the oral, pharyngeal and laryngeal regions in the nucleus tractus solitarii of the rat: a study using transganglionic transport of cholera toxin. Neurosci. Res. 2001;39:221–232. doi: 10.1016/s0168-0102(00)00218-2. [DOI] [PubMed] [Google Scholar]
- Helliwell RJA, McLatchie LM, Clarke MJ, Winter JB, Bevan S, McIntyre P. Capsaicin sensitivity is associated with the expression of vanilloid (capsaicin) receptor (VR1) mRNA in adult rat sensory ganglia. Neurosci. Lett. 1998;250:177–180. doi: 10.1016/s0304-3940(98)00475-3. [DOI] [PubMed] [Google Scholar]
- Hong Z-Y, Huang Z-L, Qu W-M, Eguchi N, Urade Y, Hayaishi O. An adenosine A2A receptor agonist induces sleep by increasing GABA release in the tuberomammillary nucleus to inhibit histaminergic systems in rats. J. Neurochem. 2005;92:1542–1549. doi: 10.1111/j.1471-4159.2004.02991.x. [DOI] [PubMed] [Google Scholar]
- Inoue S, Murakami N. Unit responses in the medulla oblongata of rabbit to changes in local and cutaneous temperature. J. Physiol. 1976;259:339–356. doi: 10.1113/jphysiol.1976.sp011469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y-H, Bailey TW, Li B, Schild JH, Andresen MC. Puringergic and vanilloid receptor activation releases glutamate from separate cranial afferent terminals in nucleus tractus solitarius. J. Neurosci. 2004;24:4709–4717. doi: 10.1523/JNEUROSCI.0753-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- Kleemann WJ, Schlaud M, Fieguth A, Hiller AS, Rothämel T, Tröger HD. Body and head position, covering of the head by bedding and risk of sudden infant death (SID) Int. J. Legal. Med. 1998;112:22–26. doi: 10.1007/s004140050192. [DOI] [PubMed] [Google Scholar]
- Kleemann WJ, Schlaud M, Poets CF, Rothämel T, Tröger HD. Hyperthermia in sudden infant death. Int. J. Legal Med. 1996;109:139–142. doi: 10.1007/BF01369674. [DOI] [PubMed] [Google Scholar]
- Lee JC, Stoll BJ, Downing SE. Properties of the laryngeal chemoreflex in neonatal piglets. Am. J. Physiol. 1977;233:R30–R36. doi: 10.1152/ajpregu.1977.233.1.R30. [DOI] [PubMed] [Google Scholar]
- Leiter JC, Böhm I. Mechanisms of pathogenesis in the Sudden Infant Death Syndrome (SIDS) Respir. Physiol. Neurobiol. 2007;159:127–138. doi: 10.1016/j.resp.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Li A, Nattie EE. Focal central chemoreceptor sensitivity in the RTN studied with a CO2 diffusion pipette in vivo. J. Appl. Physiol. 1997;83:420–428. doi: 10.1152/jappl.1997.83.2.420. [DOI] [PubMed] [Google Scholar]
- Lindgren C, Grogaard J. Reflex apnoea response and inflammatory mediators in infants with respiratory tract infection. Acta Paediatr. 1996;85:798–803. doi: 10.1111/j.1651-2227.1996.tb14154.x. [DOI] [PubMed] [Google Scholar]
- Lindgren C, Jing L, Graham B, Grogaard J, Sundell H. Respiratory syncytial virus infection reinforces reflex apnea in young lambs. Pediatri. Res. 1992;31:381–385. doi: 10.1203/00006450-199204000-00015. [DOI] [PubMed] [Google Scholar]
- Luna LG. Histopathologic Methods and Color Atlas of Special Stains and Tissue Artifacts. American Histolabs, Inc.; Gaithersburg: 1992. [Google Scholar]
- Mezey A, Toth ZE, Cortright DN, Arzubi MK, Krause JE, Elde R, Guo A, Blumberg PM, Szallasi A. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc. Natl. Acad. Sci. 2000;97:3655–3660. doi: 10.1073/pnas.060496197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie EE, Li A. Fluorescence location of RVLM kainate microinjections that alter the control of breathing. J. Appl. Physiol. 1990;68:1157–1166. doi: 10.1152/jappl.1990.68.3.1157. [DOI] [PubMed] [Google Scholar]
- Niblock MM, Luce CJ, Belliveau RA, Paterson DS, Kelley ML, Sleeper LA, Filiano J, Kinney HC. Comparative anatomical assessment of the piglet as a model for the developing human medullary serotonergic system. Brain Res. Rev. 2005;50:169–183. doi: 10.1016/j.brainresrev.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Ochi M, Koga K, Kurokawa M, Kase H, Nakamura J-I, Kuwana Y. Systemic administration of adenosine A2A receptor antagonist reverses increased GABA release in the globus pallidus of unilateral 6-hydroxydopamine-lesioned rats: A microdialysis study. Neurosci. 2000;100:53–62. doi: 10.1016/s0306-4522(00)00250-5. [DOI] [PubMed] [Google Scholar]
- Ohta T, Ikemi Y, Murakami M, Imagawa T, Otsuguro K, Ito S. Potentiation of transient receptor potential V1 functions by the activation of metabotropic 5-HT receptors in rat primary sensory neurons. J. Physiol. 2006;576.3:809–822. doi: 10.1113/jphysiol.2006.112250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson DS, Trachtenberg FL, Thompson EG, Belliveau RA, Beggs AH, Darnall RA, Chadwick AE, Krous HF, Kinney HC. Multiple serotonergic brainstem abnormalities in the sudden infant death syndrome. J.A.M.A. 2006;296:2124–2132. doi: 10.1001/jama.296.17.2124. [DOI] [PubMed] [Google Scholar]
- Patrickson JW, Smith TE, Zhou S-S. Afferent projections of the superior and recurrent laryngeal nerves. Brain Res. 1991;539:169–174. doi: 10.1016/0006-8993(91)90702-w. [DOI] [PubMed] [Google Scholar]
- Patterson LM, Zheng H, Ward SM, Berthoud H-R. Vanilloid receptor (VR1) expression in vagal afferent neurons innervating the gastrointestinal tract. Cell Tissue Res. 2003;311:277–287. doi: 10.1007/s00441-002-0682-0. [DOI] [PubMed] [Google Scholar]
- Peters JH, McDougall SJ, Fawley JA, Smith SM, Andresen MC. Primary afferent activation of thermosensitive TRPV1 triggers asynchronous glutamate release at central neurons. Neuron. 2010;65:657–669. doi: 10.1016/j.neuron.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrillo GA, Glass L, Trippenbach T. Phase locking of the respiratory rhythm in cats to a mechanical ventilator. Can. J Physiol. Pharmacol. 1983;61:599–607. doi: 10.1139/y83-092. [DOI] [PubMed] [Google Scholar]
- Phillis JW. Inhibitory action of CGS 21680 on cerebral cortical neurons is antagonized by bicuculline and picrotoxin - is GABA involved? Brain Res. 1998;807:193–198. doi: 10.1016/s0006-8993(98)00756-2. [DOI] [PubMed] [Google Scholar]
- Ponsonby A-L, Dwyer T, Couper D, Cochrane JA. Association between use of a quilt and sudden infant death syndrome: case-control study. BMJ. 1998;316:195–196. doi: 10.1136/bmj.316.7126.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remmers JE, Richter DW, Ballantyne D, Bainton CR, Klein JP. Reflex prolongation of stage I of expiration. Pflugers Arch. 1986;407:190–198. doi: 10.1007/BF00580675. [DOI] [PubMed] [Google Scholar]
- Sasaki CT. Development of laryngeal function: etiologic significance in the sudden infant death syndrome. Laryngoscope. 1979;89:1964–1982. doi: 10.1288/00005537-197912000-00010. [DOI] [PubMed] [Google Scholar]
- Sheers-Masters JR, Schootman M, Thach BT. Heat stress and Sudden Infant Death Syndrome incidence: A United States population epidemiologic study. Pediatrics. 2004;113:e586–e592. doi: 10.1542/peds.113.6.e586. [DOI] [PubMed] [Google Scholar]
- Shimizu I, Iida T, Horiuchi N, Caterina MJ. 5-iodoresiniferatoxin evokes hypothermia in mice and is a partial transient receptor potential vanilloid 1 agonist in vitro. J. Pharmacol. Exp. Therap. 2005;314:1378–1385. doi: 10.1124/jpet.105.084277. [DOI] [PubMed] [Google Scholar]
- Shoudai K, Peters JH, McDougall SJ, Fawley JA, Andresen MC. Thermally active TRPV1 tonically drives central spontaneous glutamate release. J. Neurosci. 2010;30:14470–14475. doi: 10.1523/JNEUROSCI.2557-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. John WM. An analysis of respiratory frequency alterations in vagotomized, decerebrate cats. Respir. Physiol. 1979;36:167–186. doi: 10.1016/0034-5687(79)90023-9. [DOI] [PubMed] [Google Scholar]
- Suguira T, Bielfeldt K, Gebhart GF. TRPV1 function in mouse colon sensory neurons is enhanced by metabotropic 5-hydroxytryptamine receptor activation. J. Neurosci. 2004;24:9521–9530. doi: 10.1523/JNEUROSCI.2639-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Li D-P, Chen S-R, Hittelman WN, Pan H-L. Sensing of blood pressure increase by transient receptor potential vanilloid 1 receptors on baroreceptors. J. Pharmacol. Exp. Therap. 2009;331:851–859. doi: 10.1124/jpet.109.160473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thach BT. Maturation and transformation of reflexes that protect the laryngeal airway from liquid aspiration from fetal to adult life. Am. J. Med. 2001;111:69S–77S. doi: 10.1016/s0002-9343(01)00860-9. [DOI] [PubMed] [Google Scholar]
- Thach BT. The brainstem and vulnerability to sudden infant death syndrome. Neurol. 2003;61:1170–1171. doi: 10.1212/wnl.61.9.1170. [DOI] [PubMed] [Google Scholar]
- Thach BT. The role of respiratory control disorders in SIDS. Respir. Physiol. Neurobiol. 2005;149:343–353. doi: 10.1016/j.resp.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Thach BT. Maturation of cough and other reflexes that protect the fetal and neonatal airway. Pulm. Pharmacol. Therap. 2007;20:365–370. doi: 10.1016/j.pupt.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Velde L, Curran A, Filiano JJ, Darnall RA, Bartlett D, Jr., Leiter JC. Prolongation of the laryngeal chemoreflex after inhibition of the rostroventral medulla in piglets: A role in SIDS? J. Appl. Physiol. 2003;94:1883–1895. doi: 10.1152/japplphysiol.01103.2002. [DOI] [PubMed] [Google Scholar]
- Venkatachalam K, Montell C. TRP channels. Annu. Rev. Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl P, Foged C, Tullin S, Thomsen C. Iodo-resiniferatoxin, a new potent vanilloid receptor antagonist. Molec. Pharmacol. 2001;59:9–15. doi: 10.1124/mol.59.1.9. [DOI] [PubMed] [Google Scholar]
- Williams SM, Taylor BJ, Mitchell EA. Sudden infant death syndrome: Insulation from bedding and clothing and its effect modifiers. Int. J. Epidemiol. 1996;25:366–375. doi: 10.1093/ije/25.2.366. [DOI] [PubMed] [Google Scholar]
- Xia L, Bartlett D, Jr., Leiter JC. An adenosine A2A antagonist injected in the NST reverses thermal prolongation of the LCR in decerebrate piglets. Respir. Physiol. Neurobiol. 2008a;164:358–365. doi: 10.1016/j.resp.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L, Crane-Godreau MA, Leiter JC, Bartlett D., Jr. Gestational cigarette smoke exposure and hyperthermic enhancement of laryngeal chemoreflex in rat pups. Respir. Physiol. Neurobiol. 2009;165:161–166. doi: 10.1016/j.resp.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L, Damon T, Niblock MM, Bartlett D, Jr., Leiter JC. Unilateral microdialysis of gabazine in the dorsal medulla reverses thermal prolongation of the laryngeal chemoreflex in decerebrate piglets. J. Appl. Physiol. 2007;103:1864–1872. doi: 10.1152/japplphysiol.00524.2007. [DOI] [PubMed] [Google Scholar]
- Xia L, Damon TA, Leiter JC, Bartlett D., Jr. Focal warming of the nucleus of the solitary tract prolongs the laryngeal chemoreflex in decerebrate piglets. J. Appl. Physiol. 2006;102:54–62. doi: 10.1152/japplphysiol.00720.2006. [DOI] [PubMed] [Google Scholar]
- Xia L, Leiter JC, Bartlett D., Jr. Laryngeal water receptors are insensitive to body temperature in neonatal piglets. Respir. Physiol. Neurobiol. 2005;150:82–86. doi: 10.1016/j.resp.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Xia L, Leiter JC, Bartlett D., Jr. Laryngeal apnea in rat pups: effects of age and body temperature. J. Appl. Physiol. 2008b;104:269–274. doi: 10.1152/japplphysiol.00721.2007. [DOI] [PubMed] [Google Scholar]
- Xia L, Leiter JC, Bartlett D., Jr. Gestational nicotine exposure exaggerates hyperthermic enhancement of laryngeal chemoreflex in rat pups. Respir. Physiol. Neurobiol. 2010;171:17–21. doi: 10.1016/j.resp.2010.01.011. [DOI] [PubMed] [Google Scholar]