Abstract
Fibrin structure and stability have been linked to many thrombotic diseases, including venous thromboembolism. Analysis of the molecular mechanisms that affect fibrin structure and stability became possible when Doolittle and colleagues solved the crystal structure of fibrinogen. Biochemical studies of natural and recombinant variant fibrinogens have examined the interactions that mediate the conversion of soluble fibrinogen to the insoluble fibrin network. These studies identified intermolecular interactions that control fibrin structure, though some critical events remain ambiguous. Studies show fibrin structure modulates the enzymatic lysis of the fibrin network, so the molecular mechanisms that control structure also control stability. Studies show the mechanical stability of the fibrin clot depends on the properties of the fibrin monomer, leading investigators to explore the molecular basis of the monomer’s mechanical properties. The work summarized here provides insight that might allow development of pharmaceuticals and treatments to modulate fibrin structure and stability in vivo and thereby prevent or limit thrombotic disease.
Keywords: fibrin network, fibrin stability, fibrinogen, molecular mechanisms, thrombotic disease
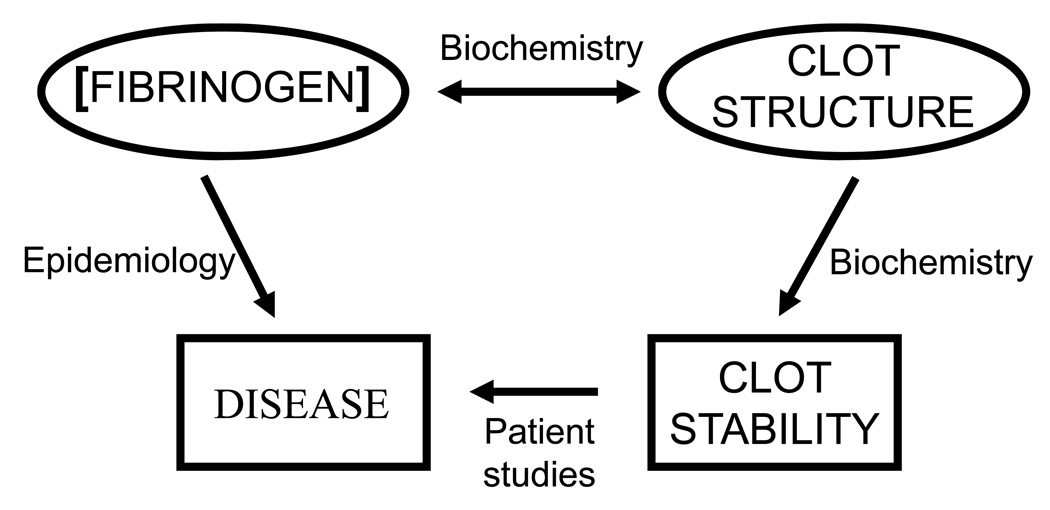
Large epidemiological studies have linked fibrinogen concentration to most, if not all, thrombotic diseases—coronary artery disease, ischemic heart disease, stroke and thromboembolic disease.1 Smaller studies have shown the structure and stability of fibrin clots formed from patient plasmas differ from clots formed from plasma from healthy individuals.2–5 Because fibrinogen concentration influences fibrin clot structure, the epidemiological correlation to concentration could logically reflect the findings in smaller studies of specific patients. (Figure 1) The recent analysis of plasmas isolated from patients with VTE, their first-degree relatives and matched controls showed a correlation between fibrin network structure and the rate of clot lysis. 5 Samples from patients with idiopathic VTE had the densest network and the slowest lysis; samples from the relatives had intermediate values, while samples from controls had the most open network and the fastest lysis. Although the implications are limited by the small number of study subjects, these results support the conclusion that the fibrin network structure is etiologic in VTE and that genetic components likely have a significant role. Because biochemical studies link fibrin structure to fibrin stability, the fundamental question becomes: what factors control fibrin structure? Here I enumerate molecular mechanisms that affect the structure, as well as identify some issues that remain unresolved. Thereafter, I describe studies that explore the correlation between fibrin structure and stability, how the structure influences resistance to shear and to enzymatic lysis.
Figure 1. Correlation of fibrinogen concentration, clot structure and thrombotic diseases.
Epidemiologic studies have shown that fibrinogen concentration is a risk factor for thrombotic disease. Biochemical studies have shown fibrinogen concentration modulates clot structure, and clot structure modulates clot stability. Case control studies link fibrin clot structure and stability to thrombotic disease.
Fibrinogen
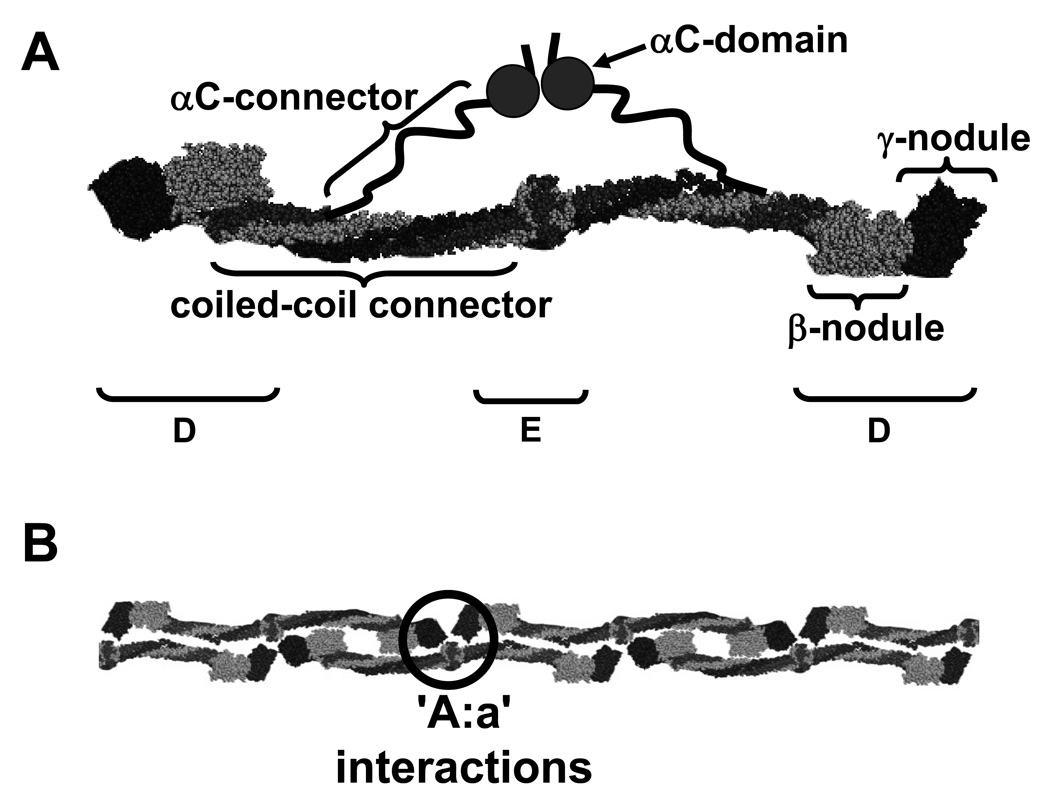
Fibrinogen is a 340-kDa glycoprotein composed of two copies each of three polypeptide chains, (AαBβγ)2. Fibrinogen’s overall structure was originally determined by multiple electron microscopy studies, as exemplified by the studies of Weisel et al.6 Several X-ray crystal structures have confirmed this overall structure and added many significant details.7–10 These studies show human fibrinogen is an elongated molecule with a unique center and two identical symmetric distal regions (Figure 2A). The center, called the E region, contains the N-termini of all six chains. The chains extend from the center as two coiled-coils composed of three chains, each coil terminating in a globular region called D. The D regions contain the carboxy-termini of the Bβ and γ chains, called the β-nodules and γ-nodules, respectively. The C-terminal segment of the Aα chain goes through the D region, folds back to join the coiled-coil for several residues, and thereafter is not visible in the X-ray structure. Presumably this region (Aα residues 213–610) is mobile within the crystal, and thus does not provide interpretable diffraction data. Other studies, including electron microscopy and NMR spectroscopy, divide this region into the αC connector (Aα 221–391) and the αC domain (Aα391–610).11, 12
Figure 2. The structure of fibrinogen (A) and the fibrin protofibril (B).
A. Fibrinogen is a dimer with two copies each of three chains: Aα, shown in dark grey, Bβ, shown in light grey, and γ, shown in black. The schematic is based on the crystal structure with the addition of the two αC regions containing the αC connector and the αC domain. B. Fibrin monomers assemble into half-staggered, double-stranded protofibrils. Protofibril formation is mediated by interactions between knobs ‘A’ in the central E region and holes ‘a’ in the distal D regions; one set of ‘A:a’ interactions is circled. The structures and nomenclature are based on published recommendations.68
Fibrin structure
Following vessel injury, a series of reactions occurs on the surfaces of cells at the site of injury, leading to the generation of the protease thrombin.13 Thrombin cleaves four short peptides from the N-termini of the Aα- and Bβ-chains releasing fibrinopeptides A (FpA) and B (FpB), respectively, to generate fibrin monomers. Fibrin monomers polymerize spontaneously into a network of fibers which stabilizes the clot. Light scattering and electron microscopy studies have shown polymerization occurs in two stages, the formation of half-staggered and double-stranded protofibrils (Figure 2B) and the assembly of protofibrils into fibers through lateral aggregation. The final structure reflects the kinetics of these reactions, and thus varies with the concentrations of fibrinogen and thrombin.14 Fibrin polymers are stabilized by the FXIIIa-catalyzed formation of γ-glutamyl-ε-lysyl amide bonds between monomers. These bonds link γ chains to form γ-γ dimers and α chains to form α polymers. Thrombin activates the FXIII zymogen, releasing the activation peptide (AP) to form the active transglutaminase. Because thrombin activates FXIII when both proteins are bound to fibrin, the conversion of fibrinogen to fibrin and the formation of covalent bonds between monomers are temporally linked.15 Together these processes determine fibrin structure.
Protofibril formation
Biochemical data have consistently shown that protofibril formation is supported by ‘A:a’ interactions. (Figure 2B)13 Crystallography studies have identified the molecular basis for these interactions and studies with natural and recombinant fibrinogen variants with changes in knob ‘A’ or hole ‘a’ show this interaction is essential for normal polymerization.16–18 Early work indicated that the ‘B:b’ interactions support the lateral aggregation of protofibrils into fibers.19–21 Subsequent studies with snake venoms demonstrated, however, that nearly normal fibers form when only FpA is cleaved, that is, in the absence of ‘B:b’ interactions.22 Studies with variant fibrinogens indicate ‘B:b’ interactions occur alongside ‘A:a’ interactions, between strands within a protofibril.23 This suggests ‘B:b’ interactions promote protofibril formation.
Lateral aggregation
Lateral aggregation controls fiber diameter. Although recent studies indicate that ‘B:b’ interactions occur within protofibrils, rather than between protofibrils, one cannot conclude that ‘B:b’ interactions have no role in lateral aggregation. Such interactions may stabilize the protofibril intermediate, and thereby shift the reaction rate to promote lateral aggregation. Alternatively the ‘B:b’ interactions between strands may alter the outer surfaces of the protofibrils, exposing new regions that interact to promote lateral aggregation. Thus, the role of ‘B:b’ remains ill-defined. Other studies suggest the αC domains have an important role in lateral aggregation. Lateral aggregation is impaired in the presence of αC-specific antibodies.24 Moreover, electron microscopy images showed the αC domains interact with one another and the E region in fibrinogen, but extend from the trinodular structure in fibrin monomers.12 Based on these images, Gorkun et al. proposed a model where lateral association is promoted by intermolecular αC:αC interactions between protofibrils. Our recent studies with recombinant fibrinogen Aα251 are consistent with this model.25 Aα251 fibrinogen, truncated at α chain residue 251, lacks the αC regions but otherwise is identical to normal recombinant fibrinogen. Microscopy studies showed the fibers in Aα251 clots were thinner and denser, with more branch points than fibers of normal clots. These results suggest that the αC domains enhance lateral aggregation to produce thicker fibers. It is important to note, however, that fiber diameters in Aα251 clots were reduced only 25% relative to normal clots. Thus, even without the αC domains, lateral aggregation was only modestly impaired. Doolittle and colleagues have proposed a detailed model of polymerization that includes two additional molecular interactions to support lateral aggregation: one between β chains called βlat, and one between γ chains called γlat.26, 27 Further studies are clearly needed to establish whether one or more specific interactions have a critical role in lateral aggregation.
Fiber network
In addition to the fiber diameter, the morphology of the fibrin clot has been described by the size of the pores in the fiber network. Pore size has been assessed morphologically from the density of fibers observed in scanning electron microscopy (SEM) images. These images have the advantage that the heterogeneity, or homogeneity, of fiber density and pore size is obvious. A tightly woven network is easily distinguished from a loosely woven network. However, samples must be dehydrated and fixed for SEM, so there is a concern that sample preparation may alter the network morphology. Even with this disadvantage, SEM has proven a powerful technique to examine relative network structures. Pore size has been measured rheologically by permeability. Permeability assesses the average pore size in a fully hydrated clot. It is reasonable to conclude that permeability is a physiologically relevant measurement, as it measures accessibility of dissolved agents to the fiber network. For example, permeability likely determines access of lytic enzymes to the fibrin network. Both SEM and permeability studies have shown that pore size is correlated with fiber diameter: clots with thin fibers have smaller pores than clots with thick fibers. The molecular mechanisms that determine pore size are much the same as those that modulate protofibril formation and lateral aggregation. For example, increasing or decreasing thrombin concentrations leads to decreased or increased pore size, respectively.28, 29 Of particular interest, recent studies performed in the presence of cells with surface generated thrombin have shown the fibrin network structure varies with distance from the cell surface.30, 31 The densest networks are closest to the cell surface.
FXIII activation
Fibrin structure is also influenced by the covalent crosslinks introduced by FXIIIa. The significant physiological role of this transglutaminase has been made evident from epidemiological studies that link a specific FXIII polymorphism, V34L, to cardiovascular disease.32 This polymorphism influences the rate of thrombin-catalyzed activation of FXIII, and consequently the rate of crosslinking.33, 34 Moreover, if clots were prepared from plasmas from individuals of differing genotypes, the network structures were different.33 Both permeability measurements and SEM showed clots from individuals homozygous for FXIII 34Val had thicker fibers and larger pores than clots from individuals homozygous for FXIII 34Leu. Recent epidemiological studies have shown that the impact of this polymorphism is modulated by the fibrinogen concentration.35–38 For example, using data from the EPIC-Norfolk prospective population Boekholdt et al. found that the FXIII polymorphism alone was not associated with the risk of future CAD, but that a significant interaction existed between the Leu allele and fibrinogen levels.37 These studies show that the interplay between two modulators of fibrin structure, fibrinogen concentration and FXIII, markedly influences risk of thrombotic disease.
Fibrinogen variants
Fibrin structure is also influenced by genetic variations in fibrinogen. Twin and family studies have shown that both heredity and environment affect fibrinogen levels, fiber diameter and clot permeability.39 Therefore, finding a direct correlation between disease and fibrinogen genotype has been elusive. Nevertheless, biochemical studies have been performed on two polymorphisms, AαThr312Ala and BβArg448Lys, that may modulate thrombotic disease. Studies with fibrinogen purified from homozygous individuals showed the Ala312 variant clots, which had been associated with an increased risk, had increased FXIIIa crosslinking and thicker fibers, but no difference in permeability relative to the Thr312 variant clots.40 Studies with recombinant fibrinogens showed Lys448 clots, associated with an increased risk, had a tighter network with thinner fibers and smaller pores compared to Arg448 clots.41
Case control studies have linked the level of γ′ fibrinogen, independent from total fibrinogen, with thrombotic disease.42 About 10% of fibrinogen molecules contain γ′ chains, almost exclusively as γA/γ′ heterodimers. The γ′ chains are encoded by an alternatively spliced γ chain mRNA, such that the four C-terminal residues of the common form, known as γA, are replaced with a 20-residue sequence with a large negative charge. Studies with recombinant fibrinogens showed the γ′ chains altered polymerization kinetics and fibrin structure.43, 44 SEM showed that fibers in γ′/γ′ clots are thinner than γ/γ′ fibers, which are similar to γ/γ fibers. A recent review summarizes the pleiotropic effects of this variant chain.42
Fibrin stability
Clot stability is regulated by both the mechanical properties of fibers and their susceptibility to enzyme-catalyzed lysis.45 In normal hemostasis, a fibrin clot must withstand the varying pressure of pulsatile blood flow so the clot must stretch without breaking, recoil to its original shape and size, and remain in place at the site of injury. Clot retraction, which likely is necessary for vessel patency and wound healing, requires the presence of cells and depends on the mechanical properties of fibrin fibers. Vessel patency and wound healing also depend on clot degradation by enzyme-catalyzed lysis of the fibrin matrix.46 Fibrin itself regulates clot lysis because it enhances the activation of plasminogen and it complexes with α2-antiplasmin.
Mechanical properties
Recent studies have examined the molecular etiology of fibrin clot mechanics.47 These studies showed the mechanical properties of clots depend on the mechanical properties of the individual fibrin monomers. The fibrin monomer contains three features that could underlie the mechanical properties of the clot: the coiled-coil connectors, the folded globular nodules and the relatively unstructured αC regions. A role for coiled-coil connectors was identified in atomic force microscopy (AFM) studies that measured the extension of single molecules within protofibrils48 or small oligomers of fibrinogen49 as a function of the force applied. Data from more recent force-extension measurements on fibrin clots were consistent with an unfolding of the coiled-coil, suggesting this change in monomer structure is relevant within a clot.50 AFM was also used to examine the strength of the ‘A:a’ interactions that mediate protofibril formation.51 These experiments showed the γ-nodule unfolds in multiple steps prior to dissociation of the ‘A:a’ interaction, suggesting that unfolding of this globular nodule could have a role in fiber mechanics. As the properties of other elastomeric fibers derive from the straightening of unstructured polypeptides, one can infer a role for the relatively unstructured αC regions in fibrin fiber stretching. Indeed, clots made with recombinant fibrinogen lacking the αC region are less stiff and show more plastic deformation than normal clots.25 Moreover, experiments with fibrinogens from different species showed fiber extensibility correlates with the length of this unstructured region.52
It has been known for many years that FXIII-crosslinked clots are less susceptible to physical deformation than non-crosslinked clots.53 Recent studies with individual fibrin fibers indicate that partial crosslinking increases fiber extensibility and elasticity, while completely crosslinked fibers are less extensible and stiffer than uncrosslinked fibers.54, 55 Because γ-γ dimers form prior to α polymers, these data suggest crosslinks that are parallel to the fiber enhance extensibility while crosslinks that are perpendicular limit extensibility. Consequently, the mechanical stability of a clot in vivo could change with the extent of FXIII activity, which varies as described above.
Fibrinolysis
Clots are degraded by plasmin-catalyzed lysis of the fibrin matrix.46 Tissue plasminogen activator (t-PA) and plasminogen bind to the surface of a fibrin clot, where t-PA cleaves plasminogen to generate the enzyme plasmin and initiate fibrinolysis. During fibrinolysis, plasmin degrades the fibrin network by cleaving specific peptide bonds in all three chains releasing soluble fragments called fibrin degradation products. C-terminal lysine residues that are exposed as the fibrin network is degraded serve as additional binding sites for plasmin, thus enhancing the rate of fibrinolysis.
The rate of activation of plasminogen is faster in the presence of fibrin, relative to fibrinogen. This finding indicates that functional fibrin sites are generated during polymerization by changes in the conformation of monomers, by the juxtaposition of monomers in the polymer, or a combination of these two.56 Relevant sites in fibrin have been localized to segments α148 – 160 and γ311–379, and the αC domain (α392–610)57–59, supporting the importance of monomer conformation. Other experiments indicate that the juxtaposition of the D nodules of one monomer with the E nodule of a second monomer induces conformational changes to expose the relevant sites.60 Analysis of variant fibrinogens, each with a single substitution within γ316–321, did not identify residues critical for plasmin generation, but did support the conclusion that the DDE complex is critical to fibrin enhanced plasminogen activation. 61
Because pore size influences permeability and fiber density determines the concentration of bound enzymes, the fibrin network architecture regulates the distribution of lytic enzymes, and thereby the rate of clot lysis.30 Clots with a tight network (small pores and high fiber density) are lysed more slowly than clots with a loose network. Thus, the molecular mechanisms that control clot structure, as described above, modulate fibrinolysis. Of particular clinical relevance, treating fibrinogen with aspirin alters fibrin network structure in a dose dependent manner to increase permeability.62, 63 The rate of clot lysis parallels permeability; aspirin-treated clots have enhanced rates of clot lysis. As stated above, clots formed in plasmas isolated from patients with VTE, from their relatives and from normal controls show the correlation between fiber network structure and rate of lysis.5 These studies support the conclusion that a tight fiber structure would cause delayed clot lysis, leading to thrombotic disease. Of note, recent studies have shown that fibrin structure also modulates tPA-catalyzed activation of plasminogen; activation was more rapid on a tight network than on a loose network.64 These studies showed that the mechanism of fibrinolysis is complex and varies with time.
Specific peptides from fibrinogen also alter clot lysis.65 These experiments demonstrated two unexpected findings. First, addition of a 12 residue peptide from the γ-nodule, WATWKTRWYSMK, did not alter fibrin assembly or lysis but rather inhibited activation of plasminogen. Second, addition of a peptide analogous to knob ‘B’, GHRPY, altered fibrin assembly leading to an altered clot structure and delayed lysis. Addition of this peptide to preformed fibrin did not alter lysis mediated by subsequent addition of t-PA and plasminogen. The authors suggest the presence of this peptide in hole ‘b’ alters the interfaces between βC-nodules preventing the normal lateral associations that were proposed in an earlier model of fibrin formation. Experiments with a variant fibrinogen, BβD432A, indicate that ‘B:b’ interactions per se influence the rate of clot lysis. This variant cannot form ‘B:b’ interactions, but the BβD432A fibrin network measured by turbidity and SEM is indistinguishable from normal.66 Nevertheless, lysis of BβD432A fibrin is enhanced relative to normal.67
In summary, epidemiological and case control studies have shown that thrombotic disease is causally related to fibrin clot structure. The work described here provides insight into the mechanisms that control fibrin structure, which might allow development of pharmaceuticals and treatments to modulate fibrin structure in vivo and thereby prevent or limit thrombotic disease. For example, agents that impair ‘A:a’ interactions could alter clot structure and stability and likely enhance tPA-induced thrombolysis. Nevertheless, many important questions remain: what interactions mediate lateral aggregation? What interactions control activation of FXIII and plasminogen on the fibrin network? What interactions link a clot’s structure to its mechanical properties? Further studies with purified reagents, plasma clots, and in vivo models are needed to characterize the molecular pathophysiology that links thrombotic disease and clot structure.
Acknowledgements
The author’s research is supported by funding from the National Institutes of Health (HL031048) and the National Science Foundation (0705977 and 1030640)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts: None.
References
- 1.Kaptoge S, White IR, Thompson SG, Wood AM, Lewington S, Lowe GD, Danesh J. Associations of plasma fibrinogen levels with established cardiovascular disease risk factors, inflammatory markers, and other characteristics: individual participant meta-analysis of 154,211 adults in 31 prospective studies: the fibrinogen studies collaboration. Am J Epidemiol. 2007;166(8):867–879. doi: 10.1093/aje/kwm191. [DOI] [PubMed] [Google Scholar]
- 2.Fatah K, Silveira A, Tornvall P, Karpe F, Blomback M, Hamsten A. Proneness to formation of tight and rigid fibrin gel structures in men with myocardial infarction at a young age. Thromb Haemost. 1996;76(4):535–540. [PubMed] [Google Scholar]
- 3.Collet JP, Allali Y, Lesty C, Tanguy ML, Silvain J, Ankri A, Blanchet B, Dumaine R, Gianetti J, Payot L, Weisel JW, Montalescot G. Altered fibrin architecture is associated with hypofibrinolysis and premature coronary atherothrombosis. Arterioscler Thromb Vasc Biol. 2006;26(11):2567–2573. doi: 10.1161/01.ATV.0000241589.52950.4c. [DOI] [PubMed] [Google Scholar]
- 4.Mills JD, Ariens RA, Mansfield MW, Grant PJ. Altered fibrin clot structure in the healthy relatives of patients with premature coronary artery disease. Circulation. 2002;106(15):1938–1942. doi: 10.1161/01.cir.0000033221.73082.06. [DOI] [PubMed] [Google Scholar]
- 5.Undas A, Zawilska K, Ciesla-Dul M, Lehmann-Kopydlowska A, Skubiszak A, Ciepluch K, Tracz W. Altered fibrin clot structure/function in patients with idiopathic venous thromboembolism and in their relatives. Blood. 2009;114(19):4272–4278. doi: 10.1182/blood-2009-05-222380. [DOI] [PubMed] [Google Scholar]
- 6.Weisel JW, Stauffacher CV, Bullitt E, Cohen C. A model for fibrinogen: domains and sequence. Science. 1985;230(4732):1388–1391. doi: 10.1126/science.4071058. [DOI] [PubMed] [Google Scholar]
- 7.Kollman JM, Pandi L, Sawaya MR, Riley M, Doolittle RF. Crystal structure of human fibrinogen. Biochemistry. 2009;48(18):3877–3886. doi: 10.1021/bi802205g. [DOI] [PubMed] [Google Scholar]
- 8.Doolittle RF. X-ray crystallographic studies on fibrinogen and fibrin. J Thromb Haemost. 2003;1(7):1559–1565. doi: 10.1046/j.1538-7836.2003.00278.x. [DOI] [PubMed] [Google Scholar]
- 9.Kostelansky MS, Betts L, Gorkun OV, Lord ST. 2.8 A crystal structures of recombinant fibrinogen fragment D with and without two peptide ligands: GHRP binding to the "b" site disrupts its nearby calcium-binding site. Biochemistry. 2002;41(40):12124–12132. doi: 10.1021/bi0261894. [DOI] [PubMed] [Google Scholar]
- 10.Pechik I, Madrazo J, Mosesson MW, Hernandez I, Gilliland GL, Medved L. Crystal structure of the complex between thrombin and the central "E" region of fibrin. Proc Natl Acad Sci U S A. 2004;101(9):2718–2723. doi: 10.1073/pnas.0303440101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burton RA, Tsurupa G, Hantgan RR, Tjandra N, Medved L. NMR solution structure, stability, and interaction of the recombinant bovine fibrinogen alphaC-domain fragment. Biochemistry. 2007;46(29):8550–8560. doi: 10.1021/bi700606v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorkun OV, Veklich YI, Medved LV, Henschen AH, Weisel JW. Role of the alpha C domains of fibrin in clot formation. Biochemistry. 1994;33(22):6986–6997. doi: 10.1021/bi00188a031. [DOI] [PubMed] [Google Scholar]
- 13.Lord ST. Fibrinogen and fibrin: scaffold proteins in hemostasis. Curr Opin Hematol. 2007;14(3):236–241. doi: 10.1097/MOH.0b013e3280dce58c. [DOI] [PubMed] [Google Scholar]
- 14.Weisel JW, Nagaswami C. Computer modeling of fibrin polymerization kinetics correlated with electron microscope and turbidity observations: clot structure and assembly are kinetically controlled. Biophys J. 1992;63(1):111–128. doi: 10.1016/S0006-3495(92)81594-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janus TJ, Lewis SD, Lorand L, Shafer JA. Promotion of thrombin-catalyzed activation of factor XIII by fibrinogen. Biochemistry. 1983;22(26):6269–6272. doi: 10.1021/bi00295a035. [DOI] [PubMed] [Google Scholar]
- 16.Spraggon G, Everse SJ, Doolittle RF. Crystal Structures of Fragment D From Human Fibrinogen and Its Crosslinked Counterpart From Fibrin. Nature. 1997;389(6650):455–462. doi: 10.1038/38947. [DOI] [PubMed] [Google Scholar]
- 17.Bowley SR, Merenbloom BK, Okumura N, Betts L, Heroux A, Gorkun OV, Lord ST. Polymerization-defective fibrinogen variant gammaD364A binds knob "A" peptide mimic. Biochemistry. 2008;47(33):8607–8613. doi: 10.1021/bi8000769. [DOI] [PubMed] [Google Scholar]
- 18.Kudryk B, Blomback B, Blomback M. Fibrinogen Detroit - an abnormal fibrinogen with non-functinal NH2-terminal polymerization domain. Thrombosis Research. 1976;9(1):25–36. doi: 10.1016/0049-3848(76)90146-8. [DOI] [PubMed] [Google Scholar]
- 19.Blomback B, Hessel B, Hogg D, Therkildsen L. A two-step fibrinogen--fibrin transition in blood coagulation. Nature. 1978;275(5680):501–505. doi: 10.1038/275501a0. [DOI] [PubMed] [Google Scholar]
- 20.Budzynski AZ, Olexa SA, Pandya BV. Fibrin polymerization sites in fibrinogen and fibrin fragments. Ann N Y Acad Sci. 1983;408:301–314. doi: 10.1111/j.1749-6632.1983.tb23253.x. [DOI] [PubMed] [Google Scholar]
- 21.Laudano AP, Doolittle RF. Studies on synthetic peptides that bind to fibrinogen and prevent fibrin polymerization. Structural requirements, number of binding sites, and species differences. Biochemistry. 1980;19(5):1013–1019. doi: 10.1021/bi00546a028. [DOI] [PubMed] [Google Scholar]
- 22.Moen JL, Gorkun OV, Weisel JW, Lord ST. Recombinant BbetaArg14His fibrinogen implies participation of N-terminus of Bbeta chain in desA fibrin polymerization. Blood. 2003;102(7):2466–2471. doi: 10.1182/blood-2003-01-0204. [DOI] [PubMed] [Google Scholar]
- 23.Okumura N, Terasawa F, Haneishi A, Fujihara N, Hirota-Kawadobora M, Yamauchi K, Ota H, Lord ST. B:b interactions are essential for polymerization of variant fibrinogens with impaired holes 'a'. J Thromb Haemost. 2007;5(12):2352–2359. doi: 10.1111/j.1538-7836.2007.02793.x. [DOI] [PubMed] [Google Scholar]
- 24.Cierniewski CS, Budzynski AZ. Involvement of the alpha chain in fibrin clot formation. Effect of monoclonal antibodies. Biochemistry. 1992;31(17):4248–4253. doi: 10.1021/bi00132a014. [DOI] [PubMed] [Google Scholar]
- 25.Collet JP, Moen JL, Veklich YI, Gorkun OV, Lord ST, Montalescot G, Weisel JW. The alphaC domains of fibrinogen affect the structure of the fibrin clot, its physical properties, and its susceptibility to fibrinolysis. Blood. 2005;106(12):3824–3830. doi: 10.1182/blood-2005-05-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Z, Mochalkin I, Doolittle RF. A model of fibrin formation based on crystal structures of fibrinogen and fibrin fragments complexed with synthetic peptides. Proc Natl Acad Sci U S A. 2000;97(26):14156–14161. doi: 10.1073/pnas.97.26.14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doolittle RF. Structural basis of the fibrinogen-fibrin transformation: contributions from X-ray crystallography. Blood Rev. 2003;17(1):33–41. doi: 10.1016/s0268-960x(02)00060-7. [DOI] [PubMed] [Google Scholar]
- 28.Wolberg AS, Monroe DM, Roberts HR, Hoffman M. Elevated prothrombin results in clots with an altered fiber structure: a possible mechanism of the increased thrombotic risk. Blood. 2003;101(8):3008–3013. doi: 10.1182/blood-2002-08-2527. [DOI] [PubMed] [Google Scholar]
- 29.He S, Blomback M, Bark N, Johnsson H, Wallen NH. The direct thrombin inhibitors (argatroban, bivalirudin and lepirudin) and the indirect Xa-inhibitor (danaparoid) increase fibrin network porosity and thus facilitate fibrinolysis. Thromb Haemost. 103(5):1076–1084. doi: 10.1160/TH09-05-0306. [DOI] [PubMed] [Google Scholar]
- 30.Weisel JW, Litvinov RI. The biochemical and physical process of fibrinolysis and effects of clot structure and stability on the lysis rate. Cardiovasc Hematol Agents Med Chem. 2008;6(3):161–180. doi: 10.2174/187152508784871963. [DOI] [PubMed] [Google Scholar]
- 31.Campbell RA, Overmyer KA, Selzman CH, Sheridan BC, Wolberg AS. Contributions of extravascular and intravascular cells to fibrin network formation, structure, and stability. Blood. 2009;114(23):4886–4896. doi: 10.1182/blood-2009-06-228940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Hylckama Vlieg A, Komanasin N, Ariens RA, Poort SR, Grant PJ, Bertina RM, Rosendaal FR, Factor XIII. Val34Leu polymorphism, factor XIII antigen levels and activity and the risk of deep venous thrombosis. Br J Haematol. 2002;119(1):169–175. doi: 10.1046/j.1365-2141.2002.03797.x. [DOI] [PubMed] [Google Scholar]
- 33.Ariens RA, Philippou H, Nagaswami C, Weisel JW, Lane DA, Grant PJ. The factor XIII V34L polymorphism accelerates thrombin activation of factor XIII and affects cross-linked fibrin structure. Blood. 2000;96(3):988–995. [PubMed] [Google Scholar]
- 34.Trumbo TA, Maurer MC. Examining thrombin hydrolysis of the factor XIII activation peptide segment leads to a proposal for explaining the cardioprotective effects observed with the factor XIII V34L mutation. J Biol Chem. 2000;275(27):20627–20631. doi: 10.1074/jbc.M000209200. [DOI] [PubMed] [Google Scholar]
- 35.Vossen CY, Rosendaal FR. The protective effect of the factor XIII Val34Leu mutation on the risk of deep venous thrombosis is dependent on the fibrinogen level. J Thromb Haemost. 2005;3(5):1102–1103. doi: 10.1111/j.1538-7836.2005.01312.x. [DOI] [PubMed] [Google Scholar]
- 36.Lim BC, Ariens RA, Carter AM, Weisel JW, Grant PJ. Genetic regulation of fibrin structure and function: complex gene-environment interactions may modulate vascular risk. Lancet. 2003;361(9367):1424–1431. doi: 10.1016/S0140-6736(03)13135-2. [DOI] [PubMed] [Google Scholar]
- 37.Boekholdt SM, Sandhu MS, Wareham NJ, Luben R, Reitsma PH, Khaw KT. Fibrinogen plasma levels modify the association between the factor XIII Val34Leu variant and risk of coronary artery disease: the EPIC-Norfolk prospective population study. J Thromb Haemost. 2006;4(10):2204–2209. doi: 10.1111/j.1538-7836.2006.02154.x. [DOI] [PubMed] [Google Scholar]
- 38.Gonzalez-Conejero R, Fernandez-Cadenas I, Iniesta JA, Marti-Fabregas J, Obach V, Alvarez-Sabin J, Vicente V, Corral J, Montaner J. Role of fibrinogen levels and factor XIII V34L polymorphism in thrombolytic therapy in stroke patients. Stroke. 2006;37(9):2288–2293. doi: 10.1161/01.STR.0000236636.39235.4f. [DOI] [PubMed] [Google Scholar]
- 39.Standeven KF, Uitte de Willige S, Carter AM, Grant PJ. Heritability of clot formation. Semin Thromb Hemost. 2009;35(5):458–467. doi: 10.1055/s-0029-1234141. [DOI] [PubMed] [Google Scholar]
- 40.Standeven KF, Grant PJ, Carter AM, Scheiner T, Weisel JW, Ariens RA. Functional analysis of the fibrinogen Aalpha Thr312Ala polymorphism: effects on fibrin structure and function. Circulation. 2003;107(18):2326–2330. doi: 10.1161/01.CIR.0000066690.89407.CE. [DOI] [PubMed] [Google Scholar]
- 41.Ajjan R, Lim BC, Standeven KF, Harrand R, Dolling S, Phoenix F, Greaves R, Abou-Saleh RH, Connell S, Smith DA, Weisel JW, Grant PJ, Ariens RA. Common variation in the C-terminal region of the fibrinogen beta-chain: effects on fibrin structure, fibrinolysis and clot rigidity. Blood. 2008;111(2):643–650. doi: 10.1182/blood-2007-05-091231. [DOI] [PubMed] [Google Scholar]
- 42.Uitte de Willige S, Standeven KF, Philippou H, Ariens RA. The pleiotropic role of the fibrinogen gamma' chain in hemostasis. Blood. 2009;114(19):3994–4001. doi: 10.1182/blood-2009-05-217968. [DOI] [PubMed] [Google Scholar]
- 43.Cooper AV, Standeven KF, Ariens RA. Fibrinogen gamma-chain splice variant gamma' alters fibrin formation and structure. Blood. 2003;102(2):535–540. doi: 10.1182/blood-2002-10-3150. [DOI] [PubMed] [Google Scholar]
- 44.Gersh KC, Nagaswami C, Weisel JW, Lord ST. The presence of gamma' chain impairs fibrin polymerization. Thromb Res. 2009;124(3):356–363. doi: 10.1016/j.thromres.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weisel JW. Structure of fibrin: impact on clot stability. J Thromb Haemost. 2007;5 Suppl 1:116–124. doi: 10.1111/j.1538-7836.2007.02504.x. [DOI] [PubMed] [Google Scholar]
- 46.Rijken DC, Lijnen HR. New insights into the molecular mechanisms of the fibrinolytic system. J Thromb Haemost. 2009;7(1):4–13. doi: 10.1111/j.1538-7836.2008.03220.x. [DOI] [PubMed] [Google Scholar]
- 47.Falvo MR, Gorkun OV, Lord ST. The molecular origins of the mechanical properties of fibrin. Biophys Chem. 152(1–3):15–20. doi: 10.1016/j.bpc.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lim BB, Lee EH, Sotomayor M, Schulten K. Molecular basis of fibrin clot elasticity. Structure. 2008;16(3):449–459. doi: 10.1016/j.str.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 49.Brown AE, Litvinov RI, Discher DE, Weisel JW. Forced unfolding of coiled-coils in fibrinogen by single-molecule AFM. Biophys J. 2007;92(5):L39–L41. doi: 10.1529/biophysj.106.101261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brown AE, Litvinov RI, Discher DE, Purohit PK, Weisel JW. Multiscale mechanics of fibrin polymer: gel stretching with protein unfolding and loss of water. Science. 2009;325(5941):741–744. doi: 10.1126/science.1172484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Averett LE, Geer CB, Fuierer RR, Akhremitchev BB, Gorkun OV, Schoenfisch MH. Complexity of "A-a" knob-hole fibrin interaction revealed by atomic force spectroscopy. Langmuir. 2008;24(9):4979–4988. doi: 10.1021/la703264x. [DOI] [PubMed] [Google Scholar]
- 52.Falvo MR, Millard D, O'Brien ET, 3rd, Superfine R, Lord ST. Length of tandem repeats in fibrin's alphaC region correlates with fiber extensibility. J Thromb Haemost. 2008;6(11):1991–1993. doi: 10.1111/j.1538-7836.2008.03147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weisel JW. The mechanical properties of fibrin for basic scientists and clinicians. Biophys Chem. 2004;112(2–3):267–276. doi: 10.1016/j.bpc.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 54.Liu W, Carlisle CR, Sparks EA, Guthold M. The mechanical properties of single fibrin fibers. J Thromb Haemost. 8(5):1030–1036. doi: 10.1111/j.1538-7836.2010.03745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Houser JR, Hudson NE, Ping L, O'Brien ET, 3rd, Superfine R, Lord ST, Falvo MR. Evidence that alphaC region is origin of low modulus, high extensibility, and strain stiffening in fibrin fibers. Biophys J. 99(9):3038–3047. doi: 10.1016/j.bpj.2010.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Doolittle RF. Searching for differences between fibrinogen and fibrin that affect the initiation of fibrinolysis. Cardiovasc Hematol Agents Med Chem. 2008;6(3):181–189. doi: 10.2174/187152508784871954. [DOI] [PubMed] [Google Scholar]
- 57.Nieuwenhuizen W, Vermond A, Voskuilen M, Traas DW, Verheijen JH. Identification of a site in fibrin(ogen) which is involved in the acceleration of plasminogen activation by tissue-type plasminogen activator. Biochimica et Biophysica Acta. 1983;748(1):86–92. doi: 10.1016/0167-4838(83)90030-4. [DOI] [PubMed] [Google Scholar]
- 58.Schielen WJ, Adams HP, van Leuven K, Voskuilen M, Tesser GI, Nieuwenhuizen W. The sequence gamma-(312–324) is a fibrin-specific epitope. Blood. 1991;77(10):2169–2173. [PubMed] [Google Scholar]
- 59.Medved L, Nieuwenhuizen W. Molecular mechanisms of initiation of fibrinolysis by fibrin. Thromb Haemost. 2003;89(3):409–419. [PubMed] [Google Scholar]
- 60.Yakovlev S, Makogonenko E, Kurochkina N, Nieuwenhuizen W, Ingham K, Medved L. Conversion of Fibrinogen to Fibrin: Mechanism of Exposure of tPA- and Plasminogen-Binding Sites. Biochemistry. 2000;39(51):15730–15741. doi: 10.1021/bi001847a. [DOI] [PubMed] [Google Scholar]
- 61.Wilhelm SE, Lounes KC, Lord ST. Investigation of residues in the fibrin(ogen) gamma chain involved in tissue plasminogen activator binding and plasminogen activation. Blood Coagul Fibrinolysis. 2004;15(6):451–461. doi: 10.1097/00001721-200408000-00003. [DOI] [PubMed] [Google Scholar]
- 62.Ajjan RA, Standeven KF, Khanbhai M, Phoenix F, Gersh KC, Weisel JW, Kearney MT, Ariens RA, Grant PJ. Effects of aspirin on clot structure and fibrinolysis using a novel in vitro cellular system. Arterioscler Thromb Vasc Biol. 2009;29(5):712–717. doi: 10.1161/ATVBAHA.109.183707. [DOI] [PubMed] [Google Scholar]
- 63.He S, Bark N, Wang H, Svensson J, Blomback M. Effects of acetylsalicylic acid on increase of fibrin network porosity and the consequent upregulation of fibrinolysis. J Cardiovasc Pharmacol. 2009;53(1):24–29. doi: 10.1097/FJC.0b013e3181953e0f. [DOI] [PubMed] [Google Scholar]
- 64.Longstaff C, Thelwell C, Williams SC, Silva MM, Szabo L, Kolev K. The interplay between tissue plasminogen activator domains and fibrin structures in the regulation of fibrinolysis: kinetic and microscopic studies. Blood. doi: 10.1182/blood-2010-06-290338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pandi L, Kollman JM, Lopez-Lira F, Burrows JM, Riley M, Doolittle RF. Two families of synthetic peptides that enhance fibrin turbidity and delay fibrinolysis by different mechanisms. Biochemistry. 2009;48(30):7201–7208. doi: 10.1021/bi900647g. [DOI] [PubMed] [Google Scholar]
- 66.Bowley SR, Lord ST. Fibrinogen variant BbetaD432A has normal polymerization but does not bind knob "B". Blood. 2009;113(18):4425–4430. doi: 10.1182/blood-2008-09-178178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Holliday BA, Bowley SR, Lord ST. Plasmin-catalyzed lysis is enhanced in fibrin variants with weakened hole 'b'. J Thromb Haemost. 2009;7 Abstract #: OC-MO-117. [Google Scholar]
- 68.Medved L, Weisel JW. Recommendations for nomenclature on fibrinogen and fibrin. J Thromb Haemost. 2009;7(2):355–359. doi: 10.1111/j.1538-7836.2008.03242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]