Abstract
BACKGROUND
Lung cancer is the leading cause of cancer death among US Asian/Pacific Islander (API) and Latina women, despite low smoking prevalence. This study examined survival patterns following non-small cell lung cancer in a population-based sample of lung cancer cases from the San Francisco Bay Area Lung Cancer Study (SFBALCS).
METHODS
Women diagnosed with lung cancer from 1998–2003 and 2005–2008 and identified through the Greater Bay Area Cancer Registry were telephone-screened for eligibility for the SFBALCS. The screener data were linked to the cancer registry data to determine follow-up. This analysis included 187 non-Hispanic White, 23 US-born Latina, 32 foreign-born Latina, 30 US-born API, and 190 foreign-born API never smokers diagnosed with lung cancer and followed through 2008.
RESULTS
All-cause survival was poorer among APIs (hazard ratio (HR) and 95% confidence interval (CI) = 1.7 (1.0–2.8) among US-born APIs; 1.2 (0.9–1.5) among foreign-born APIs), and Latinas (HR (95% CI) = 2.1 (1.2–3.6) among US-born Latinas; 1.4 (0.9–2.3) among foreign-born Latinas), relative to non-Hispanic Whites. These survival differences were not explained by differences in selected sociodemographic or clinical factors.
CONCLUSIONS
Further research should focus on factors such as cultural behaviors, access to or attitudes toward health care, and genetic variations, as possible explanations for these striking racial/ethnic differences.
IMPACT
Latina and API female never smokers diagnosed with lung cancer were up to two-times more likely to die than non-Hispanic Whites, highlighting the need for additional research to identify the underlying reasons for the disparities, as well as heightened clinical awareness.
Keywords: lung cancer survival, Asian, Latina, Hispanic, never smokers, nativity
Lung cancer is the leading cause of cancer death both worldwide (1) and in the United States (US) (2). Among US Asian/Pacific Islander (API) and Latina women, lung cancer is also the leading cause of cancer death; however, this disease burden is particularly striking in light of the fact that more than 80% of API and Latina women have never smoked cigarettes (3).
Even though trends in lung cancer incidence are predominantly dictated by patterns of tobacco smoking, there remains a substantial proportion of non-smoking-associated lung cancer that is not attributable to either firsthand or secondhand smoke exposure (4–6). Other possible risk factors for lung cancer include exposure to cooking fumes, radon, arsenic, heavy metals, certain viruses, steroid hormones, and genetic susceptibility, although few of these have been confirmed (4–6). An estimated 20% of lung cancer in US women now occurs among never-smokers (7). However, in Asia, this proportion is as high as 80% (8), and the same may be true of US Asian women (9). The exceptionally high burden of non-smoking-associated lung cancer among US Asian and possibly also Latina women is a health disparity that has received little attention. In particular, few studies of lung cancer survival have paid notice to this sizeable group of patients, in whom survival patterns, as well as clinicopathological characteristics (6), may differ from those in ever smokers.
Therefore, given the predominance of lung cancer among Asian and Latina women who have never smoked, we focused this study on better understanding the survival patterns in this specific group. Taking advantage of the availability of patient-reported data on smoking status and secondhand-smoke exposure, this study examined the impact of demographic and clinical factors on survival patterns following a first primary diagnosis of non-small cell lung cancer (NSCLC) in a population-based sample of lung cancer cases from the San Francisco Bay Area Lung Cancer Study (SFBALCS).
METHODS
Cancer cases
Lung cancer cases included in this study were from the SFBALCS, a population-based case-control study for which the original aim was to examine molecular, behavioral, and occupational factors in lung cancer etiology (10). The present study incorporates information from the screening interviews conducted with newly diagnosed lung cancer cases (ICD-O-3 C340-C349) living in Alameda, San Francisco, Contra Costa, Santa Clara, or San Mateo counties, diagnosed between September 1998-March 2003 (phase I) or July 2005-March 2008 (phase II), and identified through rapid case ascertainment from the Greater Bay Area Cancer Registry, in addition to Alta Bates/Summit Hospital in phase I and Northern California Kaiser Permanente Medical Group in phase II. Because race/ethnicity information is generally not available through pathology records, the primary source for rapid case ascertainment, all cases potentially eligible for the SFBALCS, regardless of race/ethnicity, were contacted by English- and Spanish-speaking interviewers and screened for eligibility on average 4.4 months following diagnosis. For those who were deceased, too ill or unable to complete the screener, interviewers attempted to conduct the interview with a proxy. The original SFBALCS was approved by the University of California, San Francisco IRB. The present study was approved by the Stanford Medical Center and Cancer Prevention Institute of California IRBs. Informed consent was obtained from the patients.
A total of 8414 cases from phase I and 4580 cases from phase II were identified for screening. Of these, 51.5% of females and 48.8% of males completed a screening interview (6499 cases). Approximately 5% of cases did not complete a screening interview because they were deceased at the time of contact and an interview could not be conducted with a proxy. 788 were excluded because they were subsequently determined to be ineligible. Of 2864 female cases, 504 were never smokers, defined on the basis of self-reported response to the question: “Have you ever smoked cigarettes, pipes, or cigars?” For the present study, focused on specific ethnic populations, we included only those lifetime non-smoker women who self-identified as API, Latina, or non-Hispanic (NH) White, for a total of 472 cases. We excluded one case with missing month and day of diagnosis and nine cases diagnosed with small-cell lung cancer, for a final sample of 462. We did not include men with lung cancer as this study specifically focused on women and because of the low proportion of male cases (14% of APIs, 7% of Latinos, and 5% of NH Whites) who were never-smokers. Among APIs, 99 (45%) were Chinese, 67 (30.5%) were Filipina, 10 (4.6%) were Japanese, and 44 (20%) were Pacific Islanders or another Asian ethnic group (not specified).
Analytic variables
Patient clinical and sociodemographic factors
Data on patient factors were collected from either the screening interview or the cancer registry. The screening interview included questions on date of birth, place of birth, race/ethnicity, birthplace and race/ethnicity of mother and father, number of people living in the household, education, usual occupation, health insurance, exposure to household and workplace secondhand smoke, consumption of selected vegetables and fruits, and first-degree family history of lung cancer. Place of birth was used to assign immigrant status as US- or foreign-born. 38% of the screening interviews were conducted with a proxy. Patient and clinical data from the cancer registry included year of diagnosis, age, marital status, residential address at diagnosis, American Joint Committee on Cancer stage 3rd edition (I, II, III, IV, unknown), histologic subtype (bronchioloalveolar carcinoma (BAC), squamous cell, large cell, non-BAC adenocarcinoma, or undifferentiated), first course of treatment (i.e., treatment administered within approximately four months following initial diagnosis and including surgical resection, radiation, and chemotherapy), and the hospital that first reported the case to the cancer registry (usually the diagnosing hospital).
Neighborhood and institutional factors
Patients’ addresses at diagnosis were geocoded to a latitude and longitude for 97.4% of cases; the remaining 2.6% were randomly assigned to a 2000 Census block group value within a ZIP code. Neighborhood socioeconomic status (SES) was then determined using US Census 2000 block-group data, as previously described (11). Neighborhood SES was classified into quintiles based on the distribution of the SES index across the state of California, then re-categorized into two groups because of small sample sizes in the quintiles: lower SES (quintiles 1–4) or high SES (quintile 5).
Hospital utilization data from California’s Office of Statewide Health and Policy Development (12) was used to determine the number of beds (as proxy for size) and ownership status of each hospital. We also classified hospitals as teaching or non-teaching, based on affiliation with a university medical school. Using cancer registry data, we computed measures of the percentage of Latino or API cancer patients in each hospital for the same years of diagnoses as the cases in this case series; these characteristics were classified independently of hospital size, ownership status, and teaching status.
Follow-up data
Cancer registry data are routinely linked to death certificate data. Cause of death was ascertained from the underlying cause of death on the death certificate based on International Classification of Diseases (ICD)-9 (162.2–162.9) or ICD-10 (C34) codes. Survival time was calculated in months from the date of diagnosis to: 1) date of death from any cause (for overall survival), 2) date of last known contact, or 3) December 31, 2008 (the end of the study period), whichever occurred earliest. Uncoded cause of death was more common in recent years, and reflects cancer registry practices in that the patient is known to be deceased, but the cause of death has not been determined because her record could not yet be linked to death records, such as state vital statistics data or the National Death Index. These causes of death could be updated with future linkages. As a result, more recent deaths (and thus, more recent diagnoses) are more likely to be coded as uncoded cause of death and less likely to be coded as lung-cancer deaths. For cases diagnosed between 1988–2004, 77% of the deaths were due to lung cancer, 18% other causes, and 4.7% not coded. In contrast, for cases diagnosed between 2005–2008, 50% were due to lung cancer, 7% other causes, and 43% not coded. Thus, we have focused our analyses on all-causes of death.
Statistical analyses
Multivariate Cox proportional hazards models with separate baseline hazards by stage (stage grouped as shown in Table 1) at diagnosis (thereby adjusting for stage by allowing the form of the underlying hazard function to vary by stage) were used to estimate hazard ratios (HR) and 95% confidence intervals (CI) for all-cause specific mortality. The proportional hazards assumption was assessed by visual inspection of the survival curves (log (−log) of the survival distribution function by log (months)) and by tests for time-dependency. Because associations with chemotherapy varied over time (i.e., violated the proportional hazards assumption), we included interactions between time and chemotherapy. All statistical tests were two-sided with significance assumed for P value < .05, and all analyses were performed with SAS software version 9.1.3. Table cells with fewer than five cases are not shown to protect patient confidentiality.
Table 1.
Characteristics of female never smoker non-small cell lung cancer non-Hispanic White, Latina, and Asian/Pacific Islander (API) patients diagnosed 1998–2003 and 2005–2008, by race/ethnicity and immigration status, San Francisco Bay Area Lung Cancer Study (N = 462)
| Characteristics | Non-Hisp White (N=187) |
Latina US-born (N=23) |
Latina foreign-born (N=32) |
API US-born (N=30) |
API foreign-born (N=190) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | |
| Year of diagnosis | ||||||||||
| 1998–2003 | 119 | 63.6 | 10 | 43.5 | 26 | 81.3 | 23 | 76.7 | 126 | 66.3 |
| 2005–2008 | 68 | 36.4 | 13 | 56.5 | 6 | 18.8 | 7 | 23.3 | 64 | 33.7 |
| Age at diagnosis | ||||||||||
| Mean (std dev) | 70.2 | (13.1) | 65.6 | (11.7) | 64.4 | (14.6) | 70.0 | (11.7) | 66.7 | (13.6) |
| < 70 | 78 | 41.7 | 12 | 52.2 | 20 | 62.5 | 12 | 40.0 | 107 | 56.3 |
| 70+ | 109 | 58.3 | 11 | 47.8 | 12 | 37.5 | 18 | 60.0 | 83 | 43.7 |
| Education** | ||||||||||
| ≤ High school | 84 | 45.2 | 13 | 56.5 | 23 | 74.2 | 12 | 42.9 | 99 | 54.1 |
| Some college or more | 102 | 54.8 | 10 | 43.5 | 8 | 25.8 | 16 | 57.1 | 84 | 45.9 |
| Unknown/(missing (n=11)) | ||||||||||
| Neighborhood socioeconomic status1 | ||||||||||
| Lower (statewide quintiles 1–4) | 67 | 35.8 | 9 | 39.1 | 25 | 78.1 | 9 | 30.0 | 104 | 54.7 |
| High (statewide quintile 5) | 120 | 64.2 | 14 | 60.9 | 7 | 21.9 | 21 | 70.0 | 86 | 45.3 |
| Marital status** | ||||||||||
| Never Married | 15 | 8.0 | 5 | 21.7 | 9 | 28.1 | -3 | - | 19 | 10.0 |
| Married | 100 | 53.5 | 10 | 43.5 | 9 | 28.1 | 18 | 60.0 | 111 | 58.4 |
| Sep/div/wid/(unknown (n=8))2 | 72 | 38.5 | 8 | 34.8 | 14 | 43.8 | 10 | 33.3 | 60 | 31.6 |
| Type of health insurance** | ||||||||||
| Public | 20 | 11.1 | - | - | 10 | 38.5 | 6 | 20.7 | 73 | 43.5 |
| Private | 99 | 54.7 | 10 | 43.5 | 15 | 57.7 | 12 | 41.4 | 74 | 44.0 |
| Both | 62 | 34.3 | 10 | 43.5 | - | - | 11 | 37.9 | 21 | 12.5 |
| No insurance/unknown/(missing (n=35)) | ||||||||||
| 1st degree4 relative w/cancer** | ||||||||||
| None | 70 | 38.0 | 10 | 43.5 | 22 | 73.3 | 12 | 42.9 | 106 | 58.2 |
| Any | 114 | 62.0 | 13 | 56.5 | 8 | 26.7 | 16 | 57.1 | 76 | 41.8 |
| Unknown/(missing (n=15)) | ||||||||||
| Smoke exposure at home (years w/another smoker)5 | ||||||||||
| None | 45 | 24.9 | 5 | 22.7 | 11 | 37.9 | 8 | 29.6 | 68 | 39.1 |
| 1–20 | 67 | 37.0 | 10 | 45.4 | 10 | 34.4 | 12 | 44.4 | 71 | 40.8 |
| 21+ | 69 | 38.1 | 7 | 31.8 | 8 | 27.6 | 7 | 25.9 | 35 | 20.1 |
| Unknown/(missing (n=29)) | ||||||||||
| Smoke exposure at work6 | ||||||||||
| None | 96 | 61.9 | 9 | 50.0 | 21 | 77.8 | 7 | 33.3 | 98 | 64.1 |
| Any | 59 | 38.1 | 9 | 50.0 | 6 | 22.2 | 14 | 66.7 | 55 | 36.0 |
| Unknown/(missing (n=88)) | ||||||||||
| AJCC stage at diagnosis | ||||||||||
| I/II | 67 | 35.8 | 6 | 26.1 | 6 | 18.8 | 13 | 43.3 | 47 | 24.7 |
| III | 43 | 23.0 | - | - | 13 | 40.6 | 5 | 16.7 | 41 | 21.6 |
| IV/(unstaged (n=19)) | 77 | 41.2 | 13 | 56.5 | 13 | 40.6 | 12 | 40.0 | 102 | 53.7 |
| Histological subtype | ||||||||||
| Large cell/non-BAC7 adenocarcinoma/undiff | 141 | 75.4 | 21 | 91.3 | 26 | 81.3 | 24 | 80.0 | 166 | 87.4 |
| Squamous cell | 13 | 7.0 | - | - | - | - | - | - | 6 | 3.2 |
| BAC | 33 | 17.7 | 18 | 9.5 | ||||||
| Chemotherapy | ||||||||||
| None/(unknown (n=12)) | 115 | 61.5 | 11 | 47.8 | 20 | 62.5 | 20 | 66.7 | 97 | 51.1 |
| Given | 72 | 28.5 | 12 | 52.2 | 12 | 37.5 | 10 | 33.3 | 93 | 49.0 |
| Radiation | ||||||||||
| None/(unknown (n=1)) | 122 | 65.2 | 17 | 73.9 | 19 | 59.4 | 19 | 63.3 | 117 | 61.6 |
| Given | 65 | 34.8 | 6 | 26.1 | 13 | 40.6 | 11 | 36.7 | 73 | 38.4 |
| Surgery to primary site | ||||||||||
| None/(unknown (n=1)) | 108 | 57.8 | 17 | 73.9 | 25 | 78.1 | 15 | 50.0 | 133 | 70.0 |
| Performed | 79 | 42.3 | 6 | 26.1 | 7 | 21.9 | 15 | 50.0 | 57 | 30.0 |
| Hospital ownership** | ||||||||||
| Public | 75 | 41.4 | 10 | 43.5 | 17 | 53.1 | 15 | 50.0 | 113 | 61.1 |
| Private | 106 | 58.6 | 13 | 56.5 | 15 | 46.9 | 15 | 50.0 | 72 | 39.0 |
| Missing (n=11) | ||||||||||
| Hospital teaching status** | ||||||||||
| Non-teaching | 164 | 87.7 | - | - | - | - | 24 | 80.0 | 131 | 69.0 |
| Teaching | 23 | 12.3 | 6 | 20.0 | 59 | 31.1 | ||||
| Hospital total beds | ||||||||||
| 0–299 | 108 | 59.7 | 14 | 60.9 | 20 | 62.5 | 12. | 40.0 | 72 | 38.9 |
| 300+ | 73 | 40.3 | 9 | 39.1 | 12 | 37.5 | 18 | 60.0 | 113 | 61.1 |
| Missing (n=11) | ||||||||||
| ≥10% cancer patients seen at hospital are Latino** | ||||||||||
| Yes | 63 | 33.7 | 13 | 56.5 | 22 | 68.8 | 13 | 43.3 | 100 | 52.6 |
| No | 124 | 66.3 | 10 | 43.5 | 10 | 31.3 | 17 | 56.7 | 90 | 47.4 |
| ≥10% cancer patients seen at hospital are API** | ||||||||||
| Yes | 112 | 59.9 | - | - | 26 | 81.3 | 22 | 73.3 | 166 | 87.4 |
| No | 75 | 40.1 | 6 | 18.8 | 8 | 26.7 | 24 | 12.6 | ||
| Proxy interview | ||||||||||
| No | 143 | 76.5 | 19 | 82.6 | 16 | 50.0 | 19 | 63.3 | 90 | 47.4 |
| Yes | 44 | 23.5 | - | 16 | 50.0 | 11 | 36.7 | 100 | 52.6 | |
| Mortality | ||||||||||
| Deceased | 111 | 59.4 | 16 | 69.6 | 20 | 62.5 | 20 | 66.7 | 120 | 63.2 |
| Lung cancer death | 76 | 40.6 | 11 | 47.8 | 17 | 53.1 | 17 | 56.7 | 88 | 53.7 |
| Cause of death (% of deaths) | ||||||||||
| Lung cancer | 76 | 67.3 | 11 | 68.8 | 17 | 85.0 | 17 | 85.0 | 88 | 73.0 |
| Other cancers | 18 | 15.9 |
|
31.2 | - | 15.0 | - | 15.0 | 11 | 9.1 |
| Other cause8 | - | - | 6 | 5.4 | ||||||
| Not coded | 14 | 12.4 | 15 | 12.5 | ||||||
| 2-year survival rates | 56.6 | 37.7 | 38.6 | 39.8 | 47.4 | |||||
| Median follow-up time (months) | 18.3 | 13.5 | 10.8 | 16.9 | 15.6 | |||||
p<0.01
neighborhood socioeconomic status derived from census block group residence at diagnosis
separated, divorced, widowed, or unknown status at time of interview
cells with fewer than five cases are not shown
first degree includes parents, brothers, sisters, and children related by blood
subject was asked “In your lifetime, how many years in total was there at least one smoker [IF SMOKER: other than yourself] living in your household?”
subject was asked “How many years in total were you exposed to the tobacco smoke of others in your indoor workplace?”
BAC = bronchioloalveolar carcinoma
Other causes include: myocardial infarction, pulmonary embolism, other lung disorders, pregnancy complications, dementia, pneumonia, pleural effusion, heart disease, and fall
RESULTS
Compared to the general population of lung cancer patients in the cancer registry, patients who participated in the SFBALCS screener were slightly (not statistically significant) more likely to be US-born. Among Latinas, 55.4% of the screened cases were US-born compared to 53.8% among the general patient population, and among APIs, 20.5% of screened cases were US-born compared to 16.2% of the general patient population. Participating cases were significantly more likely than the general patient population to be diagnosed with early stage disease, but were similar with regards to race/ethnicity and age at diagnosis.
Among all screened lung cancer cases, nearly 70% of API women, 35% of Latina women, and 10% of non-Hispanic White women with lung cancer were never smokers. Although we did not analyze data for NH Whites by immigration status, in this sample 18% of NH Whites reported being foreign-born. Never smoker Latinas and APIs had a higher proportion of deaths due to any cause and deaths due to lung cancer than NH Whites (Table 1). Foreign-born Latinas were more likely to have low education and live in lower SES neighborhoods. Latinas were more likely than other racial/ethnic groups to be never married. Foreign-born Latinas and foreign-born APIs were more likely to have only public health insurance, and to report no first-degree family history of cancer. None of the examined tumor characteristics were significantly different among the groups. Foreign-born Latinas and foreign-born APIs were more likely to have been diagnosed in a public hospital, whereas US-born APIs were more likely than all other groups to have been diagnosed in a teaching hospital. Two-year survival rates were higher among NH Whites (56.6%) than Latinas and APIs, comparable between US-born (37.7%) and foreign-born Latinas (38.6%), and higher among foreign-born (47.4%) than US-born APIs (39.8%). Overall, 70% of the deaths were due to lung cancer as the underlying cause, 12% were due to an unspecified or uncoded cause of death, 6% were due to a non-cancer cause, and the remaining 12% were due to a variety of other causes, listed in the footnote of Table 1. The proportion of deaths due to lung cancer was higher among Latinas and Asians relative to NH Whites.
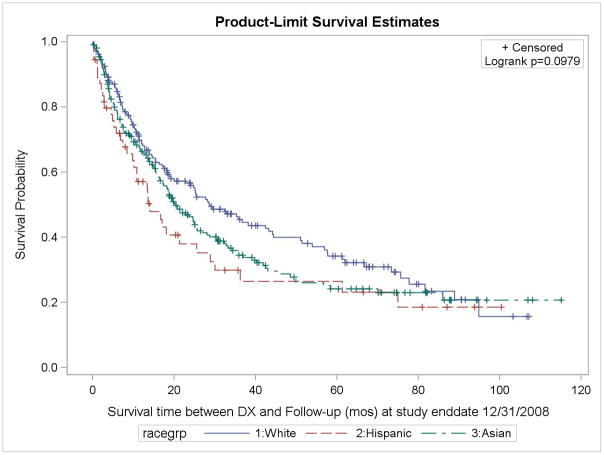
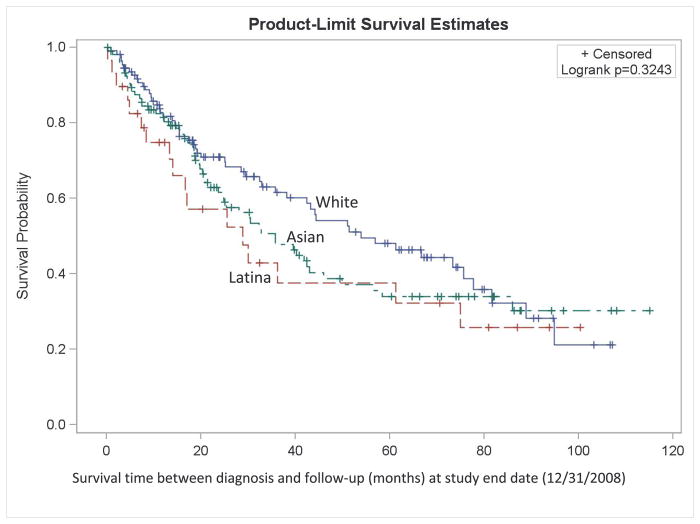
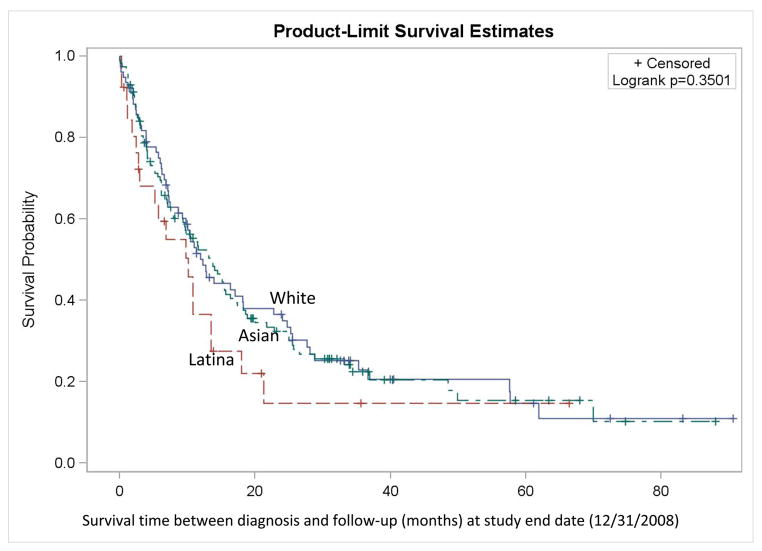
Kaplan-Meier curves (Figure 1) show that for stages I to III, overall survival was consistently better among NH Whites, followed by Asians, then Latinas. For stage IV+unstaged disease, overall survival over time was similar between NH Whites and Asians, and poorer among Latinas. Proportional hazards regression shows that overall mortality rates among Latinas and APIs, particularly the US-born, were as much as 2.1 and 1.7 times higher than those among NH White women, after adjusting for other covariates (Table 2). Cases who were separated, divorced, widowed, or of unknown marital status at the time of diagnosis had 1.5-times higher mortality relative to those who were married. Patients diagnosed with squamous cell carcinoma had 2 times higher mortality than those with large cell, non-BAC adenocarcinoma, or undifferentiated tumors, whereas there were no appreciable differences in mortality between the BAC cases and the large cell/non-BAC adenocarcinoma/undifferentiated cases. Radiation therapy as the first course of treatment was associated with slightly higher mortality, whereas surgery was associated with lower mortality.
Figure 1.
Kaplan-Meier survival curve (all-cause survival) among female never-smoker non-small cell lung cancer non-Hispanic White, Latina, and Asian/Pacific Islander (API) patients diagnosed 1998–2003 and 2005–2008, by race/ethnicity, San Francisco Bay Area Lung Cancer Study (N = 462)
A. All stages combined
B. Stages I-II
C. Stages IV & unstaged
Table 2.
Multivariate hazard ratios (HR) with 95% confidence intervals (CI) for associations with overall mortality rates after non-small cell lung cancer diagnosis in Hispanic and Asian/Pacific Islander (API) women never smokers, 1988–2003, 2005–2008, with follow-up through 2008
| Characteristic | HR (overall)1 | 95% CI |
|---|---|---|
| Age at diagnosis (single year) | 1.01 | 1.00–1.02 |
| Race/ethnicity | ||
| Non-Hispanic White | 1.00 | |
| Latina US-born | 2.05 | 1.18–3.55 |
| Latina foreign-born | 1.41 | 0.85–2.33 |
| API US-born | 1.69 | 1.01–2.75 |
| API foreign-born | 1.17 | 0.89–1.54 |
| Marital status | ||
| Married (at diagnosis) | 1.00 | |
| Never married | 0.83 | 0.53–1.27 |
| Separated/divorced/widowed/unknown | 1.54 | 1.17–2.02 |
| Histology | ||
| Large cell/non-BAC adenocarcinoma/undiff | 1.00 | |
| BAC | 0.72 | 0.45–1.14 |
| Squamous cell | 2.05 | 1.29–3.26 |
| Radiation | ||
| Not given | 1.00 | |
| Given | 1.48 | 1.13–1.92 |
| Surgery | ||
| None | 1.00 | |
| Performed | 0.30 | 0.20–0.44 |
HRs adjusted for other factors shown in the table including chemotherapy-by-time interaction; separate baseline hazards were estimated by stage at diagnosis (thereby adjusting for stage by allowing the form of the underlying hazard function to vary by stage)
DISCUSSION
In California, 87.1% of API women and 83.1% of Latina women have never smoked, compared with 56.3% of NH White and 65.9% of NH Black women (13). It has previously been documented that risks of lung cancer by level of smoking is modified by race/ethnicity (14). We found substantially poorer lung-cancer specific survival among APIs and Latinas, particularly among the US-born, compared with NH White women. These survival disparities were not explained by differences in selected sociodemographic or clinical factors, indicating that unmeasured factors associated with race/ethnicity and immigration status, such as cultural behaviors, access to or attitudes toward health care, genetic variation, and/or treatment response, may in part explain these striking differences.
Few studies have examined survival following a diagnosis of lung cancer among Latina women who have never smoked. The slight survival advantage among foreign-born compared to US-born Latina women is consistent with other studies of cancer mortality (15, 16) and may reflect the “Latino paradox” (17–19), which refers to better health among immigrants despite their being of lower SES. The better survival in foreign-born Latinos could be a result of under-ascertainment of deaths or loss to follow-up, when foreign-born Latinos with cancer return to their native countries and die there. However, prior studies have shown that while the migratory effect may exist to some extent, its magnitude is too small to explain the mortality paradox (20, 21). In our data, loss to follow-up was not significantly different between US- and foreign-born Latinas, suggesting that emigration does not explain our results. Alternatively, this paradox could be due to a true health advantage among Latinos in general due to more favorable health behaviors, use of traditional medicines, and greater extended family support; or it could reflect selective migration of healthier individuals to the US. The relative importance of these factors would be worth evaluating further with regards to lung cancer survival among Latinas.
Our results for APIs are contrary to those from southern California cancer registry data showing that survival after NSCLC was better among Asian males and females than other racial/ethnic groups, regardless of smoking status (9, 22, 23). The discrepant findings may be due to gender differences and/or population differences between southern and northern California, as it is known that Asian settlement patterns differ across regions within California (24). For instance, while Chinese comprised 45% of our population, they comprised only 15% of the female never-smoker population in the southern California analysis (25). The differences in findings may also be due to methodologic differences. In the prior analyses, smoking status was inferred based on a text-mining program that abstracted patients’ medical record information (9, 22, 23), and nearly 30% of patients were excluded because smoking status could not be determined (9). By contrast, smoking status in our study was based on self-report, which corresponds well with cotinine levels in most populations (26–31), whereas medical records can be inaccurate or lacking in any smoking information at all (32, 33). In the southern California study, the proportions of women with NSCLC who had never smoked were approximately 15% among all races/ethnicities and 55% among Asians. The former proportion is slightly lower than the 20% that we recently documented (7), and the latter proportion is considerably lower than the 70% of API never smokers in the SFBALCS. These discrepancies suggest that never smokers may have been disproportionately excluded from the southern California analyses due to missing smoking data in the medical record. Although the survival benefit among Asians relative to non-Hispanic Whites found in the southern California study was independent of smoking status (25), there was likely differential exclusion of Asian never-smokers. It is perhaps more likely that the differences between the two studies are due to the population differences noted above, as well as to effect modification by gender, as our effects were noted only among women, who were not evaluated separately in the southern California study. It is worth noting that the method for follow-up was the same in both studies.
Our study revealed other interesting associations with survival after NSCLC among female never smokers. Consistent with prior findings in lung (9, 34) and other cancers (35, 36), higher mortality was evident for patients who were widowed, divorced, or separated, compared with those who were married, even with adjustment for age, indicating that greater spousal social support improves survival. We also observed that first course radiation therapy was associated with slightly higher mortality, whereas surgery was associated with lower mortality; however, given the known limitations with cancer registry data on treatment, these patterns are likely reflective of residual confounding due to extent of disease.
Regardless of therapy, NSCLC patients who are never-smokers seem to have a small survival benefit compared to those with a smoking history (6), although this pattern is not confirmed in all studies (37). The studies that show better survival among never-smokers may be reflective of a greater number of comorbidities among patients with a history of smoking. In addition, for advanced-stage NSCLC, some studies have shown a clear difference in chemotherapy response between smokers and non-smokers (38), although others have not (39). Clinically important differences in treatment response are seen with EGFR tyrosine kinase inhibitors (erlotinib, gefitinib). Higher treatment response rates and better survival have been reported in never-smokers with these drugs compared to ever smokers (40–42). It is likely, however, that never-smoking status is a surrogate for EGFR activating mutations, as indicated by the results from the iPASS and First-Signal trials of first line gefitinib versus chemotherapy for never-smoking Asian patients with advanced stage NSCLC (43, 44). In both studies, only patients with EGFR activating mutations had a progression-free survival benefit. However, as these are relatively new drug regimens, it is unlikely that they would impact the results seen in our study, based on cases diagnosed from 1998 through 2008. Asian race/ethnicity has also been shown to be associated with better survival following NSCLC (45, 46), even adjusted for smoking status and gender (46). However, survival differences across racial/ethnic groups may be modified by smoking status and/or gender, as suggested by our results, which are discordant with those reported from the National Cancer Institute of Canada Clinical Trials Study Group BR.21 (46).
This study has its strengths in being population-based and in its availability of patient-level variables, such as self-reported smoking status and second-hand smoke exposure. The major limitation in this study is the limited sample size, especially after stratification by race/ethnicity and immigrant status, and thus, the results should be interpreted with caution. As a result, we were not able to examine survival patterns for detailed API or Latina ethnic groups, which have documented differences in lung cancer survival (45). Self-reported smoking status may be misclassified, possibly to a slightly higher extent among racial/ethnic minorities (47), and among proxy reports (although, in the literature, minorities are not consistently more likely to be misclassified on smoking status than non-Hispanic Whites) (48–50). Even though patients in this study were identified through rapid case ascertainment, patients sometimes were deceased or too ill to participate by the time they were contacted for this study; this is affirmed by the fact that participating patients were more likely to have early stage disease than the general patient population. The screening interview was conducted in English or Spanish only, therefore possibly restricting participation for subjects who speak a different language; however, participating APIs and Latinas were not significantly more likely to be US-born than the registry patient population. The findings for race/ethnicity may also be impacted by our inability to fully adjust for treatment (51). Our study may also be limited by possible biases in loss to follow-up, such that certain patients, particularly foreign-born APIs and Latinas, may have been more likely to return to their countries of origin upon learning of their serious illness (19, 52, 53). This effect would result in underestimation of the hazard ratios for foreign-born APIs and Latinas. We could not evaluate lung cancer-specific mortality due to how cause of death is coded in the cancer registries. However, given the rapid fatality of this disease and that of deaths of known causes of death, nearly 80% are due to lung cancer as the underlying cause, it is likely that the racial/ethnic and nativity results will be similar for lung cancer-specific mortality.
In summary, this study identified disparities in survival among API and Latina women never smokers who have been diagnosed with lung cancer, particularly those who were born in the US. These disparities present public health and clinical issues of concern, given the large and growing population of never smokers who present with lung cancer in these racial/ethnic groups. These results highlight the need for additional research to identify the underlying reasons for the disparities, as well as heightened clinical awareness.
Acknowledgments
The authors thank Dr. Daphne Lichtensztajn for her help with this manuscript. This study was supported by a population sciences developmental seed grant from the Stanford Cancer Center and grant NIEHS ES06717. The collection of cancer incidence data used in this study was supported by the California Department of Health Services as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract N01-PC-35136 awarded to the Cancer Prevention institute of California. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Health Services, the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors and subcontractors is not intended nor should be inferred.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Horner MJ, Ries LAG, Krapcho M, Neyman N, Aminou R, Howlader N, Altekruse SF, Feuer EJ, Huang L, Mariotto A, Miller BA, Lewis DR, Eisner MP, Stinchcomb DG, Edwards BK. Institute NC. SEER Cancer Statistics Review, 1975–2006. Bethesda, MD: 2009. http://seer.cancer.gov/csr/1975_2006/, based on November 2008 SEER data submission, posted to the SEER web site. [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) Prevalence of cigarette use among 14 racial/ethnic populations--United States, 1999–2001. MMWR Morb Mortal Wkly Rep. 2004;53(3):49–52. [PubMed] [Google Scholar]
- 4.Rudin CM, Avila-Tang E, Samet JM. Lung cancer in never smokers: a call to action. Clin Cancer Res. 2009;15(18):5622–5. doi: 10.1158/1078-0432.CCR-09-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samet JM, Avila-Tang E, Boffetta P, Hannan LM, Olivo-Marston S, Thun MJ, Rudin CM. Lung cancer in never smokers: clinical epidemiology and environmental risk factors. Clin Cancer Res. 2009;15(18):5626–45. doi: 10.1158/1078-0432.CCR-09-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Subramanian J, Govindan R. Lung cancer in never smokers: a review. J Clin Oncol. 2007;25(5):561–70. doi: 10.1200/JCO.2006.06.8015. [DOI] [PubMed] [Google Scholar]
- 7.Wakelee HA, Chang ET, Gomez SL, Keegan TH, Feskanich D, Clarke CA, Holmberg L, Yong LC, Kolonel LN, Gould MK, West DW. Lung cancer incidence in never smokers. J Clin Oncol. 2007;25(5):472–8. doi: 10.1200/JCO.2006.07.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers--a different disease. Nat Rev Cancer. 2007;7(10):778–90. doi: 10.1038/nrc2190. [DOI] [PubMed] [Google Scholar]
- 9.Ou SH, Ziogas A, Zell JA. Asian ethnicity is a favorable prognostic factor for overall survival in non-small cell lung cancer (NSCLC) and is independent of smoking status. J Thorac Oncol. 2009;4(9):1083–93. doi: 10.1097/JTO.0b013e3181b27b15. [DOI] [PubMed] [Google Scholar]
- 10.Chang JS, Wrensch MR, Hansen HM, Sison JD, Aldrich MC, Quesenberry CP, Jr, Seldin MF, Kelsey KT, Kittles RA, Silva G, Wiencke JK. Nucleotide excision repair genes and risk of lung cancer among San Francisco Bay Area Latinos and African Americans. Int J Cancer. 2008;123(9):2095–104. doi: 10.1002/ijc.23801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12(8):703–11. doi: 10.1023/a:1011240019516. [DOI] [PubMed] [Google Scholar]
- 12.The Annual Utilization Report of Hospitals. Office of Statewide Health Planning and Development; 2001. [Google Scholar]
- 13.California Health Interview Survey. [Accessed March 6, 2007];California Health Interview Survey (CHIS) 2005 www.chis.ucla.edu.
- 14.Haiman CA, Stram DO, Wilkens LR, Pike MC, Kolonel LN, Henderson BE, Le Marchand L. Ethnic and racial differences in the smoking-related risk of lung cancer. N Engl J Med. 2006;354(4):333–42. doi: 10.1056/NEJMoa033250. [DOI] [PubMed] [Google Scholar]
- 15.Singh GK, Miller BA. Health, life expectancy, and mortality patterns among immigrant populations in the United States. Canadian Journal of Public Health. 2004;95(3):14–21. doi: 10.1007/BF03403660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh GK, Siahpush M. Ethnic-immigrant differentials in health behaviors, morbidity, and cause-specific mortality in the United States: an analysis of two national data bases. Hum Biol. 2002;74(1):83–109. doi: 10.1353/hub.2002.0011. [DOI] [PubMed] [Google Scholar]
- 17.Markides KS, Coreil J. The health of Hispanics in the southwestern United States: an epidemiologic paradox. Public Health Rep. 1986;101(3):253–65. [PMC free article] [PubMed] [Google Scholar]
- 18.Scribner R. Paradox as paradigm--the health outcomes of Mexican Americans. Am J Public Health. 1996;86(3):303–5. doi: 10.2105/ajph.86.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franzini L, Ribble JC, Keddie AM. Understanding the Hispanic paradox. Ethn Dis. 2001;11(3):496–518. [PubMed] [Google Scholar]
- 20.Borrayo EA, Guarnaccia CA. Differences in Mexican-born and U.S. -born women of Mexican descent regarding factors related to breast cancer screening behavior. Health Care Women Int. 2000;21(7):599–613. doi: 10.1080/07399330050151842. [DOI] [PubMed] [Google Scholar]
- 21.Turra CM, Elo IT. The Impact of Salmon Bias on the Hispanic Mortality Advantage: New Evidence from Social Security Data. Popul Res Policy Rev. 2008;27(5):515–530. doi: 10.1007/s11113-008-9087-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ou SH, Zell JA, Ziogas A, Anton-Culver H. Prognostic factors for survival of stage I nonsmall cell lung cancer patients : a population-based analysis of 19,702 stage I patients in the California Cancer Registry from 1989 to 2003. Cancer. 2007;110(7):1532–41. doi: 10.1002/cncr.22938. [DOI] [PubMed] [Google Scholar]
- 23.Zell JA, Ou SH, Ziogas A, Anton-Culver H. Survival improvements for advanced stage nonbronchioloalveolar carcinoma-type nonsmall cell lung cancer cases with ipsilateral intrapulmonary nodules. Cancer. 2008;112(1):136–43. doi: 10.1002/cncr.23146. [DOI] [PubMed] [Google Scholar]
- 24.Takaki R. Strangers from a Different Shore: a History of Asian Americans. New York, NY: Penguin Books; 1989. [Google Scholar]
- 25.Ou SH, Ziogas A, Zell JA. A comparison study of clinicopathologic characteristics of Southern California Asian American Non-small Cell Lung Cancer (NSCLC) patients by smoking status. J Thorac Oncol. 5(2):158–68. doi: 10.1097/JTO.0b013e3181c8cc62. [DOI] [PubMed] [Google Scholar]
- 26.Studts JL, Ghate SR, Gill JL, Studts CR, Barnes CN, LaJoie AS, Andrykowski MA, LaRocca RV. Validity of self-reported smoking status among participants in a lung cancer screening trial. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1825–8. doi: 10.1158/1055-9965.EPI-06-0393. [DOI] [PubMed] [Google Scholar]
- 27.Pierce JP, Dwyer T, DiGiusto E, Carpenter T, Hannam C, Amin A, Yong C, Sarfaty G, Shaw J, Burke N. Cotinine validation of self-reported smoking in commercially run community surveys. J Chronic Dis. 1987;40(7):689–95. doi: 10.1016/0021-9681(87)90105-6. [DOI] [PubMed] [Google Scholar]
- 28.Haddow JE, Palomaki GE, Knight GJ. Use of serum cotinine to assess the accuracy of self reported non-smoking. Br Med J (Clin Res Ed) 1986;293(6557):1306. doi: 10.1136/bmj.293.6557.1306-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perez-Stable EJ, Marin BV, Marin G, Brody DJ, Benowitz NL. Apparent underreporting of cigarette consumption among Mexican American smokers. Am J Public Health. 1990;80(9):1057–61. doi: 10.2105/ajph.80.9.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee PN. Lung cancer and passive smoking: association of an artefact due to misclassification of smoking habits? Toxicol Lett. 1987;35(1):157–62. doi: 10.1016/0378-4274(87)90102-0. [DOI] [PubMed] [Google Scholar]
- 31.Wagenknecht LE, Burke GL, Perkins LL, Haley NJ, Friedman GD. Misclassification of smoking status in the CARDIA study: a comparison of self-report with serum cotinine levels. Am J Public Health. 1992;82(1):33–6. doi: 10.2105/ajph.82.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schofield PE, Hill DJ. How accurate is in-patient smoking status data collected by hospital admissions staff? Aust N Z J Public Health. 1999;23(6):654–6. doi: 10.1111/j.1467-842x.1999.tb01555.x. [DOI] [PubMed] [Google Scholar]
- 33.McCullough A, Fisher M, Goldstein AO, Kramer KD, Ripley-Moffitt C. Smoking as a vital sign: prompts to ask and assess increase cessation counseling. J Am Board Fam Med. 2009;22(6):625–32. doi: 10.3122/jabfm.2009.06.080211. [DOI] [PubMed] [Google Scholar]
- 34.Ou SH, Zell JA, Ziogas A, Anton-Culver H. Low socioeconomic status is a poor prognostic factor for survival in stage I nonsmall cell lung cancer and is independent of surgical treatment, race, and marital status. Cancer. 2008;112(9):2011–20. doi: 10.1002/cncr.23397. [DOI] [PubMed] [Google Scholar]
- 35.Gomez SL, O’Malley CD, Stroup A, Shema SJ, Satariano WA. Longitudinal, population-based study of racial/ethnic differences in colorectal cancer survival: impact of neighborhood socioeconomic status, treatment and comorbidity. BMC Cancer. 2007;7:193. doi: 10.1186/1471-2407-7-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lai H, Lai S, Krongrad A, Trapido E, Page JB, McCoy CB. The effect of marital status on survival in late-stage cancer patients: an analysis based on surveillance, epidemiology, and end results (SEER) data, in the United States. Int J Behav Med. 1999;6(2):150–76. doi: 10.1207/s15327558ijbm0602_4. [DOI] [PubMed] [Google Scholar]
- 37.Subramanian J, Velcheti V, Gao F, Govindan R. Presentation and stage-specific outcomes of lifelong never-smokers with non-small cell lung cancer (NSCLC) J Thorac Oncol. 2007;2(9):827–30. doi: 10.1097/JTO.0b013e318145af79. [DOI] [PubMed] [Google Scholar]
- 38.Tsao AS, Liu D, Lee JJ, Spitz M, Hong WK. Smoking affects treatment outcome in patients with advanced nonsmall cell lung cancer. Cancer. 2006;106(11):2428–36. doi: 10.1002/cncr.21884. [DOI] [PubMed] [Google Scholar]
- 39.Herbst RS, Prager D, Hermann R, Fehrenbacher L, Johnson BE, Sandler A, Kris MG, Tran HT, Klein P, Li X, Ramies D, Johnson DH, Miller VA. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2005;23(25):5892–9. doi: 10.1200/JCO.2005.02.840. [DOI] [PubMed] [Google Scholar]
- 40.Tsao MS, Sakurada A, Cutz JC, Zhu CQ, Kamel-Reid S, Squire J, Lorimer I, Zhang T, Liu N, Daneshmand M, Marrano P, da Cunha Santos G, Lagarde A, Richardson F, Seymour L, Whitehead M, Ding K, Pater J, Shepherd FA. Erlotinib in lung cancer - molecular and clinical predictors of outcome. N Engl J Med. 2005;353(2):133–44. doi: 10.1056/NEJMoa050736. [DOI] [PubMed] [Google Scholar]
- 41.Thatcher N, Chang A, Parikh P, Rodrigues Pereira J, Ciuleanu T, von Pawel J, Thongprasert S, Tan EH, Pemberton K, Archer V, Carroll K. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer) Lancet. 2005;366(9496):1527–37. doi: 10.1016/S0140-6736(05)67625-8. [DOI] [PubMed] [Google Scholar]
- 42.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R, van Kooten M, Dediu M, Findlay B, Tu D, Johnston D, Bezjak A, Clark G, Santabarbara P, Seymour L. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353(2):123–32. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 43.Mok TS, et al. Clinical Outcomes of patients with epidermal growth factor receptor (EGFR) mutations (mut) in IPASS. Paper presented at: International Association for the Study of Lung Cancer 13th World Conference on Lung Cancer Proceedings; July 31-Aug 4, 2009; San Francisco, CA. [Google Scholar]
- 44.Lee JS, et al. A randomized phase III study of gefitinib versus standard chemotherapy (gemcitabine plus cisplatin) as a first-line treatment for never-smokers with advanced or metastatic adenocarcinoma of the lung. International Association for the Study of Lung Cancer 13th World Conference on Lung Cancer Proceedings; San Francisco, CA. July 31-Aug 4, 2009. [Google Scholar]
- 45.Chang ET, Shema SJ, Wakelee HA, Clarke CA, Gomez SL. Uncovering disparities in survival after non-small-cell lung cancer among Asian/Pacific Islander ethnic populations in California. Cancer Epidemiol Biomarkers Prev. 2009;18(8):2248–55. doi: 10.1158/1055-9965.EPI-09-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Florescu M, Hasan B, Seymour L, Ding K, Shepherd FA. A clinical prognostic index for patients treated with erlotinib in National Cancer Institute of Canada Clinical Trials Group study BR.21. J Thorac Oncol. 2008;3(6):590–8. doi: 10.1097/JTO.0b013e3181729299. [DOI] [PubMed] [Google Scholar]
- 47.Wells AJ, English PB, Posner SF, Wagenknecht LE, Perez-Stable EJ. Misclassification rates for current smokers misclassified as nonsmokers. Am J Public Health. 1998;88(10):1503–9. doi: 10.2105/ajph.88.10.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gilpin EA, Pierce JP, Cavin SW, Berry CC, Evans NJ, Johnson M, Bal DG. Estimates of population smoking prevalence: self-vs proxy reports of smoking status. Am J Public Health. 1994;84(10):1576–9. doi: 10.2105/ajph.84.10.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Navarro AM. Smoking status by proxy and self report: rate of agreement in different ethnic groups. Tob Control. 1999;8(2):182–5. doi: 10.1136/tc.8.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hyland A, Cummings KM, Lynn WR, Corle D, Giffen CA. Effect of proxy-reported smoking status on population estimates of smoking prevalence. Am J Epidemiol. 1997;145(8):746–51. doi: 10.1093/aje/145.8.746. [DOI] [PubMed] [Google Scholar]
- 51.Giordano SH, Kuo YF, Duan Z, Hortobagyi GN, Freeman J, Goodwin JS. Limits of observational data in determining outcomes from cancer therapy. Cancer. 2008;112(11):2456–66. doi: 10.1002/cncr.23452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Markides KS, Eschbach K. Aging, migration, and mortality: current status of research on the Hispanic paradox. J Gerontol B Psychol Sci Soc Sci. 2005;60(Spec No 2):68–75. doi: 10.1093/geronb/60.special_issue_2.s68. [DOI] [PubMed] [Google Scholar]
- 53.Patel KV, Eschbach K, Ray LA, Markides KS. Evaluation of mortality data for older Mexican Americans: implications for the Hispanic paradox. Am J Epidemiol. 2004;159(7):707–15. doi: 10.1093/aje/kwh089. [DOI] [PubMed] [Google Scholar]