Abstract
Low vitamin D blood levels are postulated to be a risk factor for worse lung function, largely based on cross-sectional data. We sought to use longitudinal data to test the hypothesis that baseline plasma 25-hydroxyvitamin D [25(OH)D] is lower in subjects with more rapid lung function decline, compared to those with slow lung function decline.
We conducted a nested, matched case-control study in the Lung Health Study 3 cohort. Cases and controls were continuous smokers with rapid and slow lung function decline, respectively, over approximately 6 years of follow-up. We compared baseline 25(OH)D levels between cases and controls, matching on date of blood draw and clinical center.
Among 196 subjects, despite rapid and slow decliners experiencing strikingly and significantly different rates of decline of forced expiratory volume in one second (−152 vs. −0.3 mL/year; p<0.001), there was no significant difference in baseline 25(OH)D levels (25.0 vs. 25.9 ng/mL; p=0.54). There was a high prevalence of vitamin D insufficiency (35%) and deficiency (31%); only 4% had a normal 25(OH)D level in the winter.
Although vitamin D insufficiency and deficiency are common among continuous smokers with established mild to moderate COPD, baseline 25(OH)D levels are not predictive of subsequent lung function decline.
Keywords: Pulmonary Disease, Chronic Obstructive; Smoking; Spirometry; Vitamin D
Introduction
Data from the Third National Health and Nutrition Examination Survey (NHANES III) showed that in a cross-sectional sample of a general U.S. population (n=14,076), lower serum vitamin D levels were associated with lower forced expiratory volume in one second (FEV1) in a graded, “dose-dependent” fashion1. The results of this report from Black and colleagues have spurred hypotheses that low vitamin D levels may be a modifiable risk factor for impaired lung function and chronic obstructive pulmonary disease (COPD).
Vitamin D has long been recognized for its effects on calcium homeostasis and skeletal health. However, its non-skeletal effects have recently received increasing scientific attention, including hypotheses on its potentially beneficial effects in patients with COPD2. The mechanisms by which vitamin D levels might affect lung function are unclear. Potential explanations include effects on respiratory infection risk (via both innate and adaptive mechanisms) and lung tissue remodeling (via matrix metalloproteinases and other pathways)2–5.
We sought to build upon the cross-sectional data of Black and colleagues by using longitudinal data to further investigate vitamin D insufficiency as a risk factor for rapid lung function decline and COPD. We hypothesized that among persons with mild COPD, those with rapid declines in longitudinal lung function would have lower baseline vitamin D levels compared to persons with minimal declines in longitudinal lung function. We tested this hypothesis with a nested, matched case-control study in the Lung Health Study 3 cohort.
Materials and Methods
Study Subjects
Participants in this nested, matched case-control study were selected from the Lung Health Study 3 (LHS 3), an observational follow-up study of participants in the Lung Health Study trial, a 5-year, 10-center, randomized trial of a smoking intervention and bronchodilator 6, 7. Following the trial, study interventions were stopped, but most participants provided informed consent to participate in LHS 3 and agreed to return to study centers for a single long-term follow-up visit. 5,887 participants enrolled in the original LHS trial, 4,517 participants enrolled in LHS 3, and 4,194 completed spirometry at an average of 6 years after LHS 3 enrollment. Thus, the follow-up rate in LHS 3 was 93%. Detailed methods and results of LHS 3 have been previously published8, 9.
Study Design
We conducted a nested, matched case-control study within the LHS 3 cohort. Stored blood was only available at the end of the original trial, at the year 5 (Y5) visit; blood was not stored from other visits in the original trial or at the long-term (LT) follow-up visit. This Y5 specimen served as our baseline vitamin D assessment. Spirometry was available at the time of blood draw (at Y5) and at the LT visit. LHS 3 did not have any intermediate visits between the Y5 and LT visits (see online supplement for figure of design). We restricted our analysis to the 1,054 LHS 3 participants who were biochemically validated continuous smokers throughout all visits in the original LHS trial and still smoking at the LT visit. Cases were continuous smokers with the most rapid declines in FEV1 between the Y5 visit and the LT visit (rapid decliners). Controls were continuous smokers with the least decline in FEV1 in the same time period (slow decliners).
The primary human source of vitamin D is ultraviolet sunlight exposure, which will vary by season and by latitude (LHS study centers varied from as far south as Birmingham, AL, USA [latitude 33° N] to as far north as Winnipeg, MB, Canada [latitude 49° N]). To control for these seasonal and latitude effects on vitamin D levels, we matched cases and controls on date of Y5 blood draw (to within 60 days) and on clinical center. We rationalized that if vitamin D affected rates of FEV1 decline, then differences in vitamin D levels should be greatest between persons with the greatest differences in rates of lung function decline. Therefore, we constructed a LHS 3 database query, such that the matched case-control pair with the largest difference in rates of FEV1 decline (as % of predicted) was selected as the first pair. This process was repeated sequentially and subsequent pairs had progressively smaller differences in rate of FEV1 decline between the cases and controls, while still remaining matched on date and clinical center (see online supplement for figure of selection process). This selection process was continued until the desired sample size was reached.
Methods
Plasma was collected at the Y5 visit in standardized fashion, and shipped on dry ice to the LHS 3 data coordinating center, where samples have been continuously stored at −70°C. Once cases and controls were identified for this particular study, plasma samples were thawed and plasma 25-hydroxyvitamin D [25(OH)D] assays were performed using liquid chromatography-tandem mass spectrometry (LC-MS/MS; ThermoFisher Scientific, Franklin, MA, USA and Applied Biosystems-MDS Sciex, Foster City, CA, USA) in the laboratory of R.J. Singh at the Mayo Clinic (Rochester, MN, USA) . Details of the 25(OH)D assays, including coefficients of variation, are provided in the online supplement.
Spirometry was performed in both the main trial and LT visit using the same rolling seal spirometers (Spirotech 500; Spirotech, Atlanta, GA, USA) and the same spirometry quality control program10. Measurements of FEV1 and FVC were made before and at least 20 minutes after two puffs (200 mcg) of inhaled albuterol administered through a metered-dose inhaler. Our analysis was restricted to post-bronchodilator measures. The largest single FEV1 and FVC were reported and converted to percentages of the predicted normal using the formulas of Crapo and colleagues11.
Analysis
The primary outcome was the paired difference in the baseline exposure variable (Y5 plasma 25(OH)D level) between matched rapid and slow decliners. Annual rates of lung function decline were calculated by subtracting spirometry values at the LT visit from the values at the Y5 visit and dividing by time elapsed between the two measures.
Our sample size of 196 (98 pairs) provided 90% power (two-tailed alpha = 0.05) to detect a difference of 2.4 ng/mL in baseline 25(OH)D levels between rapid and slow decliners using paired t-testing. We therefore had excellent power to detect small differences in 25(OH)D levels. Details of the sample size determination are provided in the online supplement.
As secondary analyses, we also investigated seasonal variation in 25(OH)D levels. Seasons were defined as Winter=January-March, Spring=April-June, Summer=July-September, Autumn=October-December. The seasonal 25(OH)D data were analyzed using one-way ANOVA, corrected for multiple comparisons with a Bonferroni-adjusted p-value significance level of 0.05. We also conducted a conditional (paired) logistic regression analysis in which the outcome was rapid vs. slow decline in FEV1 and which included the following covariates from the Y5 visit (in addition to total vitamin D level): age, gender, number of cigarettes smoked per day, FEV1 % pred, FVC % pred, bronchodilator response, and methacholine response. We also included time from the Y5 visit to the LT visit.
All statistical analyses were performed using SAS 9.1 (SAS Institute, Cary, NC, USA) and STATA 9.2 (StataCorp, College Station, TX, USA). Figures were created using SigmaPlot 11.0 (Systat Software Inc., San Jose, CA, USA).
Results
Rapid and slow decliners (cases and controls) were similar in Y5 age, gender distribution, and smoking intensity (Table 1). Most participants were Caucasian, due to the sample recruited in the original Lung Health Study trial, and there was a statistically significant difference in ethnicity of rapid and slow decliners. Matching resulted in a mean difference in clinic visit days between rapid and slow decliners of 25 ± 16.5 days (range 0–60 days). The distribution of participants matched on clinical center was as follows: Baltimore, MD, USA (n = 24), Birmingham, AL, USA (n = 10), Cleveland, OH, USA (n = 14), Detroit, MI, USA (n= 24), Los Angeles, CA, USA (n = 20), Pittsburgh, PA, USA (n = 24), Portland, OR, USA (n = 22), Rochester, MN, USA (n = 12), Salt Lake City, UT, USA (n = 18), and Winnipeg, MB, Canada (n = 28).
Table 1.
Characteristics of study participants. All participants were continuous smokers from the first visit in the main Lung Health Study trial to the long-term follow-up visit. Data are presented as mean ± standard deviation for continuous variables or as number (%) for categorical variables. p-values calculated using paired t-testing. FEV1 = forced expiratory volume in one second, FVC = forced vital capacity, LT = Long-term follow-up visit, Y5 = Year-5 visit.
| Rapid Decliners (n=98) | Slow Decliners (n=98) | p-value | |
|---|---|---|---|
| Male | 62 (63%) | 60 (61%) | 0.77 |
| Caucasian ethnicity | 86 (88%) | 97 (99%) | 0.002 |
| Age, years | 53.2 ± 6.9 | 52.2 ± 6.6 | 0.31 |
| Cigarettes/day reported at Y5 | 25.1 ± 12.6 | 21.5 ± 11.6 | 0.04 |
| Y5 FEV1 (L) | 2.35 ± 0.63 | 2.49 ± 0.65 | 0.09 |
| Y5 FEV1 (% predicted) | 71.0 ± 12.1 | 73.2 ± 11.5 | 0.15 |
| Y5 FVC (L) | 4.10 ± 1.02 | 3.94 ± 0.99 | 0.23 |
| Y5 FVC (% predicted) | 98.6 ± 13.7 | 92.1 ± 11.4 | <0.001 |
| Time between Y5 to LT spirometry visits (years) | 6.0 ± 0.64 | 5.8 ± 0.66 | 0.06 |
| LT FEV1 (L) | 1.44 ± 0.52 | 2.49 ± 0.66 | <0.001 |
| LT FEV1 (% predicted) | 45.2 ± 13.3 | 77.2 ± 11.6 | <0.001 |
| LT FVC (L) | 3.31 ± 1.01 | 3.92 ± 1.05 | <0.001 |
| LT FVC (% predicted) | 81.7 ± 17.0 | 94.2 ± 12.6 | <0.001 |
Our selection criteria for cases and controls resulted in clinically and statistically significant differences in the rate of FEV1 decline between rapid and slow decliners. While FEV1 was similar between rapid and slow decliners at the Y5 visit, rapid decliners had a mean FEV1 that was >1 L worse than slow decliners at the LT visit (table 1). This resulted in a rate of FEV1 decline of −152 mL/year (−4.3% of predicted/year) in rapid decliners vs. −0.3mL/year (+0.7% of predicted/year) in slow decliners (p<0.001) (table 2).
Table 2.
Comparison of lung function decline and vitamin D status between rapid decliners and slow decliners, matched on date of blood draw (to within 60 days) and clinical center. Data are presented as mean ± standard deviation. p-values calculated using paired t-testing. 25(OH)D = 25-hydroxyvitamin D, FEV1 = forced expiratory volume in one second, LT = Long-term follow-up visit, Y5 = Year-5 visit.
| Rapid Decliners (n=98) | Slow Decliners (n=98) | p-value | |
|---|---|---|---|
| Rate of FEV1 decline from Y5 to LT (mL/year) | −151.6 ± 47.7 | −0.28 ± 24.0 | <0.0001 |
| Rate of FEV1 decline from Y5 to LT (%predicted/year) | −4.3 ± 1.2 | +0.7 ± 0.7 | <0.0001 |
| Y5 25(OH)D levels (ng/mL) | 25.0 ± 10.4 | 25.9 ± 10.2 | 0.54 |
Despite the large differences in rate of FEV1 decline, and appropriate control of latitude (through matching on clinical center) and time of year (through matching on date of blood draw), the difference in Y5 25(OH)D level between rapid decliners and slow decliners was not statistically significant (25.0 ng/mL vs. 25.9 ng/mL, respectively; p=0.54) (Table 2). Additional multiple regression and paired logistic regression analysis for baseline Y5 covariates also demonstrated no association between Y5 25(OH)D level and rapid or slow decliner status (see online supplementary material for details).
We applied current widely accepted definitions of vitamin D insufficiency and deficiency12, which classify patients as vitamin D deficient with 25(OH)D levels <20 ng/mL and insufficient with levels ≥ 20ng/mL but <30 ng/mL. Applying such criteria, we found 35% (n=69) of this LHS 3 sample was vitamin D insufficient and 31% (n=60) were vitamin D deficient. Only 34% (n=67) of the sample would be currently classified as sufficient in vitamin D status with levels ≥30 ng/mL. 14 participants (7%) had severe vitamin D deficiency, such that their 25(OH)D levels were ≤10 ng/mL.
There was also significant seasonal variation in 25(OH)D levels (fig.1 and online supplementary figure). As expected, 25(OH)D levels peaked in late summer, with nadir levels observed in the winter months. The magnitude of the seasonal variation was both clinically and statistically significant, with a mean winter 25(OH)D level of 18.3 ng/mL compared to 31.7 ng/mL in the summer (Bonferroni-corrected p<0.001). Of 48 samples drawn in the winter months, 46 (96%) were under the recommended goal level of ≥30 ng/mL.
Figure 1.
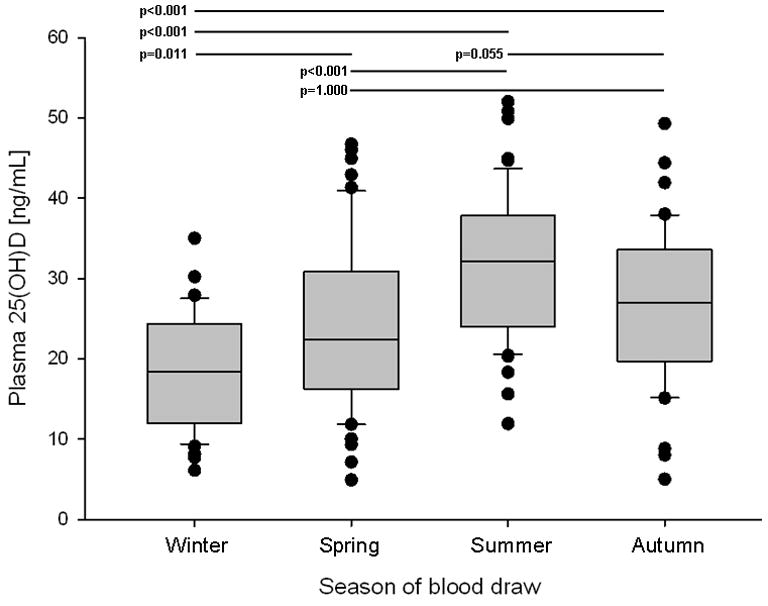
Boxplots of 25(OH)D levels by season. Line=median, shaded box=interquartile range, whiskers=range, dots=outliers. Winter=January-March, Spring=April-June, Summer=July-September, Autumn=October-December. Results of statistical testing of means listed in Table 3 (one-way ANOVA with Bonferroni-corrected p-value) displayed at top with p-value and corresponding line to indicate the comparison tested. 25(OH)D = 25-hydroxyvitamin D.
Discussion
We found no differences in baseline 25(OH)D levels between continuously smoking LHS 3 participants with rapid and slow declines in lung function over approximately 6 years of prospective follow-up. Therefore, our data do not support the notion that low 25(OH)D levels lead to faster rates of lung function decline.
Our study was primarily prompted by the study of Black and colleagues which examined cross-sectional data from 14,076 NHANES III participants1. They demonstrated a graded relationship between lower 25(OH)D levels and lower lung function, such that those in the lowest 25(OH)D quintile (≤16.2 ng/mL) had a mean FEV1 that was 126mL lower than those in the highest quintile (≥ 34.3 ng/ml), after adjusting for gender, age, ethnicity, body mass index, and cigarette smoking. Among a small subgroup with self-reported emphysema (n=251), the differences were even greater, such that when comparing those with 25(OH)D ≤16.2 ng/mL to those ≥34.3 ng/ml, FEV1 was 344mL worse in the low 25(OH)D group. The actual spirometry values from these 251 patients were not reported, so confirmation of COPD and assessment of COPD severity could not be made. While intriguing, a major limitation of these data is the cross-sectional nature of NHANES data. To our knowledge, ours is the first study examining relationships between baseline 25(OH)D levels and subsequent prospective, longitudinal rates of lung function decline.
Our study design allowed us to compare two groups of COPD patients of significant clinical interest—those who continuously smoke and have rapid lung function decline (rapid decliners) and those who continuously smoke, yet have preserved lung function over time (slow decliners). Because smoking is controlled for in both of these groups, we were able to investigate the hypothesis that the rapid decliners would have lower 25(OH)D levels as one potential mechanism by which their lung function rapidly declines. However, our data do not support this hypothesis.
Our study has several strengths. The longitudinal assessment of lung function was rigorously standardized with the same equipment and procedures used by experienced study staff (who had performed annual spirometry for 5 years prior to the Y5 measure in this study). Our matching criteria and the seasonal variation observed suggest that misclassification of 25(OH)D levels is unlikely. We had excellent power to detect small differences in 25(OH)D levels: 90% power to detect a difference as small as 2.4 ng/mL. It seems unlikely that a difference any smaller than this could explain differences in rates of subsequent lung function decline.
Our study has several important limitations. One limitation is that assessment of 25(OH)D levels was only possible from a single study visit. Therefore, this single assessment may not be fully reflective of an individual’s overall vitamin D status. For example, a wintertime assessment could be a poor indicator of overall vitamin D status throughout the year, especially in more extreme latitudes. We attempted to correct for seasonal variation and latitude effects as best as we could by matching rapid and slow decliners on date of blood draw and clinical center, but this can not correct for seasonal changes which might vary significantly both within and between individuals. We were also unable to assess whether or not the presence of low 25(OH)D levels at Y5 were associated with persistent low levels at the LT follow-up visit, as there was no blood draw at the LT follow-up visit. It is possible that some participants might have begun activities during that time interval which could have affected their subsequent 25(OH)D levels. For example, participants could have begun vitamin D supplementation and subsequently increased their 25(OH)D levels after the Y5 visit. Conversely, they could have begun using sunscreen products which could have decreased their 25(OH)D levels after the Y5 visit.
Another limitation of our data is that these analyses were restricted to continuous smokers with evidence of mild to moderate COPD at baseline. Because smoking has such a significant impact on rate of lung function decline, smoking is important to control for in a study such as ours. We chose to restrict our analysis to continuous smokers in order to focus on those COPD patients at greatest risk of progressive lung function decline and to reduce effects of variables other than vitamin D (such as intermittent smoking) that might also affect rate of lung function decline. Thus, we can not extrapolate these findings to non-smokers or to intermittent smokers. We also can not extrapolate these findings to persons without COPD confirmed by spirometry nor to persons with very advanced COPD.
We feel it important to highlight the high prevalence of vitamin D insufficiency and deficiency we found, such that only 34% of these LHS 3 participants had 25(OH)D levels that would currently be considered as adequate. In wintertime, we found only 2 of 48 25(OH)D measures to be in the accepted normal range. Riancho and colleagues studied 44 men with COPD (mean FEV1 of 39% of predicted) between 1983–1985 and showed the mean 25(OH)D level was <10 ng/mL for most of the year, with peak mean 25(OH)D level in late summer still <20 ng/mL13. They measured 25(OH)D using a competitive protein binding assay after HPLC purification—a method that is now rarely used, so a direct comparison to more current 25(OH)D assay methods may be limited. Shane and colleagues reported a mean 25(OH)D level of 20 ng/mL in 28 patients with COPD awaiting lung transplantation between 1993–199514. 10 of these patients (36%) had levels ≤10 ng/mL. Forli and colleagues reported vitamin D deficiency (<20 ng/ml) in over 50% of 71 consecutive non-smoking patients (of whom 46 had COPD) undergoing lung transplantation evaluation between 1993 and 199815.
These data are of particular concern in light of recent NHANES data demonstrating that between the surveys conducted in 1988–1994 and 2001–2004, the mean population 25(OH)D level decreased by 6 ng/mL and the percentage with inadequate 25(OH)D levels (<30 ng/mL) increased from 55% to 77%16. Because our 25(OH)D data are based on samples collected between 1991–1994, it seems likely that the current prevalence of inadequate 25(OH)D levels in patients with mild to moderate COPD is even higher than 66% we found.
In support of this, Franco and colleagues recently reported a mean springtime of 2005 25(OH)D level of 20.8 ng/mL in a small cohort of 49 Brazilian patients with mostly mild and moderate COPD17. Of these 49 patients, only 3 (6%) had 25(OH)D levels ≥30ng/mL; 29 (59%) were vitamin D insufficient, and 17 (35%) were vitamin D deficient. Janssens and colleagues also recently reported that among 262 Belgian patients with COPD, the mean 25(OH)D level was 19.9 ng/mL and 52% were vitamin D deficient with levels <20 ng/mL18.
Our cohort also demonstrated significant seasonal variation in 25(OH)D levels, which varied around the accepted cut-points of normal, insufficient, and deficient levels. As such, there was a substantial seasonal shift in the distribution of participants classified as normal or vitamin D deficient. While these blood samples from 1991–1994 are no longer a contemporary assessment, clinicians and researchers may need to consider the substantial effect of seasonality on 25(OH)D measures. It is important to note that LHS 3 participants were generally quite healthy with mostly mild COPD. One might hypothesize that in patients with more severe COPD, there may be less of a seasonal effect due to being more confined to the home and hence, less exposed to sunlight. However, we are unaware of any such contemporary data to either support or refute such a hypothesis. In addition, the mechanisms leading to vitamin D insufficiency/deficiency may be quite complex. Dietary vitamin D intake in patients with COPD has been shown to be low19, but multiple other mechanisms may lead to inadequate vitamin D status12.
Although we found no association between 25(OH)D levels and subsequent rates of lung function decline, patients with COPD suffer from many co-morbidities potentially associated with low 25(OH)D levels. The one COPD co-morbidity with well-studied links to low 25(OH)D levels is osteoporosis20. Multiple other COPD complications and co-morbidities have been linked to vitamin D insufficiency, including respiratory infections21–23, cardiovascular disease24, 25, and muscle dysfunction26, 27. However, it is important to note that there are no clinical trial data to support to the hypothesis that improving 25(OH)D levels in patients with COPD will improve any of these COPD co-morbidities, but these remain topics requiring further investigation.
In conclusion, although we found a high prevalence of low 25(OH)D levels in continuous smokers with established mild and moderate COPD, we found no difference between baseline 25(OH)D levels among those with subsequent rapid declines in lung function and slow declines in lung function. Our data suggest that normalization of 25(OH)D levels is not likely to affect subsequent rates of lung function decline in such patients.
Supplementary Material
Table 3.
Year 5 plasma 25(OH)D levels by season. Seasons were defined as: Winter=January-March, Spring=April-June, Summer=July-September, Autumn=October-December. Data are presented as mean ± standard deviation for 25(OH)D and number (%) for the categorical data. 25(OH)D = 25-hydroxyvitamin D. See Figure 1 for p-values from pairwise statistical testing of mean 25(OH)D levels by season.
| Winter | Spring | Summer | Autumn | |
|---|---|---|---|---|
| Participants | 48 | 51 | 57 | 40 |
| 25(OH)D level [ng/mL] | 18.3 ± 7.0 | 24.1 ± 10.3 | 31.7 ± 9.2 | 26.8 ± 9.6 |
| Normal vitamin D levela | 2 (4%) | 16 (31%) | 34 (60%) | 15 (38%) |
| Vitamin D insufficientb | 19 (40%) | 15 (29%) | 20 (35%) | 15 (38%) |
| Vitamin D deficientc | 27 (56%) | 20 (39%) | 3 (5%) | 10 (25%) |
Normal vitamin D level = ≥ 30 ng/mL
Vitamin D insufficiency = 25(OH)D level ≥20 ng/mL, <30 ng/mL
Vitamin D deficiency = 25(OH)D level <20 ng/mL
Acknowledgments
Study supported by the Minnesota Medical Foundation (faculty research award #3857-9227-08 to Dr. Kunisaki) and National Institutes of Health (K12 RR023247 to Dr. Kunisaki and UL1 RR024150 to Mayo Clinic).
We thank H. Blair (Mayo Clinic, Rochester, MN, USA) for technical assistance with the vitamin D assays, and H. Voelker and A. Boggess (both University of Minnesota, Minneapolis, MN, USA) for assistance with the identification and retrieval of samples. We also thank the Upper Midwest CTSA Consortium for fostering this study’s institutional collaboration.
Abbreviations
- 25(OH)D
25-hydroxyvitamin D
- COPD
chronic obstructive pulmonary disease
- FEV1
forced expiratory volume in one second
- FVC
forced vital capacity
- LHS 3
Lung Health Study 3
- LT
Long-term follow-up
- NHANES III
Third National Health and Nutrition Examination Survey
- SD
standard deviation
- Y5
Year-5 follow-up
Footnotes
The views expressed in this article are those of the authors and do not necessarily represent the views of the U.S. Department of Veterans Affairs, the University of Minnesota, the Mayo Clinic, nor the National Institutes of Health.
This article has an online supplement.
References
- 1.Black PN, Scragg R. Relationship between serum 25-hydroxyvitamin d and pulmonary function in the third national health and nutrition examination survey. Chest. 2005;128:3792–3798. doi: 10.1378/chest.128.6.3792. [DOI] [PubMed] [Google Scholar]
- 2.Janssens W, Lehouck A, Carremans C, et al. Vitamin D Beyond Bones in Chronic Obstructive Pulmonary Disease: Time to Act. Am J Respir Crit Care Med. 2009;179:630–636. doi: 10.1164/rccm.200810-1576PP. [DOI] [PubMed] [Google Scholar]
- 3.Koli K, Keski-Oja J. 1alpha,25-dihydroxyvitamin D3 and its analogues down-regulate cell invasion-associated proteases in cultured malignant cells. Cell Growth Differ. 2000;11:221–229. [PubMed] [Google Scholar]
- 4.Bao BY, Yeh SD, Lee YF. 1alpha,25-dihydroxyvitamin D3 inhibits prostate cancer cell invasion via modulation of selective proteases. Carcinogenesis. 2006;27:32–42. doi: 10.1093/carcin/bgi170. [DOI] [PubMed] [Google Scholar]
- 5.Timms PM, Mannan N, Hitman GA, et al. Circulating MMP9, vitamin D and variation in the TIMP-1 response with VDR genotype: mechanisms for inflammatory damage in chronic disorders? QJM. 2002;95:787–796. doi: 10.1093/qjmed/95.12.787. [DOI] [PubMed] [Google Scholar]
- 6.Connett JE, Kusek JW, Bailey WC, et al. Design of the Lung Health Study: a randomized clinical trial of early intervention for chronic obstructive pulmonary disease. Control Clin Trials. 1993;14:3S–19S. doi: 10.1016/0197-2456(93)90021-5. [DOI] [PubMed] [Google Scholar]
- 7.Anthonisen NR, Connett JE, Kiley JP, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA. 1994;272:1497–1505. [PubMed] [Google Scholar]
- 8.Anthonisen NR, Connett JE, Murray RP. Smoking and Lung Function of Lung Health Study Participants after 11 Years. Am J Respir Crit Care Med. 2002;166:675–679. doi: 10.1164/rccm.2112096. [DOI] [PubMed] [Google Scholar]
- 9.Anthonisen NR, Skeans MA, Wise RA, et al. The Effects of a Smoking Cessation Intervention on 14.5-Year Mortality: A Randomized Clinical Trial. Ann Intern Med. 2005;142:233–239. doi: 10.7326/0003-4819-142-4-200502150-00005. [DOI] [PubMed] [Google Scholar]
- 10.Enright PL, Johnson LR, Connett JE, et al. Spirometry in the Lung Health Study. 1. Methods and quality control. Am Rev Respir Dis. 1991;143:1215–1223. doi: 10.1164/ajrccm/143.6.1215. [DOI] [PubMed] [Google Scholar]
- 11.Crapo RO, Morris AH, Gardner RM. Reference spirometric values using techniques and equipment that meet ATS recommendations. Am Rev Respir Dis. 1981;123:659–664. doi: 10.1164/arrd.1981.123.6.659. [DOI] [PubMed] [Google Scholar]
- 12.Holick MF. Vitamin D Deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 13.Riancho JA, Gonzalez Macias J, Del Arco C, et al. Vertebral compression fractures and mineral metabolism in chronic obstructive lung disease. Thorax. 1987;42:962–966. doi: 10.1136/thx.42.12.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shane E, Silverberg SJ, Donovan D, et al. Osteoporosis in lung transplantation candidates with end-stage pulmonary disease. Am J Med. 1996;101:262–269. doi: 10.1016/S0002-9343(96)00155-6. [DOI] [PubMed] [Google Scholar]
- 15.Forli L, Halse J, Haug E, et al. Vitamin D deficiency, bone mineral density and weight in patients with advanced pulmonary disease. J Intern Med. 2004;256:56–62. doi: 10.1111/j.1365-2796.2004.01337.x. [DOI] [PubMed] [Google Scholar]
- 16.Ginde AA, Liu MC, Camargo CA., Jr Demographic Differences and Trends of Vitamin D Insufficiency in the US Population, 1988–2004. Arch Intern Med ; Arch Intern Med. 2009;169:626–632. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franco CB, Paz-Filho G, Gomes PE, et al. Chronic obstructive pulmonary disease is associated with osteoporosis and low levels of vitamin D. Osteoporos Int. 2009 doi: 10.1007/s00198-009-0890-5. [DOI] [PubMed] [Google Scholar]
- 18.Janssens W, Bouillon R, Claes B, et al. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax. 2010;65:215–220. doi: 10.1136/thx.2009.120659. [DOI] [PubMed] [Google Scholar]
- 19.de Batlle J, Romieu I, Antó JM, et al. Dietary habits of firstly admitted Spanish COPD patients. Respir Med. 2009;103:1904–1910. doi: 10.1016/j.rmed.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Cranney A, Weiler HA, O'Donnell S, et al. Summary of evidence-based review on vitamin D efficacy and safety in relation to bone health. Am J Clin Nutr. 2008;88:513S–519. doi: 10.1093/ajcn/88.2.513S. [DOI] [PubMed] [Google Scholar]
- 21.Ginde AA, Mansbach JM, Camargo CA., Jr Vitamin D, respiratory infections, and asthma. Curr Allergy Asthma Rep. 2009;9:81–87. doi: 10.1007/s11882-009-0012-7. [DOI] [PubMed] [Google Scholar]
- 22.Laaksi I, Ruohola J, Tuohimaa P, et al. An association of serum vitamin D concentrations < 40 nmol/L with acute respiratory tract infection in young Finnish men. Am J Clin Nutr. 2007;86:714–717. doi: 10.1093/ajcn/86.3.714. [DOI] [PubMed] [Google Scholar]
- 23.Aloia JF, Li-Ng M. Re: epidemic influenza and vitamin D. Epidemiol Infect. 2007;135:1095–6. doi: 10.1017/S0950268807008308. author reply 1097–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kendrick J, Targher G, Smits G, et al. 25-Hydroxyvitamin D deficiency is independently associated with cardiovascular disease in the Third National Health and Nutrition Examination Survey. Atherosclerosis. 2009;205:255–260. doi: 10.1016/j.atherosclerosis.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 25.Lee JH, O'Keefe JH, Bell D, et al. Vitamin D Deficiency: An Important, Common, and Easily Treatable Cardiovascular Risk Factor? Journal of the American College of Cardiology. 2008;52:1949–1956. doi: 10.1016/j.jacc.2008.08.050. [DOI] [PubMed] [Google Scholar]
- 26.Bischoff-Ferrari HA, Dietrich T, Orav EJ, et al. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged >=60 y. Am J Clin Nutr. 2004;80:752–758. doi: 10.1093/ajcn/80.3.752. [DOI] [PubMed] [Google Scholar]
- 27.Sato Y, Iwamoto J, Kanoko T, et al. Low-dose vitamin D prevents muscular atrophy and reduces falls and hip fractures in women after stroke: a randomized controlled trial. Cerebrovasc Dis. 2005;20:187–192. doi: 10.1159/000087203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


