Abstract
Propionibacterium acnes is a key pathogen involved in the progression of inflammation in acne vulgaris. We examined whether vaccination against P. acnes suppressed P. acnes-induced skin inflammation. Inactivation of P. acnes with heat was employed to create a P. acnes-based vaccine. Intranasal immunization in mice with this inactivated vaccine provoked specific antibodies against P. acnes. Most notably, immunization with inactivated vaccines generated in vivo protective immunity against P. acnes challenge and facilitated the resolution of ear inflammation in mice. In addition, antibodies elicited by inactivated vaccines effectively neutralized the cytotoxicity of P. acnes and attenuated the production of proinflammatory cytokine IL-8 in human sebocyte SZ95 cells. Intranasal immunization using heat-inactivated P. acnes-based vaccines provided a simple modality to develop acne vaccines. These observations highlight the concept that development of vaccines targeting microbial products may represent an alternative strategy to conventional antibiotic therapy.
INTRODUCTION
Propionibacterium acnes, a gram-positive anaerobic bacterium, is a member of the resident bacterial flora and mostly resides in pilosebaceous follicles. It has been known that the bacterium plays a critical role in the development of inflammatory acne vulgaris, which is the most common disease of human skin afflicting up to 80% of individuals through their lives (Cunliffe and Gollnick, 2001b; Leyden, 2001; Bojar and Holland, 2004). In inflammatory acne lesions, the follicular epithelium is damaged and a dermal inflammation occurs. So far, several mechanisms of developing acne associated with P. acnes have been proposed. The mechanism for the onset of acne can generally be divided into two stages. The first stage is comedo formation, which is characterized by the initiation of inflammatory events before hyperproliferation of the follicular epithelia (microcomedo) (Jeremy et al., 2003); the second stage is the occurrence of inflammation and disruption of follicular epithelia. Although the involvement of P. acnes in the initiation of comedogenesis is still controversial (Cunliffe and Gollnick, 2001a), they can aggravate or intensify abnormal desquamation once overgrowth and colonization of P. acnes in the microcomedo occur (Leeming et al., 1988; William J Cunliffe, 2001). P. acnes produces a number of extracellular enzymes and metabolites that can directly damage host tissues (Hoeffler, 1977; Holland et al., 1981; Cove et al., 1983; Hoffler et al., 1985). In addition, it was also widely accepted that inflammation in acne lesions may be induced by host immune reactions to P. acnes. Chemotactive substances released from the bacteria attract polymorphonuclear leukocytes (Webster and Leyden, 1980). It has also been reported that P. acnes stimulates production of proinflammatory cytokines, including IL-1β, IL-8, IL-12, and tumor necrosis factor-α. The P. acnes-induced cytokine productions have known to be mediated by toll-like receptor (TLR) 2 (Kim et al., 2002; Kim, 2005; Nagy et al., 2006).
Reduction in P. acnes numbers by antimicrobial agents correlates with clinical improvement of acne in patients, therefore antibiotic therapy has been a mainstay of treatment for acne over the past 25 years. However, P. acnes strains with clinically significant antibiotic resistance, and multiple drug resistance were identified from acne patients with long-term antibiotic treatment (Eady et al., 2003; Nord and Oprica, 2006). More recently, it has been demonstrated that biofilm formation by P. acnes increases resistance against antimicrobial agents (Coenye et al., 2007). In addition, P. acnes resists killing by phagocytes and is able to survive in macrophages (Webster et al., 1985b). These problems may be the roots of clinical failure to treat acne. In the cases of mild and severe acne, acne lesions are observed not only on the face, but also on the chest, shoulders, or back of the patient. In such cases, topical treatment with anti-acne agents in large areas or for long periods of time may produce side effects, and systemic antibiotic therapy may nonspecifically kill skin bacteria, which impacts the homeostasis of skin-resident microflora (Ochsendorf, 2006). Isotretinoin, 13-cis-retinoic acid, is a vitamin A-derived retinoid that has been widely prescribed for systemic treatment of severe acne. This agent acts primarily by decreasing the size of the sebaceous glands and reducing sebum production by up to 90%, thereby inhibiting the growth of P. acnes and subsequent inflammation (Clarke et al., 2007). However, its use has now been highly regulated because of its adverse effects, and it is not appropriate for most acne patients. Currently available treatments for acne lesions are often only palliative and minimally effective while treatment is maintained. In most cases, when treatments are discontinued, recurrence of acne often results. Therefore, acne is still one of the skin diseases that require alternative approach of treatments, although various antibiotics, medicines, and therapies for acne have been developed.
It has been demonstrated that IgG-coated bacteria were found in comedones of acne patients, suggesting that IgG in comedones was derived from the serum and selectively accumulated in the follicle (Knop et al., 1983). This observation inspired us to hypothesize that suppression of P. acnes by anti-P. acnes antibody may have a potential to prevent its progression and pathogenicity. In this study, we demonstrated protective effects of vaccination with heat-killed P. acnes on P. acnes-induced inflammation. More importantly, the model employing the production of proinflammatory cytokines from human sebocytes provides a relevance to the vaccine therapy for acne vulgaris. Furthermore, inactivated P. acnes-based vaccines afford a straight-forward modality for vaccine manufacturing.
RESULTS
P. acnes-induced inflammation in mice
To induce a model of P. acnes inflammation, we injected 107 colony-forming units (CFU) of living P. acnes intradermally into mouse ears. Significant cutaneous erythema (Figure 1a, left ear), ear swelling, and granulomatous response (Figure 1b) were observed in P. acnes-injected ear 24 hours after the bacterial injection, but neither of them were induced by phosphate-buffered saline (PBS) injection (Figure 1a, right ear, and c). Histological observation revealed that injection of P. acnes induced a considerable increase in the number of infiltrated inflammatory cells (Figure 1b).
Figure 1. The inflammation in mouse ears after P. acnes injection.
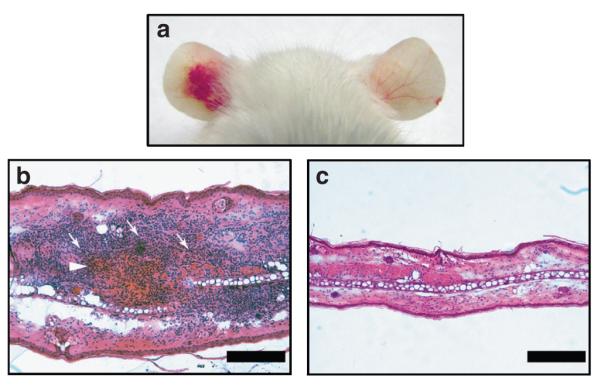
Ears of ICR mice were injected subcutaneously with 107 CFU per 20 μl of P. acnes (left ear) or 20 μl of PBS (right ear). (a) Inflammation-induced ear redness was visualized 24 hours after injection. (b, c) Increase in ear thickness and infiltrated inflammatory cells (arrows) surrounding the injected site of P. acnes (arrowhead) were observed in (b) a hematoxylin-and-eosin-stained frozen section of P. acnes-injected ear. (c) Staining PBS-injected ear serves as a control. Bars = 0.2 mm.
Immunogenicity of heat-killed P. acnes
To generate sufficient and specific antibody against P. acnes, mice were intranasally immunized with heat-killed P. acnes three times at a 3-week interval. Serum was collected 1 week after the third inoculation. Data from western blot indicated that two main components (approximately 64 and 250 kDa) of P. acnes were immunoreactive to antibodies elicited by heat-killed inactivated P. acnes (Figure 2, lane 1). No immunoreactivity to P. acnes lysates was detected if serum from PBS-injected mice was used (Figure 2, lane 3). In addition, antibodies in the sera from P. acnes-immunized mice did not cross-react with proteins in Staphylococcus epidermidis (ATCC 12228) (Figure 2, lane 2), indicating the specificity of anti-P. acnes antiserum. Intranasal administration also demonstrated the competence of inactivated P. acnes-based vaccines in inducing mucosal immunity.
Figure 2. Antibody production after injection with heat-killed P. acnes.
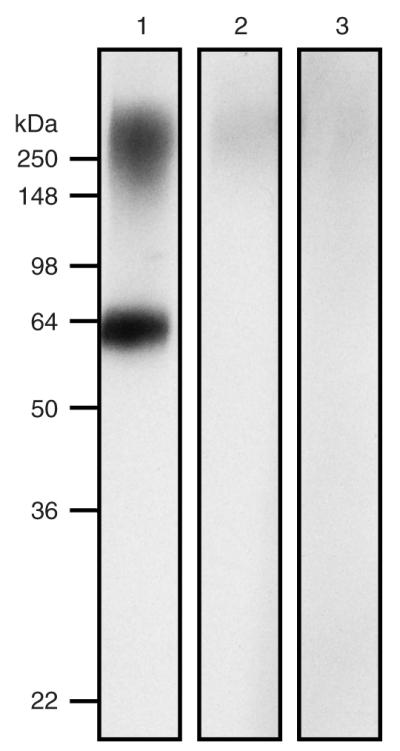
Lysate of P. acnes (7 μg) was separated by SDS-PAGE (10% acrylamide) and then subjected to western blotting using an antiserum obtained from heat-killed P. acnes (lane 1), S. epidermidis-immunized mice (lane 2), or PBS-injected mice (lane 3) as a primary antibody; immunoreactivity was developed using goat anti-mouse IgG/horseradish peroxidase complex and western lighting chemiluminescence kit (PerkinElmer, Boston, MA). Molecular weights (kDa) are indicated.
In vivo protective effect of inactivated P. acnes-based vaccines
To determine the protective immunity elicited by vaccination with heat-killed P. acnes, the ear of the vaccinated mouse was intradermally challenged with living P. acnes (1 × 107 CFU). The increase in P. acnes-induced ear thickness was recorded until thickness subsided (Figure 3). A biphasic pattern of changes in ear thickness was observed. In the PBS-inoculated mice, ear thickness increased rapidly more than two-fold (215.8%) on the first day, decreased on the second day, then rebounded (225.0%) on the seventh day, and recovered 78 days after bacterial challenge. The increase in ear thickness in both phases (P = 0.04 at 24 hours and P = 0.0013 on 7 days post-challenge) was significantly suppressed when mice were immunized with inactivated P. acnes-based vaccines. In P. acnes-immunized mice, the increase in ear thickness completely subsided 22 days after bacterial challenge. These data strongly demonstrated that vaccination with inactivated P. acnes-based vaccines suppressed the bacterial progression and facilitated the recovery of P. acnes-induced inflammation.
Figure 3. In vivo protective immunity in the mice immunized with inactivated P. acnes-based vaccines.
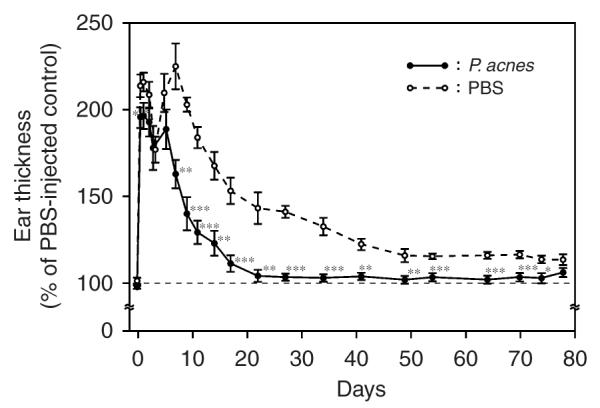
A total of 20 μl aliquot of living P. acnes (1 × 107 CFU) suspended in PBS was intradermally injected into left ears of mice immunized with heat-killed P. acnes or PBS (n = 8 or 7, respectively). As a control, an equal volume of PBS was injected into the right ears of the same mice. Ear thickness was measured with a micro-caliper at the indicated times post-bacterial challenge. The ear thickness of P. acnes-injected ear was calculated as % of a PBS-injected control. Bars represent mean±SE (*P<0.05, **P<0.005, ***P<0.0005 by Student’s t-test).
In vitro neutralization of P. acnes-induced proinflammatory cytokine production in human sebocytes
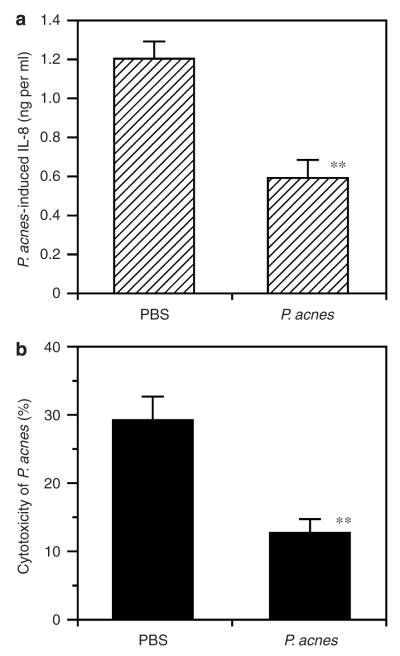
A human SZ-95 sebocyte was utilized with the aim of establishing a model for evaluating the potency of anti-P. acnes antiserum to neutralize the cytotoxicity of P. acnes. It has been shown that activation of TLR2 expressed in human sebocytes with P. acnes notably increased cytokine IL-8 production (Nagy et al., 2006). In addition, elevation of IL-8 and its gene expression was observed in skin biopsies of patients with inflammatory acne vulgaris (Trivedi et al., 2006; Abd El All et al., 2007). Thus, the capability of anti-P. acnes antiserum to neutralize P. acnes-induced IL-8 production in human sebocytes was examined. Sebocytes produced 1.2 ng ml−1 of IL-8 when they were treated with the bacteria that were pre-incubated with serum from PBS-inoculated mice (Figure 4a). By contrast, pre-incubation with anti-P. acnes antiserum effectively decreased P. acnes-induced IL-8 production to 0.59 ng ml−1 (P = 0.0015). Pre-incubation of 2 hours with antisera did not influence the growth of P. acnes as determined by CFU (Figure S2). These results suggest that anti-P. acnes antiserum attenuated P. acnes-induced IL-8 production in sebocytes without affecting bacterial survival. The ability of anti-P. acnes antiserum to neutralize the cytotoxicity of P. acnes was also determined. After incubation with serum from PBS-inoculated mice, P. acnes triggered 29.3% of sebocyte death (Figure 4b). On the other hand, the dead sebocytes were dramatically reduced to 12.7% (P = 0.003) when cells were treated with neutralized P. acnes. These data indicated that antibodies evoked by inactivated P. acnes-based vaccines efficiently counteracted the P. acnes-induced inflammation.
Figure 4. Neutralization of cytotoxicity of P. acnes and P. acnes-induced IL-8 production in human sebocytes by anti-P. acnes antiserum.
P. acnes was pre-incubated with anti-P. acnes antiserum or anti-PBS control serum (2.5% (v/v)), in which complements were deactivated by heating for 2 hours. (a) For neutralization of IL-8 production, the immortalized human sebocytes, SZ-95 (3 × 106 cells), were co-cultured with 300 μl of the pre-incubation mixtures containing 1.5 × 108 CFU of P. acnes and 7.5 μl antiserum for 8 hours. Measurement of IL-8 in the culture medium was carried out by ELISA assays. P. acnes-induced IL-8 production was calculated as the difference of amount between with and without P. acnes.(b) For neutralization of cytotoxicity of P. acnes, the sebocytes (2 × 105 cells per well) were co-cultured with 100 μl of the neutralization reaction mixtures containing 2 × 106 CFU of P. acnes and 2.5 μl antiserum for 18 hours. As a control, an equal amount of PBS was added instead of P. acnes. As a background, Triton-X was added to get a final concentration of 0.1% (v/v) to kill sebocytes. After incubation, cell viability of sebocytes was determined with P-nitrophenyl phosphate disodium, and cytotoxicity of neutralizing mixture was calculated as follows: (no P. acnes group–P. acnes added group)÷***(no P. acnes group–background group) × 100 (%). Bars represent mean±SE (n = 5). Data were analyzed by Student’s t-test (**P<0.005).
DISCUSSION
In acne lesions, a partially occluded follicle creates an ideal anaerobic environment for P. acnes to multiply. Consequently, the increase of P. acnes and its enzymes, virulence factors, and pattern recognition ligands stimulated the skin resulting in inflammation and acne lesions (Leyden, 2001; William J Cunliffe, 2001; Bojar and Holland, 2004). Our results indicated that injection of P. acnes into ICR mouse ears induced an increase in the ear thickness (Figure 1) and granulomatous response (Figure 1b). One day after injection, we observed that P. acnes was surrounded by a densely packed granulomatous infiltrate. Although ears injected with S. epidermidis (ATCC 12228; 108 CFU), an aerobic skin commensal, induced a minor swelling, this swelling rapidly subsided within 4 days (data not shown). Although multiple injections of P. acnes into mouse ears may cause tissue necrosis, it is worth investigating whether TLR2 tolerance induced by a repeat of intraperitoneal injection of P. acnes could alter host sensitivity to bacterial infection (Medvedev et al., 2006). Mice produce little or no triglycerides, a fact that has hindered the development of an animal model for studying the lipogenesis in acne lesions (Webster et al., 1981b). The rabbit ear model has a lack of bacterial colonization and inflammation (Mirshahpanah and Maibach, 2007). In addition, the use of rabbits may be inconvenient for vast vaccinations. Rhino mice with utricles that create larger follicle size have been widely used for screening anti-acne drugs (Takaoki and Kawaji, 1980). However, as Rhino mice with deficits in immune system cannot produce antibodies against thymus-dependent antigens (Takaoki and Kawaji, 1980), we thus created inflammatory responses in an ICR mouse strain as an animal model (De Young et al., 1984, 1985) to evaluate the potency of inactivated P. acnes-based vaccines. In most cases of acne, P. acnes is thought to colonize on the skin surface and/or within sebaceous follicles. Bacteria could enter the dermis once the follicular wall was ruptured (Kligman, 1974). Injection of P. acnes into mouse ears may represent an animal model for the granulomatous type of acne inflammation that follows follicular rupture.
Many vaccine development approaches are under investigation, but the one straightforward method is the use of intranasally administered killed whole pathogen preparations. It has known that P. acnes itself is a potent immunomodulator (Mussalem et al., 2006). Our data illustrated that mice immunized with heat-killed P. acnes produced antibodies against two P. acnes-specific proteins with molecular weights at approximately 64 and 250 kDa. Antibodies against P. acnes can be frequently found in severe acne patients (Webster et al., 1985a). In some cases, there is a correlation between the severity of acnes and titers of antibodies against P. acnes in patients’ sera (Holland et al., 1986; Ingham et al., 1987; Ashbee et al., 1997). The antibodies are generated against P. acnes exocellular enzymes, or cell wall/membrane fractions, such as polysaccharide, carbohydrate, or membrane-binding proteins in acne patients (Dalen et al., 1980; Iversen et al., 1985; Webster et al., 1985a; Ingham et al., 1987; Lodes et al., 2006). However, acne lesions recurred often, despite antibodies against P. acnes having been generated in those patients. One possible explanation could be that those acne patients may not produce sufficient protective antibodies against key virulence factors of P. acnes for the suppression of bacterial progression and the prevention of recurrence. Additionally, it is worth examining whether acne patients produced antibodies against the two antigens (approximately 64 and 250 kDa) that were elicited in the mice vaccinated with heat-killed P. acnes (Figure 2). As mucosal vaccination with most inactivated organisms induced a relatively weak immune response, adjuvant such as Vibrio cholerae cholera toxin or E. coli heat-labile enterotoxin was frequently administrated along with antigen to nasal cavity. However, these adjuvants may cause adverse effects (Spangler, 1992). The high immunogenicity of heat-killed P. acnes without adjuvants suggested that inactivated P. acnes exerted a self-adjuvant effect. Most importantly, the use of inactivated P. acnes-based vaccines eliminates the time-consuming steps required for antigen purification. Furthermore, intranasal immunization circumvents the intrinsic problems associated with multiple needle injections. Thus, P. acnes-based vaccines are beneficial for large-scale and rapid vaccine production.
The vaccination effectively suppressed P. acnes-induced ear swelling and expedited the recovery of ear inflammation (Figure 3). Ear thickness was measured regularly for 78 days, revealing a biphasic ear-swelling pattern. This is consistent with previous findings illustrating a biphasic change in the activity of the mouse reticuloendothelial system after intraperitoneal injection with phenol-treated P. acnes (Kobayashi et al., 1980). The biphasic pattern was interpreted by the fluctuation in the number of macrophages and other host cells in the inflamed skins. We also vaccinated ICR mice with heat-killed P. acnes (1 × 108 CFU) one day after intradermally challenging with living P. acnes (1 × 107 CFU) (Figure S1). Although ear swelling was suppressed during vaccination, a substantial increase in ear thickness still existed 22 days post-bacterial challenge. The result was different with that of mice vaccinated with heat-killed P. acnes for 10 weeks (Figure 3) before bacterial challenge. Ear swelling in mice vaccinated for 10 weeks was rapidly subsided 22 days after bacterial challenge. One possible explanation for this distinction is that vaccination after bacterial challenge may not evoke sufficient antibodies to effectively combat bacteria. It has been documented that inflammation in acne lesions was initiated by the production of proinflammatory cytokines after interaction of P. acnes to TLR2 (Kim et al., 2002; Kim, 2005; Nagy et al., 2006). In addition, it has been known that activation of TLR2 expressed in human sebocytes, one of target cells of P. acnes, with P. acnes released tremendous cytokine IL-8 (Nagy et al., 2006; Abd El All et al., 2007). The prevalence of P. acnes in the sebocytes of sebaceous glands is highly associated with acne progression. Sebocytes were involved in skin immunity by producing antimicrobial substances and proinflammatory cytokines (Nagy et al., 2006). Our data revealed that anti-P. acnes antiserum effectively neutralized the cytotoxicity of P. acnes and lowered P. acnes-induced IL-8 production in human sebocytes. The peptideglycan–polysaccharide complexes and lipoteichoic acids, two major cell wall components in the gram-positive bacteria, can stimulate the release of proinflammatory cytokines from monocytes (Mattsson et al., 1993). Webster et al. (1985a) demonstrated that a carbohydrate-structured component of P. acnes was highly antigenic in severe acne patients. Moreover, Basal et al. (2004) found a major 96 kDa antigenic component, which stimulated the production of IL-8 and tumor necrosis factor-α from human peripheral blood mononuclear cells. Taken together, inactivated P. acnes-based vaccines may diminish inflammation via blocking the TLR2 signaling in sebocytes. Ingham et al. (1987) detected antibodies against P. acnes exocellular enzymes, that is, lipase and hyaluronate lyase, in normal subjects and acne patients. They, however, could not find any differences of the antibody titers between these two groups, suggesting that the amounts of antigens secreted from P. acnes existing in the sebaceous follicles of acne patient may not be enough for humans to elevate antibody titers. Thus, boosting sufficient antibodies against P. acnes may be a future direction to develop therapeutic acne vaccines.
It has been reported that mice administrated with P. acnes enhanced the resistance to influenza virus (Gangemi et al., 1983) and tumor formation (Murano and Cummins, 1989; Mussalem et al., 2006) as well as augmented phagocytic activities (Webster et al., 1979, 1981a; Gangemi et al., 1983). These data implied that inactivated P. acnes vaccines may increase host immunity. Adversely, vaccination targeting whole bacterium may lack specificity (Kobayashi et al., 1980). Genomic (Bruggemann et al., 2004; Bruggemann, 2005) and/or proteomics approached may help in antigen selection for the development of component acne vaccines. We recently selected a surface sialidase from the P. acnes genome as an antigen for the development of a component acne vaccine (Nakatsuji et al., 2008). The bacterial specificities of P. acnes- and sialidase-based vaccines will be addressed in the future studies.
Data from bacterial counting on agar plates showed that P. acnes did not change its growth after incubation with anti-P. acnes antiserum (Figure S2). Additionally, counting bacteria from homogenized ear skins indicated that intranasal vaccination did not statically alter the colonization of P. acnes (data not shown). To confirm this phenomenon, we counted P. acnes in fluids extracted from an implanted tissue chamber in vaccinated and nonvaccinated mice (Nakatsuji et al., 2008). Consistently, vaccination did not change the multiplication of P. acnes in mice (Figure S3). These results suggested that intranasal immunization with inactivated P. acnes-based vaccines may not produce sufficient bactericidal antibodies. On the other hand, the immunization with an acne vaccine that has no bactericidal action may perform no risk of destroying the balance of skin microflora. Although the killed strains of P. acnes and/or Staphlococci have been used as acne vaccines and named as acnevac or autovaccines (Zaluga, 1998), their potency in inhibiting the production of proinflammatory cytokines in human skin cells is undetermined. In conclusion, we demonstrated that vaccination with inactivated P. acnes suppressed inflammation in vitro and in vivo. The fact that antibodies effectively suppressed the production of proinflammatory cytokine IL-8 in human sebocytes highlights inactivated P. acnes-based vaccines as a novel treatment for acne vulgaris.
MATERIALS ANS METHODS
Culture of P. acnes
Propionibacterium acnes ATCC 6919 (American Type Culture Collection, Manassas, VA) was cultured on Brucella broth agar (BD, Sparks, MD), supplemented with 5% (v/v) defibrinated sheep blood (LAMPIRE Biological Laboratories, Pipersville, PA), vitamin K (5 μgml−1, Remel, Lenexa, KS), and hemin (50 μgml−1, Remel, Lenexa, KS), under an anaerobic condition using Gas-Pak (BD, Sparks, MD) at 37°C. Single colony was inoculated in Reinforced Clostridium Medium (Oxford, Hampshire, England) and cultured at 37°C until reaching OD600 = 1.0–3.0 (logarithmic growth phase) under the anaerobic condition. Bacteria were harvested by centrifugation at 5,000 × g for 10 minutes, washed with PBS three times, and suspended to appropriate amount of PBS for the experiments.
Intranasal vaccination of mice with heat-killed P. acnes
Female 8-week-old ICR mice (Harlan, Indianapolis, IN) were used in all experiments. Mice were housed according to institutional guidelines. P. acnes was suspended in PBS and inactivated by heating bacteria at 60°C for 30 minutes. After inactivation, P. acnes was unable to grow on an agar plate (data not shown). Inactivated P. acnes was harvested by centrifuging at 5,000 × g for 5 minutes and re-suspended to appropriate amount of PBS. An amount of 25 μl aliquot of inactivated-P. acnes suspension (1 × 108 CFU) was intranasally inoculated to an ICR mouse every 3 weeks. The second and third boosts were given at the same manner as the first inoculation. Control mice were inoculated with an equal volume of PBS or S. epidermidis (ATCC 12228).
Propionibacterium acnes-induced inflammation
An amount of 20 μl aliquots of living P. acnes (1 × 107 CFU) suspended in PBS was intradermally injected in the central portion of the left ear. As a control, 20 μl of PBS was injected into the right ear of the same mice. For histological observation, the ear was cross-sectioned, stained with hematoxylin and eosin (Sigma diagnostics, St Louis, MO), and viewed on a Zeiss Axioskop2 plus microscope (Carl Zeiss, Thornwood, NY). To examine in vivo protective effects, ears of immunized mice and their controls were challenged with P. acnes. The increase in ear thickness was measured using a micro caliper (Mitutoyo, Japan) after the bacterial challenge. The increase in ear thickness of P. acnes-challenged ear was calculated as % of a PBS-injected control.
Detection of antibody against P. acnes by western blotting
After centrifugation, P. acnes pellet was suspended in 8 M urea, homogenized by beating with 0.1 mm glass beads for 2 minutes, diluted with sample buffer (125 mM Tris-HCl buffer, pH 6.8, containing 4% (w/v) SDS, 10% (w/v) glycerol, 5% (v/v) 2-mercaptoethanol and 0.002% bromophenol blue), and boiled for 2 minutes. The sample (7 μg proteins) was electrophoresed in 10% (w/v) polyacrylamide gel (Bio-Rad, Hercules, CA) and electrophoretically transferred onto poly-vinyliden difluoride membranes (Millipore, Billerica, MA) for 60 minutes at a current of 100 V. The membranes were pre-incubated for 30 minutes in PBS containing 5% (w/v) skim milk, and then incubated with anti-P. acnes antiserum or control sera (1:1,000 dilution) at 4°C overnight. Bound antibodies were detected with goat anti-rabbit peroxidase conjugated IgG (1:5,000 dilution, Promega, Madison, WI), and the peroxidase activity was developed with Western lighting chemiluminescence kit (PerkinElmer, Boston, MA).
In vitro neutralization
The immortalized human sebocyte line, SZ95 (Zouboulis et al., 1999), was cultured in Sebomed basal medium (Biochrom, Berlin, Germany) supplemented with 5 ng ml−1 human recombinant epidermal growth factor (Sigma, St Louis, MO), 10% (v/v) heat-inactivated fetal bovine serum (Mediatech Inc., Herndon, VA), at 37°C under an atmosphere of 5% (v/v) CO2 in air. P. acnes was cultured as described above, washed with PBS by centrifuging, and pre-incubated with 2.5% (v/v) anti-P. acnes antiserum or control sera, in which complements were deactivated by heating at 56°C for 30 minutes, at 37°C for 2 hours. For neutralization of IL-8 production, the sebocytes (3 × 106 cells) were co-cultured with 300 μl of the pre-incubation mixtures containing 1.5 × 108 CFU of P. acnes and 7.5 μl antiserum for 8 hours. As a background, sebocytes were incubated with antiserum alone. After centrifuging to remove bacteria, the concentrations of IL-8 in the culture medium were determined by ELISA using a Quantikine human IL-8 set (R & D Systems Inc., Minneapolis, MN). P. acnes-induced IL-8 production was calculated as the difference of amounts between with and without P. acnes. For neutralization of cytotoxicity of P. acnes, sebocytes (2 × 105 cells per well) were co-cultured with 100 μl of a pre-incubation mixture containing 2 × 106 CFU of P. acnes and 2.5 μl of antiserum for 18 hours. As a control, an equal amount of PBS was added instead of P. acnes. As a background, Triton-X was added to get a final concentration of 0.1% (v/v) to lyse sebocytes. After incubation, cell viability of sebocytes was determined with acid phosphatase assay (Martin and Clynes, 1991). Cells were washed with PBS three times and incubated with 100 μl of 10 mm P-nitrophenyl phosphate in acid phosphatase assay buffer (1 M sodium acetate buffer, pH 5.5, containing 0.1% (w/v) Triton X-100) for 1 hour at 37°C. Then, 10 μl of 1 n NaOH was added to stop the reaction, and absorbance at 405 nm was measured. Cytotoxicity of neutralizing mixture was calculated as follows: (the OD405 difference between without and with P. acnes treatment)÷(the OD405 difference between without P. acnes and with Triton-X treatment) × 100 (%).
Statistical analysis
Data are presented as mean±SE. The Student’s t-test was used to assess the significance of independent experiments. The criterion P<0.05 was used to determine statistical significance.
Supplementary Material
Figure S1. Vaccination after bacterial challenge moderately decreased P. acnes-induced ear swelling.
Figure S2. Incubation of anti-P. acnes antiserum did not affect the growth of P. acnes.
Figure S3. Vaccination did not influence the growth of P. acnes in tissue chambers.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health Grants (R01-AI067395-01, R21-R022754-01, R21-I58002-01 (C.-M.H.) and P30-AI36214-12S1 (Y-TL)). We thank Drs Kenshi Yamasaki and Amanda Büchau for their critical reading of the manuscript.
Abbreviations
- CFU
colony-forming units
- PBS
phosphate-buffered saline
- TLR
Toll-like receptor
Footnotes
CONFLICT OF INTEREST The authors state no conflict of interest.
REFERENCES
- Abd El All HS, Shoukry NS, El Maged RA, Ayada MM. Immunohis-tochemical expression of interleukin 8 in skin biopsies from patients with inflammatory acne vulgaris. Diagn Pathol. 2007;2:4. doi: 10.1186/1746-1596-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashbee HR, Muir SR, Cunliffe WJ, Ingham E. IgG subclasses specific to Staphylococcus epidermidis and Propionibacterium acnes in patients with acne vulgaris. Br J Dermatol. 1997;136:730–3. [PubMed] [Google Scholar]
- Basal E, Jain A, Kaushal GP. Antibody response to crude cell lysate of Propionibacterium acnes and induction of pro-inflammatory cytokines in patients with acne and normal healthy subjects. J Microbiol. 2004;42:117–25. [PubMed] [Google Scholar]
- Bojar RA, Holland KT. Acne and Propionibacterium acnes. Clin Dermatol. 2004;22:375–9. doi: 10.1016/j.clindermatol.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Bruggemann H. Insights in the pathogenic potential of Propionibacterium acnes from its complete genome. Semin Cutan Med Surg. 2005;24:67–72. doi: 10.1016/j.sder.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Bruggemann H, Henne A, Hoster F, Liesegang H, Wiezer A, Strittmatter A, et al. The complete genome sequence of Propionibacterium acnes, a commensal of human skin. Science. 2004;305:671–3. doi: 10.1126/science.1100330. [DOI] [PubMed] [Google Scholar]
- Clarke SB, Nelson AM, George RE, Thiboutot DM. Pharmacologic modulation of sebaceous gland activity: mechanisms and clinical applications. Dermatol Clin. 2007;25:137–46. v. doi: 10.1016/j.det.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Coenye T, Peeters E, Nelis HJ. Biofilm formation by Propionibacterium acnes is associated with increased resistance to antimicrobial agents and increased production of putative virulence factors. Res Microbiol. 2007;158:386–92. doi: 10.1016/j.resmic.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Cove JH, Holland KT, Cunliffe WJ. Effects of oxygen concentration on biomass production, maximum specific growth rate and extracellular enzyme production by three species of cutaneous propionibacteria grown in continuous culture. J Gen Microbiol. 1983;129:3327–34. doi: 10.1099/00221287-129-11-3327. [DOI] [PubMed] [Google Scholar]
- Cunliffe WJ, Gollnick HP. Follicular keratinization. In: Cunliffe WJ, Gollnick HP, editors. Acne: Diagnosis and Management. Martin Dunitz; Kent: 2001a. pp. 15–28. [Google Scholar]
- Cunliffe WJ, Gollnick HP. Microbiology of acne. In: Cunliffe WJ, Gollnick HP, editors. Acne: Diagnosis and Management. Martin Dunitz; Kent: 2001b. pp. 29–36. [Google Scholar]
- Dalen A, Hellgren L, Iversen OJ, Vincent J. Antibodies against extractable components from Propionibacterium acnes in humans with and without acne vulgaris. Arch Dermatol Res. 1980;269:253–9. doi: 10.1007/BF00406418. [DOI] [PubMed] [Google Scholar]
- De Young LM, Spires DA, Ballaron SJ, Cummins CS, Young JM, Allison AC. Acne-like chronic inflammatory activity of Propionibacterium acnes preparations in an animal model: correlation with ability to stimulate the reticuloendothelial system. J Invest Dermatol. 1985;85:255–8. doi: 10.1111/1523-1747.ep12276732. [DOI] [PubMed] [Google Scholar]
- De Young LM, Young JM, Ballaron SJ, Spires DA, Puhvel SM. Intradermal injection of Propionibacterium acnes: a model of inflammation relevant to acne. J Invest Dermatol. 1984;83:394–8. doi: 10.1111/1523-1747.ep12264715. [DOI] [PubMed] [Google Scholar]
- Eady EA, Gloor M, Leyden JJ. Propionibacterium acnes resistance: a worldwide problem. Dermatology. 2003;206:54–6. doi: 10.1159/000067822. [DOI] [PubMed] [Google Scholar]
- Gangemi JD, Hightower JA, Jackson RA, Maher MH, Welsh MG, Sigel MM. Enhancement of natural resistance to influenza virus in lipopolysaccharide-responsive and -nonresponsive mice by Propionibacterium acnes. Infect Immun. 1983;39:726–35. doi: 10.1128/iai.39.2.726-735.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffler U. Enzymatic and hemolytic properties of Propionibacterium acnes and related bacteria. J Clin Microbiol. 1977;6:555–8. doi: 10.1128/jcm.6.6.555-558.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffler U, Gehse M, Gloor M, Pulverer G. Enzyme production of propionibacteria from patients with acne vulgaris and healthy persons. Acta Derm Venereol. 1985;65:428–32. [PubMed] [Google Scholar]
- Holland DB, Ingham E, Gowland G, Cunliffe WJ. IgG subclasses in acne vulgaris. Br J Dermatol. 1986;114:349–51. doi: 10.1111/j.1365-2133.1986.tb02826.x. [DOI] [PubMed] [Google Scholar]
- Holland KT, Ingham E, Cunliffe WJ. A review, the microbiology of acne. J Appl Bacteriol. 1981;51:195–215. doi: 10.1111/j.1365-2672.1981.tb01234.x. [DOI] [PubMed] [Google Scholar]
- Ingham E, Gowland G, Ward RM, Holland KT, Cunliffe WJ. Antibodies to P. acnes and P. acnes exocellular enzymes in the normal population at various ages and in patients with acne vulgaris. Br J Dermatol. 1987;116:805–12. doi: 10.1111/j.1365-2133.1987.tb04899.x. [DOI] [PubMed] [Google Scholar]
- Iversen OJ, Dalen AB, Svindland HB. Isolation of an acidic polysaccharide antigen from Propionibacterium acnes. Arch Dermatol Res. 1985;277:225–9. doi: 10.1007/BF00404321. [DOI] [PubMed] [Google Scholar]
- Jeremy AH, Holland DB, Roberts SG, Thomson KF, Cunliffe WJ. Inflammatory events are involved in acne lesion initiation. J Invest Dermatol. 2003;121:20–7. doi: 10.1046/j.1523-1747.2003.12321.x. [DOI] [PubMed] [Google Scholar]
- Kim J. Review of the innate immune response in acne vulgaris: activation of Toll-like receptor 2 in acne triggers inflammatory cytokine responses. Dermatology. 2005;211:193–8. doi: 10.1159/000087011. [DOI] [PubMed] [Google Scholar]
- Kim J, Ochoa MT, Krutzik SR, Takeuchi O, Uematsu S, Legaspi AJ, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002;169:1535–41. doi: 10.4049/jimmunol.169.3.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kligman AM. An overview of acne. J Invest Dermatol. 1974;62:268–87. doi: 10.1111/1523-1747.ep12676801. [DOI] [PubMed] [Google Scholar]
- Knop J, Ollefs K, Frosch PJ. Anti-P. acnes antibody in comedonal extracts. J Invest Dermatol. 1983;80:9–12. doi: 10.1111/1523-1747.ep12530860. [DOI] [PubMed] [Google Scholar]
- Kobayashi F, Nagoya T, Koshi T, Saino Y. Biphasic protection against bacterial infection in mice induced by vaccination of Propionibacterium acnes. Infect Immun. 1980;27:391–6. doi: 10.1128/iai.27.2.391-396.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeming JP, Holland KT, Cuncliffe WJ. The microbial colonization of inflamed acne vulgaris lesions. Br J Dermatol. 1988;118:203–8. doi: 10.1111/j.1365-2133.1988.tb01775.x. [DOI] [PubMed] [Google Scholar]
- Leyden JJ. The evolving role of Propionibacterium acnes in acne. Semin Cutan Med Surg. 2001;20:139–43. doi: 10.1053/sder.2001.28207. [DOI] [PubMed] [Google Scholar]
- Lodes MJ, Secrist H, Benson DR, Jen S, Shanebeck KD, Guderian J, et al. Variable expression of immunoreactive surface proteins of Propionibacterium acnes. Microbiology. 2006;152:3667–81. doi: 10.1099/mic.0.29219-0. [DOI] [PubMed] [Google Scholar]
- Martin A, Clynes M. Acid phosphatase: endpoint for in vitro toxicity tests. In Vitro Cell Dev Biol. 1991;27A:183–4. doi: 10.1007/BF02630912. [DOI] [PubMed] [Google Scholar]
- Mattsson E, Verhage L, Rollof J, Fleer A, Verhoef J, van Dijk H. Peptidoglycan and teichoic acid from Staphylococcus epidermidis stimulate human monocytes to release tumour necrosis factor-alpha, interleukin-1 beta and interleukin-6. FEMS Immunol Med Microbiol. 1993;7:281–7. doi: 10.1111/j.1574-695X.1993.tb00409.x. [DOI] [PubMed] [Google Scholar]
- Medvedev AE, Sabroe I, Hasday JD, Vogel SN. Tolerance to microbial TLR ligands: molecular mechanisms and relevance to disease. J Endotoxin Res. 2006;12:133–50. doi: 10.1179/096805106X102255. [DOI] [PubMed] [Google Scholar]
- Mirshahpanah P, Maibach HI. Models in acnegenesis. Cutan Ocul Toxicol. 2007;26:195–202. doi: 10.1080/15569520701502815. [DOI] [PubMed] [Google Scholar]
- Murano EA, Cummins CS. Role of respiratory-burst products from polymorphonuclear leukocytes in the antitumor activity of Propionibacterium acnes vaccine. Cancer Immunol Immunother. 1989;29:7–16. doi: 10.1007/BF00199910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussalem JS, Vasconcelos JR, Squaiella CC, Ananias RZ, Braga EG, Rodrigues MM, et al. Adjuvant effect of the Propionibacterium acnes and its purified soluble polysaccharide on the immunization with plasmidial DNA containing a Trypanosoma cruzi gene. Microbiol Immunol. 2006;50:253–63. doi: 10.1111/j.1348-0421.2006.tb03791.x. [DOI] [PubMed] [Google Scholar]
- Nagy I, Pivarcsi A, Kis K, Koreck A, Bodai L, McDowell A, et al. Propionibacterium acnes and lipopolysaccharide induce the expression of antimicrobial peptides and proinflammatory cytokines/chemokines in human sebocytes. Microbes Infect. 2006;8:2195–205. doi: 10.1016/j.micinf.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Nakatsuji T, Liu YT, Huang CP, Gallo RL, Huang CM. Vaccination targeting a surface sialidase of P. acnes: implication for new treatment of acne vulgaris. PLoS ONE. 2008;3:e1551. doi: 10.1371/journal.pone.0001551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nord CE, Oprica C. Antibiotic resistance in Propionibacterium acnes. Microbiological and clinical aspects. Anaerobe. 2006;12:207–10. doi: 10.1016/j.anaerobe.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Ochsendorf F. Systemic antibiotic therapy of acne vulgaris. J Dtsch Dermatol Ges. 2006;4:828–41. doi: 10.1111/j.1610-0387.2006.06053.x. [DOI] [PubMed] [Google Scholar]
- Spangler BD. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiol Rev. 1992;56:622–47. doi: 10.1128/mr.56.4.622-647.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaoki M, Kawaji H. Impaired antibody response against T-dependent antigens in rhino mice. Immunology. 1980;40:27–32. [PMC free article] [PubMed] [Google Scholar]
- Trivedi NR, Gilliland KL, Zhao W, Liu W, Thiboutot DM. Gene array expression profiling in acne lesions reveals marked upregulation of genes involved in inflammation and matrix remodeling. J Invest Dermatol. 2006;126:1071–9. doi: 10.1038/sj.jid.5700213. [DOI] [PubMed] [Google Scholar]
- Webster GF, Indrisano JP, Leyden JJ. Antibody titers to Propionibacterium acnes cell wall carbohydrate in nodulocystic acne patients. J Invest Dermatol. 1985a;84:496–500. doi: 10.1111/1523-1747.ep12273462. [DOI] [PubMed] [Google Scholar]
- Webster GF, Leyden JJ. Characterization of serum-independent polymorphonuclear leukocyte chemotactic factors produced by Propionibacterium acnes. Inflammation. 1980;4:261–9. doi: 10.1007/BF00915027. [DOI] [PubMed] [Google Scholar]
- Webster GF, Leyden JJ, Musson RA, Douglas SD. Susceptibility of Propionibacterium acnes to killing and degradation by human neutrophils and monocytes in vitro. Infect Immun. 1985b;49:116–21. doi: 10.1128/iai.49.1.116-121.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster GF, Leyden JJ, Nilsson UR. Complement activation in acne vulgaris: consumption of complement by comedones. Infect Immun. 1979;26:183–6. doi: 10.1128/iai.26.1.183-186.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster GF, Nilsson UR, McArthur WP. Activation of the alternative pathway of complement in human serum by Propionibacterium acnes (Corynebacterium parvum) cell fractions. Inflammation. 1981a;5:165–76. doi: 10.1007/BF00914205. [DOI] [PubMed] [Google Scholar]
- Webster GF, Ruggieri MR, McGinley KJ. Correlation of Propionibacterium acnes populations with the presence of triglycerides on nonhuman skin. Appl Environ Microbiol. 1981b;41:1269–70. doi: 10.1128/aem.41.5.1269-1270.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- William J, Cunliffe HPG. Microbiology of acne. In: William J, Cunliffe HPG, editors. Acne. Martin Dunitz; Kent: 2001. pp. 29–36. [Google Scholar]
- Zaluga E. Skin reactions to antigens of Propionibacterium acnes in patients with acne vulgaris treated with autovaccine. Ann Acad Med Stetin. 1998;44:65–85. [PubMed] [Google Scholar]
- Zouboulis CC, Seltmann H, Neitzel H, Orfanos CE. Establishment and characterization of an immortalized human sebaceous gland cell line (SZ95) J Invest Dermatol. 1999;113:1011–20. doi: 10.1046/j.1523-1747.1999.00771.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Vaccination after bacterial challenge moderately decreased P. acnes-induced ear swelling.
Figure S2. Incubation of anti-P. acnes antiserum did not affect the growth of P. acnes.
Figure S3. Vaccination did not influence the growth of P. acnes in tissue chambers.