Abstract
Merkel cell carcinoma (MCC) is an uncommon cutaneous malignant tumor that presents as a rapidly growing skin nodule on sun-exposed areas of the body. MCC is aggressive with regional nodal and distant metastases to the skin, lung, and bones. There have been no reports of metastatic MCC to the mesentery and 6 reports describing metastasis to the small intestine. We present a case of metastatic MCC to the mesentery with infiltration to the small bowel, 8 years after original tumor resection. This is the 5th metastasis and it encased the small bowel resulting in a hair-pin loop contributing to the unusual clinical presentation. Although MCC metastatic to the bowel is uncommon, it is not rare. It is important to recognize the unusual manifestations of this disease as they are becoming more common in the future. Routine radiologic surveillance and thorough review of systems are important to patient follow-up.
Key words: Merkel cell carcinoma, mesentery, small intestine, metastasis, Cytokeratin 20.
Introduction
Merkel cell carcinoma of the skin (MCC) was first described by Toker1 in 1972. It is a rare, aggressive cutaneous malignancy that predominantly affects elderly Caucasians and immunocompromised populations. The incidence of MCC has increased in the last 15 years, yet its pathogenesis is still debated.2,3 Ultraviolet radiation,2,4,5 immunosuppression,4,7 and a novel polyomavirus6 have been implicated as important etiologic agents. The clinical presentation of MCC is nonspecific, with the majority of cases presenting as a rapidly growing, painless, firm, shiny, flesh-colored to bluish-red, intracutaneous nodule on a sun-exposed surface of the body. Histologically, MCC usually arises in the dermis and extends into the subcutis with infrequent epidermal involvement. The differential of this small blue cell tumor includes other poorly differentiated neoplastic tumors such as small cell carcinoma of the lung, lymphoma, metastatic carcinoid, amelanotic melanoma and Ewing sarcoma. MCC is defined by both neuroendocrine and epithelial differentiation as demonstrated by immunopositivity for perinuclear cytokeratin 20 expression and neuroendocrine markers like chromogranin and synaptophysin. The disease course is difficult to predict and ranges from relatively indolent to highly aggressive, with a propensity for local recurrence, regional lymph node metastases as well as metastasis to distant sites. Overall, the prognosis for patients with MCC is poor with 5-year survival rates of ∼75%, 59%, and 25% for localized, regional, and distant MCC, respectively. Metastasis usually occurs in the skin, lymph nodes, liver, lung, bone, and the brain. Metastasis can also involve the gastrointestinal tract in sporadic cases. Due to the rarity of the disease, there are no randomized treatment trials for MCC, hence no standardized treatment exists. At present, treatment options include surgery alone or with adjuvant radiation therapy, and systemic chemotherapy reserved for recurrent or disseminated disease. Earlier detection of this neoplasm with multidisciplinary management may achieve a better prognosis and an improved outcome as demonstrated by the following case.
Clinical features and histologic findings
The patient is a 73-year old Caucasian man with a past medical history most significant for coronary artery disease, hypertension, and diabetes mellitus who presented with a raised, violacious skin lesion of the middle third of his left radial forearm in December of 2001. A punch biopsy at an outside institution revealed MCC. A 2.0 cm wide local excision of the biopsy scar and tumor bed was performed along with sentinel lymph node biopsy of the left epitrochlear and left axillary basins. The margins were widely negative, as were all four sentinel nodes. The patient was treated postoperatively with adjuvant radiation to the primary site and 4 cycles of carboplatin and etoposide.
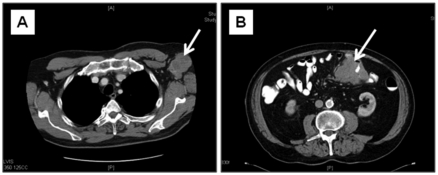
One year after surgery, a follow-up CT scan of the chest, abdomen, and pelvis was within normal limits. However, a CT scan of the chest in May of 2003 revealed a large, 5.6×4.2 cm left axillary mass along with an adjacent 1.9×1.8 cm mass (Figure 1A). Fine needle aspiration of the larger mass was positive for metastatic MCC. With no evidence of distant metastases, the patient underwent a level III left axillary lymph node dissection. Histologic examination showed metastatic MCC to four of twenty (4/20) discrete and matted lymph nodes. Microscopically, the normal lymph node architecture had been replaced by a population of monotonous, round tumor cells with frequent apoptotic nuclei and mitotic figures. These cells had scant eosinophilic cytoplasm as well as round and vesicular nuclei with finely granular chromatin and multiple nucleoli. Adjuvant radiation therapy was administered to the axilla, and the patient was followed at regular intervals. In October of 2003, the patient developed a 1.4×1.2×0.6 cm left anterior chest wall mass at the margin of his radiation port, which again was confirmed to be MCC on fine needle aspiration. He underwent a wide local excision of the left anterior chest wall and the left inferior axilla. The margins of resection were negative, and the patient again received postoperative radiation therapy, this time to the chest wall and mediastinum, with concurrent taxol-based chemotherapy.
Figure 1.
Preoperative computed tomography scans of the patients metastatic lesions. A scan from May 2003 demonstrates a 5.6 cm lobulated mass (white arrow) in the left axilla (A). In May 2009, a CT scan reveals a short segment of circumferential mural thickening of the jejunum and an eccentric, contiguous 4.3 cm serosal-based mass (arrow) with no evidence of bowel obstruction (B).
The patient was followed every 6 months with pelvic and abdominal CT scans. These tests were unremarkable until April of 2007, when he presented with a 6.0×5.6×4.5 cm right anterior chest wall mass deep to the pectoralis muscle. Fine needle aspiration of the mass was positive for metastatic MCC. The patient was taken to the operating room for a fourth time for radical resection of the right chest wall mass with en bloc resection of the pectoralis major and minor muscles. Histologic examination demonstrated metastatic MCC to one of two (1/2) lymph nodes with negative deep and peripheral margins. Postoperatively, the patient received additional adjuvant radiation therapy. PET imaging in November of 2007 showed increased metabolic activity in 2 sites: the mid-right anterolateral chest wall and in the lower left anterolateral abdominal wall near the anterior iliac spine. Radical resection of two soft tissue masses was performed with abdominal reconstruction using Prolene mesh. Postoperative histologic examination confirmed both masses to be metastatic MCC with negative margins.
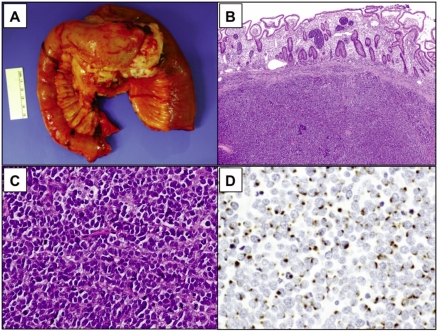
In May of 2009, the patient presented with intermittent, crampy abdominal pain associated with decreased appetite and a 15 pound weight loss. A CT scan of the abdomen revealed a new, enhancing lesion encasing the distal jejunum and proximal ileum (Figure 1B). The patient underwent laparoscopic-assisted jejunal resection. On gross examination, the small bowel was remarkable for a 10.5×9.5×8.0 cm lobulated mesenteric tumor forming a hair-pin loop in the bowel (Figure 2A). Microscopically, there was an infiltrate of small blue neoplastic cells undermining the mucosa (Figure 2B). The morphology and cytology of these tumor cells was identical to that previously identified in the lymph node (Figure 2C). In addition, perinuclear dot-like expression of CK20 (Figure 2D) confirmed the diagnosis of MCC metastatic to the mesentery and small bowel. All resection margins were free of tumor. The patient was discharged after an uneventful, three-day hospital stay.
Figure 2.
Macroscopic and histologic appearance of metastatic Merkel cell carcinoma. In May 2009, the patient complained of vague abdominal pain and was found to have a 6.5 cm mesenteric mass involving the jejunum and ileum and resulting in a hair-pin loop (A). Low power (B; H&E, ×20) and high power (C; H&E, ×200) microscopic examination demonstrates the small intestinal wall is infiltrated by small, blue neoplastic cells focally involving the mucosa. There is a monotonous population of small, loosely cohesive tumor cells. The cells have round to oval nuclei, well-defined nuclear membranes, finely granular chromatin with inconspicuous nucleoli, scanty eosinophilic cytoplasm and frequent apoptotic nuclei and mitotic figures. Immunohistochemistry performed on the tumor mass shows dot-like perinuclear expression of cytokeratin 20 in the neoplastic cells, confirming the diagnosis of metastatic Merkel cell carcinoma (D; CK20, ×200).
Discussion
MCC is an uncommon skin cancer of neuroendocrine origin effecting primarily elderly Caucasians which has a high rate of local recurrence and regional metastases first described by Toker in 1972.1 Despite being rare, the incidence of MCC has tripled over the last 15 years with nearly 1,200 new cases diagnosed in the US each year.2,3 The pathogenesis of MCC is not fully understood. MCC is thought to arise from Merkel cells that are located in the basal layer of the epidermis, however the majority of MCCs are intradermal and only 10% arise in the epidermis. In addition, several factors such as ultraviolet (UV) radiation exposure,2,4,5 Merkel cell polyomavirus,6 and immunosuppression4,7 have been associated with the development of MCC.
The classic initial presentation of MCC is a painless, indurated, solitary dermal nodule with a slightly erythematous to deeply violaceous color, on sun-exposed areas of the body. Because of the benign, nonspecific clinical appearance of the initial lesion, MCC is rarely suspected prior to biopsy. The majority of primary MCC lesions occur in the head and neck region, but a large proportion also involve the extremities.1,3 MCC arises in the dermis, can extend into the subcutaneous tissue, and can infiltrate locally via dermal lymphatics, resulting in multiple satellite lesions. Metastasis is most common to the distant lymph nodes and skin, lung, central nervous system and bone, in order of decreasing frequency.5 As spread of MCC occurs via lymphatic drainage, nodal status appears to be the strongest predictor of distant metastases.8
Metastasis to the mesenteric soft tissue has not been reported and metastasis to the bowel being extremely rare, with only six cases described to date. Naunton Morgan et al. reported the first case in 1985 of a 42-year-old man with an MCC skin lesion on his right shin which first metastasized to his right femoral lymph nodes.9 The patient later presented with melena and anemia. A barium follow-through study revealed a stricture of the proximal jejunum caused by a 6.5×5×4 cm tumor which was found to be metastatic MCC. Canales et al. encountered a case of metastatic MCC to the stomach and duodenum from a right buttock lesion.10 Foster et al. reported a male with MCC of the right cheek which spread to the small bowel, as well as to the right lung, right adrenal gland, and left perirenal space.11 Shalhub et al. described a 62 year old man who presented with right axillary and left groin lymphadenopathy and a colonic mass consistent with metastatic MCC. The patient's primary neck lesion had been excised 6 years earlier. One month after presentation, the patient developed metastasis to his stomach, duodenum, and right hip.12 In 2007, Hizawa et al. described the case of a woman with MCC of the right eyelid who presented with an intraabdominal mass directly invading the stomach, pancreas, and distal duodenum.13 Most recently, Cheung et al. described the case of a woman who presented with a 2 cm lesion in the right antecubital fossa followed by metastatic MCC to the ileocecal valve 18 months after initial diagnosis.14
Due to the rarity of the disease, there have been no randomized trials for the treatment of MCC and current therapies are based on observational studies alone. At presentation, 60–75% of patients with MCC have only local disease, 10–25% have regional lymph node involvement and 2–8% have metastatic disease.5,15 Although metastasis is uncommon at presentation, ∼33–50% of patients with MCC will eventually develop systemic metastasis.5 Due to the high propensity for local recurrence and metastasis, MCC has been cited as having an overall 5-year survival rate of ∼60% with only 50% of patients with advanced disease alive at 9 months. The disparity in survival is most likely due to early detection, aggressive treatment and a shift from monotherapy to a multidisciplinary approach. Outcomes are correlated with location and size of the primary lesion, regional metastasis, gender and age.2,16 However, despite improvements in survival, optimal treatment of MCC has yet to be well established. Currently, surgical treatment centers on margin-negative excision of the primary tumor.15,16 Surgical staging with sentinel lymph node biopsy has also been shown to be important, as up to one-third of patients with clinically negative lymph nodes had lymph node metastases.17 Adjuvant radiation therapy also plays a significant role in MCC treatment by reducing recurrence and improving local/ regional control of disease.3,15,18 Thus, for localized disease, surgical excision with adjuvant radiotherapy is recommended, achieving a local control rate of 60–90%. Treatment of distant metastases involves palliative surgery, radiotherapy, and chemotherapy. Despite its use, the role of chemotherapy remains controversial because MCC has been found to be chemosensitive, but chemotherapy rarely results in a cure. Close radiologic follow-up is recommended for patients with MCC due to the high recurrence rate even with aggressive therapy. Nuclear studies are used for sentinel lymph node biopsy for initial staging of MCC, and positron emission tomography (PET) imaging is helpful in monitoring treatment response and recurrence.
In conclusion, we report a case of MCC with metastases found in five separate sites despite aggressive surgical treatment combined with adjuvant radiation and chemotherapy. The most recent metastasis was to the mesentery with infiltration of the small bowel, which is a very rare site of MCC spread. Therefore, it is important to recognize metastatic MCC as a potential source of abdominal symptoms in patients with a previous diagnosis of MCC. Additionally, this case highlights the malignant nature of MCC and emphasizes the need for close follow-up despite aggressive treatment.
References
- 1.Toker C. Trabecular carcinoma of the skin. Arch Dermatol. 1972;105:107–10. [PubMed] [Google Scholar]
- 2.Agelli M, Clegg LX. Epidemiology of primary Merkel cell carcinoma in the United States. J Am Acad Dermatol. 2003;49:832–41. doi: 10.1016/s0190-9622(03)02108-x. [DOI] [PubMed] [Google Scholar]
- 3.Albores-Saavedra J, Batich K, Chable-Montero F, et al. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population based study. J Cutan Pathol. 2010;37:20–7. doi: 10.1111/j.1600-0560.2009.01370.x. [DOI] [PubMed] [Google Scholar]
- 4.Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375–81. doi: 10.1016/j.jaad.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhan FQ, Packianathan VS, Zeitouni NC. Merkel cell carcinoma: a review of current advances. J Natl Compr Canc Netw. 2009;7:333–9. doi: 10.6004/jnccn.2009.0025. [DOI] [PubMed] [Google Scholar]
- 6.Feng H, Shuda M, Chang Y, et al. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096–100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Penn I, First MR. Merkel's cell carcinoma in organ recipients: report of 41 cases. Transplantation. 1999;68:1717–21. doi: 10.1097/00007890-199912150-00015. [DOI] [PubMed] [Google Scholar]
- 8.Gupta SG, Wang LC, Peñas PF, et al. Sentinel lymph node biopsy for evaluation and treatment of patients with Merkel cell carcinoma: The Dana-Farber experience and meta-analysis of the literature. Arch Dermatol. 2006;142:685–90. doi: 10.1001/archderm.142.6.685. [DOI] [PubMed] [Google Scholar]
- 9.Naunton Morgan TC, Henderson RG. Small bowel metastases from a merkel cell tumour. Br J Radiol. 1985;58:1212–3. doi: 10.1259/0007-1285-58-696-1212. [DOI] [PubMed] [Google Scholar]
- 10.Canales LI, Parker A, Kadakia S. Upper gastrointestinal bleeding from merkel cell carcinoma. Am J Gastroenterol. 1992;87:1464–6. [PubMed] [Google Scholar]
- 11.Foster R, Stevens G, Egan M. An unusual pattern of metastases from merkel cell carcinoma. Australas Radiol. 1994;38:231–2. doi: 10.1111/j.1440-1673.1994.tb00184.x. [DOI] [PubMed] [Google Scholar]
- 12.Shalhub S, Clarke L, Morgan M. Metastatic merkel cell carcinoma masquerading as colon cancer. Gastrointest Endosc. 2004;60:856–8. doi: 10.1016/s0016-5107(04)02173-x. [DOI] [PubMed] [Google Scholar]
- 13.Hizawa K, Kurihara S, Nakamori M, et al. An autopsy case of Merkel cell carcinoma presenting aggressive intraabdominal metastasis and duodenal obstruction. Nippon Shokakibyo Gakkai Zasshi. 2007;104:1383–6. [PubMed] [Google Scholar]
- 14.Cheung M, Lee H, Purkayastha S, et al. Ileocaecal recurrence of Merkel cell carcinoma of the skin: a case report. J Med Case Reports. 2010;4:43–7. doi: 10.1186/1752-1947-4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allen PJ, Bowne WB, Jaques DP, et al. Merkel cell carcinoma: prognosis and treatment of patients from a single institution. J Clin Oncol. 2005;23:2300–9. doi: 10.1200/JCO.2005.02.329. [DOI] [PubMed] [Google Scholar]
- 16.Tai PT, Yu E, Tonita J, et al. Merkel cell carcinoma of the skin. J Cutan Med Surg. 2000;4:186–95. doi: 10.1177/120347540000400403. [DOI] [PubMed] [Google Scholar]
- 17.Senchenkov A, Barnes SA, Moran SL. Predictors of survival and recurrence in the surgical treatment of merkel cell carcinoma of the extremities. J Surg Oncol. 2007;95:229–34. doi: 10.1002/jso.20647. [DOI] [PubMed] [Google Scholar]
- 18.Mojica P, Smith D, Ellenhorn JD. Adjuvant radiation therapy is associated with improved survival in Merkel cell carcinoma of the skin. J Clin Oncol. 2007;25:1043–7. doi: 10.1200/JCO.2006.07.9319. [DOI] [PubMed] [Google Scholar]