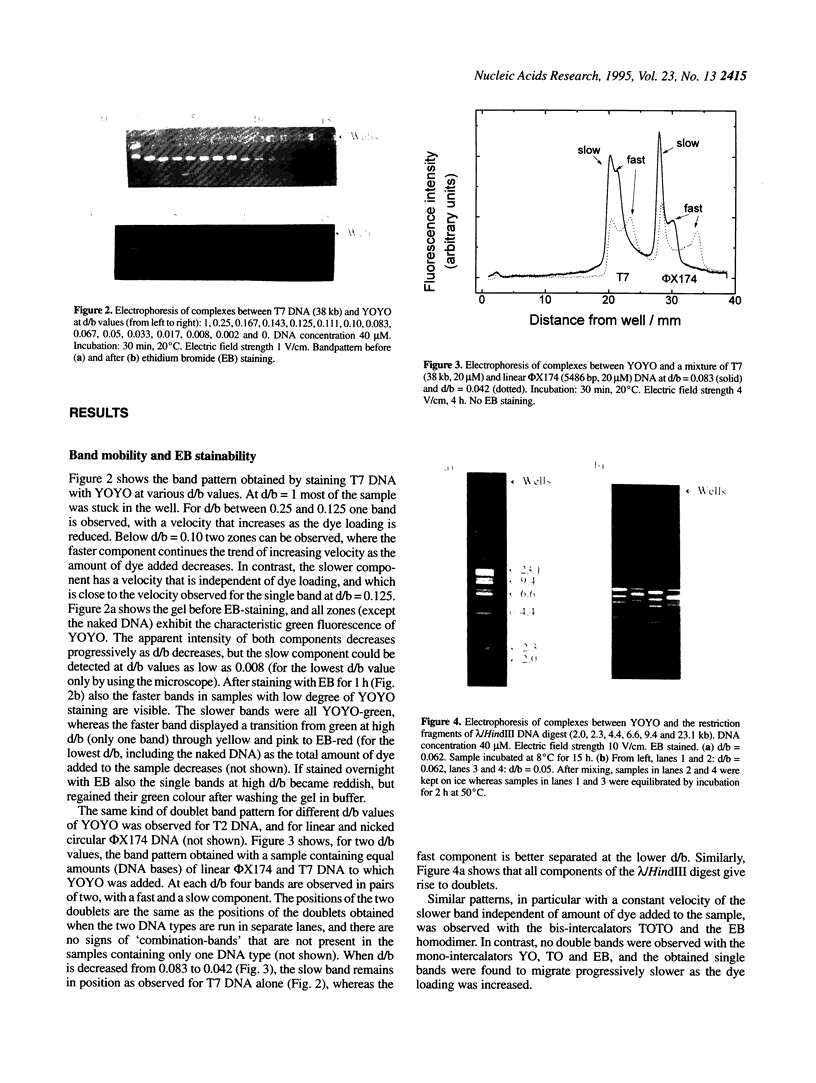
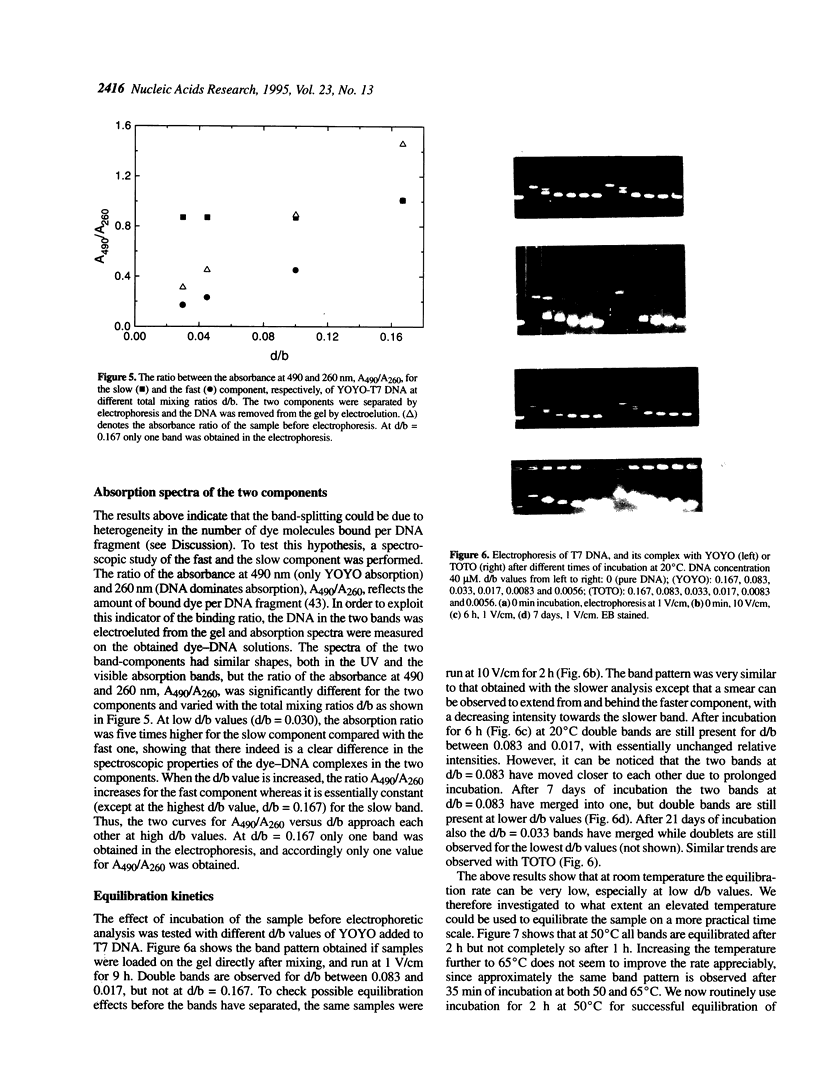
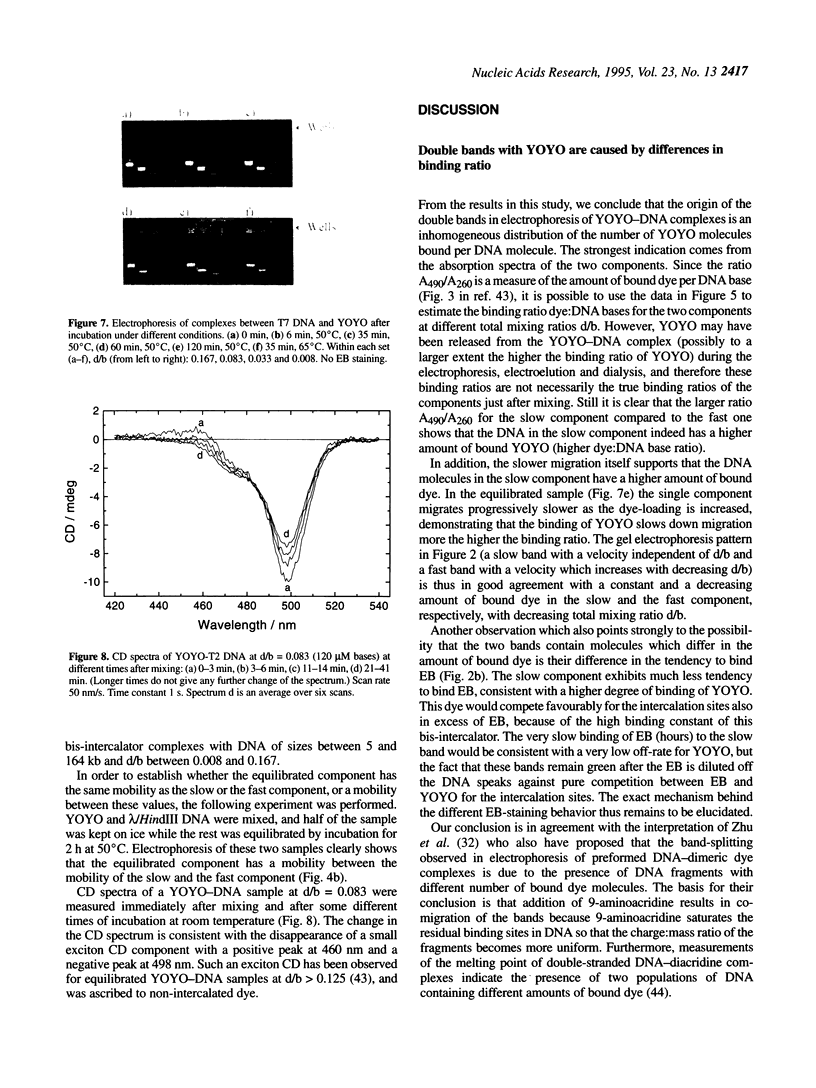
Abstract
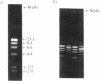
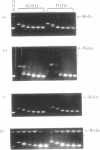
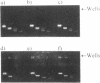
Many bis-intercalating dyes used for fluorescence detection of DNA in electrophoresis have been reported to give band-splitting and band-broadening, which results in poor resolution and a decreased detection sensitivity. We have studied the dimeric dye YOYO-1, and to some extent also TOTO-1 and EthD-1, and found that in complex with DNA these dyes give rise to two components with different electrophoretic mobilities. Electrophoresis experiments and spectroscopic measurements on the two components show that they differ in that the DNA molecules have different amounts of dye bound. Our results exclude that the extra bands are caused by intermolecular cross-linking. Incubation of the samples for increasing times before electrophoresis makes the bands move closer and closer to each other as the dye molecules become more homogeneously distributed among the DNA molecules. Finally, the two bands merge into one at an intermediate position. This equilibration process is extremely slow at room temperature (days), and is therefore not a practical method to eliminate band-splitting in routine analysis. However, we find that if the temperature is raised to 50 degrees C, the dye-DNA complexes equilibrate completely in only 2 h.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addess K. J., Sinsheimer J. S., Feigon J. Solution structure of a complex between [N-MeCys3,N-MeCys7]TANDEM and [d(GATATC)]2. Biochemistry. 1993 Mar 16;32(10):2498–2508. doi: 10.1021/bi00061a006. [DOI] [PubMed] [Google Scholar]
- Annan N. K., Cook P. R., Mullins S. T., Lowe G. Evidence for cross-linking DNA by bis-intercalators with rigid and extended linkers is provided by knotting and catenation. Nucleic Acids Res. 1992 Mar 11;20(5):983–990. doi: 10.1093/nar/20.5.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assa-Munt N., Denny W. A., Leupin W., Kearns D. R. 1H NMR study of the binding of Bis(acridines) to d(AT)5.d(AT)5. 1. Mode of binding. Biochemistry. 1985 Mar 12;24(6):1441–1449. doi: 10.1021/bi00327a024. [DOI] [PubMed] [Google Scholar]
- Auzanneau I., Barreau C., Salomé L. Imaging by fluorescence videomicroscopy of individual single stranded DNA molecules in solution. C R Acad Sci III. 1993;316(5):459–462. [PubMed] [Google Scholar]
- Benson S. C., Mathies R. A., Glazer A. N. Heterodimeric DNA-binding dyes designed for energy transfer: stability and applications of the DNA complexes. Nucleic Acids Res. 1993 Dec 11;21(24):5720–5726. doi: 10.1093/nar/21.24.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D. F., Wolf M. K. AGGREGATION OF DYES BOUND TO POLYANIONS. Proc Natl Acad Sci U S A. 1959 Jul;45(7):944–952. doi: 10.1073/pnas.45.7.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canellakis E. S., Shaw Y. H., Hanners W. E., Schwartz R. A. Diacridines: bifunctional intercalators. I. Chemistry, physical chemistry and growth inhibitory properties. Biochim Biophys Acta. 1976 Feb 5;418(3):277–289. doi: 10.1016/0005-2787(76)90290-2. [DOI] [PubMed] [Google Scholar]
- Crater G. D., Gregg M. R., Holzwarth G. Mobility surfaces for field-inversion gel electrophoresis of linear DNA. Electrophoresis. 1989 May-Jun;10(5-6):310–315. doi: 10.1002/elps.1150100507. [DOI] [PubMed] [Google Scholar]
- Delepierre M., Milhe C., Namane A., Dinh T. H., Roques B. P. 1H- and 31P-NMR studies of ditercalinium binding to a d(GCGC)2 and d(CCTATAGG)2 minihelices: a sequence specificity study. Biopolymers. 1991 Feb 15;31(3):331–353. doi: 10.1002/bip.360310307. [DOI] [PubMed] [Google Scholar]
- Gao Q., Williams L. D., Egli M., Rabinovich D., Chen S. L., Quigley G. J., Rich A. Drug-induced DNA repair: X-ray structure of a DNA-ditercalinium complex. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2422–2426. doi: 10.1073/pnas.88.6.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaugain B., Barbet J., Capelle N., Roques B. P., Le Pecq J. B. DNA Bifunctional intercalators. 2. Fluorescence properties and DNA binding interaction of an ethidium homodimer and an acridine ethidium heterodimer. Biochemistry. 1978 Nov 28;17(24):5078–5088. doi: 10.1021/bi00617a002. [DOI] [PubMed] [Google Scholar]
- Geierstanger B. H., Mrksich M., Dervan P. B., Wemmer D. E. Design of a G.C-specific DNA minor groove-binding peptide. Science. 1994 Oct 28;266(5185):646–650. doi: 10.1126/science.7939719. [DOI] [PubMed] [Google Scholar]
- Glazer A. N., Peck K., Mathies R. A. A stable double-stranded DNA-ethidium homodimer complex: application to picogram fluorescence detection of DNA in agarose gels. Proc Natl Acad Sci U S A. 1990 May;87(10):3851–3855. doi: 10.1073/pnas.87.10.3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer A. N., Rye H. S. Stable dye-DNA intercalation complexes as reagents for high-sensitivity fluorescence detection. Nature. 1992 Oct 29;359(6398):859–861. doi: 10.1038/359859a0. [DOI] [PubMed] [Google Scholar]
- Hirons G. T., Fawcett J. J., Crissman H. A. TOTO and YOYO: new very bright fluorochromes for DNA content analyses by flow cytometry. Cytometry. 1994 Feb 1;15(2):129–140. doi: 10.1002/cyto.990150206. [DOI] [PubMed] [Google Scholar]
- Huang C. H., Mirabelli C. K., Mong S., Crooke S. T. Intermolecular cross-linking of DNA through bifunctional intercalation of an antitumor antibiotic, luzopeptin A (BBM-928A). Cancer Res. 1983 Jun;43(6):2718–2724. [PubMed] [Google Scholar]
- Kim Y., Morris M. D. Separation of nucleic acids by capillary electrophoresis in cellulose solutions with mono- and bis-intercalating dyes. Anal Chem. 1994 Apr 1;66(7):1168–1174. doi: 10.1021/ac00079a035. [DOI] [PubMed] [Google Scholar]
- McFadyen W. D., Wakelin L. P., Roos I. A., Hillcoat B. L. Binuclear platinum (II)-terpyridine complexes. A new class of bifunctional DNA-intercalating agent. Biochem J. 1986 Sep 15;238(3):757–763. doi: 10.1042/bj2380757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen P. E., Zhen W. P., Henriksen U., Buchardt O. Sequence-influenced interactions of oligoacridines with DNA detected by retarded gel electrophoretic migrations. Biochemistry. 1988 Jan 12;27(1):67–73. doi: 10.1021/bi00401a012. [DOI] [PubMed] [Google Scholar]
- Ogura M., Mitsuhashi M. Screening method for a large quantity of polymerase chain reaction products by measuring YOYO-1 fluorescence on 96-well polypropylene plates. Anal Biochem. 1994 May 1;218(2):458–459. doi: 10.1006/abio.1994.1207. [DOI] [PubMed] [Google Scholar]
- Ohler L. D., Zollo M., Mansfield E. S., Rose E. A. Use of a sensitive fluorescent intercalating dye to detect PCR products of low copy number and high molecular weight. PCR Methods Appl. 1993 Oct;3(2):115–119. doi: 10.1101/gr.3.2.115. [DOI] [PubMed] [Google Scholar]
- Peek M. E., Lipscomb L. A., Bertrand J. A., Gao Q., Roques B. P., Garbay-Jaureguiberry C., Williams L. D. DNA distortion in bis-intercalated complexes. Biochemistry. 1994 Apr 5;33(13):3794–3800. doi: 10.1021/bi00179a002. [DOI] [PubMed] [Google Scholar]
- Perkins T. T., Quake S. R., Smith D. E., Chu S. Relaxation of a single DNA molecule observed by optical microscopy. Science. 1994 May 6;264(5160):822–826. doi: 10.1126/science.8171336. [DOI] [PubMed] [Google Scholar]
- Perkins T. T., Smith D. E., Chu S. Direct observation of tube-like motion of a single polymer chain. Science. 1994 May 6;264(5160):819–822. doi: 10.1126/science.8171335. [DOI] [PubMed] [Google Scholar]
- Popa L. M., Winter S., Löber G. Some new properties of DNA-YOYO-3 homodimer complexes revealed by electrophoresis and fluorescence lifetime measurements. Biochem Mol Biol Int. 1994 Dec;34(6):1189–1196. [PubMed] [Google Scholar]
- Reese H. R. Effects of DNA charge and length on the electrophoretic mobility of intercalated DNA. Biopolymers. 1994 Oct;34(10):1349–1358. doi: 10.1002/bip.360341007. [DOI] [PubMed] [Google Scholar]
- Rye H. S., Quesada M. A., Peck K., Mathies R. A., Glazer A. N. High-sensitivity two-color detection of double-stranded DNA with a confocal fluorescence gel scanner using ethidium homodimer and thiazole orange. Nucleic Acids Res. 1991 Jan 25;19(2):327–333. doi: 10.1093/nar/19.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rye H. S., Yue S., Quesada M. A., Haugland R. P., Mathies R. A., Glazer A. N. Picogram detection of stable dye-DNA intercalation complexes with two-color laser-excited confocal fluorescence gel scanner. Methods Enzymol. 1993;217:414–431. doi: 10.1016/0076-6879(93)17080-o. [DOI] [PubMed] [Google Scholar]
- Rye H. S., Yue S., Wemmer D. E., Quesada M. A., Haugland R. P., Mathies R. A., Glazer A. N. Stable fluorescent complexes of double-stranded DNA with bis-intercalating asymmetric cyanine dyes: properties and applications. Nucleic Acids Res. 1992 Jun 11;20(11):2803–2812. doi: 10.1093/nar/20.11.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen N. C. Effect of the electric field on the apparent mobility of large DNA fragments in agarose gels. Biopolymers. 1985 Dec;24(12):2243–2255. doi: 10.1002/bip.360241207. [DOI] [PubMed] [Google Scholar]
- Wakelin L. P. Polyfunctional DNA intercalating agents. Med Res Rev. 1986 Jul-Sep;6(3):275–340. doi: 10.1002/med.2610060303. [DOI] [PubMed] [Google Scholar]
- Wakelin L. P., Romanos M., Chen T. K., Glaubiger D., Canellakis E. S., Waring M. J. Structural limitations on the bifunctional intercalation of diacridines into DNA. Biochemistry. 1978 Nov 14;17(23):5057–5063. doi: 10.1021/bi00616a031. [DOI] [PubMed] [Google Scholar]
- Wang A. H., Ughetto G., Quigley G. J., Hakoshima T., van der Marel G. A., van Boom J. H., Rich A. The molecular structure of a DNA-triostin A complex. Science. 1984 Sep 14;225(4667):1115–1121. doi: 10.1126/science.6474168. [DOI] [PubMed] [Google Scholar]
- Waring M. J. Complex formation between ethidium bromide and nucleic acids. J Mol Biol. 1965 Aug;13(1):269–282. doi: 10.1016/s0022-2836(65)80096-1. [DOI] [PubMed] [Google Scholar]
- Waring M. J., Wakelin L. P., Lee J. S. A solvent-partition method for measuring the binding of drugs to DNA. Application to the quinoxaline antibiotics echinomycin and triostin A. Biochim Biophys Acta. 1975 Oct 1;407(2):200–212. doi: 10.1016/0005-2787(75)90285-3. [DOI] [PubMed] [Google Scholar]
- Wilson W. D., Keel R. A., Jones R. L., Mosher C. W. Viscometric analysis of the interaction of bisphenanthridinium compounds with closed circular supercoiled and linear DNA. Nucleic Acids Res. 1982 Jul 10;10(13):4093–4106. doi: 10.1093/nar/10.13.4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright R. G., Wakelin L. P., Fieldes A., Acheson R. M., Waring M. J. Effects of ring substituents and linker chains on the bifunctional intercalation of diacridines into deoxyribonucleic acid. Biochemistry. 1980 Dec 9;19(25):5825–5836. doi: 10.1021/bi00566a026. [DOI] [PubMed] [Google Scholar]
- Zhang X. L., Patel D. J. Solution structure of the luzopeptin-DNA complex. Biochemistry. 1991 Apr 23;30(16):4026–4041. doi: 10.1021/bi00230a030. [DOI] [PubMed] [Google Scholar]
- Zhu H., Clark S. M., Benson S. C., Rye H. S., Glazer A. N., Mathies R. A. High-sensitivity capillary electrophoresis of double-stranded DNA fragments using monomeric and dimeric fluorescent intercalating dyes. Anal Chem. 1994 Jul 1;66(13):1941–1948. doi: 10.1021/ac00085a004. [DOI] [PubMed] [Google Scholar]