Abstract
SMOC1 and SMOC2 are matricellular proteins thought to influence growth factor signaling, migration, proliferation and angiogenesis. We examined the expression and regulation of Smoc1 and Smoc2 in fetal gonad/mesonephros complexes to discover possible roles for these genes in gonad and mesonephros development. Smoc1 was upregulated at ~E10.75 in a center-to-poles wave in pre-Sertoli and pre-granulosa cells and its expression was greatly reduced in Wt1, Sf1 and Fog2 mutants. After E13.5, Smoc1 was downregulated in an anterior-to-posterior wave in granulosa cells but persisted in Sertoli cells, suggesting a sexually dimorphic requirement in supporting cell lineage differentiation. Smoc2 was expressed in Leydig cells, mesonephroi, and Wnt4 mutant ovaries, but not wildtype ovaries. Using organ culture, we determined that Smoc2 expression was dependent on Hedgehog signaling in testes, mesonephroi, and kidneys. Overall, these results demonstrate that SMOC1 and SMOC2 may mediate intercellular signaling and cell type-specific differentiation during gonad and reproductive tract development.
Keywords: gonad development, ovary, testis, reproductive tract, Smoc1, Smoc2, Hedgehog, Sertoli cell, Leydig cell, granulosa cell
INTRODUCTION
SMOC1 (SPARC related modular calcium binding 1) and SMOC2 (SPARC related modular calcium binding 2) are two recently identified matricellular proteins that belong to the SPARC (Secreted acidic cysteine rich glycoprotein; also known as BM-40/osteonectin) protein family, which includes SPARCL1 (SPARC-like1, SC1/hevin), FSTL1 (Follistatin-like 1, TSC-36), SPOCK1, SPOCK2 and SPOCK3 (Sparc/osteonectin, cwcv and kazal-like domains proteoglycan 1–3, testican1-3). Matricellular proteins are thought to influence a variety of cellular functions including growth factor signaling, cell migration, cell adhesion and proliferation (Bornstein and Sage, 2002). SPARC is the best characterized member of this class and has been shown to interfere with receptor-mediated signaling of many growth factors including PDGF (Raines et al., 1992), VEGF (Kupprion et al., 1998), FGF2 (Motamed et al., 2003), IGF1 (Francki et al., 2003), and TGFβ (Francki et al., 2004). SMOC1 and SMOC2 are widely expressed in the embryonic and adult mouse with SMOC1 expressed mainly in organ basement membranes (Vannahme et al., 2002; Gersdorff et al., 2006) and SMOC2 expressed mainly in the extracellular matrix (Vannahme et al., 2003; Maier et al., 2008).
Recent studies have begun to uncover the molecular functions of SMOC1 and SMOC2. SMOC1 has been shown to inhibit BMP signaling downstream of the BMPR1B receptor during Xenopus development, and a loss-of-function mutant results in developmental arrest at neurulation (Thomas et al., 2009). Studies in vitro have shown that SMOC2 interacts with αvβ1 and αvβ6 integrins (Maier et al., 2008) and maintains integrin linked kinase (ILK) activity during the G1 phase of the cell cycle thereby contributing to cell cycle progression (Liu et al., 2008). These data suggest that SMOC2 could mediate interactions between integrins and the extracellular matrix and contribute to signaling to their intracellular effector, ILK. Other experiments show that SMOC2 mediates the mitogenic and angiogenic effects of VEGF, PDGF, and FGF thereby suggesting that SMOC2 could mediate interactions between these growth factors and their receptors, or act in a parallel, synergistic pathway (Rocnik et al., 2006; Liu et al., 2008). To our knowledge, functions for SMOC1 and SMOC2 during mammalian development have not yet been described.
The development of ovaries or testes from the bipotential genital ridge involves sex-specific responses to, or expression of, growth factors, morphogens, and secreted signaling molecules. For example, disruption of FGF9 signaling in the mouse leads to XY sex reversal, and disruption of WNT4 signaling compromises both ovarian and testicular development (Vainio et al., 1999; Colvin et al., 2001; Jeays-Ward et al., 2004). Additionally, disruption of Desert Hedgehog (DHH) or PDGF signaling compromises Leydig cell differentiation (Yao et al., 2002; Brennan et al., 2003). Given that SMOC1 and SMOC2 have been shown to mediate growth factor signaling in vivo and in vitro, respectively, and the fact that Smoc2 was shown to be expressed in the reproductive tract at E12.5-13 (Liu et al., 2008), we examined the possibility that they may mediate growth factor and morphogen signaling in developing gonads and mesonephroi. We examined the spatiotemporal expression of Smoc1 and Smoc2 in normal and mutant fetal mouse gonad/mesonephros complexes using whole-mount in situ hybridization (WISH) and quantitative real-time RT-PCR (qRT-PCR). We found that these genes were expressed in dynamic, cell-type and sex specific spatiotemporal patterns. Smoc1 was expressed specifically in supporting cell progenitors (pre-Sertoli and pre-granulosa cells), was downregulated in an RA-independent manner in E13.5 ovaries, and its expression was reduced in Sf1 (officially Nr5a1, nuclear receptor subfamily 5, group A, member 1, also known as Ad4BP), Wt1 (Wilms’ tumor 1) and Fog2 (officially Zfpm2, zinc finger protein, multitype 2) mutant gonads. In contrast, Smoc2 was expressed in developing testicular Leydig cells and in the mesonephros in both sexes, and its expression was dependent on Hedgehog signaling. Overall, these data suggest that SMOC1 and SMOC2 may have important roles in cell type-specific differentiation during gonad and mesonephros development and that Smoc2 is a downstream target of Hedgehog signaling.
RESULTS
Smoc1 and Smoc2 expression in E10.5-15.5 gonad/mesonephros complexes
Using WISH, Smoc1 expression was first detected at E11.5 in both sexes in cells that populated the length of the gonad (Fig. 1A). Expression persisted in testes from E12.5-15.5 and was localized to cells within the developing cords (Fig. 1A). Expression in ovaries continued throughout the gonad at E12.5, but at E13.5 high-level expression was present only in the posterior half and at E14.5 high-level expression was only visible at the posterior-most end. By E15.5, only low-level expression was visible in the ovary. Therefore, it appears that Smoc1 is downregulated in the ovary in an anterior-to-posterior (A-P) wave beginning at about E13.5. Smoc1 expression was absent in the coelomic epithelium and mesonephros in both sexes at all timepoints examined.
Figure 1. Analysis of Smoc1 expression in embryonic gonad/mesonephros complexes.
A) WISH analysis of Smoc1 expression in E10.5-15.5 wildtype gonad/mesonephros complexes. Expression was first detected at E11.5 in cells along the length of the gonad in both sexes. This expression pattern persisted until E13.5 when a wave of Smoc1 downregulation was observed in the anterior half of the ovary and by E15.5 only a low level of expression was detected. In testes, expression was detected within cords from E12.5-15.5. Expression was not detected in the mesonephros. B) qRT-PCR analysis of Smoc1 expression in E10.5-14.5 wildtype gonad/mesonephros complexes. Expression peaked in ovaries at E12.5 and in testes at E13.5. Each sample represents at least 3 gonad/mesonephros complexes. C) WISH analysis of Smoc1 expression in E13.5 WV/WV gonad/mesonephros complexes. Expression persisted in E13.5 WV/WV germ cell-deficient ovaries and testes. In each image, the gonad is located above, and the mesonephros below, the black dotted line, and complexes are oriented with the anterior (A) to the left and posterior (P) to the right.
Quantitative RT-PCR analysis was used to determine Smoc1 expression levels in E10.5–E14.5 gonad/mesonephros complexes. Expression was first detected at E10.5 (9–11 ts) (Fig. 1B) indicating the Smoc1 expression is initiated prior to E11.5. Expression was equal in ovaries and testes at E11.5; however, Smoc1 expression peaked at E12.5 in ovaries, and at E13.5 in testes (Fig. 1B). These results are consistent with what was observed by WISH (Fig. 1A).
Expression was examined by WISH in gonads from germ cell-deficient WV/WV E13.5 fetuses to determine if Smoc1 was expressed in somatic or germ cells (Fig. 1C). In testes, expression persisted within cords, indicating that Smoc1 was expressed in Sertoli cells, the only cell type present within cords in germ cell-deficient testes. In WV/WV ovaries, expression did not noticeably differ from wildtype ovaries, and the downregulation of expression in an A-P wave was retained. These results indicate that in both sexes, Smoc1 expression is not dependent on the presence of germ cells and that it is expressed in gonadal somatic cells. Additionally, a microarray analysis comparing global gene expression in wildtype and WV/WV E13.5 ovaries, revealed that the Smoc1 expression level did not significantly change in the absence of germ cells, suggesting that it is not expressed in ovarian germ cells (Pazin & Albrecht, unpublished).
Expression of Smoc2 was not detected by WISH in the ovary at any stage tested (Fig. 2A). In XY gonads, Smoc2 was first detected in a subset of cells at E11.5 (Fig. 2A). By E12.5, expression was restricted to the interstitium where Leydig cells, vasculature, peritubular myoid and other somatic cell types reside (Fig. 2A). This expression pattern persisted in testes until at least E15.5 (Fig. 2A). In the mesonephros of both sexes, Smoc2 was expressed at E11.5 in the Wolffian duct and in a region that extends medially towards the gonad. Expression was not detected in the mesonephric region just below the gonad from E11.5–15.5. By E12.5, Smoc2 expression was detected in the developing Müllerian duct, which has extended along the length of the mesonephros (Orvis and Behringer, 2007), and in the region between the Müllerian and Wolffian ducts. This pattern persists until E15.5 in XY mesonephroi while in XX mesonephroi expression appeared to be downregulated in the Wolffian duct region beginning at E14.5 (see arrows in Fig. 2A).
Figure 2. Analysis of Smoc2 expression in embryonic gonad/mesonephros complexes.
A) WISH analysis of Smoc2 expression in E10.5-15.5 wildtype gonad/mesonephros complexes. Expression was not detected in ovaries at these stages. In testes, expression began at E11.5, was localized to the interstitium by E12.5, and persisted through E15.5. Expression also was detected in the mesonephros from E11.5-15.5 in the Müllerian and Wolffian ducts and the region in between them. However, by E14.5 expression was downregulated in the Wolffian duct region of XX mesonephroi (white arrows). In each image, the gonad is located above, and the mesonephros below, the black dotted line, and complexes are oriented with the anterior (A) to the left and posterior (P) to the right. B) qRT-PCR analysis of Smoc2 expression in E10.5-14.5 wildtype gonad/mesonephros complexes. Expression in ovary/mesonephros complexes and testis/mesonephros complexes peaked at E12.5. Each sample represents at least 3 gonad/mesonephros complexes.
By qRT-PCR analysis, Smoc2 expression was equal in E10.5 and E11.5 XX and XY gonad/mesonephros complexes (Fig. 2B). Expression peaked in ovary/mesonephros and testis/mesonephros complexes at E12.5, although expression in ovary/mesonephros complexes only reached a level that was about 50% of testis/mesonephros complexes. The reduced expression in ovary/mesonephros complexes after E12.5 is consistent with the reduced expression in the Wolffian duct region detected by WISH.
Smoc1 expression is upregulated in a center-to-poles wave
Because Smoc1 expression was first detected at about E11.5, and because the experiments described above suggested that it was expressed in supporting cell progenitors (SCPs: pre-Sertoli and pre-granulosa cells), we hypothesized that its expression might be upregulated in a center-to-poles (C-P) wave. It was previously shown that SCP differentiation in ovaries and testes occurs in a C-P wave marked by the expression of genes such as Sry (sex determining region of chromosome Y), Fst (follistatin), 1700106J16Rik, and Sprr2d (small proline-rich protein 2D) (Albrecht and Eicher, 2001; Bullejos and Koopman, 2001; Menke et al., 2003; Lee et al., 2009). A careful examination of Smoc1 expression in gonad/mesonephros complexes from closely spaced developmental stages around E11.5 was performed using WISH to determine the spatiotemporal pattern of its initial expression (Fig. 3). Expression was first detected at approximately E10.75 (10–11 tail somites) in cells in the central gonadal region in both sexes. Expression in progressively later stages spread toward both the anterior and posterior poles in a wave-like pattern so that by E11.5, Smoc1 was expressed in cells that populated the length of the gonad.
Figure 3. WISH analysis of Smoc1 expression in E10.75–E11.6 gonad/mesonephros complexes.
Expression was initiated in the center of XX and XY gonads at 10–11 tail somites (E10.75) and spread towards the anterior and posterior poles. Expression was detected throughout the entire length of the gonad by 19 tail somites (E11.6). Dotted lines demarcate gonadal tissue and black arrowheads mark the boundary of Smoc1 expression. In all images, gonad/mesonephros complexes are oriented with the anterior (A) to the top and posterior (P) to the bottom.
Smoc1 expression is reduced in Wt1, Sf1, and Fog2, but not Wnt4 mutants
Because our analysis suggested that Smoc1 might have an important role at the earliest stages of gonad development, we were interested in determining whether its expression was altered in Wt1, Sf1, Fog2, and Wnt4 (wingless-related MMTV integration site 4) mutants. These genes play important roles in early ovary and testis development (Kreidberg et al., 1993; Sadovsky et al., 1995; Vainio et al., 1999; Tevosian et al., 2002; Jeays-Ward et al., 2004). Using qRT-PCR, we determined that Smoc1 expression was greatly reduced in E11.5 XX and XY homozygous mutant Wt1, Sf1, and Fog2 gonad/mesonephros complexes (Fig. 4A and B). The reduction in expression was significant (p<0.005) in all groups except XY Wt1 −/− and Fog2 −/−. This result could be due to the small sample size (n=3 in both cases). Additionally, the Fog2 mutant mice were on a mixed 129/B6 background, leaving open the possibility that modifier genes that affect gonad development were segregating among the different samples. Expression of Smoc1 was also significantly reduced in E11.5 Wt1 +/− XX and XY gonad/mesonephros complexes and in Sf1 +/− ovary/mesonephros complexes (p<0.05). There was no significant change in Smoc1 expression in E11.5 XX and XY Wnt4 +/− or −/− gonads (Fig. 4C).
Figure 4. qRT-PCR analysis of Smoc1 expression in E11.5 gonad/mesonephros complexes from Wt1, Sf1, Fog2 and Wnt4 mutant mice.
A) Expression was reduced in XX and XY Wt1 +/− and Wt1 −/− mice, as well as XX and XY Sf1 +/− and Sf1 −/− gonad/mesonephros complexes compared to +/+ control gonad/mesonephros complexes of the same sex. B) Expression was also reduced in XX and XY Fog2 −/− gonad/mesonephros complexes. C) However, expression was unchanged regardless of Wnt4 status. Each bar represents the average fold change in expression compared to wildtype samples of the same sex, where a value of 1 equals no change in expression, and error bars represent the standard error of the mean. Sample size is indicated in parentheses above each bar. Each sample represents a pair of gonad/mesonephros complexes (*=p<0.005; #=p<0.05).
To determine if the reduction in Smoc1 expression occurs in a specific region or cell type, Smoc1 expression was examined in Sf1 mutant gonad/mesonephros complexes using WISH. As expected, Smoc1 was expressed in cells along the length of E11.5 XX and XY Sf1 +/+ gonads, and expression was not detected in mesonephroi (Fig. 5). Expression was noticeably reduced in E11.5 XX and XY Sf1 +/− gonads, and the reduction was uniform and not spatially restricted. Expression of Smoc1 was not detected in E11.5 XX and XY Sf1 −/− gonads, most likely due to the decreased sensitivity of this method compared to qRT-PCR.
Figure 5. WISH analysis of Smoc1 expression in E11.5 XX and XY gonad/mesonephros complexes from Sf1 mutant mice.
An overall reduction in expression was observed in XX and XY Sf1 +/− mice in cells that populate the length of the gonad. Expression was not detected in Sf1 −/− gonads by this method. In each image, the gonad is located above, and the mesonephros below, the black dotted line, and complexes are oriented with the anterior (A) to the left and posterior (P) to the right.
Downregulation of Smoc1 expression in E13.5 ovaries in an anterior-to-posterior wave is independent of retinoic acid signaling
The A-P wave of Smoc1 downregulation in E13.5 ovaries is similar in timing to the A-P wave of meiotic initiation in ovarian germ cells, which is preceded by an A-P wave of Stra8 (stimulated by retinoic acid gene 8) upregulation that is dependent on retinoic acid (RA) signaling (Bowles et al., 2006; Koubova et al., 2006). Therefore, we investigated whether Smoc1 downregulation is dependent on RA signaling. Gonad/mesonephros complexes dissected from E11.5 mice were cultured in the presence or absence of exogenous all-trans RA for 24 hours. This developmental stage was chosen for analysis because if endogenous RA induces Smoc1 downregulation beginning at E12.5, RA treatment at E11.5 should induce premature Smoc1 downregulation. qRT-PCR analysis revealed that the addition of exogenous RA to the culture medium had no effect on Smoc1 expression (Fig. 6A). In contrast, Stra8 expression was increased 3.2 fold compared to untreated complexes, as expected (Fig. 6A) (Koubova et al., 2006). Using WISH, the spatiotemporal expression pattern of Smoc1 was analyzed in gonad/mesonephros complexes dissected at E11.5 and cultured for 48 h in the presence or absence of exogenous all-trans RA. The A-P wave of Smoc1 downregulation was not affected despite the addition of RA (Fig. 6B). Next, gonad/mesonephros complexes from E12.5 mice were cultured for 24 h in the presence of a pan-retinoic acid receptor (RAR) antagonist (BMS-204493), which blocks RA signaling at the receptor level (Germain et al., 2002). This inhibitor has been used previously to prevent Stra8 upregulation in cultured embryonic ovaries (Koubova et al., 2006). This developmental stage was chosen for analysis because if endogenous RA induces Smoc1 downregulation at E12.5, inhibitor treatment at E12.5 should result in persistent expression of Smoc1. We found that treatment with RAR inhibitor did not affect Smoc1 expression (Fig. 6C). Expression of Stra8, however, was reduced by 89%, as expected (Fig. 6C) (Koubova et al., 2006). Taken together, these results suggest that Smoc1 downregulation is not dependent on RA signaling.
Figure 6. Analysis of Smoc1 expression in ovary/mesonephros complexes treated with all-trans retinoic acid or with a pan-RAR inhibitor.
A) E11.5 ovary/mesonephros complexes treated for 24 h with all-trans RA. Expression, as measured by qRT-PCR, was unchanged compared to controls despite the presence of ectopic RA (n=6). B) The A-P wave of downregulation was not affected by the addition of exogenous RA when E12.5 ovary/mesonephros complexes were cultured for 48 h. C) E12.5 ovary/mesonephros complexes treated for 24 h with a pan-RAR inhibitor. Expression, as measured by qRT-PCR, was unchanged when RAR signaling was inhibited (n=6). Expression of Stra8 was induced in RA treated samples and inhibited in the presence of RAR inhibitor indicating that the treatments were effective, at least in germ cells. Each bar represents the average fold change in expression compared to untreated contralateral controls, where a value of 1 equals no change in expression, and error bars represent the standard error of the mean. In each image, the gonad is located above, and the mesonephros below, the black dotted line, and complexes are oriented with the anterior (A) to the left and posterior (P) to the right.
Smoc2 expression is regulated by Hedgehog signaling in the testis, mesonephros, and kidney
Because Smoc2 expression was first detected at E11.5 and was localized to the interstitial region once testis cords formed at E12.5, we suspected that it was expressed in developing Leydig cells. Leydig cell differentiation is dependent upon Dhh expression in Sertoli cells, which begins at E11.5 (Yao et al., 2002). The Hedgehog receptor PATCHED1 is expressed in Leydig cells and in the mesonephros, where its expression also overlaps with Smoc2 expression. To determine if Smoc2 is regulated by DHH signaling in testes, gonad/mesonephros complexes were cultured in the presence of cyclopamine. Cyclopamine is an inhibitor of Hedgehog signaling that works by binding to SMOOTHENED, a downstream effector in the Hedgehog pathway (Chen et al., 2002) and it has previously been used to inhibit DHH signaling in cultured testes (Yao et al., 2002). Gonad/mesonephros complexes harvested from E11.5 mice and cultured in the presence of cyclopamine for 24 h exhibited an approximately 70% decrease in Smoc2 expression compared to untreated control complexes (p<0.005; Fig. 7A). WISH analysis revealed that expression was decreased in testes, and in XX and XY mesonephroi (Fig. 7B). Expression of Cyp11a1, a gene previously shown to be downregulated in Leydig cells in the presence of cyclopamine (Yao et al., 2002), was decreased by 59% (p<0.005; Fig. 7A). Expression of Cyp17a1, another Leydig cell marker, was examined by WISH and also was decreased by cyclopamine treatment, as expected (Fig. 7B). The downregulation of Smoc2 in the testis could be due to two possibilities: 1) Smoc2 is regulated by DHH signaling or 2) Smoc2 is expressed as a consequence of Leydig cell differentiation and its expression is reduced when cell differentiation is inhibited.
Figure 7. Analysis of Smoc2 expression in cyclopamine-treated cultured gonad/mesonephros complexes.
A) qRT-PCR analysis demonstrated that Smoc2 expression was downregulated when E11.5 and E12.5 gonad/mesonephros complexes were treated for 24 h with the Hedgehog signaling inhibitor cyclopamine. Each bar represents the average fold change in expression compared to untreated contralateral controls, and error bars represent the standard error of the mean (n=6, *p<0.01). Sample size is indicated in parentheses above each bar. B) WISH analysis showed that expression was reduced in the testes and mesonephroi of both sexes (n>4 for each group). Cyp11a1 is a Leydig cell marker whose expression was previously determined to be inhibited in E11.5 cyclopamine-treated gonads, but not in E12.5 gonads (Yao et al., 2002). In each image, the gonad is located above, and the mesonephros below, the black dotted line, and complexes are oriented with the anterior (A) to the left and posterior (P) to the right.
To distinguish between these two possibilities, gonad/mesonephros complexes were harvested from E12.5 mice and cultured in the presence of cyclopamine, as above. At this developmental stage, Leydig cell differentiation does not require continued Hedgehog signaling (Yao et al., 2002). As expected, Cyp17a1 (WISH) and Cyp11a1 (qRT-PCR) expression was not altered by cyclopamine treatment (Fig. 7A and 7B). In contrast, Smoc2 expression was decreased by 51% in cyclopamine-treated ovary/mesonephros complexes and was decreased by 56% in testis/mesonephros complexes (p<0.005; Fig. 7A). Similar to E11.5 complexes, decreased expression was observed in both the testis, and XX and XY mesonephroi. Cultured gonad/mesonephros complexes from E13.5 mice treated with cyclopamine gave results similar to those from E12.5 mice (data not shown). These experiments suggest that Smoc2 is expressed in Leydig cells and not in other interstitial cell types such as the vasculature, and that Smoc2 expression is regulated by DHH signaling in the testis.
The reduction in Smoc2 expression in the mesonephros of both sexes by cyclopamine treatment suggested that mesonephric expression also was dependent on Hedgehog signaling. Because Dhh is not expressed in the mesonephros, we were interested in determining if another Hedgehog family member was expressed in this tissue. Expression of Shh was observed in the Wolffian duct of E13.5 mesonephroi, consistent with published reports (Little et al., 2007) (Fig. 8A) Additionally, the spatiotemporal expression pattern of the transcriptional effectors of the Hedgehog pathway, Gli1-Gli3, was examined by WISH. Expression of Gli1 and Gli2, was detected in E11.5-13.5 gonad/mesonephros complexes. Both genes had a similar pattern in that they were expressed in XX and XY mesonephroi from E11.5-13.5, and in the testicular interstitium at E12.5 and E13.5 (Fig. 8B). This spatiotemporal expression pattern overlaps with that of Smoc2 (Fig. 2A). Expression of Gli3 was not detected in the gonad or mesonephros (data not shown). Together, these data show that Smoc2 expression is dependent on Hedgehog signaling in at least two developing organs.
Figure 8. WISH analysis of Shh expression in E13.5 gonad/mesonephros complexes, and Gli1 and Gli2 expression in E11.5-13.5 gonad/mesonephros complexes.
A) Expression of Shh was detected in E13.5 mesonephroi specifically in the Wolffian duct. B) Expression of Gli1 and Gli2 was detected in E11.5-13.5 mesonephroi and the testicular interstitium from E12.5-13.5. The gonad is located above, and the mesonephros below, the black dotted line, and complexes are oriented with the anterior (A) to the left and posterior (P) to the right in each image
Because we knew that kidney development is dependent upon SHH signaling (Yu et al., 2002), we were interested in determining if Smoc2 expression also is dependent on SHH signaling. We found that Smoc2, but not Smoc1, was expressed in E13.5 kidneys, and in the cortical region of the adrenal gland (Fig. 9A). Additionally, we found that Shh was expressed in the adrenal cortex (Fig. 9A). To determine if Smoc2 expression was regulated by SHH in the kidneys, we cultured E13.5 kidneys for 24 h in the presence of cyclopamine and found that cyclopamine treatment resulted in a 31% reduction in Smoc2 expression (p<0.01) (Fig. 9B). Reduced expression also was observed by WISH and may be spatially restricted (Fig. 9C). As a control, the expression of Gli1 was analyzed and was found to be reduced.
Figure 9. WISH analysis of Smoc2 expression in E13.5 kidneys and adrenal glands and analysis of Smoc2 expression in cyclopamine-treated cultured kidneys.
A) Expression of Smoc1 was detected in the E13.5 adrenal gland medulla and ureter, but was absent from the kidney. Expression of Smoc2 was detected in the E13.5 adrenal gland cortex and kidney. Expression of Shh was detected in the E13.5 adrenal cortex. B) qRT-PCR analysis demonstrated that Smoc2 expression was downregulated in E13.5 kidneys that were treated for 24 h with cyclopamine. Each bar represents the average fold change in expression compared to untreated contralateral controls, and error bars represent the standard error of the mean (n=6, *p<0.01). C) WISH analysis suggested that the downregulation may occurr in a spatially restricted manner.
To further elucidate whether Smoc2 is a downstream target of Hedgehog signaling, gonad/mesonephros complexes were cultured in the presence of purmorphamine, a Hedgehog pathway agonist. Purmorphamine is thought to act at the level of SMOOTHENED and has been shown to activate Hedgehog target genes in cultured cells (Wu et al., 2004; Sinha and Chen, 2006). Gonad/mesonephros complexes were harvested at E11.5 and cultured for 48 hours. This timepoint was chosen because endogenous Leydig cells differentiate in response to DHH between E11.5 and E12.5. Induction of Smoc2 expression was observed in purmorphamine treated ovaries, but not in untreated ovaries (Fig. 10). Additionally, Smoc2 was upregulated in the testis and mesonephros. As a control, the expression of Gli1 was analyzed and also was found to be induced in the ovary, and upregulated in the testis and mesonephros (Fig. 10). The induction of Smoc2 and Gli1 in the ovary appeared to be restricted to the surface of the gonad, whereas in the testis and mesonephros, induction was observed throughout the tissue. The expression of Cyp17a1 was also analyzed and was upregulated in the ovary, indicating that purmorphamine treatment induced ectopic Leydig cell differentiation (Fig. 10). These results are consistent with data showing that stabilization of Smoothened in ovarian somatic cells causes ectopic Leydig cell differentiation (Barsoum et al., 2009). Taken together, these results suggest that Smoc2 expression is regulated by DHH signaling in the embryonic testis, and by SHH signaling in the mesonephros and kidney, and potentially in the adrenal cortex.
Figure 10. Analysis of Smoc2 expression in purmorphamine-treated cultured gonad/mesonephros complexes.
Expression of Smoc2 was induced in the ovary and upregulated in the testis and mesonephros when E11.5 gonad/mesonephros complexes were treated for 48h with the Hedgehog pathway agonist, purmorphamine. As a control, the expression of Gli1, a known Hedgehog target gene, was also examined and found to be induced in the ovary and upregulated in the testis and mesonephros. Expression of the Leydig cell marker Cyp17a1 was also induced in the ovary indicating ectopic differentiation of this cell type. In each image, the gonad is located above, and the mesonephros below, the black dotted line, and complexes are oriented with the anterior (A) to the left and posterior (P) to the right.
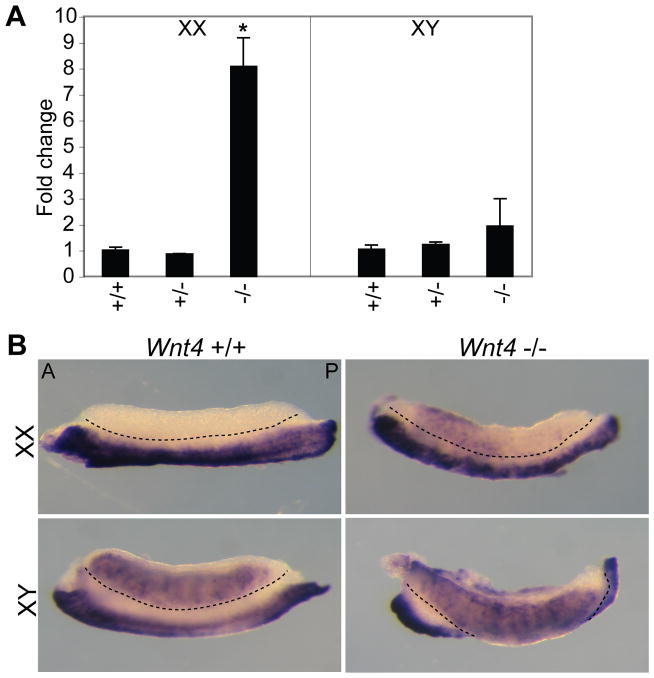
Ectopic Smoc2 expression in Wnt4 mutant ovaries
Wnt4 mutants have defects in gonad and mesonephros development in both sexes (Vainio et al., 1999; Jeays-Ward et al., 2004). Of particular interest here is that mutant ovaries are masculinized, displaying ectopic expression of steroidogenic enzymes and the formation of a coelomic vessel (Vainio et al., 1999; Jeays-Ward et al., 2003). Given that Smoc2 was expressed in steroidogenic Leydig cells, we were interested in determining if it was expressed ectopically in E12.5 Wnt4 −/− ovaries. Expression of Smoc2 was upregulated about 8-fold in Wnt4 −/−ovary/mesonephros complexes compared to Wnt4 +/+ ovary/mesonephros complexes (Fig. 11A). Expression was not significantly altered in Wnt4 +/− ovaries or testes, or in Wnt4 −/− mutant testes. [In contrast, Smoc1 expression was unchanged regardless of the Wnt4 genotype in either sex (data not shown)]. WISH analysis confirmed the Smoc2 qRT-PCR results and revealed that Smoc2 was expressed ectopically in a cluster of cells in the anterior region of the Wnt4 −/− ovary (Fig. 11B). This spatial expression pattern is similar to the ectopic expression of the normally male-specific steroidogenic enzyme 3β-hydroxysteroid dehydrogenase (Jeays-Ward et al., 2003). These data suggests that the ectopic steroidogenic cells found in Wnt4 −/− ovaries also express Smoc2. Given that the ectopic steroidogenic cells in Wnt4 −/− ovaries are thought to be of adrenal origin (Heikkila et al., 2002), it is interesting to note that Smoc2 is expressed in the cortex of E13.5 wildtype adrenal glands (Smoc1 is expressed within the medullary region) (Fig. 9A).
Figure 11. Analysis of Smoc2 expression in E12.5 Wnt4 −/− gonad/mesonephros complexes.
A) qRT-PCR analysis indicated that expression was dramatically upregulated in E12.5 Wnt4 −/−ovary/mesonephros complexes compared to Wnt4 +/+ complexes (n=3 for each genotype and sex; p<0.05). Expression was unchanged in XY gonad/mesonephros complexes regardless of Wnt4 status. Each bar represents the average fold change in expression compared to wildtype samples of the same sex, and error bars represent the standard error of the mean. B) WISH analysis revealed an anterior cluster of ectopic Smoc2 expressing cells in Wnt4 −/− ovaries (n=6). In each image, the gonad is located above, and the mesonephros below, the black dotted line, and complexes are oriented with the anterior (A) to the left and posterior (P) to the right.
DISCUSSION
Here we present an analysis of the expression and regulation of Smoc1 and Smoc2, which encode related matricellular proteins, during the initial stages of fetal gonad and mesonephros differentiation. Our data suggest that Smoc1 is expressed in supporting cell progenitors (SCPs), which differentiate into ovarian granulosa cells and testicular Sertoli cells. We and others have shown that SCP differentiation occurs in a C-P wave beginning between E10.5-11, as illustrated by the expression of Sry, Sox9, Sprr2d and 1700106J16Rik. We now add Smoc1 to this growing list of genes.
To gain insight into possible roles for Smoc1 in gonad development we examined if its expression was altered in E11.5 Fog2, Sf1, Wt1 and Wnt4 mutant gonad/mesonephros complexes. The results showed that Smoc1 expression was greatly reduced in Fog2, Sf1, and Wt1, but not Wnt4 mutant gonad/mesonephros complexes. These data show that Smoc1 expression depends on the correct expression of Sf1, Wt1, and Fog2, and that Smoc1 is downstream of these genes. Further experiments are necessary, however, to determine if this regulation is direct, or indirect via action of these three genes on SCP differentiation and/or expansion. We note that the expression of other SCP-specific genes such as Sry, 1700106J16Rik and Sprr2d also is greatly reduced in E11.5 Fog2, Sf1, and Wt1 mutant gonad/mesonephros complexes (Capel, 1998; Hammes et al., 2001; Tevosian et al., 2002; Lee et al., 2009) (Correa, Pazin and Albrecht, unpublished results).
In wildtype ovaries, Smoc1 expression was downregulated in an A-P wave beginning between E12.5 and E13.5. Because this spatiotemporal expression pattern is similar to that observed for Stra8 induction in germ cells, which is RA-dependent, we examined if Smoc1 downregulation was dependent on RA signaling. However, we found that addition of neither exogenous RA nor a pan-RAR inhibitor altered Smoc1 expression in cultured ovaries. This finding is similar to our previous results for Sprr2d, which is downregulated in a comparable RA-independent spatiotemporal manner (Lee et al., 2009) and is consistent with the absence of canonical retinoic acid-response elements (RAREs) within 2 kb upstream of the Smoc1 translational start site (Pazin and Albrecht, unpublished observation). These data suggest that the A-P pattern of gene expression in somatic cells and germ cells may occur independently of each other, or that one wave of expression may facilitate the other (Lee et al., 2009). These results also suggest that the downregulation of Smoc1 and Sprr2d in ovaries potentially is controlled by the same unknown factor. In contrast, the downregulation of Smoc1 and Sprr2d in testes differs in that Smoc1 expression persists until at least E15.5, while Sprr2d expression is downregulated in a C-P wave beginning just after E11.5. The persistent expression of Smoc1 in Sertoli cells also suggests that the requirement for Smoc1 in developing Sertoli and granulosa cells differs after E13.5. It is currently unknown whether persistent expression in Sertoli cells and/or if downregulation in granulosa cells is critical for normal development. However, we speculate that downregulation of Smoc1 expression in granulosa cells could be important for the initiation of meiosis in germ cells or perhaps in other aspects of germ cell differentiation. It will be interesting to determine what effect persistent Smoc1 expression after E13.5 has on ovary and germ cell differentiation.
Overall, analysis of the expression and regulation of Smoc1 suggests that it belongs to a set of SCP genes that display similar expression patterns and may be similarly regulated. Like Sry, 1700106J16Rik and Sprr2d, Smoc1 expression is initiated in a C-P wave and is downregulated in Wt1, Sf1, and Fog2 mutants. Unlike these genes however, Smoc1 is not downregulated in a C-P wave in XY gonads suggesting that it may have a role in Sertoli cell/testis differentiation. In the ovary, Smoc1 and Sprr2d are downregulated in an A-P wave suggesting that they may be co-regulated, and that their absence is necessary for normal ovarian differentiation.
In the gonads, Smoc2 was expressed only in testes, was first detected at about E11.5 and was restricted to the interstitial region at the onset of cord development, suggesting that it is expressed by differentiating Leydig cells and regulated by DHH signaling. The fact that testicular Smoc2 expression was downregulated in gonads cultured in the presence of cyclopamine suggests that Smoc2 is indeed expressed in Leydig cells and that it is a downstream target of DHH signaling. This idea is further supported by the fact that Smoc2 is induced in ovaries, and upregulated in the testis and mesonephros in the presence of purmorphamine. We also found that Smoc2 expression overlaps with Shh in the mesonephros, kidney, and adrenal gland. Expression of Smoc2 was downregulated by cyclopamine treatment in these organs suggesting that it is regulated by SHH signaling. Further experiments are required to determine whether this regulation is direct or indirect, and if regulation by Hedgehog signaling is a general feature of Smoc2 expression.
In Wnt4 −/− ovaries steroidogenic cells derived from the adrenal precursor migrate ectopically into the anterior part of the gonad (Heikkila et al., 2002), and we show here that Smoc2 also is expressed ectopically in this region. Given that Smoc2 is expressed in steroidogenic Leydig cells, we think that it is very likely that Smoc2 is expressed in the ectopic steroidogenic adrenal cells. Consistent with this idea, we found that Smoc2 is expressed in the embryonic adrenal gland. These data suggest that Smoc2 may have a role in steroidogenic cell development or differentiation in multiple tissues.
Previous experiments in vitro suggested that Smoc2 is involved in regulating cell proliferation and angiogenesis, and that it modulates the mitogenic effects of PDGF and VEGF (Rocnik et al., 2006; Liu et al., 2008). In developing mouse gonads, PDGFs are important for testis cord formation, Leydig cell development, and vasculature organization, and VEGF is important for vascularization in rat testes (Brennan et al., 2003; Bott et al., 2006). Given that SMOC2 is likely to be secreted by Leydig cells within the interstitum, it is possible that it has a role in mediating signaling involving PDGF and/or VEGF that leads to testicular vascularization.
Smoc2 is also expressed in males and females in a Hedgehog-dependent manner in the mesonephros, specifically in the region where the Müllerian and Wolffian ducts form. This mesonephric region undergoes extensive growth and remodeling from E13-18 as the Müllerian ducts regress in males, and the Wolffian ducts regress in females. In particular, Smoc2 appeared to be downregulated in the Wolffian duct region of XX mesonephroi beginning at E14.5, which coincides with Wolffian duct regression in females, suggesting that Smoc2 could be important for maintenance of this duct in males.
We are intrigued by the fact that the expression of two closely related genes, SMOC1 and SMOC2, are completely non-overlapping in the gonad. This cell-type specific expression pattern suggests that they may have unique functions. However, given the high degree of similarity in amino acid sequence (75%) and their similar protein domain structure, it will be interesting to determine if they have similar functions, and if SMOC1 can compensate for SMOC2 function or vice versa (Vannahme et al., 2003). Expression of at least two other SPARC family members, SPARC and FSTL1, has been described in embryonic gonad development. SPARC is expressed in embryonic Leydig, Sertoli, and germ cells, however, SPARC null mice have no reported gonadal phenotype (Norose et al., 1998; Wilson et al., 2006). FSTL1 is expressed in testicular interstitial cells and ovarian stromal cells, but not germ cells at E17.5, and a knockout has not been reported (Adams et al., 2007). It is possible that a SPARC family member could functionally compensate for the loss of another SPARC family protein. However, although the SPARC-like proteins are similar, they also have distinct domains. For example, SMOC1 and SMOC2 contain a SMOC domain that is unique among family members and may confer a specific function.
Given their spatiotemporal expression patterns in fetal gonads and mesonephroi, SMOC1 and SMOC2 have the potential to mediate growth factor signaling during the development of these organs. The results presented here suggest that in both sexes, Smoc1 may be involved in the differentiation of the supporting cell lineage and in the interactions between supporting and germ cells. On the other hand, our data suggest that Smoc2 might be involved in the differentiation of steroidogenic Leydig cells cells and the testicular vasculature. Potentially, Smoc2 also may be involved in the development of the urogenital tract. A better understanding of the role of Smoc1 and Smoc2 during embryogenesis awaits the development of mice carrying both gain- and loss-of-function mutations.
EXPERIMENTAL PROCEDURES
Mice and Genotyping
CD-1 and C57BL/6J-KitW-v (B6-WV) mice were purchased from Charles River Laboratories and The Jackson Laboratory, respectively. Wnt4 knockout mice on the 129S1/SvImJ background were a gift from Dr. Andy McMahon (Harvard University) (Stark et al., 1994). Fog2 knockout mice were a gift from Dr. Sergei Tevosian (Dartmouth University) (Tevosian et al., 2000). Sf1 knockout mice were a gift from Dr. Keith Parker (University of Texas Southwestern Medical Center) (Luo et al., 1994). Wt1 knockout mice were a gift from Dr. Jordan Kreidberg (Harvard Medical School) (Kreidberg et al., 1993). Fog2, Sf1 and Wt1 mice were received on mixed genetic backgrounds and subsequently backcrossed to the B6/J background. Sf1 and Wt1 mice were backcrossed for >20 generations, and Fog2 mice were at the N4 and N5 generations. Timed matings, where noon on the day of vaginal plug detection was designated embryonic day (E)0.5, were used for all experiments. E12.5 and younger fetuses were staged by counting the number of tail somites posterior to the hind limb (Hacker et al., 1995). Fetuses older than E12.5 were staged by examining limb morphology (Kaufman, 2003). The chromosomal sex of fetuses from Wnt4 matings and those younger than E12.5 was determined using a PCR-based assay (Capel et al., 1999). Genotyping of mutants was performed using PCR assays as previously described (Stark et al., 1994; Lee et al., 2009). The Boston University Laboratory Animal Science Center is AAALAC accredited and all animal procedures were approved by the BUMC Institutional Animal Care and Use Committee.
Whole-mount in situ hybridization
Sense and antisense digoxigenin-labeled riboprobes were generated by in vitro transcription in the presence of digoxigenin-labeled dUTP (Roche). Plasmids containing full-length cDNAs from either Smoc1 (Image ID 3673732) or Smoc2 (Liu et al., 2008) were a gift from Dr. Cyrus Vaziri (Boston University School of Medicine). Antisense and sense Smoc1 probes were generated by BamHI digest and subsequent transcription using T7 polymerase, or NcoI digest and subsequent transcription using SP6 polymerase, respectively. Antisense and sense Smoc2 probes were generated by EcoRI digest and subsequent transcription using T3 polymerase, or BamHI digest and subsequent transcription using SP6 polymerase, respectively. The plasmid used to generate the Cyp17a1 probe was a gift from Dr. Sergei Tevosian (Tevosian et al., 2002). The plasmids used to generate Gli1-3 probes were a gift from Dr. Alex Joyner (Sloan Kettering Institute) (Hui et al., 1994). The plasmid used to generate the Shh probe was a gift from Dr. Andy McMahon (Bitgood and McMahon, 1995). WISH was performed using standard protocols with minor modifications (Henrique et al., 1995; Lee et al., 2009). Only signal specific to the antisense probe is shown as gene-specific expression. Each result shown represents a minimum of four samples.
RNA isolation, cDNA synthesis and qRT-PCR
RNA was isolated from gonad/mesonephros complexes using the Qiagen RNeasy kit. Samples were DNase treated and subsequently determined to be DNA-free (Lee et al., 2009). For expression analysis of Sf1, Wt1, Fog2, and Wnt4 mutant complexes, and of organ culture/cyclopamine treatment experiments, cDNA was generated using Superscript II reverse transcriptase (Invitrogen) and real-time PCR was performed using Power SYBR Green PCR Master Mix (Applied Biosystems). For temporal expression analyses and organ culture/retinoic acid treatment experiments, cDNA was synthesized using Verso RT (Thermo Scientific) and real-time PCR was performed using Absolute Blue SYBR Green Master Mix (Thermo Scientific). Real-time PCR was performed using a 7900HT Sequence Detection System (Applied Biosystems) and the following primers: Smoc1: 5’-CACCACAGACATGGTTCAGG and 5’-TGGCTGAAGTACCAGTGTGC; Smoc2: 5’-GACCCCTCTTCCTCTTCTGG a n d 5 ’-TCCTTCTTGCCAATGTCTCC; Cyp11a1: 5’-AAAGACCGAATCGTCCTAAACC and 5’-CTTGATGCGTCTGTGTAAGACT. Primers for Stra8 (Koubova et al., 2006) and Hprt (Bouma et al., 2004) were used as previously published. Primers produced single products of the expected size without primer dimers and were validated according to Applied Biosystems guidelines (Applied Biosystems, 2004). For temporal expression analyses, expression relative to HPRT (2−ΔCt) was determined. For all other analyses, fold change values were calculated using the ΔΔCT method (Livak and Schmittgen, 2001). Two-tailed Student’s t-tests were performed to determine statistical significance.
Gonad organ culture
Gonad/mesonephros complexes were dissected from E11.5-13.5 CD-1 mice and kidneys from E13.5 CD-1 mice. One complex or kidney from each animal served as an untreated control while the other was treated. Gonad/mesonephros complexes were incubated in Dulbecco’s Modified Eagle Medium (DMEM; Invitrogen) supplemented with 10% fetal bovine serum and ampicillin (50 μg/ml). Kidneys were incubated in DMEM-F12 (Hyclone) supplemented with 5 μg/ml transferrin (Sigma). Isolated complexes or kidneys were placed on TMTP Isopore membrane filters (5 μm, Millipore) floating on media in 4-well cell culture plates and incubated μM cyclopamine (Toronto at 37°C and 5% CO2. Tissues were treated for 24–48 h with 25 Research Chemicals) (Yao et al., 2002), 5 μM purmorphamine (Cayman Chemical Company), 0.7 μM all-trans retinoic acid (Sigma), or 5 μM of pan-retinoic acid receptor inhibitor (BMS- 204493, Bristol-Myers-Squibb) (Koubova et al., 2006), or with vehicle control (DMSO for cyclopamine and purmorphamine; ethanol for retinoic acid and BMS-204493).
Acknowledgments
National Institute of Child Health and Human Development; R01-HD042779 American Cancer Society Research Scholar Grant; 06-033-01-DDC
We thank Stephanie Correa and Caryn Navarro for reading a previous version of this manuscript and for providing helpful insights. We thank Cyrus Vaziri for helpful discussions and for providing the Smoc1 and Smoc2 plasmids, Alex Joyner for providing the Gli1-3 plasmids, Sergei Tevosian for providing the Cyp17a1 plasmid and Fog2 mice, Andy McMahon for providing the Shh plasmid and Wnt4 mice, Jordan Kreidberg for providing Wt1 mice, Keith Parker for providing Sf1 mice, and Wellington Cardoso, Christopher Zusi and Bristol Myers Squib for providing the RAR antagonist.
References
- Adams D, Larman B, Oxburgh L. Developmental expression of mouse Follistatin-like 1 (Fstl1): Dynamic regulation during organogenesis of the kidney and lung. Gene Expr Patterns. 2007;7:491–500. doi: 10.1016/j.modgep.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht KH, Eicher EM. Evidence that Sry is expressed in pre-Sertoli cells and Sertoli and granulosa cells have a common precursor. Dev Biol. 2001;240:92–107. doi: 10.1006/dbio.2001.0438. [DOI] [PubMed] [Google Scholar]
- Barsoum IB, Bingham NC, Parker KL, Jorgensen JS, Yao HH. Activation of the Hedgehog pathway in the mouse fetal ovary leads to ectopic appearance of fetal Leydig cells and female pseudohermaphroditism. Dev Biol. 2009 doi: 10.1016/j.ydbio.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitgood MJ, McMahon AP. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev Biol. 1995;172:126–138. doi: 10.1006/dbio.1995.0010. [DOI] [PubMed] [Google Scholar]
- Bornstein P, Sage EH. Matricellular proteins: extracellular modulators of cell function. Curr Opin Cell Biol. 2002;14:608–616. doi: 10.1016/s0955-0674(02)00361-7. [DOI] [PubMed] [Google Scholar]
- Bott RC, McFee RM, Clopton DT, Toombs C, Cupp AS. Vascular endothelial growth factor and kinase domain region receptor are involved in both seminiferous cord formation and vascular development during testis morphogenesis in the rat. Biol Reprod. 2006;75:56–67. doi: 10.1095/biolreprod.105.047225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouma GJ, Hart GT, Washburn LL, Recknagel AK, Eicher EM. Using real time RT-PCR analysis to determine multiple gene expression patterns during XX and XY mouse fetal gonad development. Gene Expr Patterns. 2004;5:141–149. doi: 10.1016/j.modgep.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Bowles J, Knight D, Smith C, Wilhelm D, Richman J, Mamiya S, Yashiro K, Chawengsaksophak K, Wilson MJ, Rossant J, Hamada H, Koopman P. Retinoid signaling determines germ cell fate in mice. Science. 2006;312:596–600. doi: 10.1126/science.1125691. [DOI] [PubMed] [Google Scholar]
- Brennan J, Tilmann C, Capel B. Pdgfr-alpha mediates testis cord organization and fetal Leydig cell development in the XY gonad. Genes Dev. 2003;17:800–810. doi: 10.1101/gad.1052503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullejos M, Koopman P. Spatially dynamic expression of Sry in mouse genital ridges. Dev Dyn. 2001;221:201–205. doi: 10.1002/dvdy.1134. [DOI] [PubMed] [Google Scholar]
- Capel B. Sex in the 90s: SRY and the switch to the male pathway. Annu Rev Physiol. 1998;60:497–523. doi: 10.1146/annurev.physiol.60.1.497. [DOI] [PubMed] [Google Scholar]
- Capel B, Albrecht KH, Washburn LL, Eicher EM. Migration of mesonephric cells into the mammalian gonad depends on Sry. Mech Dev. 1999;84:127–131. doi: 10.1016/s0925-4773(99)00047-7. [DOI] [PubMed] [Google Scholar]
- Chen JK, Taipale J, Cooper MK, Beachy PA. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev. 2002;16:2743–2748. doi: 10.1101/gad.1025302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin JS, Green RP, Schmahl J, Capel B, Ornitz DM. Male-to-female sex reversal in mice lacking fibroblast growth factor 9. Cell. 2001;104:875–889. doi: 10.1016/s0092-8674(01)00284-7. [DOI] [PubMed] [Google Scholar]
- Francki A, McClure TD, Brekken RA, Motamed K, Murri C, Wang T, Sage EH. SPARC regulates TGF-beta1-dependent signaling in primary glomerular mesangial cells. J Cell Biochem. 2004;91:915–925. doi: 10.1002/jcb.20008. [DOI] [PubMed] [Google Scholar]
- Francki A, Motamed K, McClure TD, Kaya M, Murri C, Blake DJ, Carbon JG, Sage EH. SPARC regulates cell cycle progression in mesangial cells via its inhibition of IGF-dependent signaling. J Cell Biochem. 2003;88:802–811. doi: 10.1002/jcb.10424. [DOI] [PubMed] [Google Scholar]
- Germain P, Iyer J, Zechel C, Gronemeyer H. Co-regulator recruitment and the mechanism of retinoic acid receptor synergy. Nature. 2002;415:187–192. doi: 10.1038/415187a. [DOI] [PubMed] [Google Scholar]
- Gersdorff N, Muller M, Schall A, Miosge N. Secreted modular calcium-binding protein-1 localization during mouse embryogenesis. Histochem Cell Biol. 2006 doi: 10.1007/s00418-006-0200-7. [DOI] [PubMed] [Google Scholar]
- Hacker A, Capel B, Goodfellow P, Lovell-Badge R. Expression of Sry, the mouse sex determining gene. Development. 1995;121:1603–1614. doi: 10.1242/dev.121.6.1603. [DOI] [PubMed] [Google Scholar]
- Hammes A, Guo JK, Lutsch G, Leheste JR, Landrock D, Ziegler U, Gubler MC, Schedl A. Two splice variants of the Wilms' tumor 1 gene have distinct functions during sex determination and nephron formation. Cell. 2001;106:319–329. doi: 10.1016/s0092-8674(01)00453-6. [DOI] [PubMed] [Google Scholar]
- Heikkila M, Peltoketo H, Leppaluoto J, Ilves M, Vuolteenaho O, Vainio S. Wnt-4 deficiency alters mouse adrenal cortex function, reducing aldosterone production. Endocrinology. 2002;143:4358–4365. doi: 10.1210/en.2002-220275. [DOI] [PubMed] [Google Scholar]
- Henrique D, Adam J, Myat A, Chitnis A, Lewis J, Ish-Horowicz D. Expression of a Delta homologue in prospective neurons in the chick. Nature. 1995;375:787–790. doi: 10.1038/375787a0. [DOI] [PubMed] [Google Scholar]
- Hui CC, Slusarski D, Platt KA, Holmgren R, Joyner AL. Expression of three mouse homologs of the Drosophila segment polarity gene cubitus interruptus, Gli, Gli-2, and Gli-3, in ectoderm- and mesoderm-derived tissues suggests multiple roles during postimplantation development. Dev Biol. 1994;162:402–413. doi: 10.1006/dbio.1994.1097. [DOI] [PubMed] [Google Scholar]
- Jeays-Ward K, Dandonneau M, Swain A. Wnt4 is required for proper male as well as female sexual development. Dev Biol. 2004;276:431–440. doi: 10.1016/j.ydbio.2004.08.049. [DOI] [PubMed] [Google Scholar]
- Jeays-Ward K, Hoyle C, Brennan J, Dandonneau M, Alldus G, Capel B, Swain A. Endothelial and steroidogenic cell migration are regulated by WNT4 in the developing mammalian gonad. Development. 2003;130:3663–3670. doi: 10.1242/dev.00591. [DOI] [PubMed] [Google Scholar]
- Kaufman M. The Atlas of Mouse Development. London: Academic Press; 2003. [Google Scholar]
- Koubova J, Menke DB, Zhou Q, Capel B, Griswold MD, Page DC. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci U S A. 2006;103:2474–2479. doi: 10.1073/pnas.0510813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreidberg JA, Sariola H, Loring JM, Maeda M, Pelletier J, Housman D, Jaenisch R. WT-1 is required for early kidney development. Cell. 1993;74:679–691. doi: 10.1016/0092-8674(93)90515-r. [DOI] [PubMed] [Google Scholar]
- Kupprion C, Motamed K, Sage EH. SPARC (BM-40, osteonectin) inhibits the mitogenic effect of vascular endothelial growth factor on microvascular endothelial cells. J Biol Chem. 1998;273:29635–29640. doi: 10.1074/jbc.273.45.29635. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Pazin DE, Kahlon RS, Correa SM, Albrecht KH. Novel markers of early ovarian pre-granulosa cells are expressed in an Sry-like pattern. Dev Dyn. 2009;238:812–825. doi: 10.1002/dvdy.21902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little MH, Brennan J, Georgas K, Davies JA, Davidson DR, Baldock RA, Beverdam A, Bertram JF, Capel B, Chiu HS, Clements D, Cullen-McEwen L, Fleming J, Gilbert T, Herzlinger D, Houghton D, Kaufman MH, Kleymenova E, Koopman PA, Lewis AG, McMahon AP, Mendelsohn CL, Mitchell EK, Rumballe BA, Sweeney DE, Valerius MT, Yamada G, Yang Y, Yu J. A high-resolution anatomical ontology of the developing murine genitourinary tract. Gene Expr Patterns. 2007;7:680–699. doi: 10.1016/j.modgep.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Lu J, Cardoso WV, Vaziri C. The SPARC-related Factor SMOC-2 Promotes Growth Factor-induced Cyclin D1 Expression and DNA Synthesis via Integrin-linked Kinase. Mol Biol Cell. 2008;19:248–261. doi: 10.1091/mbc.E07-05-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Luo X, Ikeda Y, Parker KL. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994;77:481–490. doi: 10.1016/0092-8674(94)90211-9. [DOI] [PubMed] [Google Scholar]
- Maier S, Paulsson M, Hartmann U. The widely expressed extracellular matrix protein SMOC-2 promotes keratinocyte attachment and migration. Exp Cell Res. 2008;314:2477–2487. doi: 10.1016/j.yexcr.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Menke DB, Koubova J, Page DC. Sexual differentiation of germ cells in XX mouse gonads occurs in an anterior-to-posterior wave. Dev Biol. 2003;262:303–312. doi: 10.1016/s0012-1606(03)00391-9. [DOI] [PubMed] [Google Scholar]
- Motamed K, Blake DJ, Angello JC, Allen BL, Rapraeger AC, Hauschka SD, Sage EH. Fibroblast growth factor receptor-1 mediates the inhibition of endothelial cell proliferation and the promotion of skeletal myoblast differentiation by SPARC: a role for protein kinase A. J Cell Biochem. 2003;90:408–423. doi: 10.1002/jcb.10645. [DOI] [PubMed] [Google Scholar]
- Norose K, Clark JI, Syed NA, Basu A, Heber-Katz E, Sage EH, Howe CC. SPARC deficiency leads to early-onset cataractogenesis. Invest Ophthalmol Vis Sci. 1998;39:2674–2680. [PubMed] [Google Scholar]
- Orvis GD, Behringer RR. Cellular mechanisms of Mullerian duct formation in the mouse. Dev Biol. 2007;306:493–504. doi: 10.1016/j.ydbio.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines EW, Lane TF, Iruela-Arispe ML, Ross R, Sage EH. The extracellular glycoprotein SPARC interacts with platelet-derived growth factor (PDGF)-AB and -BB and inhibits the binding of PDGF to its receptors. Proc Natl Acad Sci U S A. 1992;89:1281–1285. doi: 10.1073/pnas.89.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocnik EF, Liu P, Sato K, Walsh K, Vaziri C. The novel SPARC family member SMOC-2 potentiates angiogenic growth factor activity. J Biol Chem. 2006;281:22855–22864. doi: 10.1074/jbc.M513463200. [DOI] [PubMed] [Google Scholar]
- Sadovsky Y, Crawford PA, Woodson KG, Polish JA, Clements MA, Tourtellotte LM, Simburger K, Milbrandt J. Mice deficient in the orphan receptor steroidogenic factor 1 lack adrenal glands and gonads but express P450 side-chain-cleavage enzyme in the placenta and have normal embryonic serum levels of corticosteroids. Proc Natl Acad Sci U S A. 1995;92:10939–10943. doi: 10.1073/pnas.92.24.10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S, Chen JK. Purmorphamine activates the Hedgehog pathway by targeting Smoothened. Nat Chem Biol. 2006;2:29–30. doi: 10.1038/nchembio753. [DOI] [PubMed] [Google Scholar]
- Stark K, Vainio S, Vassileva G, McMahon AP. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature. 1994;372:679–683. doi: 10.1038/372679a0. [DOI] [PubMed] [Google Scholar]
- Tevosian SG, Albrecht KH, Crispino JD, Fujiwara Y, Eicher EM, Orkin SH. Gonadal differentiation, sex determination and normal Sry expression in mice require direct interaction between transcription partners GATA4 and FOG2. Development. 2002;129:4627–4634. doi: 10.1242/dev.129.19.4627. [DOI] [PubMed] [Google Scholar]
- Tevosian SG, Deconinck AE, Tanaka M, Schinke M, Litovsky SH, Izumo S, Fujiwara Y, Orkin SH. FOG-2, a cofactor for GATA transcription factors, is essential for heart morphogenesis and development of coronary vessels from epicardium. Cell. 2000;101:729–739. doi: 10.1016/s0092-8674(00)80885-5. [DOI] [PubMed] [Google Scholar]
- Thomas JT, Canelos P, Luyten FP, Moos M., Jr Xsmoc-1 inhibits BMP signaling downstream of receptor binding and is essential for post-gastrulation development in Xenopus. J Biol Chem. 2009 doi: 10.1074/jbc.M807759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainio S, Heikkila M, Kispert A, Chin N, McMahon AP. Female development in mammals is regulated by Wnt-4 signalling. Nature. 1999;397:405–409. doi: 10.1038/17068. [DOI] [PubMed] [Google Scholar]
- Vannahme C, Gosling S, Paulsson M, Maurer P, Hartmann U. Characterization of SMOC-2, a modular extracellular calcium-binding protein. Biochem J. 2003;373:805–814. doi: 10.1042/BJ20030532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannahme C, Smyth N, Miosge N, Gosling S, Frie C, Paulsson M, Maurer P, Hartmann U. Characterization of SMOC-1, a novel modular calcium-binding protein in basement membranes. J Biol Chem. 2002;277:37977–37986. doi: 10.1074/jbc.M203830200. [DOI] [PubMed] [Google Scholar]
- Wilson MJ, Bowles J, Koopman P. The matricellular protein SPARC is internalized in Sertoli, Leydig, and germ cells during testis differentiation. Mol Reprod Dev. 2006;73:531–539. doi: 10.1002/mrd.20394. [DOI] [PubMed] [Google Scholar]
- Wu X, Walker J, Zhang J, Ding S, Schultz PG. Purmorphamine induces osteogenesis by activation of the hedgehog signaling pathway. Chem Biol. 2004;11:1229–1238. doi: 10.1016/j.chembiol.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Yao HH, Whoriskey W, Capel B. Desert Hedgehog/Patched 1 signaling specifies fetal Leydig cell fate in testis organogenesis. Genes Dev. 2002;16:1433–1440. doi: 10.1101/gad.981202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Carroll TJ, McMahon AP. Sonic hedgehog regulates proliferation and differentiation of mesenchymal cells in the mouse metanephric kidney. Development. 2002;129:5301–5312. doi: 10.1242/dev.129.22.5301. [DOI] [PubMed] [Google Scholar]