Abstract
Inadequate blood flow and increased vasoconstriction of the placenta contribute to pregnancy associated disorders such as preeclampsia (PE). Because placental vessels lack autonomic innervation, humoral effects of the placenta must play critical roles in regulation of fetal-placental vascular contractility. In this study, we examined the nature of humoral factors produced by PE trophoblasts on placental vessel contractility using an organ bath perfusion model. Vasomotor responses were studied in vitro using placental chorionic plate arteries. Vessel rings from third branch chorionic plate arteries were dissected from human placentas following normal or PE delivery. The arterial rings were equilibrated in Krebs Henseleit buffer and exposed to placental conditioned medium, which was prepared by culture of villous tissue from PE placentas. Receptor antagonists for angiotensin II (ANG II), thromboxane (TX), and endothelin (ET) were used to determine which humoral factor produced by placental tissue (trophoblasts) was more effective in promoting vasoconstriction. The role of angiotensin converting enzyme (ACE) and non-ACE ANG II generating enzymes in regulation of placental vasomotor tone were also investigated. A total of 80 arterial rings from 48 placentas were studied. Our results showed: 1) enhanced vasomotor tone in arteries from PE placentas compared to those from normal placentas; 2) PE-CM induced vaso-constrictive activity could be partially attenuated by receptor antagonists for TX, ANG II and ET, respectively; and 3) chymostatin (a chymase inhibitor) produced a stronger inhibitory effect than captopril (ACE inhibitor) on PE conditioned medium induced vasoconstriction. Our data demonstrate increased vasocontractility in PE placentas and suggest that the non-ACE pathway is probably a major source of ANG II produced in the human placenta.
Keywords: Placenta, Vasoactivity, ANG II, ET, TX, Preeclampsia
1. Introduction
Preeclampsia (PE) is a multiple system disorder unique to human pregnancy. Although the etiology remains unclear, increased vasoconstriction is one of the major underlying pathophysiological events in this pregnancy disorder. During normal pregnancy, vascular resistance is reduced, allowing adequate maternal blood perfusion into the placenta to support fetal development. However, in PE there is increased vascular resistance leading to maternal hypertension and reduced blood flow in the placenta. The placenta is a key component in the pathophysiology of PE particularly those with reduced placental perfusion. Many investigators believe that preeclampsia is the result of vasoactive and inflammatory mediators secreted by the placenta acting on the vascular endothelium leading to vasoconstriction of the smooth muscle [1,2]. These mediators are likely secreted from trophoblast cells (TCs) in response to placental hypoxia/ischemia and shallow cytotrophoblast invasion acting on vascular endothelium [3].
It has been demonstrated that multiple mechanisms contribute to the increased vascular resistance during PE, including increased vascular sensitivity to angiotensin II (ANG II) [4,5], the presence of angiotensin II receptor-1 (AT1) autoantibody [2,6], an imbalance between the vasoconstrictor thromboxane (TX) and decreased vasodilator prostacyclin (PGI2) [7,8], and altered endothelin (ET) [9,10] and nitric oxide (NO) [11] levels in both the maternal circulation and in the placenta in women with PE. These alterations may result in a detrimental positive feedback system that promotes inflammation and further vasoconstriction. Since the human placenta lacks autonomic innervation, its vascular tone must be controlled by circulating or locally released vasoactive substances. Therefore, vasoconstrictors produced by placental trophoblast cells may play a critical role in regulating vasomotor tone in the placental vasculature.
Past studies have described that placental tissues produce ANG II [12-16], TX [17,18] and ET [19-21] and they are major contributors to the vasoconstriction in PE. Receptors for ANG II [2,6,22-25], TX [26,27], and ET [28] are present in placental trophoblasts and vasculature. However, the differential effects of each vasoconstrictor in the regulation of placental vasomotor tone have not been simultaneously studied. In the present study we examined vasomotor responses using placental chorionic plate artery organ bath perfusion as a testing model. Receptor antagonists for ANG II, TX, ET were used and the role of ACE and non-ACE ANG II generating systems in the regulation of placental vasomotor tone were also investigated. The purpose of this study was to determine if placenta trophoblast-produced vasoactivate agents could induce a vasomotor response in placental chorionic arteries and what soluble vasoconstrictor agents produced by preeclamptic placentas were involved.
2. Materials and methods
2.1. Placenta and patient information
Placentas were obtained immediately after delivery from normal pregnant women and from women with PE. Normal pregnancies are defined as mothers with normal blood pressure (<140/90 mmHg), absence of proteinuria, and without medical and obstetrical complications. Preeclampsia was defined as gestational blood pressure of greater than 140/90 mmHg on two occasions measured 6 h apart and with urinary protein >300 mg over a 24 h period. This study was approved by the Institutional Review Board for Human Research at LSUHSC-Shreveport, LA. A total of 48 placentas were used, 43 from normal and 5 from PE pregnancies. In the PE group, the mean gestation age was 34+5 weeks with 4 out of 5 delivered by cesarean section. In the normal group, the mean gestation age was 39+1 weeks with 5 out of 43 delivered by cesarean section.
2.2. Conditioned medium preparation
Placental conditioned media (CM) were prepared from villous tissue explant culture from the placentas of preeclamptic patients as previously described [29,30]. Briefly, placentas were obtained immediately after delivery from preeclamptic pregnancies. Placental villous tissue, excluding chorionic basal plates, was removed from these placentas under sterile conditions. Villous tissues were washed with phosphate buffered saline (PBS) containing 1% penicillin (v/v 10,000 units/ml, streptomycin 10,000 μg/ml) and placed in 6 well plates with 500 mg tissue/well in 7 ml serum free Dulbecco's Modified Eagles Medium (DMEM, Sigma Chemical Inc., St Louis, MO). The plates were then placed in a cell culture incubator (5% CO2 in air) for 48 h culture. At the end of incubation, the culture medium was collected and stored at −70 °C until use. Before use, the cultured medium was thawed and centrifuged for 5 min at 3000 to remove cellular debris in the conditioned medium.
2.3. Chorionic artery myography
Placental vessel contractility was examined by placing placental chorionic arterial rings in an organ bath apparatus. Rings were tested with placental conditioned media derived from PE villous tissue culture (PE-CM), which was used to examine the humoral effects of trophoblasts on placental vascular contractility. Chorionic plate arteries chosen were tertiary branches of the umbilical arteries just before they submerge into the chorionic plate, with an internal diameter of 1 mm. The vessels were gently dissected then immediately placed in cold Krebs buffer. The vessels were then transferred into warm Krebs-Henseleit (KH) physiologic saline solution (37 °C, pH 7.4) continuously aerated with a gas mixture consisting of 95% O2 and 5% CO2. The formulation of the KH buffer was as follows (in mmol/L): 118 NaCl; 4.8 KCl; 2.5 MgSO4.7H2O; 1KH2PO4; 27.2 NaHCO3; and 2 g/L of glucose [31].
The vessels were cut into 3 mm long rings and threaded onto wire clips attached to isometric force transducers via silk thread. The size of the vessel rings were selected based on previously published works [32-35]. In general, two sister vessels from the same placenta were studied in parallel in each experiment. The chorionic plate artery rings were then placed in organ baths with U-shaped chambers, and the clips attached to the isometric force-displacement transducers, which were in turn connected to a Grass Model 7 D polygraph recorder.
Vessels were equilibrated without passive tension for 60 min. Thereafter, 1 g of tension was applied to the arterial rings for a 1.5 h period followed with periodic resetting of the 1 g passive tension until they could maintain consistent tone. Before, between and after each application of placental conditioned media and inhibitors/blockers, the vessel rings were washed with Krebs buffer for at least 90 min. This procedure ensured that the vessels return to their baseline tension and that all media or antagonist effects were washed out.
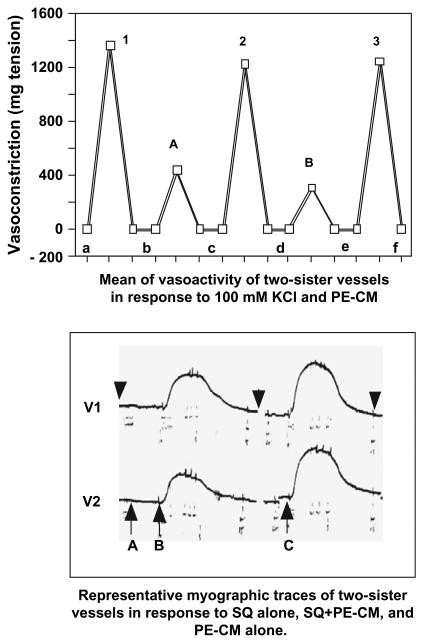
In each experiment, a KH buffer with a concentration of 100 mmol KCl was used to determine the maximal contraction capable of being produced by the vessel [32]. The vessels were exposed to 100 mmol KCl until the response plateaued and maximal contraction was achieved. The vessels were then washed with KH until the vessels relaxed to the baseline level. Vessels incapable of producing 500 mg tension by 100 mmol KCl were considered to be unacceptable for data collection. After a wash period the vessels were perfused with PE-CM. This was used to examine the humoral effects of trophoblasts secretions on placental vascular contractility. This also acted as a comparative control against media mixed with receptor antagonists or enzyme inhibitors. Fig. 1 (upper panel) displays the means of two sister vessels from a normal placenta in response to either 100 mmol KCl or PE-CM. The responses of the vessels to PE-CM with or without a selective receptor antagonist were also determined. DMEM and all inhibitors/receptor antagonists in KH buffer were perfused alone for fifteen minutes prior to addition of PE-CM. Neither DMEM alone nor receptor antagonist or inhibitor alone affected baseline vasomotor tone in tested vessels (Fig. 1 lower panel). In general, a 90 min wash between CM and CM plus a selected receptor antagonist was applied in all experiments. At the end of each experiment the tissues were again exposed to 100 mmol KCl to determine the contractile activity of the artery over the course of the experiment.
Fig. 1.
Upper Panel: Representative vasomotor responses of two sister vessels from a normal placenta during the duration of a 6 h organ bath perfusion experiment in response to 100 mmol KCl and PE-CM stimulation. Data is expressed in milligram tension. This data illustrate that over a 6 h period, the vessels are still healthy and responsive to stimulation. 1–3: maximum contraction induced by 100 mmol KCl; A and B: maximum contraction induced by PE-CM; and a–f: baseline tension of vessels before, between and after 100 mmol KCl and PE-CM stimulation. Lower Panel: Myographic traces of two sister vessels from the same placenta: V1 and V2. A: SQ29548 alone for 15 min; B: SQ29548 + PE-CM; and C: PE-CM alone. Arrowheads: wash period before, between, and after each perfusion with inhibitor or PE-CM, respectively.
2.4. Chemicals
The receptor antagonists and inhibitors used in this study included the selective thromboxane receptor (TP) antagonist SQ-29548 [Cayman Chemicals; Ann Arbor, MI], a specific AT1 receptor antagonist losartan [a generous gift from Dr. Neil Granger; LSUHSC-S], a selective non-peptide AT2 receptor antagonist PD-123,319 [Sigma Chemical Inc. St. Louis, MO.], an ACE inhibitor captopril [ICN biochemicals Costa Mesa, CA], the chymase inhibitor chymostatin [Sigma Chemical Inc.], a selective ETA and B receptor antagonists PD-151,242 and BQ-788 [Sigma Chemical Inc.], respectively.
2.5. Statistical analysis
Data were expressed as the mean (±SE) of % maximum contraction elicited by 100 mmol/L KCl and expressed as % contraction normalized to mg tension during CM perfusion. Statistical analyses were performed with non-parametric Mann–Whitney test or a paired t-test. A computer software program StatView (Cary, NC) was used. A probability level of p < 0.05 was considered statistically significant.
3. Results
3.1. Vasoconstrictor response by vessels from normal and preeclamptic placenta
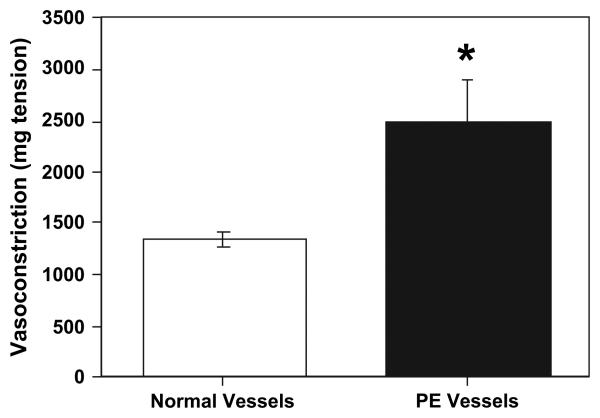
The maximum vasoconstrictive response to 100 mmol KCl by artery rings from normal and PE placentas are shown in Fig. 2. For this data, 40 vessels rings from normal placentas and 7 vessel rings from PE placentas were used. Our data showed that the vasoconstrictive response to KCl was greater in PE vessels than those of normal vessels, indicating that vessels from PE placentas are more sensitive to KCl stimulation than normal vessels (p < 0.05).
Fig. 2.
Maximal contraction of vessel rings from normal and PE placentas in response to 100 mmol KCl. Data are expressed as mean ±SE of mg tension developed. Vasoconstriction was significantly increased in vessel rings 7 from PE placentas compared to 40 from normal placentas, *p < 0.05.
3.2. Placentas release vasoconstrictors
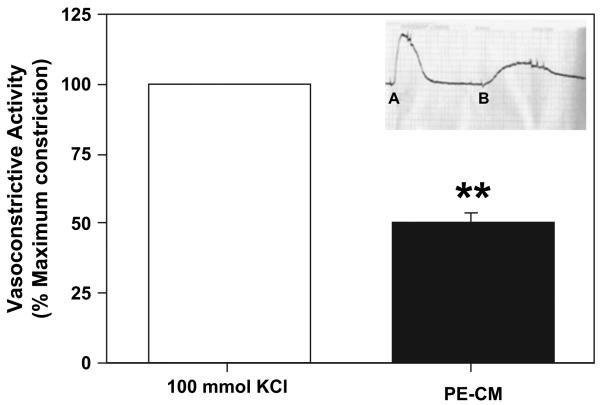
Organ bath myography was then used to determine if vasoactive agents produced by PE placentas could elicit vasoconstrictive effects on the placenta vessels. To study this, placental conditioned media (CM) derived from PE placental explant culture (PE-CM) was used. As shown in Fig. 3, compared to 100 mmol KCl stimulation as 100% of maximal contraction, PE-CM perfusion of placental vessel rings could induce 50 ± 5% of maximal contraction (P < 0.01).
Fig. 3.
Percent of maximal contraction of chorionic vessel rings from normal placentas in response to PE-CM perfusion compared to maximal contraction induced by 100 mmol KCl perfusion (vessel rings, n = 20). PE-CM induced contraction of normal placental vessels, which indicate placentas from preeclampsia release vasoconstrictors. ** Significantly different compared to 100 mmol KCl, p < 0.01. (Insert: Example of an organ bath trace, A: 100 mmol KCl; B: PE-CM).
3.3. Role of angiotensin converting enzymes in the vasomotor responses to placental derived vasoactivators
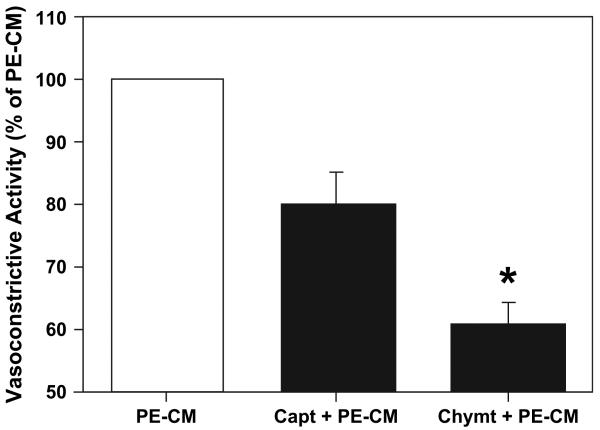
To determine if ANG II was released from PE placentas and to determine which pathway was responsible for the production of ANG II, artery rings were exposed to the ACE inhibitor captopril at a concentration of 10−5 M or the chymase inhibitor chymostatin at a concentration of 10−4 M prior to perfusion of the vessel rings to PE-CM. The concentrations for captopril and chymostatin were selected based on organ bath perfusion studies [36,37]. The results illustrated in Fig. 4 showed that the treatment with the inhibitor captopril or chymostatin resulted in significantly less vasoconstrictive effects of PE-CM with a 20 ± 4% attenuation of PE-CM by captopril, and a 40% attenuation by chymostatin. These data suggest that chymase may play a more significant role than ACE in ANG II generation.
Fig. 4.
Effect of captopril and chymostatin on PE-CM induced vasoconstriction of placental vessels. Captopril: ACE inhibitor (capt; n = 6); chymostatin: chymase inhibitor (chymt; n = 5). Chymase is a non-ACE ANG II generating enzyme. Data is presented as mean % ± SE of PE-CM induced contraction, *p < 0.05.
3.4. The involvement of ANG II AT1 and AT2 receptors in the vasoconstrictve response to placental derived vasoactivators
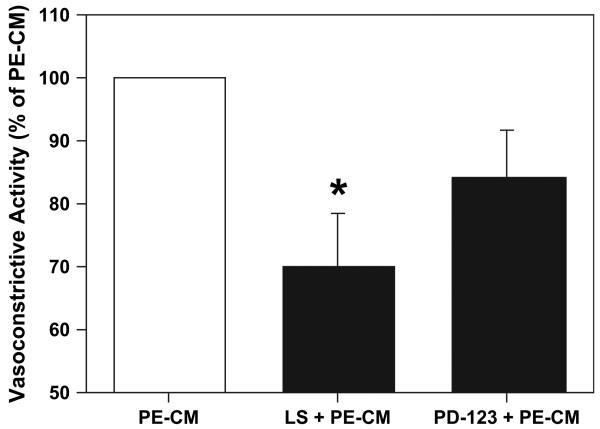
To further investigate the involvement of ANG II pathway in the vasoconstrictor response produced by PE placentas, losartan (a selective AT1 blocker) and PD-123319 (a selective AT2 blocker) were used. Losartan (10−5 M) significantly attenuated the contractile response to PE-CM by 30 ± 8% (P < 0.05), while the AT2 receptor antagonist PD-123319 (10−6M) only reduced the contractile response of the vessels to PE-CM by 16 ± 8% (Fig. 5).
Fig. 5.
Blockade of angiotensin AT1 or AT2 receptors with losartan (LS) and PD123319 (PD-123) respectively attenuates PE-CM induced vasoconstriction in chorionic vessels (n = 6). Data is presented as mean % ± SE of PE-CM induced contraction, *p < 0.05.
3.5. The involvement of endothelin-1 in the vasoconstrictive response to placental derived vasoactivators
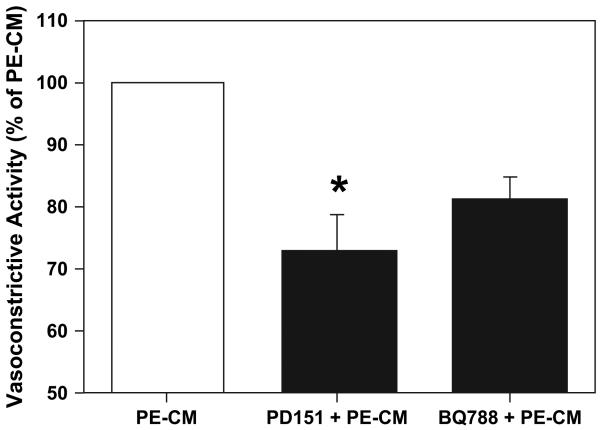
The ETA antagonist PD-151242 and the ETB antagonist BQ788 were used to study the effects of endothelin on placental vasoconstriction. PD-151242 (10−5 M) attenuated the contractile response of PE-CM significantly (28 ± 6%, P < 0.05), whereas, BQ788 (10−6 M) produced a non-significant degree of attenuation (Fig. 6).
Fig. 6.
Blocking of endothelin ETA and ETB receptors with PD151242 and BQ788 respectively attenuates PE-CM induced vasoconstriction in chorionic vessels (n = 6). PD151242 (PD151) = ETA receptor antagonist; BQ788 = ETB receptor antagonist. Data is presented as mean % ± SE of PE-CM induced contraction, *p < 0.05.
3.6. The involvement of thromboxane in the vasoconstrictive response to placental derived vasoactivators
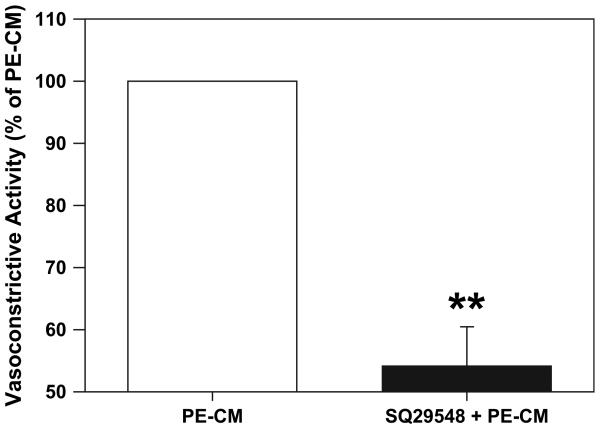
In this experiment, the TXA2 receptor antagonist, SQ-29548 at a concentration of 10−6 M was used. SQ-29548 significantly attenuated the contractile response induced by PE-CM (Fig. 7). The mean contraction of chorionic vessels with PE-CM ± SQ29548 was reduced by approximately 50 ± 6% of that compared to CM alone (P < 0.01).
Fig. 7.
Thromboxane receptor antagonist SQ29548 greatly attenuates the chorionic vessel vasocontractility produced by PE-CM perfusion in the organ bath (vessel rings, n = 6). Data is presented as mean % ± SE of PE-CM induced contraction, **p < 0.01.
4. Discussion
In the present study, the vasomotor responses of chorionic plate arteries from placentas delivered by normal pregnant women and by pregnant women complicated by preeclampsia were examined. One of the most important findings in our study was that chorionic plate arteries from preeclamptic placentas produced a significantly stronger contractile response to 100 mmol KCl challenge than the arteries from normal placentas, which demonstrated that increased vasocontractile activity is present in the placental vasculature in preeclampsia. The finding of increased vasomotor response in preeclamptic vessels is consistent with the current view that increased vasoconstriction occurs in the placental vasculature during preeclampsia. Our results agree with those of Bertrand et al. where they found that chorionic plate arteries and veins developed a greater active tension than those from normal placentas [32]. The ability of preeclamptic vessels to develop a greater tension than normal vessels suggests that there are physical and biochemical differences in vasomotor responses to contractile stimuli between the vessels of normal placentas and those of preeclamptic placentas.
Since the placenta lacks autonomic innervation, it is believed that placental vascular tone must be regulated by circulating or locally released vasoactive substances. To study whether placental trophoblast-derived vasoactivators could be responsible for the increased vasomotor tone observed in preeclamptic placental vessels, chorionic plate artery rings from normal placentas were perfused with conditioned media, which was prepared by culture of villous tissue from preeclamptic placentas. Our results showed that when normal vessels were perfused with preeclamptic placental conditioned media, they could produce approximately 50% of maximum tension of that produced by 100 mmol KCl. These data suggest that placental tissue-produced vasoactivators could, at least in part, be responsible for the increased vasomotor activity in the placental vasculature during preeclampsia.
The placenta produces ANG II [12-16], TX [17,18] and ET [19-21]. To study which vasoactivators and their pathways produced by PE placenta were more responsible for regulating the vasomotor response in the placental vasculature, selected receptor antagonists or inhibitors of three major vasoconstrictors, ANG II, TX and ET-1, were used. Our results showed that all these inhibitors or receptor blockers produced a more or less suppressive effect on PE-CM induced vasomotor response with TX receptor blocker as the most potent one in chronic plate artery vessels.
Our data indicated that TX is probably the strongest vasoconstrictor produced by the preeclamptic placenta since the thromboxane receptor blocker, SQ29548, elicited the greatest inhibitory effects on vasoconstriction induced by PE-CM. SQ29548 was used at a concentration of 10−6 M, as based on previously published works [20,38]. This concentration was shown to be able to completely block TXB induced vasoconstriction stimulated by bradykinin in a perfused placental cotyledon study [38].
During normal pregnancy, the placenta produces equivalent amounts of TXA2 and PGI2, and thus their activities are balanced. In PE women, TXA2 production is significantly increased while PGI2 and PGE2 production are significantly decreased and results in vasoconstriction [17]. It has been reported that placental trophoblast cells are the major source of TX production in placental tissue and that trophoblast cells from PE placentas produce significantly more TX than normal placentas [8,17], although the possibility exists that vasoconstrictors may or may not exert their actions through TXA receptor [20,38].
Several studies have demonstrated that ANG I [13] and ANG II [39] are present in the placenta and the uteroplacental unit possesses all components of the RAS necessary for their generation [12]; renin [14], angiotensin [40] and ACE [15]. However, the impact of the altered RAS on the fetal side of the placenta is not well defined [41,42]. Most of the vasoactive effects of ANG II are produced depending on which angiotensin II receptor is expressed in the smooth muscle of the particular tissue of interest. AT1 receptor activation produces vasoconstriction and AT2 receptor activation produces vasoconstriction or dilation depending on the cell type. It is likely that ANG II mainly binds to AT1 receptor and elicits downstream vasocontrictor effects in the placenta. Li X et al. [23] examined AT1 and AT2 receptor expression in PE and normal placental trophoblast cells and found that both receptors are present in the placenta and that AT1 receptor predominates [23]. However, Li and colleagues observed that in placentas from PE and intrauterine growth restriction pregnancies the AT1 receptor expression is decreased compared to normal placental expression. Our data demonstrated that the AT1 receptor antagonist, losartan, inhibited the effect of PE-CM induced vascular vasocontractility by 30% (Fig. 5), while the AT2 antagonist, PD123319, was less effective. This suggests that AT1 receptor exerts predominate function in the placental arteries. In addition, our results confirm the observation made by MaasenVanDenBrink et al., that the AT2 receptor antagonist, PD123319, had no effect on ANG II induced contraction in human coronary artery (HCA) rings [37].
Interestingly, our results showed that chymostatin had a stronger inhibitory effect on PE-CM induced vascontraction. Chymostatin is a specific inhibitor for chymase which has been demonstrated to be a major non-ACE angiotensin II generating enzyme in the human heart [43]. Our results indicate that ANG II can be generated by both ACE and non-ACE systems in the placenta. These non-ACE and ACE inhibitory results imply that the strong vasoconstrictive effects produced by preeclamptic placentas may be regulated via the ANG II pathway and that the non-ACE generating system may be more dominant in regulation of vasoactivity in the placental vasculature during PE. The observations of enhanced chymase expression and activity in PE placental tissue than that in normal placental tissue supports this notion [44].
The importance of endothelin in the vascular consequences of PE was examined pharmacologically with endothelin ETA and ETB receptor antagonists. We found that the ETA receptor antagonist produced a significant reduction in the response of chorionic tertiary level arteries to PE-CM. Sand et al. also studied endothelin induced contraction in placental arteries [45]. They found that block of the ETA receptor can attenuate approximately 75% of ET-1 induced vasoconstriction, whereas ETB receptor block produced a 58% decrease in the effect of ET-1. Using the same concentrations of ETA and ETB receptor blockers showed that the ETB receptor does not significantly alter the vasoconstriction produced by PE-CM, and only 27.5% of the vasoconstrictor response to PE-CM was altered with the ETA blocker PD-151242 (Fig. 6), indicating that ET-1 induced vasoconstriction is not the major component produced by placentas in the vascoconstriction seen in PE.
Placental trophoblast cells release TX, ANG II and ET into intervillous space. These vasoactivators could enter the maternal circulation and affect vascular function. Although at the present time there is no information available regarding basolateral release of vasoactivators by placental trophoblast cells, an in vitro cell culture study by Larry Guilbert group did show that more than 90% of MMP2 and MMP9 produced by trophoblast cells were released from the basolateral surface compared to epithelial surface by cultured human placental syncytiotrophoblasts [46]. Our published works using cell co-culture model: co-culture of endothelial cells and trophoblast cells [47,48] also support the idea that trophoblast-produced bioactive agents could be released not only into the epithelial direction (inter-villous space) but also into the basolateral direction (villous stromal compartment). This concept is also supported by the fact that the maternal circulation is separated from the fetal circulation in the placenta, so vasoactivity of placental vasomotor tone must be regulated by locally produced vasoactive agents or come from the maternal circulation shuttled across (diffuse) the trophoblast layer to villous core fetal vessels. Results from the present study suggest that paracrine regulation of trophoblast-derived vasoactivators might play an important role in control of placental vessel motor tension and contractile function. Further studies on paracrine regulation of trophoblast-derived vasoactivitors would provide insight into mechanisms in vessel motor tone of placental vasculature.
In summary, in the present study using organ bath perfusion model, we tested ANG II, TX, ET-1 receptors and ACE and non-ACE angiotensin generating systems in the regulation of chorionic plate artery vasoactivity as altered by preeclampsia. We found that: 1) PE placental vessels exert an increased vasocontractile activity; 2) placental tissues (trophoblasts) release vasoconstrictors; 3) thromboxane might be the strongest vasoconstrictor produced by placental tissue in PE; and 4) the most significant finding of the study is that the non-ACE angiotensin II generating system is dominate in the placenta and may play a critical role in regulation of placental vascular contractile function. Further study is needed to determine the interplay of these vasoconstrictors in the regulation of vasomotor tone of the placental vasculature in normal pregnancy and in preeclampsia.
Acknowledgement
This study was supported in part by grants from the National Institute of Health, National Institute of Child Health Development (HD36822) and National Heart Blood Lung institute (HL65997).
Footnotes
This study was supported in part by grants from the National Institute of Health, National Institute of Child Health Development (HD36822) and National Heart Blood Lung Institute (HL65997).
References
- 1.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–4. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 2.Dechend R, Homuth V, Wallukat G, Kreuzer J, Park JK, Theuer J, et al. AT(1) receptor agonistic antibodies from preeclamptic patients cause vascular cells to express tissue factor. Circulation. 2000;101:2382–7. doi: 10.1161/01.cir.101.20.2382. [DOI] [PubMed] [Google Scholar]
- 3.Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol. 1989;161:1200–4. doi: 10.1016/0002-9378(89)90665-0. [DOI] [PubMed] [Google Scholar]
- 4.Gant NF, Chand S, Worley RJ, Whalley PJ, Crosby UD, MacDonald PC. A clinical test useful for predicting the development of acute hypertension in pregnancy. Am J Obstet Gynecol. 1974;120:1–7. doi: 10.1016/0002-9378(74)90170-7. [DOI] [PubMed] [Google Scholar]
- 5.Gant NF, Daly GL, Chand S, Whalley PJ, MacDonald PC. A study of angiotensin II pressor response throughout primigravid pregnancy. J Clin Invest. 1973;52:2682–9. doi: 10.1172/JCI107462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xia Y, Wen H, Bobst S, Day MC, Kellems RE. Maternal autoantibodies from preeclamptic patients activate angiotensin receptors on human trophoblast cells. J Soc Gynecol Ivestig. 2003;10:82–93. doi: 10.1016/s1071-5576(02)00259-9. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Walsh SW, Guo JD, Zhang J. The imbalance between thromboxane and prostacyclin in preeclampsia is associated with an imbalance between lipid peroxides and vitamin E in maternal blood. Am J Obstet Gynecol. 1991;165:1695–700. doi: 10.1016/0002-9378(91)90017-l. [DOI] [PubMed] [Google Scholar]
- 8.Walsh SW. Preeclampsia: an imbalance in placental prostacyclin and thromboxane production. Am J Obstet Gynecol. 1985;152:335–40. doi: 10.1016/s0002-9378(85)80223-4. [DOI] [PubMed] [Google Scholar]
- 9.Schiff E, Ben-Baruch G, Peleg E, Rosenthal T, Alcalav M, Devir M, et al. Immunoreactive circulating endothelin-1 in normal and hypertensive pregnancies. Am J Obstet Gynecol. 1992;166:624–8. doi: 10.1016/0002-9378(92)91688-7. [DOI] [PubMed] [Google Scholar]
- 10.Myatt L. Current topic: control of vascular resistance in the human placenta. Placenta. 1992;13:329–41. doi: 10.1016/0143-4004(92)90057-z. [DOI] [PubMed] [Google Scholar]
- 11.Granger JP, Alexander BT, Bennett WA, Khalil RA. Pathophysiology of pregnancy-induced hypertension. Am J Hypertens. 2001;14:178S–85. doi: 10.1016/s0895-7061(01)02086-6. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen AH, Schauser KH, Poulsen K. Current topic: the uteroplacental renin-angiotensin system. Placenta. 2000;21:468–77. doi: 10.1053/plac.2000.0535. [DOI] [PubMed] [Google Scholar]
- 13.Taira H, Mizutani S, Narita O, Tomoda Y. Angiotensin I-converting enzyme in human placenta. Placenta. 1985;6:543–9. doi: 10.1016/s0143-4004(85)80008-4. [DOI] [PubMed] [Google Scholar]
- 14.Skinner SL, Lumbers ER, Symonds EM. Renin concentration in human fetal and maternal tissues. Am J Obstet Gynecol. 1968;101:529–33. doi: 10.1016/0002-9378(68)90564-4. [DOI] [PubMed] [Google Scholar]
- 15.Ito M, Itakura A, Ohno Y, Nomura M, Senga T, Nagasaka T, et al. Possible activation of the renin-angiotensin system in the feto-placental unit in preeclampsia. J Clin Endocrinol Metab. 2002;87:1871–8. doi: 10.1210/jcem.87.4.8422. [DOI] [PubMed] [Google Scholar]
- 16.Broughton Pipkin F, Craven DJ, Symonds EM. The uteroplacental renin-angiotensin system in normal and hypertensive pregnancy. Contributions Nephrol. 1981;25:49–52. doi: 10.1159/000396012. [DOI] [PubMed] [Google Scholar]
- 17.Walsh SW, Wang Y. Trophoblast and placental villous core production of lipid peroxides, thromboxane, and prostacyclin in preeclampsia. J Clin Endocrinol Metab. 1995;80:1888–93. doi: 10.1210/jcem.80.6.7775637. [DOI] [PubMed] [Google Scholar]
- 18.Bowen RS, Zhang Y, Gu Y, Lewis DF, Wang Y. Increased phospholipase A2 and thromboxane but not prostacyclin production by placental trophoblast cells from normal and preeclamptic pregnancies cultured under hypoxia conditions. Placenta. 2005;26:402–9. doi: 10.1016/j.placenta.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Levin E. Endothelins. N Engl J Med. 1995;333:356–62. doi: 10.1056/NEJM199508103330607. [DOI] [PubMed] [Google Scholar]
- 20.Le SQ, Wasserstrum N, Mombouli JV, Vanhoutte PM. Contractile effect of endothelin in human placental veins: role of endothelium prostaglandins and thromboxane. Am J Obstet Gynecol. 1993;169:919–24. doi: 10.1016/0002-9378(93)90027-g. [DOI] [PubMed] [Google Scholar]
- 21.Singh HJ, Rahman A, Larmie ET, Nila A. Endothelin-1 in feto-placental tissues from normotensive pregnant women and women with preeclampsia. Acta Obstet Gynecol Scand. 2001;80:99–103. doi: 10.1034/j.1600-0412.2001.080002099.x. [DOI] [PubMed] [Google Scholar]
- 22.Roberts J. Angiotensin-1 receptor autoantibodies: a role in the pathogenesis of preeclampsia? Circulation. 2000;101:2335–7. doi: 10.1161/01.cir.101.20.2335. [DOI] [PubMed] [Google Scholar]
- 23.Li X, Shams M, Zhu J, Khalig A, Wilkes M, Whittle M, et al. Cellular localization of AT1 Receptor mRNA and Protein in Normal Placenta and its Reduced Expression in Intrauterine Growth Restriction. J Clin Invest. 1998;101:442–54. doi: 10.1172/JCI119881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knock GA, Sullivan MH, McCarthy A, Elder MG, Polak JM, Wharton J. Angiotensin II (AT1) vascular binding sites in human placentae from normal-term, preeclamptic and growth retarded pregnancies. J Pharmacol Exp Ther. 1994;271:1007–15. [PubMed] [Google Scholar]
- 25.Kalenga MK, de Gasparo M, de Hertogh R, Whitebread S, Vankrieken L, Thomas K. Angiotensin II receptors in the human placenta are type AT1. Reprod Nutr Dev. 1991;31:257–67. [PubMed] [Google Scholar]
- 26.Boura AL, Gude NM, King RG, Mak KK, Walters WA. Characterization of thromboxane A2 receptors in the human fetal placental vessels and umbilical vein. Clin Exp Pharmacol Physiol. 1986;13:83–6. doi: 10.1111/j.1440-1681.1986.tb00319.x. [DOI] [PubMed] [Google Scholar]
- 27.Hirata M, Hayashi Y, Ushikubi F, Yokota Y, Kadeyama R, Nakanishi S, et al. Cloning and expression of cDNA for human thromboxane A2 receptor. Nature. 1991;349:617–20. doi: 10.1038/349617a0. [DOI] [PubMed] [Google Scholar]
- 28.Wilkes BM, Macica C, Mento PF. Endothelin conversion and receptor characterization in human placental arteries. Am J Physiol. 1994;267:325–415. doi: 10.1152/ajpendo.1994.267.2.E242. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Adair C, Weeks JW, Lewis DF, Alexander JS. Increased neutrophil-endothelial adhesion induced by placental factors is mediated by platelet-activating factor in preeclampsia. J Soc Gynecol Investig. 1999;6:136–41. doi: 10.1016/s1071-5576(99)00004-0. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Gu Y, Philibert L, Lucas MJ. Neutrophil activation induced by placental factors in normal and pre-eclamptic pregnancies in vitro. Placenta. 2001;22:560–5. doi: 10.1053/plac.2001.0691. [DOI] [PubMed] [Google Scholar]
- 31.Zavecz JH, Bueno O, Maloney RE, O'Donnell JM, Roerig SC, Battarbee HD. Cardiac excitation-contraction coupling in the portal hypertensive rat. Am J Physiol Gastointest Liver Physiol. 2000;279:g28–39. doi: 10.1152/ajpgi.2000.279.1.G28. [DOI] [PubMed] [Google Scholar]
- 32.Bertrand C, Duperron L, St-Louis J. Umbilical and placental vessels: modifications of their mechanical properties in preeclampsia. Am J Obstet Gynecol. 1993;168:1537–46. doi: 10.1016/s0002-9378(11)90795-9. [DOI] [PubMed] [Google Scholar]
- 33.Wareing M, Baker PN. Vasoconstriction of small arteries isolated from the human placental chorionic plate in normal and compromised pregnancy. Hypertens Pregnancy. 2004;23:237–46. doi: 10.1081/PRG-200030297. [DOI] [PubMed] [Google Scholar]
- 34.Jain V, Lim M, Longo M, Fisk N. Inhibitory effect of erythropoietin on contractility of human chorionic plate vessels. Am J Obstet Gynecol. 2006;194:246.e1–7. doi: 10.1016/j.ajog.2005.05.037. [DOI] [PubMed] [Google Scholar]
- 35.Estan L, Abad A, Morales-Olivas FJ, Serra-Serra V. Isolated human chorionic vascular reactivity: Technical considerations for fresh preparations. Gen Pharmac. 1998;30:89–93. doi: 10.1016/s0306-3623(97)00079-7. [DOI] [PubMed] [Google Scholar]
- 36.Tom B, Gerrelds IM, Scalbert E, Stegmann AP, Boomsma F, Saxena PR, et al. ACE-versus chymase-dependent antiotensin II generation in human coronary arteries: a Matter of Efficiency? Arterioscler Thromb Vasc Biol. 2003;23:251–6. doi: 10.1161/01.atv.0000051875.41849.25. [DOI] [PubMed] [Google Scholar]
- 37.MaasenVanDenBrink A, de Vries R, Saxena PR, Schalekamp M, Danser AHJ. Vasoconstriction by in situ formed angiotensin II: role of ACE and chymase. Cardiovascular research. 1999;44:407–15. doi: 10.1016/s0008-6363(99)00249-7. [DOI] [PubMed] [Google Scholar]
- 38.Wilkes BM, Mento P. Bradykinin-induced vasoconstriction and thromboxane release in perfused human placenta. Am J Physiol. 1988;254:E681–6. doi: 10.1152/ajpendo.1988.254.6.E681. [DOI] [PubMed] [Google Scholar]
- 39.Naruse M, Naruse K, Kurimoto F, Sakurai H, Yoshida S, Toma H, et al. Evidence for the existence of des-Asp1-angiotensin II in human uterine and adrenal tissues. J Clin Endocrinol Metab. 1985;61:480–3. doi: 10.1210/jcem-61-3-480. [DOI] [PubMed] [Google Scholar]
- 40.Paul M, Wagner J, Dzau VJ. Gene expression of the renin-angiotensin system in human tissues: quantitative analysis by the polymerase chain reaction. J Clin Invest. 1993;91:2058–64. doi: 10.1172/JCI116428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shah D. Role of the renin-angiotensin system in the pathogenesis of preeclampsia. Am J Physiol Renal Physiol. 2005;288:614–25. doi: 10.1152/ajprenal.00410.2003. [DOI] [PubMed] [Google Scholar]
- 42.Khalil RA, Granger JP. Vascular mechanisms of increased arterial pressure in preeclampsia: lessons from animal models. Am J Physiol Regul Integr Comp Physiol. 2002;283:R29–45. doi: 10.1152/ajpregu.00762.2001. [DOI] [PubMed] [Google Scholar]
- 43.Urata H, Ganten D. Cardiac angiotensin II formation: the angiotensin-1 converting enzyme and human chymase. Eur Heart J. 1993;14:177–82. [PubMed] [Google Scholar]
- 44.Wang Y, Gu Y, Zhang Y, Lewis DF, Alexander JS, Granger DN. Increased chymotrypsin-like protease (chymase) expression and activity in placentas from women with preeclampsia. Placenta. 2006 doi: 10.1016/j.placenta.2006.03.012. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 45.Sand AE, Ostlund E, Andersson E, Fried G. Endothelin-induced contractions in placental arteries is mediated by both ETa- and ETb receptors. Acta Physiol Scand. 1998;163:227–34. doi: 10.1046/j.1365-201x.1998.00368.x. [DOI] [PubMed] [Google Scholar]
- 46.Sawicki G, Radomski MW, Winkler-Lowen B, Krzymien A, Guilbert LJ. Polarized release of matrix metalloproteinase-2 and -9 from cultured human placental syncytiotrophoblasts. Biol Reprod. 2000;63(5):1390–5. doi: 10.1095/biolreprod63.5.1390. [DOI] [PubMed] [Google Scholar]
- 47.Wang Y, Zhang Y, Lewis DF, Gu Y, Li H, Granger DN, et al. Protease chymotrypsin mediates the endothelial expression of P- and E-selectin, but not ICAM and VCAM, induced by placental trophoblasts from pre-eclamptic pregnancies. Placenta. 2003;24:851–61. doi: 10.1016/s0143-4004(03)00132-2. [DOI] [PubMed] [Google Scholar]
- 48.Wang Y, Lewis DF, Gu Y, Zhang Y, Alexander JS, Granger DN. Placental trophoblast-derived factors diminish endothelial barrier function. J Endocrinol. 2004;89(5):2421–8. doi: 10.1210/jc.2003-031707. [DOI] [PubMed] [Google Scholar]