Abstract
Quantum dot conjugates of compounds capable of inhibiting the serotonin transporter (SERT) could form the basis of fluorescent probes for live cell imaging of membrane bound SERT. Additionally, quantum dot-SERT antagonist conjugates may be amenable to fluorescence-based, high-throughput assays for this transporter. This paper describes the synthesis of SERT-selective ligands amenable to conjugation to quantum dots via a biotin-streptavidin binding interaction. SERT selectivity and affinity were incorporated into the ligand via a tetrahydropyridine or cyclohexylamine derivative and the affinity of these compounds for SERT was measured by their ability to produce SERT-dependent currents in Xenopus laveis oocytes.
Keywords: Quantum Dot, Serotonin, SERT antagonist, homotryptamine, Leak current
Neurotransmitters such as dopamine, norepinephrine and 5-hydroxytryptamine (serotonin, 5-HT) modulate neuronal signaling in response to a variety of sensory and physiological stimuli. Synaptic concentrations of these neurotransmitters are regulated by presynaptic transporters, specifically the dopamine transporter (DAT), the norepinephrine transporter (NET) and the serotonin transporter (SERT).1,2 Methodologies used to study the location and temporal dynamics of these transporters within the membrane may provide important insights to their dysfunction, which is suspected to contribute to disorders such as autism, OCD, depression, ADHD and addiction. Our efforts currently center on the development of fluorescent probes based upon DAT and SERT antagonists that can be conjugated to quantum dots.3-6 Ultimately, these probes will be used to study the expression, location and dynamics of these transporters within the presynaptic membrane7-9 and to identify abnormalities and therapeutics pertinent to transporter-related illnesses.
In earlier studies, we identified 3-(1,2,3,6-tetrahydropyrindin-4-yl)-1H-indole (2) as a high affinity SERT antagonist that may be conjugated to quantum dots.8,9 A linker arm may be attached to this tetrahydropyridyl nitrogen atom, this biological activity is retained (Figure 1, Table 1). The ability of these derivatives to inhibit the uptake of tritiated serotonin (5-HT) in human SERT (hSERT) expressing HEK cells was measured. After conjugation to quantum dots, the uptake inhibition by these conjugates was measured and IC50 values were calculated following titration of conjugated dot concentrations (Table 1). Studies of non-specific binding of quantum dots to cellular membranes suggested a long polyethylene glycol chain on the surface of the dot was necessary to reduce non-specific adsorption of the quantum dots to cellular membranes.10 Such compounds were shown to retain antagonist activity.
Figure 1.
High affinity SERT ligands for conjugation to quantum dots.
Table 1. Biological activity of free and conjugated ligands against hSERT.
| Compound No | IC50 (nM) | Conjugate IC50 (nM) |
|---|---|---|
| 2 | 80* | not applicable |
| 3 | 2 | 30 |
| 4 | 1 | 100 |
| 5 | 2,000 | not conjugated |
| 6 | 30 | 10 |
| 7 | 25 | not conjugated |
Literature value11
Although we have demonstrated our ligands have a high affinity for hSERT in uptake inhibition assays, we sought further evidence that ligand-conjugated dots bind tightly to hSERT in intact cells in real-time physiological assays. Using hSERT expressing oocytes, it is possible to detect antagonist suppressed hSERT leak currents (which appear as outward currents above a pretreatment baseline), from these measurements it is possible to gain insights regarding the relative potency of hSERT ligands. In this report, the synthesis of a series of high-affinity hSERT ligands (Figure 3.) and their ability to suppress hSERT leak currents in hSERT expressing oocytes was studied.
Figure 3.

The ratio of antagonist revealed leak current to 5-HT induced current for the analogs used in this study.
Derivatives of both (2) and cyclohexenyl amine were synthesized with an alkyl spacer attached to biotin-PEG5000 (Figure 2). The alkyl spacer length was changed to study the effect on binding (compounds (8), (10) and (11)). The effect of substituting on the indole ring was studied by synthesizing compounds (8), (9) and (12). Compounds (13) and the intermediate (14) were synthesized extending the basic nitrogen from the tetrahydropyridine ring to study the effects of this modification on binding. Finally, a methyl caped PEG ligand (15) was synthesized and used as a negative control.
Figure 2. Ligands used in this study.
Compounds (8)-(12) were synthesized as shown in Scheme 1. Initially (2), the methoxy, and the cyano analogs were synthesized using the method described by Guillaume et al11; these were then attached to 2-(bromoalkyl)isoindoline-1,3-dione (15a), (15b) or (15c), resulting in (16a)-(16f) and were converted to the amines (17a)-(17f)9, after coupling to Biotin-PEG5000-NHS compounds (8)-(12) were obtained.12 The synthesis of (16a) and (16b) are previously described by Tomlinson et al.9
Scheme 1.
(i) Et3N; (ii) Hydrazine monohydrate; (iii) Biotin-PEG5000-NHS
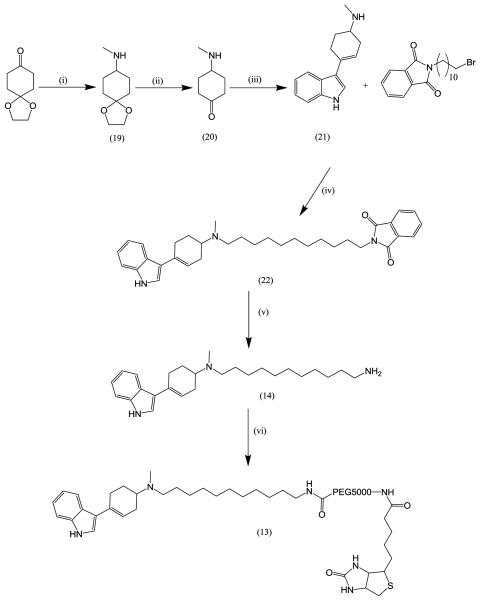
Compound (13) was synthesized as outlined in Scheme 2. The mono ethyl ketal of commercially available cyclohexane dione was converted to N-methyl-1,4-dioxaspiro[4.5]decan-8-amine (19) by a reductive amination with sodium triacetoxy borohydride and methylamine. The ketal was hydrolysed to yield 4-(methylamino)cyclohexanone (20) and this was reacted with indole to yield 4-(1H-indol-3-yl)-N-methylcyclohex-3-enamine (21) in a 20% overall yield.13 The 11 carbon spacer was attached to this using the same methodology as previously described to give (22) in a 64% yield.14 The phthalimide protecting group was removed by stirring in ethanol in the presence of hydrazine mono hydrate using the method previously described9 to give (14) in a 93% yield. This was coupled to Biotin-PEG5000-NHS to give compound (13).15 Compound (15) was obtained by reacting Biotin-PEG5000-NHS with methylamine.16
Scheme 2.
(i) Methylamine, Na(AcO)3BH; (ii) TFA; (iii) Indole, NaOMe; (iv) Et3N; (v) Hydrazine monohydrate; (vi) Biotin-PEG5000-NHS
The ability of compounds (8)-(15) to suppress hSERT-dependent leak currents in Xenopus oocytes was measured by perfusing oocytes with a 1μM solution of (8)-(14) in buffer. Electrophysiological recordings for each ligand were obtained using a low-pass filter at 10 Hz and digitized at 20 Hz. All analyses were performed using Origin 7 (OriginLab, Northampton, MA). The magnitude of the leak currents for (8)-(14) was obtained using 5 replicates and the magnitudes of the leak currents for compounds (8)-(13) are shown in Figure 3. An example raw trace of leak suppression and 5-HT induced currents is shown in Figure 4. Compound (15) was a control compound and induced no suppression of leak current indicating that the indole derivative is necessary for biological activity.
Figure 4.

A raw data trace showing a representative leak current obtained for the intermediate IDT373 (14).
Figure 4 shows a raw data trace obtained for the intermediate IDT373 (14).
In addition to measuring leak current suppression, the ability of the ligands to inhibit the uptake of tritiated 5-HT in hSERT expressing Xenopus oocytes was also measured and IC50 values were obtained using 5 replicates. Figure 5 shows a correlation of the IC50 value obtained using this oocyte system with the observed leak current suppression.
Figure 5.

Leak current correlations with 5-HT uptake inhibition IC50 in oocytes obtained for compounds (8)-(14).
Compounds (8)-(14) showed leak currents that are typical of SERT antagonists. It is crucial to understand how tightly our ligands are binding to the SERT since we intend to utilize them in a variety of biological assays. Ligands that bind tightly and do not diffuse into the surrounding media during an assay may be required for assays to track the location and temporal dynamics of SERT within live cell membranes. On the other hand, ligands that do not bind as tightly may be more suitable for displacement assays which could be the basis of a quantum dot based high throughput (HTS) assay. The magnitude of the measured leak currents and IC50 value will enable the selection of the appropriate ligand.
The magnitude of the leak current is proportional to the potency of the ligand for hSERT interactions. IDT374 (13) exhibited the greatest activity while IDT317 (11) exhibited the lowest leak current suppression, and consequently, the weakest binding to hSERT. These data were mirrored by the measured IC50 values for SERT uptake inhibition. The relative potencies of our SERT ligands appears to be determined by three factors. The 1st of these is the length of the alkyl spacer in the linker arm; when the alkyl spacer is increased in length from 2 carbon atoms in IDT317 (11) to 6 carbon atoms in IDT366 (10), an increase in leak current suppression can be observed. This trend continues when the alkyl spacer is increased to 11 carbon atoms (IDT357 (8)), indicating that the alkyl spacer in these molecules participates in binding. One hypothesis is that hydrophobic interactions within SERT itself support ligand interactions, or another possibility may be that hydrophobic interactions between the alkyl spacer and the lipid membrane increase the tightness of binding. Substitution on the indole ring has a less significant effect on the magnitude of the leak current. Derivatives including electron withdrawing substituents on the 5-position of the indole ring, such as the cyano derivative (IDT361 (12)), have a slightly larger leak current suppression than derivatives where an electron donating substituent, such as a methoxy derivative is on the ring (IDT318 (9)). However these differences do not seem to correlate well with IC50 values since it has been reported that the cyano substituted parent drug has a potency that is approximately 20 fold greater than the methoxy substituted parent drug.17 A large increase in the magnitude of the leak current suppression was obtained by moving the basic nitrogen out of the tetrahydropyridyl ring to give IDT374 (13), suggesting this basic nitrogen may be interacting with the binding site in hSERT. The basic nitrogen may be acting as a hydrogen bond acceptor and the binding strength is likely dependent upon the orientation of the lone pair of electrons on the hydrogen bond acceptor. Consequently, it is not surprising any modification in the structure of the homotryptamine, which changes the orientation of this nitrogen atom and its lone pair of electrons, will have a significant impact upon the tightness of binding to hSERT if it is acting as a hydrogen bond acceptor.
In conclusion, the relative potency for interactions with hSERT for our ligands follows the order: IDT374 (13)∼IDT373 (14) > IDT361 (12) ∼ IDT357 (8) = IDT318 (9) > IDT366 (10) > IDT317(11). In addition to these leak current experiments, we also demonstrated that IDT317 (11) binds relatively weakly to hSERT since it can be easily washed off, restoring a normal 5-HT induced influx current (data not shown). The leak current revealed by incubating IDT317 with hSERT expressing oocytes was similar in magnitude to the leak current obtained with fluoxetine, while the antagonists with longer alkyl spacers had leak currents of similar magnitude to the higher affinity hSERT antagonist paroxetine. These data suggest that the other ligands have a higher affinity for hSERT, bind tighter than IDT317, and one or more of these compounds may be amenable to the development of quantum dot-based fluorescent assays using hSERT-expressing mammalian cells (preliminary data not shown).
Acknowledgments
This work was supported by grants from the National Institutes of Health (RO1EB003728-02 and GM72048-02). We would also like to thank Professor Louis De Felice for providing assistance with measuring the leak currents and IC50 values in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Qian Y, Melikian HE, Rye DB, Levey AI, Blakely RD. J Neurosci. 1995;15:1261–1274. doi: 10.1523/JNEUROSCI.15-02-01261.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jayanthi LD, Samuvel DJ, Ramamoorthy S. J Biol Chem. 2004;279:19315–19326. doi: 10.1074/jbc.M311172200. [DOI] [PubMed] [Google Scholar]
- 3.McBride J, Treadway J, Feldman LC, Pennycook SJ, Rosenthal SJ. Nano Lett. 2006;6:1496–1501. doi: 10.1021/nl060993k. [DOI] [PubMed] [Google Scholar]
- 4.Rosenthal SJ, McBride J, Pennycook SJ, Feldman LC. Surface Science Reports. 2007;62:111–157. doi: 10.1016/j.surfrep.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruchez M, Jr, Moronne M, Gin P, Weiss S, Alivisatos AP. Science. 1998;281:2013–2016. doi: 10.1126/science.281.5385.2013. [DOI] [PubMed] [Google Scholar]
- 6.Chan WCW, Nie S. Science. 1998;281:2016–2018. doi: 10.1126/science.281.5385.2016. [DOI] [PubMed] [Google Scholar]
- 7.Rosenthal SJ, Tomlinson ID, Schroter S, Adkins E, Swafford L, Wang Y, DeFelice LJ, Blakely RD. J Am Chem Soc. 2002;124:4586–4594. doi: 10.1021/ja003486s. [DOI] [PubMed] [Google Scholar]
- 8.Tomlinson ID, Mason JN, Blakely RD, Rosenthal SJ. Bioorganic and medicinal chemistry letters. 2005;15:5307–5310. doi: 10.1016/j.bmcl.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 9.Tomlinson ID, Warnerment MR, Mason JN, Vergne MJ, Hercules DM, Blakely RD, Rosenthal SJ. Bioorganic and Medicinal Chemistry Letters. 2007;17:5656–5660. doi: 10.1016/j.bmcl.2007.07.061. [DOI] [PubMed] [Google Scholar]
- 10.Bentzen EL, Tomlinson ID, Mason JN, Gresch P, Wright D, Sanders-Busch E, Blakely R, Rosenthal SJ. Bioconjugate Chemistry. 2005;16:1488–1494. doi: 10.1021/bc0502006. [DOI] [PubMed] [Google Scholar]
- 11.Guillaume J, Dumont C, Laurent J, Ne'de'lec I. Eur J Med Chem. 1987;22:33. [Google Scholar]
- 12.IDT357 (8) was synthesized by dissolving Biotin-PEG5000-NHS (0.2g) in methylene chloride (100ml) and 11-(4-(1H-indol-3-yl)-5,6-dihydrpyridin- 1(2H)-yl)undecylamine (17a) 0.015g was added. This solution was stirred for 18 hours then evaporated under reduced pressure. The product was repeatedly washed with diethylether (5 × 100ml) to yield 0.14g of (8) as a pale yellow solid. Compounds (9)-(11) were synthesized using the same protocol.
- 13.4-(1H-indol-3-yl)-N-methylcyclohex-3-enamine (21). 1,4-cyclohexanedione monoethyl ketal (5g, 32 mmols), methylamine 33% in ethanol (10ml) and triacetoxy sodium borohydride (9g, 42 mols) were mixed in methylene chloride (100ml) and stirred at room temperature under nitrogen for 18 hours. Sodium hydroxide (2M, 100ml) was added and the organic solution was separated and dried over magnesium sulfate. This was filtered and evaporated to yield 5.7g of N-methyl-1,4-dioxaspiro[4.5]decan-8-amine (19) which was used without further purification. The N-methyl-1,4-dioxaspiro[4.5]decan-8-amine (19) was dissolved in tetrahydrofuran (50ml) and trifluoroacetic acid (50ml) was added. This mixture was heated at reflux for 18 hours cooled and evaporated to yield crude 4-(methylamino)cyclohexanone (20). The crude 4-(methylamino)cyclohexanone (20) was dissolved in methanolic KOH solution (2M, 200ml) and indol (4.1g, 45 mmol) was added. The mixture was heated at reflux for 8 hours cooled to ambient temperature and evaporated. The product was purified by column chromatography on silica gel eluted with ethyl acetate (98%) : triethyl amine (2%) to give 1.4g of 4-(1H-indol-3-yl)-N-methylcyclohex-3-enamine (21) as a colorless solid in a 20% yield. 1H nmr (CDCl3) δ 8.55 (s, 1H), 7.90 (d, 1H), 7.34 (d, 1H), 7.13 (m, 3H), 6.21 (s, 1H), 2.77 (m, 1H), 2.55 (m, 4H), 1.88 (m, 2H), 1.64 (m, 4H). 13C nmr (CDCl3) δ 136.78, 131.08, 125.22, 122.00, 121.14, 120.72, 119.86, 119.65, 118.47, 111.27, 54.70, 33.63, 32.57, 28.99, 27.53
- 14.2-(11-((4-(1H-indol-3-yl)cyclohex-3-enyl)(methyl)aminoundecyl)isoindoline-1,3-dione (22). 4-(1H-indol-3-yl)-N-methylcyclohex-3-enamine (1.2g, 5.3 mmols) and 11-(1,3-Dioxo-1,3-dihydro-isoindol-2-yl)-undecanyl bromide (2g, 5.3 mmols) were mixed in acetonitrile (100ml) and triethyl amine (5ml) was added. The mixture was heated at reflux for 18 hours cooled and evaporated. The product was recrystalized from acetonitrile to yield 1.8g (64%) of 2-(11-((4-(1H-indol-3-yl)cyclohex-3-enyl)(methyl)aminoundecyl)isoindoline-1,3-dione (22) as a pale brown solid. 1H nmr (DMSO-d6) δ 11.18 (s, 1H), 7.85 (m, 4H), 7.42 (m, 2H), 7.10 (m, 2H), 6.11 (s, 1H), 3.55 (t, 2H), 3.36 (s, 6H), 3.11 (m, 4H), 2.77 (s, 2H), 1.70 (m, 4H), 1.29 (m, 14H). 13C nmr (DMSO-d6) δ 167.95, 136.89, 134.44, 134.40, 131.56, 126.00, 124.54, 123.10, 122.99, 119.98, 119.33, 116.80, 115.53, 111.81, 45.61, 37.35, 29.36, 28.80, 28.50, 27.84, 26.20, 26.08, 23.70
- 15.IDT374 (13) was synthesized by dissolving Biotin-PEG5000-NHS (0.2g) in dry dimethyl formamide (10ml) and 2-(11-((4-(1H-indol-3-yl)cyclohex-3-enyl)(methyl)aminoundecyl)isoindoline-1,3-dione (0.016g) was added. The solution was stirred for 18 hours then tenta gel derivatized with NHS was added (1g) and stirred for 6 hours the solution was filtered and evaporated and the resulting material was dissolved in deionised water then filtered and evaporated to yield IDT374 (0.16g) as a pale brown solid.
- 16.Compound (14). Using the same synthetic protocol as was used to synthesize (8); 120mg of IDT364 (12) was obtained from 150mg of Biotin-PEG5000-NHS and methylamine (33%,5ml) dissolved in methanol.
- 17.Deskus JA, Epperson JR, Sloan CP, Cipollina JA, Dextraze P, Qian-Cutrone J, Gao Q, Ma B, Beno BR, Mattson GK, Molski TF, Krause RG, Taber MT, Lodge NJ, Mattson RJ. Bioorganic and Medicinal Chemistry Letters. 2007;17:3099–3104. doi: 10.1016/j.bmcl.2007.03.040. [DOI] [PubMed] [Google Scholar]