Abstract
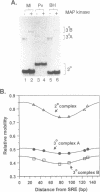
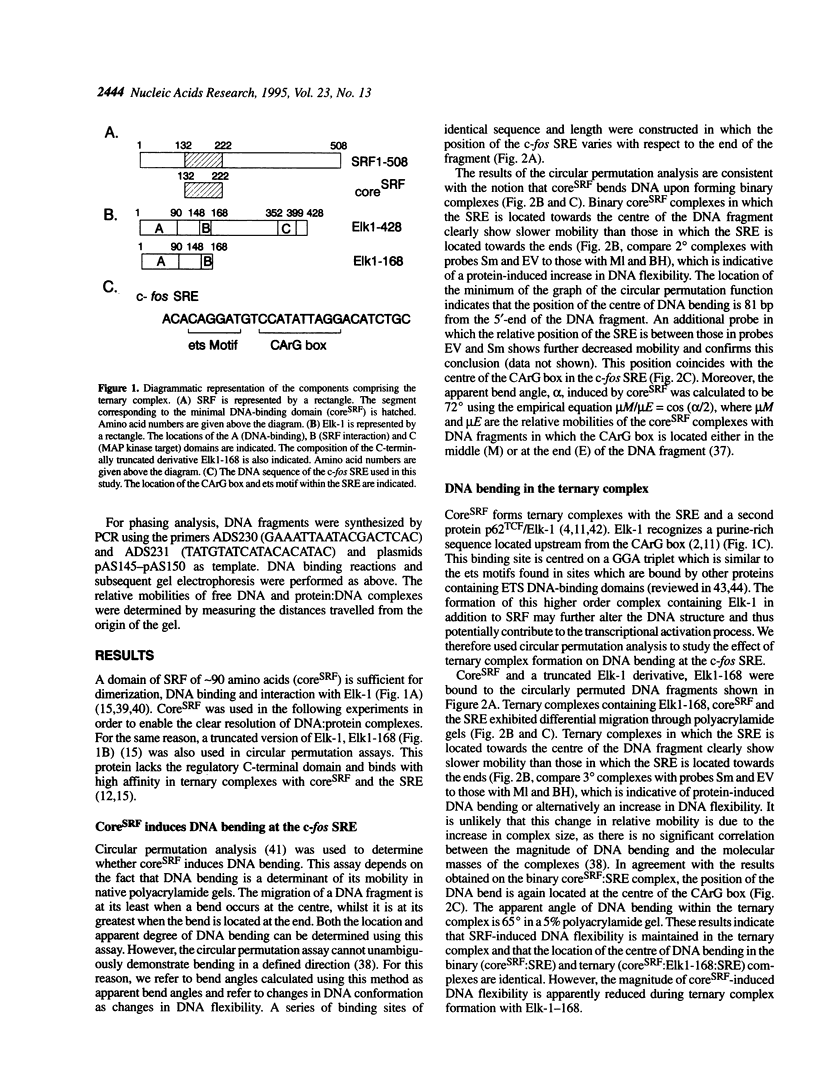
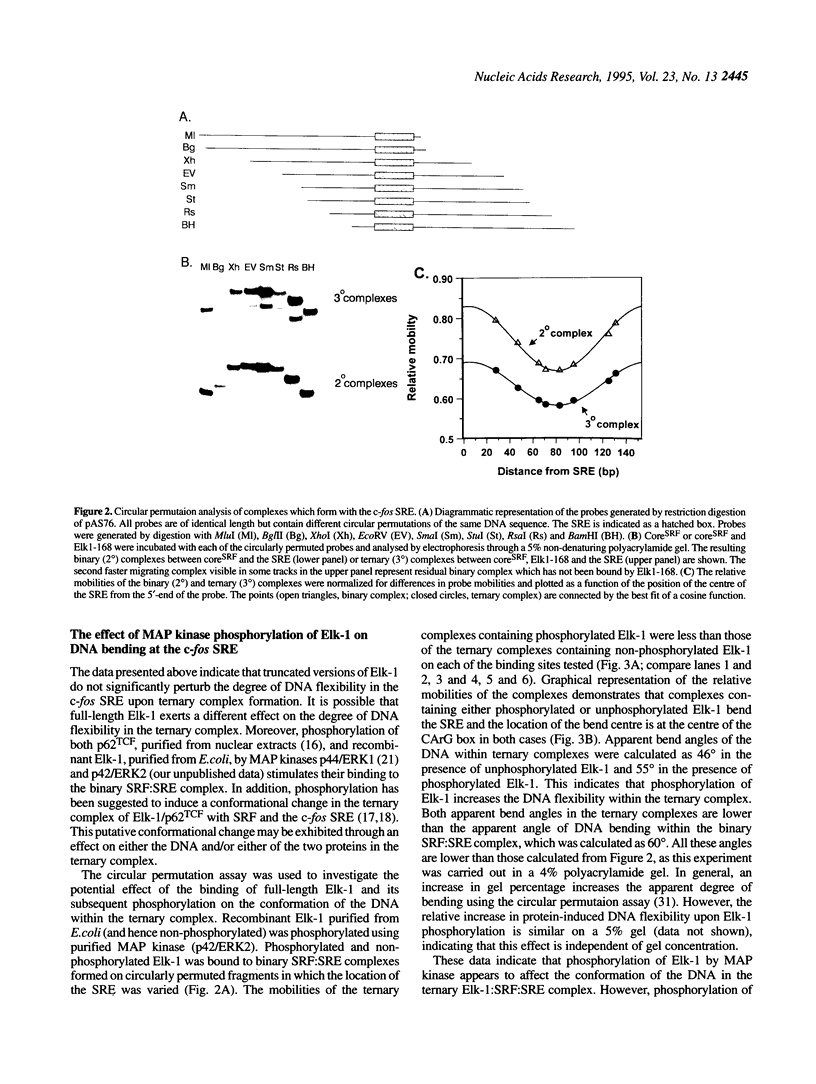
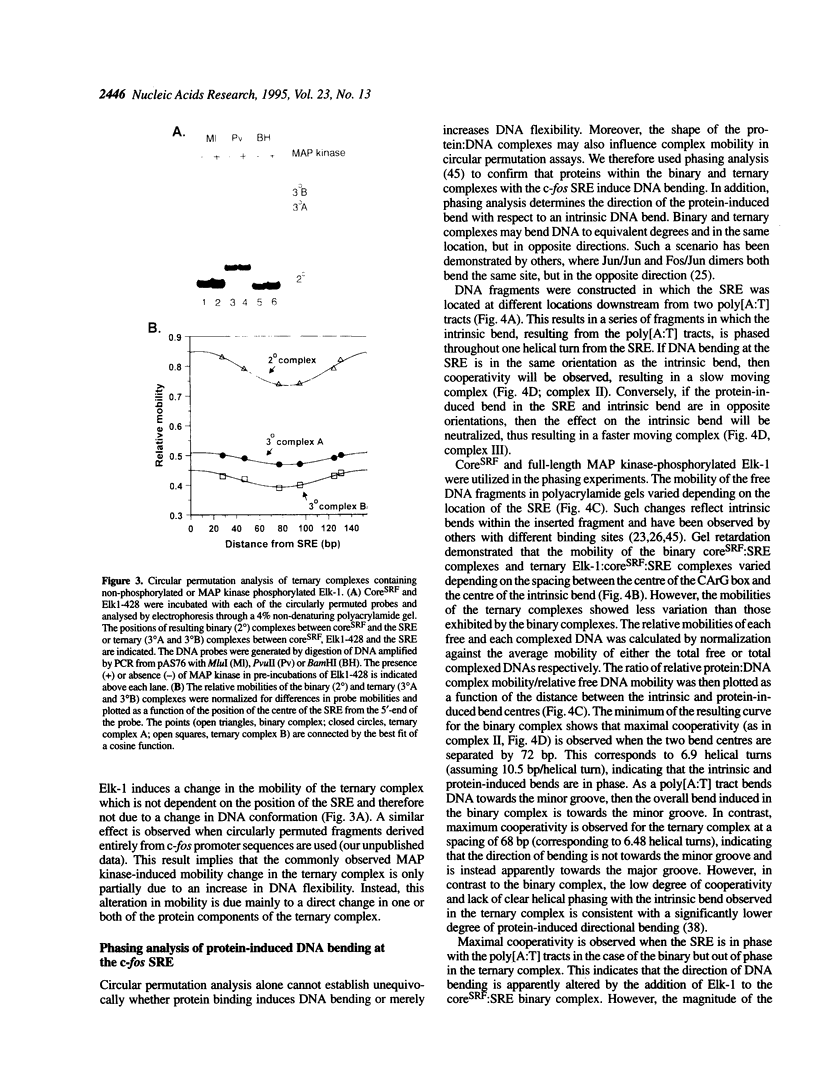
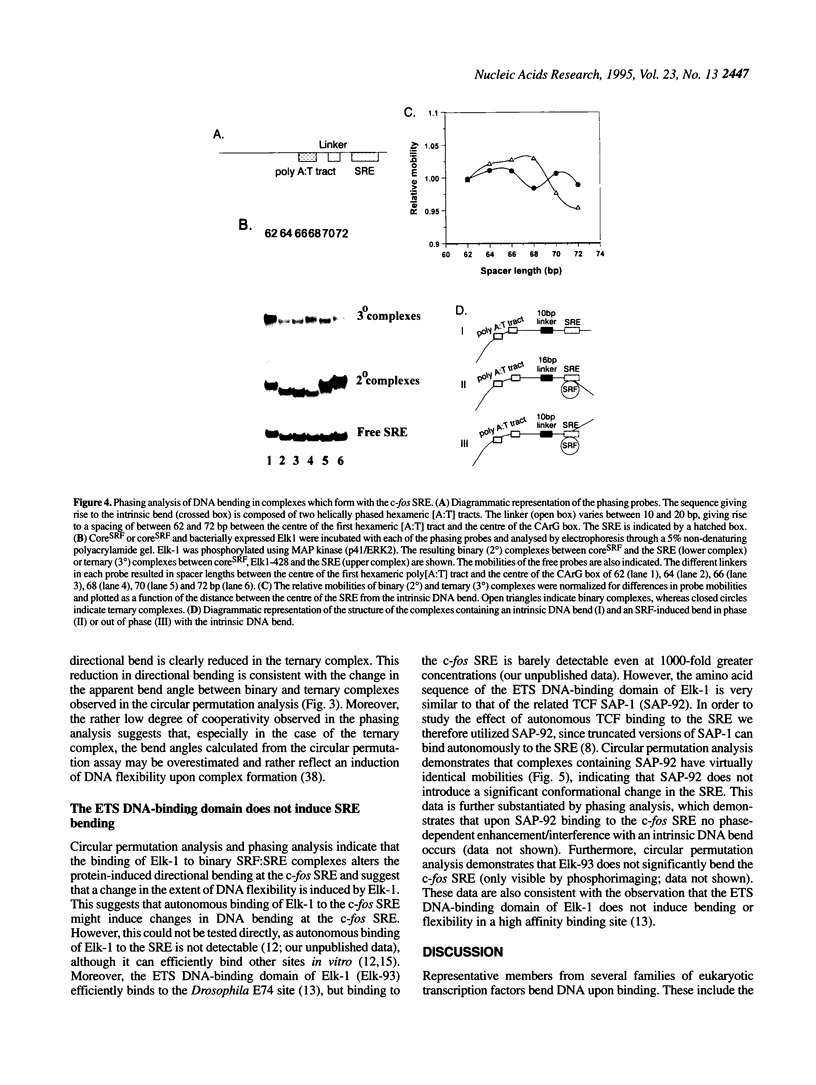
Transcriptional induction of the c-fos proto-oncogene in response to serum growth factors is mediated in part by a ternary complex that forms on the serum response element (SRE) within its promoter. This complex consists of Elk-1, serum response factor (SRF) and the SRE. Elk-1 is phosphorylated by MAP kinase, which correlates with the induction of c-fos transcription. In this study we have investigated the protein-induced DNA bending which occurs during the formation and post-translational modification of the ternary complex that forms at the c-fos SRE. Circular permutation analysis demonstrates that the minimal DNA-binding domain of SRF, which contains the MADS box, is sufficient to induce flexibility into the centre of its binding site within the SRE. Phasing analysis indicates that at least part of this flexibility results in the production of a directional bend towards the minor groove. The isolated ETS domains from Elk-1 and SAP-1 induce neither DNA bending nor increased DNA flexibility. Formation of ternary complexes by binding of Elk-1 to the binary SRF:SRE complex results in a change in the flexibility of the SRE. Phosphorylation of Elk-1 by MAP kinase (p42/ERK2) induces further minor changes in this DNA flexibility. However, phasing analysis reveals that the recruitment of Elk-1 to form the ternary complex affects the SRF-induced directional DNA bend in the SRE. The potential roles of DNA bending at the c-fos SRE are discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansari A. Z., Bradner J. E., O'Halloran T. V. DNA-bend modulation in a repressor-to-activator switching mechanism. Nature. 1995 Mar 23;374(6520):371–375. doi: 10.1038/374370a0. [DOI] [PubMed] [Google Scholar]
- Crothers D. M., Haran T. E., Nadeau J. G. Intrinsically bent DNA. J Biol Chem. 1990 May 5;265(13):7093–7096. [PubMed] [Google Scholar]
- Dalton S., Treisman R. Characterization of SAP-1, a protein recruited by serum response factor to the c-fos serum response element. Cell. 1992 Feb 7;68(3):597–612. doi: 10.1016/0092-8674(92)90194-h. [DOI] [PubMed] [Google Scholar]
- Drak J., Crothers D. M. Helical repeat and chirality effects on DNA gel electrophoretic mobility. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3074–3078. doi: 10.1073/pnas.88.8.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D. E., Parent L. A., Sharp P. A. Myc/Max and other helix-loop-helix/leucine zipper proteins bend DNA toward the minor groove. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11779–11783. doi: 10.1073/pnas.89.24.11779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gille H., Kortenjann M., Thomae O., Moomaw C., Slaughter C., Cobb M. H., Shaw P. E. ERK phosphorylation potentiates Elk-1-mediated ternary complex formation and transactivation. EMBO J. 1995 Mar 1;14(5):951–962. doi: 10.1002/j.1460-2075.1995.tb07076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gille H., Sharrocks A. D., Shaw P. E. Phosphorylation of transcription factor p62TCF by MAP kinase stimulates ternary complex formation at c-fos promoter. Nature. 1992 Jul 30;358(6385):414–417. doi: 10.1038/358414a0. [DOI] [PubMed] [Google Scholar]
- Giovane A., Pintzas A., Maira S. M., Sobieszczuk P., Wasylyk B. Net, a new ets transcription factor that is activated by Ras. Genes Dev. 1994 Jul 1;8(13):1502–1513. doi: 10.1101/gad.8.13.1502. [DOI] [PubMed] [Google Scholar]
- Gustafson T. A., Taylor A., Kedes L. DNA bending is induced by a transcription factor that interacts with the human c-FOS and alpha-actin promoters. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2162–2166. doi: 10.1073/pnas.86.7.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera R. E., Shaw P. E., Nordheim A. Occupation of the c-fos serum response element in vivo by a multi-protein complex is unaltered by growth factor induction. Nature. 1989 Jul 6;340(6228):68–70. doi: 10.1038/340068a0. [DOI] [PubMed] [Google Scholar]
- Hill C. S., Marais R., John S., Wynne J., Dalton S., Treisman R. Functional analysis of a growth factor-responsive transcription factor complex. Cell. 1993 Apr 23;73(2):395–406. doi: 10.1016/0092-8674(93)90238-l. [DOI] [PubMed] [Google Scholar]
- Janknecht R., Ernst W. H., Pingoud V., Nordheim A. Activation of ternary complex factor Elk-1 by MAP kinases. EMBO J. 1993 Dec 15;12(13):5097–5104. doi: 10.1002/j.1460-2075.1993.tb06204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janknecht R., Nordheim A. Elk-1 protein domains required for direct and SRF-assisted DNA-binding. Nucleic Acids Res. 1992 Jul 11;20(13):3317–3324. doi: 10.1093/nar/20.13.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janknecht R., Nordheim A. Gene regulation by Ets proteins. Biochim Biophys Acta. 1993 Dec 23;1155(3):346–356. doi: 10.1016/0304-419x(93)90014-4. [DOI] [PubMed] [Google Scholar]
- Kerppola T. K., Curran T. DNA bending by Fos and Jun: the flexible hinge model. Science. 1991 Nov 22;254(5035):1210–1214. doi: 10.1126/science.1957173. [DOI] [PubMed] [Google Scholar]
- Kerppola T. K., Curran T. Selective DNA bending by a variety of bZIP proteins. Mol Cell Biol. 1993 Sep;13(9):5479–5489. doi: 10.1128/mcb.13.9.5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Zwieb C., Wu C., Adhya S. Bending of DNA by gene-regulatory proteins: construction and use of a DNA bending vector. Gene. 1989 Dec 21;85(1):15–23. doi: 10.1016/0378-1119(89)90459-9. [DOI] [PubMed] [Google Scholar]
- Kortenjann M., Thomae O., Shaw P. E. Inhibition of v-raf-dependent c-fos expression and transformation by a kinase-defective mutant of the mitogen-activated protein kinase Erk2. Mol Cell Biol. 1994 Jul;14(7):4815–4824. doi: 10.1128/mcb.14.7.4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez M., Oettgen P., Akbarali Y., Dendorfer U., Libermann T. A. ERP, a new member of the ets transcription factor/oncoprotein family: cloning, characterization, and differential expression during B-lymphocyte development. Mol Cell Biol. 1994 May;14(5):3292–3309. doi: 10.1128/mcb.14.5.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox J. Towards synthetic self-replication. Nature. 1991 Dec 5;354(6352):351–351. doi: 10.1038/354351a0. [DOI] [PubMed] [Google Scholar]
- Marais R., Wynne J., Treisman R. The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell. 1993 Apr 23;73(2):381–393. doi: 10.1016/0092-8674(93)90237-k. [DOI] [PubMed] [Google Scholar]
- Mueller C. G., Nordheim A. A protein domain conserved between yeast MCM1 and human SRF directs ternary complex formation. EMBO J. 1991 Dec;10(13):4219–4229. doi: 10.1002/j.1460-2075.1991.tb05000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natesan S., Gilman M. Z. DNA bending and orientation-dependent function of YY1 in the c-fos promoter. Genes Dev. 1993 Dec;7(12B):2497–2509. doi: 10.1101/gad.7.12b.2497. [DOI] [PubMed] [Google Scholar]
- Pollock R., Treisman R. A sensitive method for the determination of protein-DNA binding specificities. Nucleic Acids Res. 1990 Nov 11;18(21):6197–6204. doi: 10.1093/nar/18.21.6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prywes R., Roeder R. G. Purification of the c-fos enhancer-binding protein. Mol Cell Biol. 1987 Oct;7(10):3482–3489. doi: 10.1128/mcb.7.10.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao V. N., Huebner K., Isobe M., ar-Rushdi A., Croce C. M., Reddy E. S. elk, tissue-specific ets-related genes on chromosomes X and 14 near translocation breakpoints. Science. 1989 Apr 7;244(4900):66–70. doi: 10.1126/science.2539641. [DOI] [PubMed] [Google Scholar]
- Rao V. N., Reddy E. S. elk-1 domains responsible for autonomous DNA binding, SRE:SRF interaction and negative regulation of DNA binding. Oncogene. 1992 Nov;7(11):2335–2340. [PubMed] [Google Scholar]
- Sadowski H. B., Shuai K., Darnell J. E., Jr, Gilman M. Z. A common nuclear signal transduction pathway activated by growth factor and cytokine receptors. Science. 1993 Sep 24;261(5129):1739–1744. doi: 10.1126/science.8397445. [DOI] [PubMed] [Google Scholar]
- Saikumar P., Gabriel J. L., Reddy E. P. The Myb oncogene product induces DNA-bending. Oncogene. 1994 Apr;9(4):1279–1287. [PubMed] [Google Scholar]
- Schultz S. C., Shields G. C., Steitz T. A. Crystal structure of a CAP-DNA complex: the DNA is bent by 90 degrees. Science. 1991 Aug 30;253(5023):1001–1007. doi: 10.1126/science.1653449. [DOI] [PubMed] [Google Scholar]
- Sharrocks A. D., Gille H., Shaw P. E. Identification of amino acids essential for DNA binding and dimerization in p67SRF: implications for a novel DNA-binding motif. Mol Cell Biol. 1993 Jan;13(1):123–132. doi: 10.1128/mcb.13.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrocks A. D., Shaw P. E. Improved primer design for PCR-based, site-directed mutagenesis. Nucleic Acids Res. 1992 Mar 11;20(5):1147–1147. doi: 10.1093/nar/20.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrocks A. D., von Hesler F., Shaw P. E. The identification of elements determining the different DNA binding specificities of the MADS box proteins p67SRF and RSRFC4. Nucleic Acids Res. 1993 Jan 25;21(2):215–221. doi: 10.1093/nar/21.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P. E., Frasch S., Nordheim A. Repression of c-fos transcription is mediated through p67SRF bound to the SRE. EMBO J. 1989 Sep;8(9):2567–2574. doi: 10.1002/j.1460-2075.1989.tb08395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P. E., Schröter H., Nordheim A. The ability of a ternary complex to form over the serum response element correlates with serum inducibility of the human c-fos promoter. Cell. 1989 Feb 24;56(4):563–572. doi: 10.1016/0092-8674(89)90579-5. [DOI] [PubMed] [Google Scholar]
- Shaw P. E. Ternary complex formation over the c-fos serum response element: p62TCF exhibits dual component specificity with contacts to DNA and an extended structure in the DNA-binding domain of p67SRF. EMBO J. 1992 Aug;11(8):3011–3019. doi: 10.1002/j.1460-2075.1992.tb05371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore P., Bisset L., Lakey J., Waltho J. P., Virden R., Sharrocks A. D. Characterization of the Elk-1 ETS DNA-binding domain. J Biol Chem. 1995 Mar 17;270(11):5805–5811. doi: 10.1074/jbc.270.11.5805. [DOI] [PubMed] [Google Scholar]
- Shore P., Sharrocks A. D. The transcription factors Elk-1 and serum response factor interact by direct protein-protein contacts mediated by a short region of Elk-1. Mol Cell Biol. 1994 May;14(5):3283–3291. doi: 10.1128/mcb.14.5.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Thompson J. F., Landy A. Empirical estimation of protein-induced DNA bending angles: applications to lambda site-specific recombination complexes. Nucleic Acids Res. 1988 Oct 25;16(20):9687–9705. doi: 10.1093/nar/16.20.9687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman R., Marais R., Wynne J. Spatial flexibility in ternary complexes between SRF and its accessory proteins. EMBO J. 1992 Dec;11(12):4631–4640. doi: 10.1002/j.1460-2075.1992.tb05565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman R. The SRE: a growth factor responsive transcriptional regulator. Semin Cancer Biol. 1990 Feb;1(1):47–58. [PubMed] [Google Scholar]
- Treisman R. The serum response element. Trends Biochem Sci. 1992 Oct;17(10):423–426. doi: 10.1016/0968-0004(92)90013-y. [DOI] [PubMed] [Google Scholar]
- Verrijzer C. P., van Oosterhout J. A., van Weperen W. W., van der Vliet P. C. POU proteins bend DNA via the POU-specific domain. EMBO J. 1991 Oct;10(10):3007–3014. doi: 10.1002/j.1460-2075.1991.tb07851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner B. J., Hayes T. E., Hoban C. J., Cochran B. H. The SIF binding element confers sis/PDGF inducibility onto the c-fos promoter. EMBO J. 1990 Dec;9(13):4477–4484. doi: 10.1002/j.1460-2075.1990.tb07898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylyk B., Hahn S. L., Giovane A. The Ets family of transcription factors. Eur J Biochem. 1993 Jan 15;211(1-2):7–18. doi: 10.1007/978-3-642-78757-7_2. [DOI] [PubMed] [Google Scholar]
- Wu H. M., Crothers D. M. The locus of sequence-directed and protein-induced DNA bending. Nature. 1984 Apr 5;308(5959):509–513. doi: 10.1038/308509a0. [DOI] [PubMed] [Google Scholar]
- Zinck R., Hipskind R. A., Pingoud V., Nordheim A. c-fos transcriptional activation and repression correlate temporally with the phosphorylation status of TCF. EMBO J. 1993 Jun;12(6):2377–2387. doi: 10.1002/j.1460-2075.1993.tb05892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkel S. S., Crothers D. M. DNA bend direction by phase sensitive detection. Nature. 1987 Jul 9;328(6126):178–181. doi: 10.1038/328178a0. [DOI] [PubMed] [Google Scholar]
- de Groot R. P., Delmas V., Sassone-Corsi P. DNA bending by transcription factors CREM and CREB. Oncogene. 1994 Feb;9(2):463–468. [PubMed] [Google Scholar]