Summary
The primary cilium is a cellular organelle that is almost ubiquitous in eukaryotes, yet its functions in vertebrates have been slow to emerge. The last fifteen years have been marked by accelerating insight into the biology of primary cilia, arising from the synergy of three major lines of research. These research programs describe a specialized mode of protein trafficking in cilia, reveal that genetic disruptions of primary cilia cause complex human disease syndromes, and establish that Sonic hedgehog (Shh) signal transduction requires the primary cilium. New lines of research have branched off to investigate the role of primary cilia in neuronal signaling, adult neurogenesis, and brain tumor formation. We review a fast expanding literature to determine what we now know about the primary cilium in the developing and adult CNS, and what new directions should lead to further clarity.
Introduction
Primary cilia were definitively identified in the vertebrate nervous system several decades ago, principally using electron microscopy (EM). Reports of primary cilia extending from neuroepithelial progenitor cells into the lumen of the neural tube (Duncan, 1957; Sotelo and Trujillo-Cenoz, 1958) were followed by descriptions of primary cilia on neurons and glia (Dahl, 1963; Karlsson, 1966; Palay, 1961; Peters et al., 1976), and by the early eighties the prevailing view was that virtually all neurons are ciliated (Wheatley, 1982). The elegant ultrastructure and broad distribution of the primary cilium captured attention, but its function in neural cells was unclear (Peters et al., 1976). Intense recent scrutiny of the primary cilium has elucidated many functions in the body and brain, and the consequences of defective cilia for human disease. Central to this progress are findings that indicate the primary cilium receives signals from the environment and transduces them to the cell. In the vertebrate nervous system, the primary cilium is increasingly viewed as hub for certain neural developmental signaling pathways, and growing data suggest this is also true for several types of adult neuronal signaling. To set the stage for understanding the functions of primary cilia in the CNS, particularly for readers new to cilia research, we begin with a summary of basic cilia biology, and a brief appraisal of the range of physiological defects that arise in mice and humans from cilia dysfunction.
Structure of the cilium
The primary cilium is a slender protrusion of the cell membrane about 1 – 5 microns in length. The ciliary membrane surrounds an axoneme, composed of nine microtubule pairs. These are anchored to a microtubule organizer, the ciliary basal body, which is a modified mother centriole. Appropriate to an organelle that propagates specialized signals, the primary cilium is partially isolated from the rest of the cell by a transition zone at its base, which acts as a ciliary pore and a docking area for proteins headed for the cilium (Rosenbaum and Witman, 2002; Pedersen and Rosenbaum, 2008; Satir and Christensen, 2008; Seely and Nachury, 2010; Sorokin, 1968). Proteins selected for entry (Emmer et al., 2010; Inglis et al., 2006) are carried along the ciliary axoneme by intraflagellar transport (IFT) (Figure 1), first discovered in the flagella of the alga Chlamydomonas reinhardtii (Kozminski et al., 1993; Kozminski et al., 1995). Cilia membrane proteins needed for signaling are prevented from leaving the cilium prematurely by a septin diffusion barrier at the base of the primary cilium, below the site at which proteins are first inserted into the ciliary membrane (Hu et al., 2010). A similar diffusion barrier is formed in budding yeast, supporting an evolutionarily conserved role for septins in maintaining separate cell compartments (Hu et al., 2010).
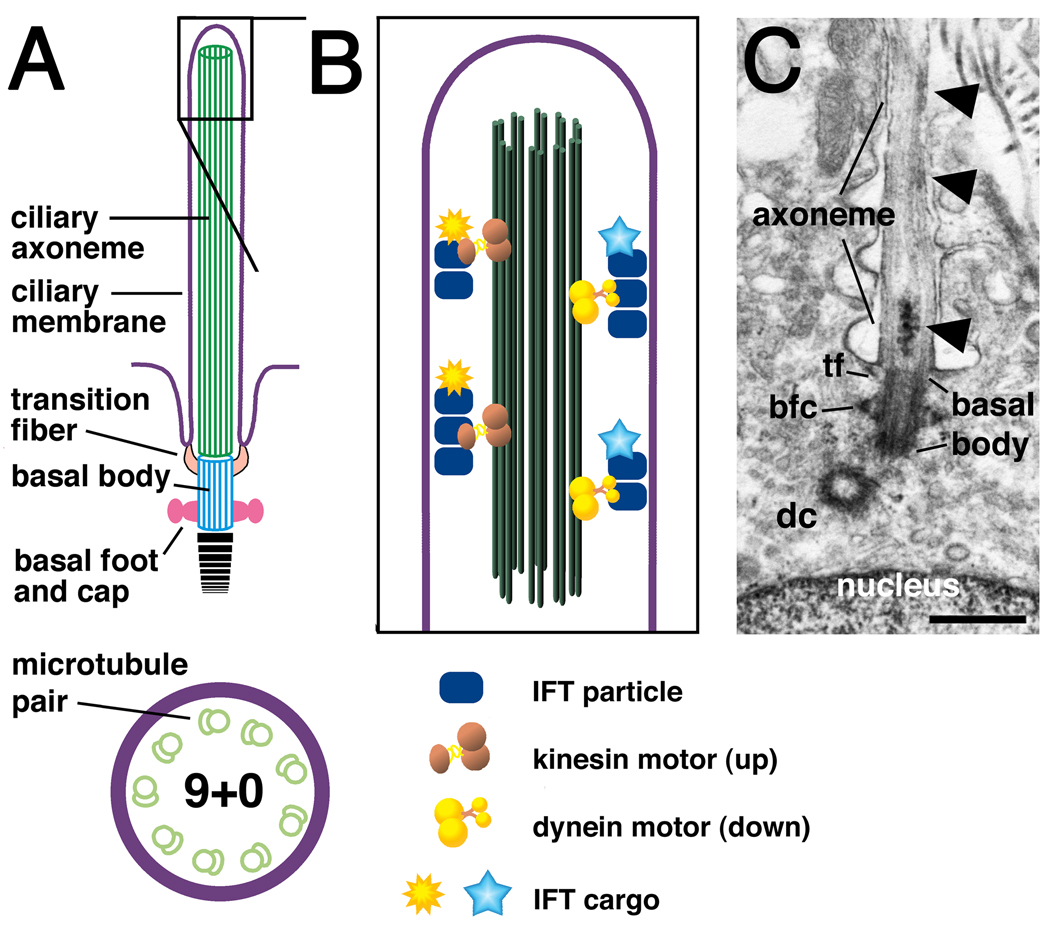
Figure 1. The structure of a primary cilium.
(A) Major components are the ciliary axoneme, composed of microtubules (green), the ciliary membrane (purple) and the basal body (blue), which is a modified mother centriole. Modifications of the basal body include transition fibers (orange) that form a permeable barrier between the cilium and the rest of the cell, the basal foot and cap (pink) and striated rootlets (black horizontal lines), which provide mechanical support (Seeley and Nachury, 2010). A cross-section through the axoneme shows nine paired microtubules (the 9+0 configuration).
(B) Macromolecules (sun shapes) important for ciliogenesis attach to IFT particles and travel along microtubules towards the ciliary tip using a kinesin motor. Turnover products (stars) are carried back to the ciliary base by IFT particles attached to a dynein motor.
(C) Electronmicrograph of a primary cilium in an adult mouse brain. Visible features schematized in (A) include the axoneme, basal body, a transition fiber (tf), the basal foot and cap (bfc) and the daughter centriole (dc), which lies close to the basal body. Arrowheads indicate possible IFT particles travelling along the cilium. Scale bar in C is 0.5 microns.
Secondary cilia, which include eukaryotic flagella, differ from primary cilia in that the axoneme contains an extra central pair of microtubules, linked by radial spokes to the nine outer microtubule pairs which are attached to a dynein motor that drives microtubule sliding and generates movement (Pedersen and Rosenbaum, 2008; Rosenbaum and Witman, 2002; Satir and Christensen, 2008) (Figure 2A). Secondary cilia are therefore motile, whereas primary cilia are generally not. Additionally, a cell possesses a single primary cilium, but may have many secondary cilia. In the CNS, the multiciliated epithelial cells lining the ventricles are tufted with secondary cilia that sway in synchrony to move cerebrospinal fluid (Banizs et al., 2005; Dalen et al., 1971) (Figure 2A). Specialized sensory cilia in the nervous system are found between the outer and inner segments (OS, IS) of retinal photoreceptors (Figure 2B), and on the dendrites of olfactory receptor neurons (ORNs) (Figure 2C). In this review we focus on the primary cilium, but consider briefly secondary cilia that regulate the flow of CSF, and hybrid cilia on ORNs that epitomize the role of cilia in sensing their environment.
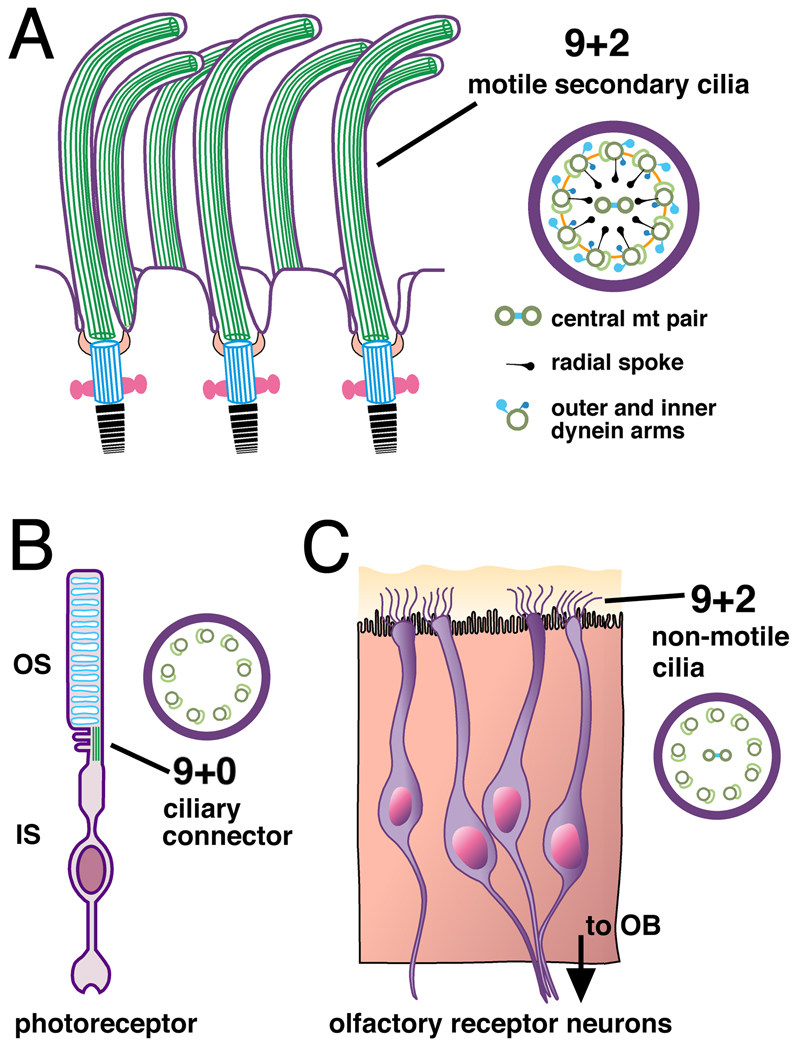
Figure 2. The structure of secondary and specialized sensory cilia.
(A) Secondary cilia structurally resemble primary cilia, except that the axonemes of secondary cilia display a 9+2 microtubule configuration. The outer nine paired microtubules are attached to outer and inner dynein arms and connected to the central pair of tubules by radial spokes; this allows the secondary cilium self-generated motility. In the CNS, multiplesecondary cilia on ependymal cells lining the ventricles regulate the flow of cerebrospinal fluid (see text). (B) A ciliary segment joins the outer and inner segments (OS and IS) of the retinal photoreceptor and has the 9+0 configuration of a primary cilium. (C) Olfactory receptor neurons (ORNs) have cilia with a hybrid character. The cilia at the dendritic tips of each ORN display the 9+2 microtubule configuration, but lack the dynein machinery needed to generate motion (Arstila and Wersall, 1967; Jenkins et al., 2009). ORN cilia sample odorants in the mucus layer (yellow) at the surface of the olfactory epithelium (orange). ORN axons project to the olfactory bulb (OB).
Intraflagellar Transport (IFT)
Three major programs of research have revealed the importance of the primary cilium in the body and nervous system. The first program comprises many years of study of how Chlamydomonas constructs, maintains and uses its flagella. In particular, the discovery of IFT, a method of protein trafficking characteristic of cilia, led to the identification of genes required to put cilia together and make them function (Figure 1, Table 1). Because IFT is used by both primary and secondary cilia, and is conserved from Chlamydomonas to Drosophila, C. elegans, zebrafish, mice, and humans (Follit et al., 2009; Inglis et al., 2007; Pedersen and Rosenbaum, 2008; Rosenbaum and Witman, 2002; Sharma et al., 2008; Tsujikawa and Malicki, 2004), this work was an essential background for interpreting the findings of the other two major research programs, and, moreover, established genetic approaches for manipulating the primary cilium in a variety of species.
Table 1. Key Components of IFT.
IFT proteins are numbered according to their molecular weight. Genes encoding proteins in bold blue have been frequently mutated to investigate primary cilia in studies reviewed here. Proteins in bold blue or bold black influence Shh signaling (see Goetz and Anderson, 2010).
| General structural component | Proteins | |
|---|---|---|
| Anterograde IFT, Complex B | IFT172 | IFT57 |
| IFT88 | IFT54 | |
| IFT81 | IFT52 | |
| IFT80 | IFT46 | |
| IFT74 | IFT27 | |
| IFT20 | ||
| Retrograde IFT, Complex A) | IFT144 | |
| IFT140 | ||
| IFT139 | ||
| IFT122 | ||
| IFTA1 | ||
| IFT43 | ||
| Anterograde kinesin motor subunits | KIF3A | |
| KIF3B | ||
| KAP3 | ||
| Retrograde dynein motor subunits | DYNC2H1 | |
| DYNC2L1 | ||
IFT particles, initially seen in the light microscope moving along living Chlamydomonas flagella (Kozminski et al., 1993), are composed of 17 proteins forming two complexes. Complex B IFT particles carry cargo in the anterograde direction from the base to the tip of the cilium, using a kinesin-2 motor. Complex A particles move turnover products retrogradely, with a dynein motor, back to the base of the cilium (Figure 1, Table 1), where IFT particles are recycled (Pedersen and Rosenbaum, 2008; Rosenbaum and Witman, 2002). At the ciliary pore, transition fibers form a pinwheel-like structure where macromolecules attach to IFT particles (Deane et al., 2001; Pedersen and Rosenbaum, 2008; Rosenbaum and Witman, 2002; Seely and Nachury, 2010). For entry into the cilium, proteins may also require specific targeting sequences that contribute to recognition as ciliary proteins (Berbari et al., 2008a; Dishinger et al., 2010; Follit et al.,2009; Jenkins et al., 2006; Mazelova et al., 2009). Notably, ciliary localization sequences have been identified for several G-protein coupled receptors (GPCRs) found in the ciliary membrane of neurons, including somatostatin receptor 3 (Berbari et al., 2008a; Berbari et al., 2008b, see below).
IFT therefore not only carries structural components needed for ciliogenesis, but also components required for signaling pathways mediated by the ciliated cell. Vertebrate photoreceptors, for example, develop from primary cilia, and retain a ciliary portion between the OS and IS (Richardson, 1969) (Figure 2). The OS, where phototransduction occurs, contains discs filled with light-sensing opsins. New opsins are constantly transported into the OS by IFT, and if IFT is disrupted in the ciliary connector photoreceptors degenerate (Deretic and Papermaster, 1991; Luby-Phelps et al., 2008; Moritz et al., 2001; Pazour et al., 2002). The signaling pathway currently most strongly linked to the vertebrate primary cilium is the Sonic hedgehog (Shh) pathway. In the prevailing model, the movement of Shh signaling components into the cilium, up and down the axoneme by IFT, and out of the cilium again, sequences and paces the steps of Shh signal transduction (Goetz and Anderson, 2010).
Human disease related to ciliary dysfunction
In the face of a growing bias towards ‘translational research’, it is a healthy lesson that studies of a unicellular alga led to profound insights into a class of human disease syndromes. These syndromes show a bewildering variety of abnormalities, such as cystic disease in the kidney, polydactyly, brain malformations, hydrocephaly, blindness, anosmia, obesity and cognitive deficits. How could single diseases involve pathology in so many different systems? The answer appears to be that affected organs contain ciliated cells, and that genetic mutations associated with the syndromes disrupt ciliary proteins, frequently those of the basal body, but also IFT, dynein motor and other proteins (Christensen et al., 2008; Fliegauf et al., 2007; Goetz and Anderson, 2010; Lancaster and Gleeson, 2009; Sharma et al., 2008; Sloboda and Rosenbaum, 2007; Veland et al., 2009). As expected, relevant genetic mutations affect both secondary and primary cilia. Many disorders, however, arise specifically from dysfunction of primary cilia. Neural cells implicated in some of the anomalies listed above, include ORNs with immotile cilia, and primary ciliated neural progenitor cells, choroid plexus cells, photoreceptors, and neurons of the mature brain.
Key experimental links between the primary cilium and human disease arose from research on polycystic kidney disease (PKD). Mutations in two human genes, PKD1 and PKD2 cause autosomal dominant PKD, and both genes were found to have homologs in C. elegans whose protein products localize to cilia (Barr et al., 2001; Barr and Sternberg, 1999). Further, the Oak Ridge Polycystic Kidney (ORPK) mouse, a model of PKD, is hypomorphic for Ift88, the mouse homolog of Chlamydomonas IFT88 (see Table 1) and has stunted primary cilia (Pazour et al., 2000). In kidney epithelial cells primary cilia respond to fluid flow by passively bending, which initiates a calcium ion (Ca++) influx, illustrating ciliary transduction of a sensory stimulus (Praetorius and Spring, 2001). The stunted cilia of the OPRK mouse cannot perform this function. Whether defective cilia mechanoreception is a central cause of cyst development in PKD is debated (Davenport et al., 2007), but these findings nonetheless reveal the primary cilium as a sensory detector. Furthermore, the ORPK mouse remains an excellent model of human ciliopathic syndromes, developing a range of other abnormalities seen in human patients, ascribed to defective cilia (Lehman et al., 2008).
These observations launched a massive program of research on the genetics and cell biology of ciliopathic syndromes, reviewed extensively elsewhere (Badano et al., 2006; Christensen et al., 2008; Fliegauf et al., 2007; Lancaster and Gleeson, 2009; Sharma et al., 2008; Sloboda and Rosenbaum, 2007; Veland et al., 2009). In this review, we focus more narrowly on ciliopathic symptoms that direct attention to cilia-dependent aspects of neural development and function. Ciliopathies showing a strong association with neural defects include Joubert Syndrome, Bardet-Biedl Syndrome (BBS), and Alström Syndrome. Joubert Syndrome is a phenotypically and genetically heterogeneous group of disorders whose defining features are hindbrain defects, and related neurological symptoms such as breathing abnormalities, ataxia and developmental delay. Joubert Syndrome can also be associated with hydrocephalus, anatomical abnormalities in the cerebral cortex, autism spectrum disorders, and retinal dystrophy. Bardet-Biedl Syndrome (BBS), also genetically heterogeneous, is marked by cognitive disabilities, anosmia, obesity, and retinal degeneration. Alström Syndrome, caused by mutations in the ALMS1 gene, is associated with obesity and retinal degeneration (Table 2) (http://www.ncbi.nlm.nih.gov/omim). The specific associations of cilia with these abnormalities will be discussed further below.
Table 2.
Select Ciliopathic Syndromes and Associated Genetic Mutations
| Syndrome | Ciliopathic symptoms related to the nervous system |
Genes | Gene/protein details |
|---|---|---|---|
| Alström Syndrome | Obesity, retinal cone-rod dystrophy leading to blindness |
ALMS1 | Cilia maintenance Localized to basal body |
| Bardet-Biedl Syndrome | Obesity, retinal dystrophy, anosmia, developmental delay, cognitive deficits |
BBS1 – BBS14 | BBS1,2,4,5,7,8,9 are part of the BBSome, a protein complex associated with the basal body BBS 6,10 and 12 are chaperonin-like molecules |
| Joubert Syndrome | Neuroradiological “molar tooth sign”, cerebellar vermis hypoplasia, developmental delay with cognitive and behavioral deficits Variably: Autism Spectrum Disorders cerebral cortex anomalies, retinal dystrophy |
AHI1 | Also Jbn; first mutation associated with JS |
| NPHP1 | Nephrocystin 1 | ||
| CEP290 | Nephrocystin 6, localized to centrosome | ||
| TMEM67 | Centriole recruitment, ciliogenesis | ||
| RPGRIP1L | Localized to centrosome, basal body | ||
| ARL13B | In cilia; mutation disrupts graded response to Shh | ||
| CC2D2A | Interacts with CEP290 | ||
| INPP5E | In axoneme and basal body, stabilizes cilia | ||
Sonic hedgehog developmental signaling
The third major program of research discloses one reason why primary cilia appear on neural progenitor cells, and illustrates the primary cilium as an organelle specialized to receive an environmental signal. In this case, the signal is the secreted molecule Shh, which specifies neuronal cell type in the ventral neural tube, and configures digits in the limb bud, as well as patterning other structures in the embryo (Chiang et al., 1996; Echelard et al., 1993; Ericson et al., 1995; Roelink et al., 1995). Evidence linking Shh signaling to cilia came from a program of forward genetics in mice that screened for neural tube defects and dorsoventral patterning abnormalities. This screen generated mutants with phenotypes similar to those caused by disruptions in Shh signaling (Huangfu et al., 2003), yet the mutations were not in genes encoding Shh signaling components, but those encoding IFT proteins and the ciliary kinesin and dynein motor proteins (Table 1).
Core players of the Hedgehog (Hh) pathway in the mouse include Shh, the transmembrane proteins Smoothened (Smo) and Patched 1 (Ptch1), and transcription factors Gli2 and Gli3. Kif7 is the vertebrate homolog of Drosophila Costal 2 (Cos2), a hub for Hh signaling in the fly, and suppressor of Fused (Sufu) is a negative regulator of the pathway (Fuccillo et al., 2006). Importantly, all these signaling components have been localized to cilia (Table 3). In future, live imaging of the movements of these components will be critical for testing the current models of their trafficking within the cilium. In essence, Shh signaling regulates the balance between Gli transcriptional activators and repressors in a manner appropriate to the tissue being patterned. In the absence of Shh, Gli2 and Gli3 are cleaved to repressor forms (Gli2R, Gli3R), whereas in its presence, proteolysis is inhibited and Gli2 and Gli3 function as activators (Gli2A, Gli3A). Gli2A is the primary activator of Shh target genes, Gli3R the main repressor (Fuccillo et al., 2006). Disruptions to this regulatory system result in tissue-specific defects. In the ventral neural tube, reduced GliA function results in misspecified ventral cell types, whereas in the limb, reduced Gli3R causes polydactyly (Franz, 1994; Hui and Joyner, 1993; Johnson, 1967; Schimmang et al., 1992). Findings from the mutant screen indicated that Shh regulation of Gli protein function depends on the ability of Shh signaling components to associate with and travel through the primary cilium. Mutations in Ift172, Ift88, Ift52, Kif3a and Dync2h1 cause losses of ventral neuron cell types, consistent with deficient GliA, and polydactyly in the limb, consistent with reduced Gli3R (Huangfu and Anderson, 2005; Huangfu et al., 2003; Liu et al., 2005a; May et al., 2005). Further evidence confirms that both Gli activator and repressor functions depend on primary cilia (Cheung et al., 2009; Endoh-Yamagami et al., 2009; Liem et al., 2009).
Table 3.
Elements of the Shh, PCP and PDGFα signaling pathways in cilia
| I. Shh signaling components | |||||
|---|---|---|---|---|---|
| System | where | details | how | reference | |
| Shh | mouse | ventral neural tube | apical basal body of neural progenitors | Shh∷gfp mouse | Chamberlain 2008 |
| Smo | mouse | ventral neural tube | in cilia projecting into the central canal | endogenous | Rohatgi 2007 |
| mouse | node | punctate staining along the axoneme | endogenous | Corbit 2005 | |
| May 2005 | |||||
| Tran 2008 | |||||
| MEFs (immortalized) | endogenous | Rohatgi 2007 | |||
| Chen 2009 | |||||
| NIH 3T3 | Shh dependent | endogenous | Rohatgi 2007 | ||
| Wang 2009 | |||||
| MDCK, | Smo-myc, | Corbit 2005 | |||
| NIH 3T3 | GFP-Smo | Kovacs 2008 | |||
| zebrafish | dorsal neural tube | adjacent to midline | Smo-myc | Aanstad 2009 | |
| Ptch1 | mouse | ventral neural tube | very low levels (some punctate staining in cilia) | endogenous | Rohatgi 2007 |
| mouse | paraxial mesoderm cells responding to Shh |
cilium base and in particles along the shaft | endogenous | Rohatgi 2007 | |
| mouse | splanchnic mesoderm | endogenous | Rohatgi 2007 | ||
| MEFs | co-localization in puncta in response to Shh | transfected | Rohatgi 2007 | ||
| endogenous | Chen 2009 | ||||
| NIH 3T3 | Rohatgi 2007 | ||||
| Gli2 | mouse | neural tube | endogenous | Cho 2008 | |
| neural tube | cilium tip | endogenous | Ko 2010 | ||
| mouse | gut endoderm | endogenous | Cho 2008 | ||
| MEFs (primary) | endogenous | Cho 2008 | |||
| MEFs (immortalized) | low levels in absence of Shh; high levels in its presence | endogenous | Chen 2009 | ||
| NIH 3T3 | primary cilium tip upon Shh stimulation | endogenous, endogenous | Kim 2009 | ||
| Gli3 | MEFs (immortalized) | low levels in absence of Shh; high levels in its presence | endogenous | Chen 2009 | |
| mouse | limb cell cultures (primary) |
cilium tip | endogenous | Haycraft 2005 | |
| Sufu | MEFs (immortalized) | cilium tip in some cells and distributed along the entire cilium in others |
endogenous | Chen 2009 | |
| mouse | limb cell cultures | cilium tip | endogenous | Haycraft 2005 | |
| Kif7 | NIH3T3, MDCK, IMCD3 | cilium base and tip | transfected Kif7∷eGFP |
Liem 2009 | |
| II. PCP signaling components | |||||
| Vangl2 | human | IMCD3, human respiratory epithelial cells from nasal brushings |
cilium base; punctate pattern along axoneme | endogenous | Ross 2005 |
| mouse | ependyma (in vivo), multiciliated ependymal cells (in culture) |
along cilium from tip to base | endogenous | Guirao 2010 | |
| Dvl2 | Frog | ciliated epidermis | cilium rootlet | endogenous | Park 2008 |
| frog | ciliated epidermis | cilium rootlet | Dvl2∷GFP | Park 2008 | |
| mouse | multiciliated ependymal cells (in culture) |
basal body | Endogenous | Hirota 2010 | |
| III. PDGF signaling | |||||
| Pdgfrα | adult rat | astrocytes with single cilia | cilium (including axoneme), centriole | endogenous | Danilov 2009 |
| mature protoplasmic astrocytes | cilium, centriole | (immuno-EM) | |||
| SVZ neuroblast | cilium | ||||
| ependymal cells | cilium, basal body, cilium root | ||||
| NIH 3T3 | along primary cilium in growth-arrested fibroblasts |
endogenous, GFP∷Pdgfra |
Schneider 2005 Schneider 2010 |
||
| NIH 3T3 | along primary cilium, asynchronously in sister cells resulting from mitotic division |
endogenous | Anderson 2009 | ||
A fundamental question regarding Shh signaling is the cellular location at which full-length Gli proteins (Gli-FL) are modified to their repressor or activator forms. In Drosophila, which does not use the primary cilium for Hh signaling, a complex of Cos2, Fused and Sufu, in the absence of Hh ligand, recruits protein kinase A (PKA), glycogen synthase kinase 3 (GSK3) and casein kinase 1 (CK1). These kinases phosphorylate full-length cubitus interruptus (Ci), the Drosophila homolog of the Gli proteins, and Ci-FL is cleaved to generate CiR (Zhang et al., 2005). The current model of conversion of Gli3-FL to Gli3R, in the absence of Shh, is strikingly similar in the mouse, except that the complex of Kif7, Sufu and protein kinases forms at the base of the primary cilium (Goetz and Anderson, 2010). Meanwhile, Ptch1, near the base of the cilium, prevents entry of functionally significant levels of Smo. In the presence of Shh, Ptch1 binds Shh and moves away from the ciliary membrane, allowing Smo to accumulate in the cilium (Chen et al., 2009; Corbit et al., 2005; Endoh-Yamagami et al., 2009; Kim et al., 2009; Rohatgi et al., 2007; Wang et al., 2009a). Smo activation, in turn, causes Kif7, Sufu and Gli proteins to travel to the tip of the cilium, with Kif7, in particular, required for efficient Gli2 and Gli3 accumulation (Cheung et al., 2009; Endoh-Yamagami et al., 2009; Liem et al., 2009). Gli-FL is thus moved away from the kinase complex that promotes conversion to GliR, and may be transformed to GliA at the ciliary tip (Goetz and Anderson, 2010). In a different model, Gli-FL translocates from the cilium to be converted to GliA only in the nucleus (Humke et al., 2010). Given that the primary cilium is required for GliA production, Gli-FL must at least be modified in the cilium to allow subsequent nuclear conversion to GliA.
Hedgehog proteins have many binding partners, and a full discussion of these is beyond our current scope. Two binding partners, however, are required for Hh binding in Drosophila, acting as co-receptors with Ptc. These are the single pass membrane proteins Ihog (Interference hedgehog) and Boi (Brother of ihog) (Beachy et al., 2010). Vertebrate homologs of the two Drosphila co-receptors, Cdo and Boc bind to Shh and positively regulate Shh signaling. Yet, in vertebrates, unlike Drosophila, Shh binds directly to Ptc, so that the functions of Cdo and Boc in vertebrate Hh signaling are unclear (Beachy et al., 2010). Determining if Cdo and Boc are localized to cilia, and if their influence on Shh signaling requires the cilium should help clarify their functions.
The recent realization that Shh signal transduction is largely restricted to one organelle has been a surprise, given that Shh has been a focus of study for twenty years. Nonetheless, the relationship between the primary cilium and Shh signaling holds in mice and zebrafish (Huang and Schier, 2009; Kim et al., 2010), and ciliopathic symptoms indicative of disrupted Shh signaling suggest the same relationship in humans (Lancaster and Gleeson, 2009; Sharma et al., 2008). An obvious question is whether Shh signaling can occur at all in vertebrates in the absence of primary cilia. The answer appears to be yes, to a degree, in that the Shh pathway shows low-level constitutive activity in the absence of both Shh and the cilium. Sufu normally represses this activity outside the cilium (Jia et al., 2009), which therefore can be derepressed by disrupting Sufu function. The primary cilium is nonetheless required for the huge amplification of Shh pathway activation when Shh ligand is present. As noted, Hh signaling in Drosophila does not require a cilium, although the fly has many ciliated cells. Current evidence suggests an ancestral association between Hh signaling and cilia in metazoans, lost in Drosophila evolution, but maintained in vertebrates (Rink et al., 2009). The species difference in Hh signaling’s dependence on the cilium prompts a related question (see Perspectives, below), why, in vertebrates, is the cilium employed by certain signaling pathways, and not by others?
PDGF signaling and cilia-dependent migration
The primary cilium also transduces Platelet-Derived Growth Factor (PDGF) signaling, demonstrating that Shh signaling is not uniquely suited to the cilium. Homodimers of PDGF-A (PDGF-AA) activate PDGFRαα receptors on the cilium of mouse embryonic fibroblasts (MEFs) (Table 3), initiating the AKT and ERK1/2 signaling cascades in the cilium (Schneider et al., 2005). Wildtype MEFs respond to PDGF-AA, by proliferating or migrating towards a source of the ligand. MEFs derived from the ORPK mouse show neither response, and in the living ORPK mouse, fibroblasts fail to close a wound normally (Schneider et al., 2010). Intriguingly, primary cilia on migrating fibroblasts orient in the direction of cell migration (Albrecht-Buehler, 1977), and in an in vitro assay of wound healing, primary cilia align towards the “wound”, a scratch made in the sheet of cells, suggesting cilia are oriented by a positional signal, and could be involved in directing cell migration (Schneider et al., 2005).
Whether cilia orient in migrating neurons or glia, or otherwise contribute to the guidance of neural cells, is unexplored. Shh is a chemoattractant for migrating neurons and axons (Angot et al., 2008; Bourikas et al., 2005; Charron et al., 2003). This activity of Shh requires the putative Hh co-receptor Boc (Okada et al., 2006), and does not appear to utilize the canonical Shh pathway, instead activating Src family kinases to regulate growth cone turning (Yam et al., 2009). Despite these unusual features, Shh chemoattractant signaling is Smo-dependant (Charron et al., 2003; Yam et al., 2009), suggesting a link with the cilium. PDGF-AA directs migration of oligodendrocyte precursor cells (Dubois-Dalcq and Murray, 2000; Kessaris et al., 2006; Kiernan and Ffrench-Constant, 1993; Woodruff et al., 2001), which could be mediated by primary cilia. Favoring this possibility, oligodendrocytes have primary cilia (A. Peters, personal communication; Cenacchi et al., 1996), and the neuroepithelial cells that generate oligodendrocyte precursors are presumed to be ciliated, given that they respond to Shh (Richardson et al., 1997). Based on current knowledge, the primary cilium is unlikely to provide motive force to a migrating cell but could potentially sense and amplify a distant guidance signal.
Wnt signaling
The relationship between the primary cilium and Wnt signal transduction is an important problem that, despite considerable study, is unresolved. Canonical, β-catenin-dependent Wnt signaling regulates cell fate and proliferation in the nervous system (Angers and Moon, 2009). The planar cell polarity (PCP) Wnt pathway orients sheets of cells, for example, regulating the convergent extension cell movements that lead to neural tube closure. The PCP pathway is increasingly implicated in neuronal migration and axon guidance, in particular in the orderly development of large axon tracts (Tissir and Goffinet, 2010). This last observation is intriguing because diffusion tensor imaging in Joubert Syndrome patients reveals that both the corticospinal tract and superior cerebellar peduncle make major mistakes in their trajectories (Poretti et al., 2007).
Reports on Wnt signaling and cilia diverge from the model of Shh signaling by indicating that primary cilia suppress, rather than mediate, the canonical Wnt pathway. Mice deficient in Kif3a or Ift88 have shown upregulated signaling (Corbit et al., 2008). Similarly, reduction of certain BBS proteins (named for their association with Bardet-Biedl Syndrome, BBS, Table 2) stabilizes β-catenin in zebrafish and mammalian cells, leading to upregulated expression of canonical Wnt pathway target genes (Gerdes et al., 2007). Finally, inversin acting in primary cilia (Watanabe et al., 2003) has been proposed to turn off canonical Wnt signaling at a critical stage of kidney morphogenesis (Simons et al., 2005). In contrast, a positive association has been reported between the cilium, BBS proteins, and PCP signaling. Mice deficient in Bbs genes show disrupted convergent extension cell movements causing a neural tube defect (Ross et al., 2005). Further, in both mice and zebrafish, Bbs1 and Bbs6 genetically interact with Vangl2 (vang-like 2, encoding a core PCP pathway protein). BBS proteins and Vangl2 are present in the basal body and axoneme (Ross et al., 2005) (Table 3). These associations between primary cilia and Wnt signaling have been questioned, however, based on recent observations that mice deficient in Kif3a, Ift88, Ift172 and Dync2h1 show normal canonical Wnt responses in several assays (Ocbina et al., 2009), and that zebrafish lacking both maternal and zygotic Ift88 display defective Hh signaling, but no overt disruption in canonical Wnt signaling or PCP-guided convergent extension cell movements (Huang and Schier, 2009).
An interesting proposal for reconciling these disparate findings is that the ciliary axoneme and basal body may not invariably function as one entity (Huang and Schier, 2009), that is, the basal body could mediate signaling in the absence of the axoneme. Supporting this solution: several BBS proteins, among the core ancestral proteins of the centriole (Hodges et al., 2010) form the BBSome complex, which associates with the basal body (Table 2), thus, the ciliopathic syndrome BBS may often be caused specifically by basal body dysfunction (Ansley et al., 2003); the zebrafish Ift88 mutant, which shows no Wnt signaling abnormalities, retains a basal body (Huang and Schier, 2009); and depleting BBSome proteins results both in defects in PCP Wnt signaling, and upregulation of canonical Wnt signaling (Gerdes et al., 2007; Ross et al., 2005).
Wnt signaling may also be associated with cilia in a different manner. PCP signaling is required, for example, for the proper organization of secondary cilia on ventricular ependymal cells, whose main known function is to regulate the circulation of cerebrospinal fluid (CSF) (Del Bigio 2010).
Cilia and the CSF
Primary cilia on radial glia and choroid plexus epithelial (CPe) cells coordinate with secondary cilia on ependymal cells lining the brain ventricles to direct CSF flow, and deliver a potentially large range of signaling factors carried in the CSF to the developing and mature brain. CPe cells bearing both primary and secondary cilia generate and regulate the contents of the CSF (Narita et al., 2010; Peters et al., 1991). Primary cilia on CPe cells modulate the transcytosis of CSF into the ventricles, and recent evidence suggests an autocrine control mechanism in which CPe cilia monitor CSF levels of a neuropeptide that CPe cells produce and release (Narita et al., 2010). Defects in CPe primary cilia can cause increased CSF volume and hydrocephalus in the embryonic brain even before ciliated ependymal cells develop (Banizs et al., 2005).
Radial glia progenitor cells transform themselves into ventricular ependymal cells beginning late in embryogenesis by giving up their single primary cilium, expanding their apical surface, and acquiring multiple basal bodies and motile cilia (Mizradeh et al., 2010; Spassky et al., 2005). When stunted or disorganized, the beating of these secondary cilia is disrupted, and CSF accumulates in the ventricles, again causing hydrocephalus (Banizs et al., 2005). To direct CSF flow, ciliary beating must be coordinated across the sheet of ependyma, an example of planar cell polarity. Accumulating evidence implicates PCP signaling in establishing organized beating of ventricular cilia (Guirao et al., 2010; Tissir et al., 2010). Anatomically, ventricular ependymal cells show two forms of planar cell polarity: basal bodies are oriented towards the downstream direction of CSF flow, and the tuft of secondary cilia on the apical surface of each cell occupies a ‘downstream’ position. Basal body orientation arises from interactions between PCP signaling and hydrodynamic forces exerted by embryonic CSF during ependymal cell maturation (Guirao et al., 2010). Positioning of basal bodies requires a non-muscle myosin II-regulated process (Hirota et al., 2010), and may also depend on pre-patterning, namely, the asymmetric position of primary cilia on the apical surface of radial glia progenitor cells (Mizradeh et al., 2010).
CSF is increasingly appreciated as a source of signaling factors that act on the developing and adult brain, and the directional beating of ependymal cilia appears to establish concentration gradients of these factors. Chemorepellants, including Slit family members, for example, are produced by the CPe and carried in the CSF (Sawamoto et al., 2006). In the subventricular zone (SVZ), a source of new neurons for the olfactory bulb that lies just inside the ependyma of the lateral ventricle, a Slit 2 gradient forms that parallels the direction of CSF flow, and is dependent on cilia function. The gradient of Slit2 then guides migration of neuroblasts generated in the adult SVZ (Sawamoto et al., 2006). As the contents of the embryonic CSF are further characterized (Zappaterra et al., 2007), new examples are likely to be found in which cilia regulate and mediate signaling from the CSF to the brain.
Primary cilia as sensory organelles
Barnes observed that, “most cilia possessing a 9+0 pattern occur in sites which strongly suggest that they are performing a sensory or conducting function” (Barnes, 1961). More recently, the sensory function of primary cilia in adult neurons has been systematically studied in C. elegans and Drosophila. Primary cilia in Drosophila are required for the functions of chemoreceptor and mechanoreceptor neurons (Kernan, 2007; Li et al, 2006). In C. elegans, neurons with chemoreceptors localized to their cilia mediate many sensory functions, including odorant and osmolarity detection (Bargmann, 2006); ciliated mechanoreceptor cells transduce touch, guiding the worm’s exploration of its environment (Kang et al., 2010). Worms with ciliary defects thus show a wide range of behavioral abnormalities (Bargmann, 2006). Studies in C. elegans and Drosophila have further brought to light two important families of ion channels present on the cilia of sensory neurons, and which mediate their sensory function: cyclic nucleotide–gated (CNG) and TRP (transient receptor potential) channels (Bargmann, 2006; Cheng et al., 2010; Kang et al., 2010). With respect to CNG channels, observations of both vertebrate and invertebrate sensory cilia highlight their use of cAMP and cGMP signaling pathways (Barzi et al., 2010; Johnson and Krieg, 1995; Johnson and Leroux, 2010; Meyer et al., 2000), prompting the hypothesis that primary cilia provide a unique compartment that localizes cAMP and cGMP signaling for specific cellular functions (Johnson and Leroux, 2010; Milenkovic and Scott, 2010). Because of the strong link between TRP channels and sensation (Clapham et al., 2001; Hardie and Minke, 1993; Tobin et al., 2002; Venkatachalam and Montell, 2007) it will be interesting to discover if TRP channels are also prominent in the vertebrate cilia proteome, and if so, whether the channels serve ciliary sensory functions.
Anosmia is a common feature of ciliopathic syndromes (Table 2), and significant insight has been obtained into the role of cilia in vertebrate olfaction. Olfactory receptor neurons (ORNs) are unusual among vertebrate neurons because of their immediate contact with the outside air. Each ORN has a tuft of 10–20 cilia, enmeshed in the mucus overlying the olfactory epithelium (Figure 2). Similar to Shh transduction, much of the olfactory signaling cascade takes place in the cilium (Hengl et al., 2009) (Figure 3). Odorants bind to olfactory receptors in the ciliary membrane, activating adenylyl cyclase type III (ACIII), and increasing cAMP. (Notably, ACIII, one of ten mammalian adenylyl cylases, is so prevalent within cilia that ACIII immunoreactivity is considered a ‘marker’ of primary cilia in the adult mouse brain (Bishop et al., 2007).) As cAMP levels rise, CNG ion channels open allowing an influx of Na+ and Ca++ ions and depolarizing the potential of the cilium. Ca++ influx opens chloride (Cl−) channels, and Cl− efflux acts as a signal amplifier, depolarizing the cilium still further. Without this amplification, odorant responsiveness in mice is severely blunted (Hengl et al., 2009). Loss of functional ACIII, the first step in the ORN ciliary cascade, causes anosmia (Wong et al., 2000). ORN activation thus emphasizes the vertebrate cilium’s ability to sustain complex intracellular signaling and to regulate vertebrate neuronal excitability.
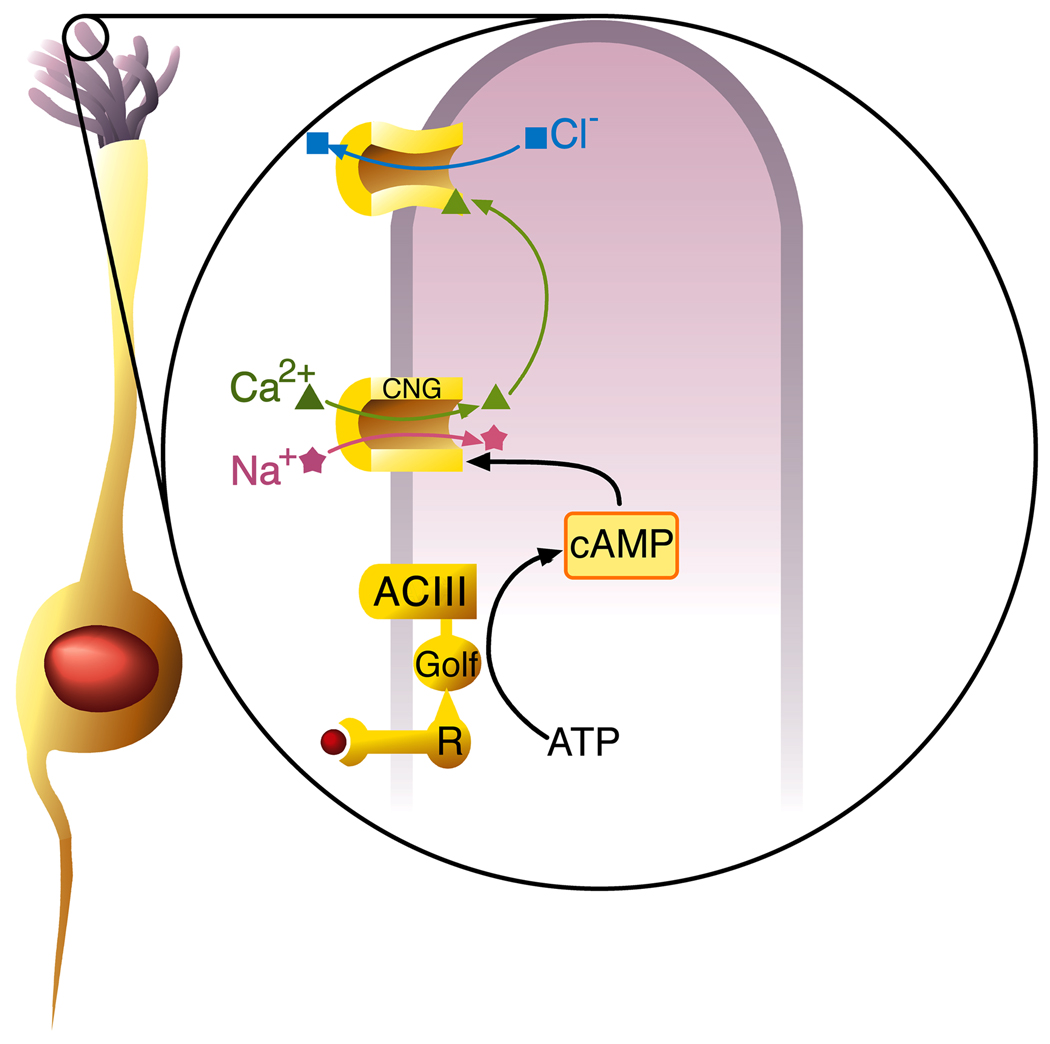
Figure 3. The cilium as a sensory transduction organelle.
At left an ORN extends a dendrite ending in a cluster of cilia (purple). To the right, signal transduction in an ORN cilium. An odorant (red ball) binds to the olfactory receptor (R), coupled to the G-protein Golf. Activation of ACIII increases cAMP. cAMP opens CNG ion channels, causing an influx of Ca++ (green) and Na+ (red) ions which depolarizes the ORN. Raised Ca++ levels open Cl− (blue) channels, allowing an efflux of Cl−, further depolarizing the cell, and amplifying the odorant signal. The depolarized potential of the cilium spreads passively to the somatic membrane of the ORN where it activates Ca++, Na+ and K- channels, leading to the firing of an action potential.
The neural control of energy balance
Obesity in ciliopathic syndromes, such as BBS and Alström Syndrome, suggests cilia are utilized in the neural circuitry that monitors food intake (Kesterson et al., 2009; Seo et al., 2009; Sharma et al., 2008). Mutations in ACIII are associated with obesity in both mice and humans (Nordman et al., 2008; Wang et al., 2009b). Direct evidence of an association between cilia dysfunction and obesity comes from deleting Kif3a or Ift88 conditionally in adult mice. Cilia are stunted and animals overeat, becoming obese. This occurs despite elevated leptin, a satiety signal, suggesting the satiety response is compromised in the absence of functional cilia (Davenport et al., 2007). Ciliated neurons that regulate feeding include a group of pro-opiomelanocortin (POMC)-expressing neurons in the hypothalamic arcuate nucleus. These cells respond to leptin by cleaving POMC to generate α-melanocyte-stimulating hormone (α-MSH), which inhibits feeding, and mice in which Kif3a is deleted selectively from POMC neurons become obese (Davenport et al., 2007; Sharma et al., 2008). What ciliary signaling pathways in POMC neurons might modulate α-MSH production, however, remains unclear. The localization of leptin receptors to POMC cell cilia might be predicted, for example, but has not been confirmed.
Cilia, postnatal neurogenesis and oncogenesis
Connecting Shh signaling with the cilium illuminates CNS defects found in cilliopathic syndromes and other human disease states. Ciliated cerebellar granule neuron precursors (GNPs) proliferate in a Shh-dependent manner in the external granule layer (EGL) of the developing cerebellum (Dahmane and Ruiz i Altaba, 1999; Wallace, 1999; Wechsler-Reya and Scott, 1999). In mice with conditional deletions of Ift88 or Kif3a, cilia on EGL precursors are stunted, cerebellar granule neurons are fewer, and the cerebellum is hypoplastic, similar to its appearance in Joubert Syndrome (Chizhikov et al., 2007; Spassky et al., 2008).
Learning disabilities are also a feature of several ciliopathies. Although associated defects in the hippocampus have yet to be reported in human patients, the hippocampal dentate gyrus (DG) in mice is highly sensitive to the disruption of primary cilia. DG progenitor cells are ciliated, and respond to Shh to generate granule neurons (Breunig et al., 2008; Han et al., 2008; Town et al., 2008). Deleting Kif3a in DG progenitor cells stunts primary cilia, decreases Shh signaling in the DG, and inhibits the normal perinatal expansion of DG progenitor population. This reduces the main wave of production of DG neurons in the first three weeks after birth, as well as the initial allocation of radial astrocyte precursors that supply new DG neurons into adulthood (Han et al., 2008). Similar results are seen in mice deficient in Ift88 (Han et al., 2008), and in the stumpy mouse mutant, in which ciliogenesis is still more impaired (Breunig et al., 2008; Town et al., 2008). The adult neural stem cells of the DG are themselves ciliated, and the role of primary cilia in these cells awaits analysis of mice with deletion of cilia in adulthood.
Ptc mutations in both humans and mice have linked Shh signaling with medulloblastoma, the most common type of brain cancer in children (Goodrich et al., 1997; Ingham, 1998). Constitutive activation of Smo in the cerebellar granule cell progenitor lineage in mice identified GNPs as the progenitors of Shh-dependent medulloblastoma tumor cells (Schuller et al., 2008). Interestingly, the contribution of cilia to tumorigenesis depends on where the Shh pathway is deregulated. Cilia are permissive for mouse medulloblastomas elicited by constitutively activated Smo, but inhibitory to tumorigenesis caused by constitutively activated Gli2 (Gli2CA) (Han et al., 2009). These findings are consistent with the ciliary model of Shh signaling (Goetz and Anderson, 2010), given that Smo acts in the ciliary membrane, whereas Gli2A moves away from the cilium into the nucleus to regulate downstream genes in the Shh pathway. Gli2CA does not need the cilium to function and, moreover, is inhibited from initiating tumorigenesis by Gli3R, which is dependent on the cilium for its production. When cilia and Gli3R are abolished, Gli2CA can become oncogenic. Cilia thus have a dual role in mediating or suppressing tumorigenesis, depending on the tumor initiator.
Cilia and the cerebral cortex
Two recent studies connect cerebral cortical development, primary cilia and Shh signaling, and support a hypothesis that Gli3R regulates cortical morphology (Stottmann et al., 2009; Willaredt et al., 2008). In the cobblestone mouse mutant, hypomorphic for Ift88, Gli3 processing is abnormal, leading to an excess of Gli3-FL relative to Gli3R (Willaredt et al., 2008). The imbalance generates a cortical malformation also seen in the absence of Gli3 in the extra-toes mutant, in which the hippocampus is missing, neocortical neurons clump together, and neocortex ultimately degenerates (Theil et al., 1999; Tole et al., 2000). A comparable phenotype appears in Ift139 mutant mice in which retrograde IFT is disrupted and the Shh pathway is overactive, leading to reduced Gli3R (Stottmann et al., 2009; Tran et al., 2008).
Mutations in the Abelson helper integration site gene-1 (AHI1), which define a subtype of Joubert Syndrome, cause different cerebral cortical abnormalities. A dramatic cortical malformation, polymicrogyria, characterized by shallow cortical sulci, can occur (Dixon-Salazar et al., 2004). AHI1 is highly expressed in the cerebral cortex, particularly in cortical neurons that project to the cerebral peduncles, and into the corticospinal tract. Consistent with this observation, the corticospinal tract fails to cross the midline in some Joubert Syndrome patients, suggesting an axon guidance defect (Poretti et al., 2007). Moreover, the cognitive impairments seen frequently in Joubert Syndrome are likely to be accompanied by less obvious defects in cerebral cortex development. Up to 40% of Joubert Syndrome patients show autism spectrum disorders (Alvarez Retuerto et al., 2008).
Encouragingly, evidence of the involvement of cilia in higher vertebrate brain functions is now emerging. Loss of function of the somatostatin receptor 3 (SSTR3), localized to cilia in the neocortex and hippocampus (Einstein et al., 2010; Handel et al., 1999) leads to impaired object recognition in mice, whereas the loss of other SSTRs, not found on cilia, does not (Einstein et al., 2010). SSTR3 is evident in the brain only after mice are born (Stanic et al., 2009), implying that the phenotype depends on loss of signaling mediated by a ciliary somatostatin receptor in mature neurons. Additionally, hippocampal long-term potentiation evoked with forskolin, a cAMP activator, is significantly diminished in the Sstr3 mutant (Einstein et al., 2010). These findings finally link primary cilia with complex mammalian behavior and brain plasticity.
Perspectives
Primary cilia research is likely to continue its rapid growth. Also likely, however, is a new phase of self-correction. Two main caveats need to be addressed. Several proteins that belong to the ciliary proteome additionally contribute to cellular processes outside the cilium, and more such extraciliary functions stand to be discovered. To state a few examples, several IFT proteins participate in the cytoplasmic vesicle pathway for exocytosis (Baldari and Rosenbaum, 2010); AHI1, associated with Joubert Syndrome, interacts with Rab8a, a small GTPase, also regulating vesicle trafficking (Hsiao et al., 2009); and certain BBS proteins are localized to additional microtubule motor complexes as well as the basal body (May Simera et al., 2009; Sen Gupta et al., 2009). Additionally, in mice, Ahi1 is found in the adult kidney where it acts outside the cilium to upregulate β-catenin-mediated Wnt signaling. In adult Ahi1 null mice, reduced Wnt signaling, not ciliary defects, leads to cystic kidney disease (Lancaster et al., 2009). Given that Ahi1 is expressed at several sites in mouse, including the forebrain, decreased Wnt signaling could prove to cause a variety of abnormalities in patients with AHI1 mutations. These findings suggest that at least some abnormalities now termed ciliopathic in mouse models and human patients will be found to result from the disruption of cellular functions outside the cilium. Future studies are likely to amend substantially our current conclusions about the primary cilium, and extend our understanding of disorders now termed ciliopathic.
A second, related caveat is that in mice with deficiencies in an IFT protein or other ciliary protein, the brain phenotype may suggest a ciliary defect, yet ultrastructurally there may be little wrong with the cilium. Does this mean that a defective cilium is not central to the phenotype? Not necessarily. In the case of the cobblestone mutant mouse, in which cerebral cortical primary cilia appear normal, the signaling defect probably occurs at the cilium base where Gli3-FL is processed to Gli3R. A structural correlate may not be visible. To determine, rather than infer, that a ciliary defect is to blame for a given phenotype needs more than an EM photomicrograph showing the presence or absence of clia. Such a determination must derive from a more thorough evaluation of cilia structure and function.
More immediately interesting are the future directions of cilia research. A recurring question is why only certain signaling pathways utilize the primary cilium. Transduction of the Hh pathway requires a localized signaling hub for which, in vertebrates, the primary cilium appears ideal. By contrast, β-catenin-based Wnt signaling interacts in the cytoplasm with other Wnt pathways and with cell adhesion machinery (Angers and Moon, 2009), making it less obviously suited to the confined cilium. No conclusions can be drawn, however, from two examples. As the known cilia proteome continues to grow further signaling pathways are likely to be identified whose components are enriched in cilia. A more systematic classification may then reveal the common features of pathways that use, or do not use, the primary cilium.
A second major question is what the primary cilium does for adult brain function. Some progress has been made on this question since primary cilia were first seen on adult neurons and glia. Yet progress here bears no comparison with the amazing recent advances in understanding the general biology of the primary cilium, and its functions in other parts of the body and the developing brain. Why is this so? To date, cilia function has been mainly studied in mouse models and human patients in which gene mutations are constitutive, affecting development from conception. The florid phenotypes that result have been the first focus of interest. Mice with cilia defects exclusive to the adult brain will allow a new focus on primary cilia in adult neural cells. Notably, to date, mouse lines engineered to lack cilia in the adult show no catastrophic neurological symptoms and the mice are viable (Davenport et al., 2007). To identify adult neural phenotypes in such mice, detailed analyses of brain function and behavior will be essential. This could be more readily accomplished by targeting specific brain structures whose behavioral contributions and physiology are well understood, and in which comparisons between wildtype and mutant mice would be most easily interpreted. Such structures could include the hippocampus and primary sensory areas of the cerebral cortex. Finally, the methods utilized in pioneering studies of electrical activity in the ORN cilium and its effects on excitability of the whole cell (Kleene, 1993; Leinders-Zufall et al., 1997; Takeuchi and Kurahashi, 2002) should be extended to neurons in the brain. Such studies may be technically more challenging than the first wave of investigations of primary cilia in the nervous system, but will be consistent with a new and more considered phase of cilia research.
Acknowledgements
We would like to acknowledge the illustrator, Sue Lundy Christopher, for her work on the figures for this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aanstad P, Santos N, Corbit KC, Scherz PJ, Trinh le A, Salvenmoser W, Huisken J, Reiter JF, Stainier DY. The extracellular domain of Smoothened regulates ciliary localization and is required for high-level Hh signaling. Curr Biol. 2009;19:1034–1039. doi: 10.1016/j.cub.2009.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht-Buehler G. Phagokinetic tracks of 3T3 cells: parallels between the orientation of track segments and of cellular structures which contain actin or tubulin. Cell. 1977;12:333–339. doi: 10.1016/0092-8674(77)90109-x. [DOI] [PubMed] [Google Scholar]
- Alvarez Retuerto AI, Cantor RM, Gleeson JG, Ustaszewska A, Schackwitz WS, Pennacchio LA, Geschwind DH. Association of common variants in the Joubert syndrome gene (AHI1) with autism. Hum Mol Genet. 2008;17:3887–3896. doi: 10.1093/hmg/ddn291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CT, Stearns T. Centriole age underlies asynchronous primary cilium growth in mammalian cells. Curr Biol. 2009;19:1498–1502. doi: 10.1016/j.cub.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol. 2009;10:468–477. doi: 10.1038/nrm2717. [DOI] [PubMed] [Google Scholar]
- Angot E, Loulier K, Nguyen-Ba-Charvet KT, Gadeau AP, Ruat M, Traiffort E. Chemoattractive activity of sonic hedgehog in the adult subventricular zone modulates the number of neural precursors reaching the olfactory bulb. Stem Cells. 2008;26:2311–2320. doi: 10.1634/stemcells.2008-0297. [DOI] [PubMed] [Google Scholar]
- Ansley SJ, Badano JL, Blacque OE, Hill J, Hoskins BE, Leitch CC, Kim JC, Ross AJ, Eichers ER, Teslovich TM, et al. Basal body dysfunction is a likely cause of pleiotropic Bardet-Biedl syndrome. Nature. 2003;425:628–633. doi: 10.1038/nature02030. [DOI] [PubMed] [Google Scholar]
- Arstila A, Wersall J. The ultrastructure of the olfactory epithelium of the guinea pig. Acta Otolaryngol. 1967;64:187–204. doi: 10.3109/00016486709139106. [DOI] [PubMed] [Google Scholar]
- Badano JL, Mitsuma N, Beales PL, Katsanis N. The ciliopathies: an emerging class of human genetic disorders. Annu Rev Genomics Hum Genet. 2006;7:125–148. doi: 10.1146/annurev.genom.7.080505.115610. [DOI] [PubMed] [Google Scholar]
- Baldari CT, Rosenbaum J. Intraflagellar transport: it's not just for cilia anymore. Curr Opin Cell Biol. 2010;22:75–80. doi: 10.1016/j.ceb.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banizs B, Pike MM, Millican CL, Ferguson WB, Komlosi P, Sheetz J, Bell PD, Schwiebert EM, Yoder BK. Dysfunctional cilia lead to altered ependyma and choroid plexus function, and result in the formation of hydrocephalus. Development. 2005;132:5329–5339. doi: 10.1242/dev.02153. [DOI] [PubMed] [Google Scholar]
- Bargmann CI. Chemosensation in C. elegans. WormBook. 2006:1–29. doi: 10.1895/wormbook.1.123.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes BG. Ciliated secretory cells in the pars distalis of the mouse hypophysis. J Ultrastruct Res. 1961;5:453–467. doi: 10.1016/s0022-5320(61)80019-1. [DOI] [PubMed] [Google Scholar]
- Barr MM, DeModena J, Braun D, Nguyen CQ, Hall DH, Sternberg PW. The Caenorhabditis elegans autosomal dominant polycystic kidney disease gene homologs lov-1 and pkd-2 act in the same pathway. Curr Biol. 2001;11:1341–1346. doi: 10.1016/s0960-9822(01)00423-7. [DOI] [PubMed] [Google Scholar]
- Barr MM, Sternberg PW. A polycystic kidney-disease gene homologue required for male mating behaviour in C. elegans. Nature. 1999;401:386–389. doi: 10.1038/43913. [DOI] [PubMed] [Google Scholar]
- Barzi M, Berenguer J, Menendez A, Alvarez-Rodriguez R, Pons S. Sonic-hedgehog-mediated proliferation requires the localization of PKA to the cilium base. J Cell Sci. 2010;123:62–69. doi: 10.1242/jcs.060020. [DOI] [PubMed] [Google Scholar]
- Beachy PA, Hymowitz SG, Lazarus RA, Leahy DJ, Siebold C. Interactions between Hedgehog proteins and their binding partners come into view. Genes Dev. 2010;24:2001–2012. doi: 10.1101/gad.1951710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbari NF, Johnson AD, Lewis JS, Askwith CC, Mykytyn K. Identification of ciliary localization sequences within the third intracellular loop of G protein-coupled receptors. Mol Biol Cell. 2008a;19:1540–1547. doi: 10.1091/mbc.E07-09-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbari NF, Lewis JS, Bishop GA, Askwith CC, Mykytyn K. Bardet-Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proc Natl Acad Sci U S A. 2008b;105:4242–4246. doi: 10.1073/pnas.0711027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop GA, Berbari NF, Lewis J, Mykytyn K. Type III adenylyl cyclase localizes to primary cilia throughout the adult mouse brain. J Comp Neurol. 2007;505:562–571. doi: 10.1002/cne.21510. [DOI] [PubMed] [Google Scholar]
- Bourikas D, Pekarik V, Baeriswyl T, Grunditz A, Sadhu R, Nardo M, Stoeckli ET. Sonic hedgehog guides commissural axons along the longitudinal axis of the spinal cord. Nat Neurosci. 2005;8:297–304. doi: 10.1038/nn1396. [DOI] [PubMed] [Google Scholar]
- Breunig JJ, Sarkisian MR, Arellano JI, Morozov YM, Ayoub AE, Sojitra S, Wang B, Flavell RA, Rakic P, Town T. Primary cilia regulate hippocampal neurogenesis by mediating sonic hedgehog signaling. Proc Natl Acad Sci U S A. 2008;105:13127–13132. doi: 10.1073/pnas.0804558105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenacchi G, Giangaspero F, Cerasoli S, Manetto V, Martinelli GN. Ultrastructural characterization of oligodendroglial-like cells in central nervous system tumors. Ultrastruct Pathol. 1996;20:537–547. doi: 10.3109/01913129609016358. [DOI] [PubMed] [Google Scholar]
- Chamberlain CE, Jeong J, Guo C, Allen BL, McMahon AP. Notochord-derived Shh concentrates in close association with the apically positioned basal body in neural target cells and forms a dynamic gradient during neural patterning. Development. 2008;135:1097–1106. doi: 10.1242/dev.013086. [DOI] [PubMed] [Google Scholar]
- Charron F, Stein E, Jeong J, McMahon AP, Tessier-Lavigne M. The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance. Cell. 2003;113:11–23. doi: 10.1016/s0092-8674(03)00199-5. [DOI] [PubMed] [Google Scholar]
- Chen MH, Wilson CW, Li YJ, Law KK, Lu CS, Gacayan R, Zhang X, Hui CC, Chuang PT. Cilium-independent regulation of Gli protein function by Sufu in Hedgehog signaling is evolutionarily conserved. Genes Dev. 2009;23:1910–1928. doi: 10.1101/gad.1794109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng LE, Song W, Looger LL, Jan LY, Jan YN. The Role of the TRP Channel NompC in Drosophila Larval and Adult Locomotion. Neuron. 2010;67:373–380. doi: 10.1016/j.neuron.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung HO, Zhang X, Ribeiro A, Mo R, Makino S, Puviindran V, Law KK, Briscoe J, Hui CC. The kinesin protein Kif7 is a critical regulator of Gli transcription factors in mammalian hedgehog signaling. Sci Signal. 2009;2:ra29. doi: 10.1126/scisignal.2000405. [DOI] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- Chizhikov VV, Davenport J, Zhang Q, Shih EK, Cabello OA, Fuchs JL, Yoder BK, Millen KJ. Cilia proteins control cerebellar morphogenesis by promoting expansion of the granule progenitor pool. J Neurosci. 2007;27:9780–9789. doi: 10.1523/JNEUROSCI.5586-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho A, Ko HW, Eggenschwiler JT. FKBP8 cell-autonomously controls neural tube patterning through a Gli2- and Kif3a–dependent mechanism. Dev Biol. 2008;321:27–39. doi: 10.1016/j.ydbio.2008.05.558. [DOI] [PubMed] [Google Scholar]
- Christensen ST, Pedersen SF, Satir P, Veland IR, Schneider L. The primary cilium coordinates signaling pathways in cell cycle control and migration during development and tissue repair. Curr Top Dev Biol. 2008;85:261–301. doi: 10.1016/S0070-2153(08)00810-7. [DOI] [PubMed] [Google Scholar]
- Clapham DE, Runnels LW, Strubing C. The TRP ion channel family. Nat Rev Neurosci. 2001;2:387–396. doi: 10.1038/35077544. [DOI] [PubMed] [Google Scholar]
- Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- Corbit KC, Shyer AE, Dowdle WE, Gaulden J, Singla V, Chen MH, Chuang PT, Reiter JF. Kif3a constrains beta-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nat Cell Biol. 2008;10:70–76. doi: 10.1038/ncb1670. [DOI] [PubMed] [Google Scholar]
- Dahl HA. Fine structure of cilia in rat cerebral cortex. Z Zellforsch Mikrosk Anat. 1963;60:369–386. doi: 10.1007/BF00336612. [DOI] [PubMed] [Google Scholar]
- Dahmane N, Ruiz i Altaba A. Sonic hedgehog regulates the growth and patterning of the cerebellum. Development. 1999;126:3089–3100. doi: 10.1242/dev.126.14.3089. [DOI] [PubMed] [Google Scholar]
- Dalen H, Schlapfer WT, Mamoon A. Cilia on cultured ependymal cells examined by scanning electron microscopy. Exp Cell Res. 1971;67:375–379. doi: 10.1016/0014-4827(71)90422-8. [DOI] [PubMed] [Google Scholar]
- Danilov AI, Gomes-Leal W, Ahlenius H, Kokaia Z, Carlemalm E, Lindvall O. Ultrastructural and antigenic properties of neural stem cells and their progeny in adult rat subventricular zone. Glia. 2009;57:136–152. doi: 10.1002/glia.20741. [DOI] [PubMed] [Google Scholar]
- Davenport JR, Watts AJ, Roper VC, Croyle MJ, van Groen T, Wyss JM, Nagy TR, Kesterson RA, Yoder BK. Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Curr Biol. 2007;17:1586–1594. doi: 10.1016/j.cub.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane JA, Cole DG, Seeley ES, Diener DR, Rosenbaum JL. Localization of intraflagellar transport protein IFT52 identifies basal body transitional fibers as the docking site for IFT particles. Curr Biol. 2001;11:1586–1590. doi: 10.1016/s0960-9822(01)00484-5. [DOI] [PubMed] [Google Scholar]
- Del Bigio MR. Ependymal cells: biology and pathology. Acta Neuropathol. 2010;119:55–73. doi: 10.1007/s00401-009-0624-y. [DOI] [PubMed] [Google Scholar]
- Deretic D, Papermaster DS. Polarized sorting of rhodopsin on post-Golgi membranes in frog retinal photoreceptor cells. J Cell Biol. 1991;113:1281–1293. doi: 10.1083/jcb.113.6.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishinger JF, Kee HL, Jenkins PM, Fan S, Hurd TW, Hammond JW, Truong YN, Margolis B, Martens JR, Verhey KJ. Ciliary entry of the kinesin-2 motor KIF17 is regulated by importin-beta2 and RanGTP. Nat Cell Biol. 2010 doi: 10.1038/ncb2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon-Salazar T, Silhavy JL, Marsh SE, Louie CM, Scott LC, Gururaj A, Al-Gazali L, Al-Tawari AA, Kayserili H, Sztriha L, et al. Mutations in the AHI1 gene, encoding jouberin, cause Joubert syndrome with cortical polymicrogyria. Am J Hum Genet. 2004;75:979–987. doi: 10.1086/425985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois-Dalcq M, Murray K. Why are growth factors important in oligodendrocyte physiology? Pathol Biol (Paris) 2000;48:80–86. [PubMed] [Google Scholar]
- Duncan D. Electron microscope study of the embryonic neural tube and notochord. Tex Rep Biol Med. 1957;15:367–377. [PubMed] [Google Scholar]
- Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, McMahon AP. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993;75:1417–1430. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- Einstein EB, Patterson CA, Hon BJ, Regan KA, Reddi J, Melnikoff DE, Mateer MJ, Schulz S, Johnson BN, Tallent MK. Somatostatin signaling in neuronal cilia is critical for object recognition memory. J Neurosci. 2010;30:4306–4314. doi: 10.1523/JNEUROSCI.5295-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmer BT, Maric D, Engman DM. Molecular mechanisms of protein and lipid targeting to ciliary membranes. J Cell Sci. 2010;123:529–536. doi: 10.1242/jcs.062968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endoh-Yamagami S, Evangelista M, Wilson D, Wen X, Theunissen JW, Phamluong K, Davis M, Scales SJ, Solloway MJ, de Sauvage FJ, et al. The Mammalian Cos2 Homolog Kif7 Plays an Essential Role in Modulating Hh Signal Transduction during Development. Curr Biol. 2009 doi: 10.1016/j.cub.2009.06.046. [DOI] [PubMed] [Google Scholar]
- Ericson J, Muhr J, Placzek M, Lints T, Jessell TM, Edlund T. Sonic hedgehog induces the differentiation of ventral forebrain neurons: a common signal for ventral patterning within the neural tube. Cell. 1995;81:747–756. doi: 10.1016/0092-8674(95)90536-7. [DOI] [PubMed] [Google Scholar]
- Fliegauf M, Benzing T, Omran H. When cilia go bad: cilia defects and ciliopathies. Nat Rev Mol Cell Biol. 2007;8:880–893. doi: 10.1038/nrm2278. [DOI] [PubMed] [Google Scholar]
- Follit JA, Li L, Vucica Y, Pazour GJ. The cytoplasmic tail of fibrocystin contains a ciliary targeting sequence. J Cell Biol. 2010;188:21–28. doi: 10.1083/jcb.200910096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follit JA, Xu F, Keady BT, Pazour GJ. Characterization of mouse IFT complex B. Cell Motil Cytoskeleton. 2009 doi: 10.1002/cm.20346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz T. Extra-toes (Xt) homozygous mutant mice demonstrate a role for the Gli-3 gene in the development of the forebrain. Acta Anatomica. 1994;150:38–44. doi: 10.1159/000147600. [DOI] [PubMed] [Google Scholar]
- Fuccillo M, Joyner AL, Fishell G. Morphogen to mitogen: the multiple roles of hedgehog signalling in vertebrate neural development. Nat Rev Neurosci. 2006;7:772–783. doi: 10.1038/nrn1990. [DOI] [PubMed] [Google Scholar]
- Gerdes JM, Liu Y, Zaghloul NA, Leitch CC, Lawson SS, Kato M, Beachy PA, Beales PL, DeMartino GN, Fisher S, et al. Disruption of the basal body compromises proteasomal function and perturbs intracellular Wnt response. Nat Genet. 2007;39:1350–1360. doi: 10.1038/ng.2007.12. [DOI] [PubMed] [Google Scholar]
- Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet. 2010;11:331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guirao B, Meunier A, Mortaud S, Aguilar A, Corsi JM, Strehl L, Hirota Y, Desoeuvre A, Boutin C, Han YG, et al. Coupling between hydrodynamic forces and planar cell polarity orients mammalian motile cilia. Nat Cell Biol. 2010;12:341–350. doi: 10.1038/ncb2040. [DOI] [PubMed] [Google Scholar]
- Han YG, Spassky N, Romaguera-Ros M, Garcia-Verdugo JM, Aguilar A, Schneider-Maunoury S, Alvarez-Buylla A. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat Neurosci. 2008;11:277–284. doi: 10.1038/nn2059. [DOI] [PubMed] [Google Scholar]
- Handel M, Schulz S, Stanarius A, Schreff M, Erdtmann-Vourliotis M, Schmidt H, Wolf G, Hollt V. Selective targeting of somatostatin receptor 3 to neuronal cilia. Neuroscience. 1999;89:909–926. doi: 10.1016/s0306-4522(98)00354-6. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Minke B. Novel Ca2+ channels underlying transduction in Drosophila photoreceptors: implications for phosphoinositide-mediated Ca2+ mobilization. Trends Neurosci. 1993;16:371–376. doi: 10.1016/0166-2236(93)90095-4. [DOI] [PubMed] [Google Scholar]
- Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, Yoder BK. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet. 2005;1:e53. doi: 10.1371/journal.pgen.0010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengl T, Kaneko H, Dauner K, Vocke K, Frings S, Mohrlen F. Molecular components of signal amplification in olfactory sensory cilia. Proc Natl Acad Sci U S A. 2009;107:6052–6057. doi: 10.1073/pnas.0909032107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y, Meunier A, Huang S, Shimozawa T, Yamada O, Kida YS, Inoue M, Ito T, Kato H, Sakaguchi M, et al. Planar polarity of multiciliated ependymal cells involves the anterior migration of basal bodies regulated by non-muscle myosin II. Development. 2010;137:3037–3046. doi: 10.1242/dev.050120. [DOI] [PubMed] [Google Scholar]
- Hodges ME, Scheumann N, Wickstead B, Langdale JA, Gull K. Reconstructing the evolutionary history of the centriole from protein components. J Cell Sci. 2010;123:1407–1413. doi: 10.1242/jcs.064873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao YC, Tong ZJ, Westfall JE, Ault JG, Page-McCaw PS, Ferland RJ. Ahi1, whose human ortholog is mutated in Joubert syndrome, is required for Rab8a localization, ciliogenesis and vesicle trafficking. Hum Mol Genet. 2009;18:3926–3941. doi: 10.1093/hmg/ddp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, Milenkovic L, Jin H, Scott MP, Nachury MV, Spiliotis ET, Nelson WJ. A septin diffusion barrier at the base of the primary cilium maintains ciliary membrane protein distribution. Science. 2010;329:436–439. doi: 10.1126/science.1191054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Schier AF. Dampened Hedgehog signaling but normal Wnt signaling in zebrafish without cilia. Development. 2009;136:3089–3098. doi: 10.1242/dev.041343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, Anderson KV. Cilia and Hedgehog responsiveness in the mouse. Proc Natl Acad Sci U S A. 2005;102:11325–11330. doi: 10.1073/pnas.0505328102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–87. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- Hui CC, Joyner AL. A mouse model of greig cephalopolysyndactyly syndrome: the extra-toesJ mutation contains an intragenic deletion of the Gli3 gene. Nature Genetics. 1993;3:241–246. doi: 10.1038/ng0393-241. [DOI] [PubMed] [Google Scholar]
- Humke EW, Dorn KV, Milenkovic L, Scott MP, Rohatgi R. The output of Hedgehog signaling is controlled by the dynamic association between Suppressor of Fused and the Gli proteins. Genes Dev. 2010;24:670–682. doi: 10.1101/gad.1902910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham PW. The patched gene in development and cancer. Curr Opin Genet Dev. 1998;8:88–94. doi: 10.1016/s0959-437x(98)80067-1. [DOI] [PubMed] [Google Scholar]
- Inglis PN, Boroevich KA, Leroux MR. Piecing together a ciliome. Trends Genet. 2006;22:491–500. doi: 10.1016/j.tig.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Inglis PN, Ou G, Leroux MR, Scholey JM. The sensory cilia of Caenorhabditis elegans. WormBook. 2007:1–22. doi: 10.1895/wormbook.1.126.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins PM, Hurd TW, Zhang L, McEwen DP, Brown RL, Margolis B, Verhey KJ, Martens JR. Ciliary targeting of olfactory CNG channels requires the CNGB1b subunit and the kinesin-2 motor protein, KIF17. Curr Biol. 2006;16:1211–1216. doi: 10.1016/j.cub.2006.04.034. [DOI] [PubMed] [Google Scholar]
- Jenkins PM, McEwen DP, Martens JR. Olfactory cilia: linking sensory cilia function and human disease. Chem Senses. 2009;34:451–464. doi: 10.1093/chemse/bjp020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AD, Krieg PA. A Xenopus laevis gene encoding EF-1 alpha S, the somatic form of elongation factor 1 alpha: sequence, structure, and identification of regulatory elements required for embryonic transcription. Dev Genet. 1995;17:280–290. doi: 10.1002/dvg.1020170313. [DOI] [PubMed] [Google Scholar]
- Johnson DR. Extra-toes: a new mutant gene causing multiple abnormalities in the mouse. Journal of Embryology & Experimental Morphology. 1967;17:543–581. [PubMed] [Google Scholar]
- Johnson JL, Leroux MR. cAMP and cGMP signaling: sensory systems with prokaryotic roots adopted by eukaryotic cilia. Trends Cell Biol. 2010;20:435–444. doi: 10.1016/j.tcb.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Kang L, Gao J, Schafer WR, Xie Z, Xu XZ. C. elegans TRP Family Protein TRP-4 Is a Pore-Forming Subunit of a Native Mechanotransduction Channel. Neuron. 2010;67:381–391. doi: 10.1016/j.neuron.2010.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson U. Three-dimensional studies of neurons in the lateral geniculate nucleus of the rat. I. Organelle organization in the perikaryon and its proximal branches. J Ultrastruct Res. 1966;16:429–481. doi: 10.1016/s0022-5320(66)80001-1. [DOI] [PubMed] [Google Scholar]
- Kernan MJ. Mechanotransduction and auditory transduction in Drosophila. Pflugers Arch. 2007;454:703–720. doi: 10.1007/s00424-007-0263-x. [DOI] [PubMed] [Google Scholar]
- Kessaris N, Fogarty M, Iannarelli P, Grist M, Wegner M, Richardson WD. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat Neurosci. 2006;9:173–179. doi: 10.1038/nn1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesterson RA, Berbari NF, Pasek RC, Yoder BK. Utilization of conditional alleles to study the role of the primary cilium in obesity. Methods Cell Biol. 2009;94:163–179. doi: 10.1016/S0091-679X(08)94008-5. [DOI] [PubMed] [Google Scholar]
- Kiernan BW, Ffrench-Constant C. Oligodendrocyte precursor (O-2A progenitor cell) migration; a model system for the study of cell migration in the developing central nervous system. Dev Suppl. 1993:219–225. [PubMed] [Google Scholar]
- Kim HR, Richardson J, van Eeden F, Ingham PW. Gli2a protein localization reveals a role for Iguana/DZIP1 in primary ciliogenesis and a dependence of Hedgehog signal transduction on primary cilia in the zebrafish. BMC Biol. 2010;8:65. doi: 10.1186/1741-7007-8-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kato M, Beachy PA. Gli2 trafficking links Hedgehog-dependent activation of Smoothened in the primary cilium to transcriptional activation in the nucleus. Proc Natl Acad Sci U S A. 2009;106:21666–21671. doi: 10.1073/pnas.0912180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleene SJ. Origin of the chloride current in olfactory transduction. Neuron. 1993;11:123–132. doi: 10.1016/0896-6273(93)90276-w. [DOI] [PubMed] [Google Scholar]
- Ko HW, Norman RX, Tran J, Fuller KP, Fukuda M, Eggenschwiler JT. Broad-minded links cell cycle-related kinase to cilia assembly and hedgehog signal transduction. Dev Cell. 18:237–247. doi: 10.1016/j.devcel.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs JJ, Whalen EJ, Liu R, Xiao K, Kim J, Chen M, Wang J, Chen W, Lefkowitz RJ. Beta-arrestin-mediated localization of smoothened to the primary cilium. Science. 2008;320:1777–1781. doi: 10.1126/science.1157983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozminski KG, Beech PL, Rosenbaum JL. The Chlamydomonas kinesin-like protein FLA10 is involved in motility associated with the flagellar membrane. J Cell Biol. 1995;131:1517–1527. doi: 10.1083/jcb.131.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc Natl Acad Sci U S A. 1993;90:5519–5523. doi: 10.1073/pnas.90.12.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA, Louie CM, Silhavy JL, Sintasath L, Decambre M, Nigam SK, Willert K, Gleeson JG. Impaired Wnt-beta-catenin signaling disrupts adult renal homeostasis and leads to cystic kidney ciliopathy. Nat Med. 2009;15:1046–1054. doi: 10.1038/nm.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA, Gleeson JG. The primary cilium as a cellular signaling center: lessons from disease. Curr Opin Genet Dev. 2009;19:220–229. doi: 10.1016/j.gde.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BH, Ashrafi K. A TRPV channel modulates C. elegans neurosecretion, larval starvation survival, and adult lifespan. PLoS Genet. 2008;4:e1000213. doi: 10.1371/journal.pgen.1000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman JM, Michaud EJ, Schoeb TR, Aydin-Son Y, Miller M, Yoder BK. The Oak Ridge Polycystic Kidney mouse: modeling ciliopathies of mice and men. Dev Dyn. 2008;237:1960–1971. doi: 10.1002/dvdy.21515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinders-Zufall T, Rand MN, Shepherd GM, Greer CA, Zufall F. Calcium entry through cyclic nucleotide-gated channels in individual cilia of olfactory receptor cells: spatiotemporal dynamics. J Neurosci. 1997;17:4136–4148. doi: 10.1523/JNEUROSCI.17-11-04136.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem KF, Jr, He M, Ocbina PJ, Anderson KV. Mouse Kif7/Costal2 is a cilia-associated protein that regulates Sonic hedgehog signaling. Proc Natl Acad Sci U S A. 2009;106:13377–13382. doi: 10.1073/pnas.0906944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, Wang B, Niswander LA. Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development. 2005a;132:3103–3111. doi: 10.1242/dev.01894. Physiol289, F978-88. [DOI] [PubMed] [Google Scholar]
- Luby-Phelps K, Fogerty J, Baker SA, Pazour GJ, Besharse JC. Spatial distribution of intraflagellar transport proteins in vertebrate photoreceptors. Vision Res. 2008;48:413–423. doi: 10.1016/j.visres.2007.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion V, Stoetzel C, Schlicht D, Messaddeq N, Koch M, Flori E, Danse JM, Mandel JL, Dollfus H. Transient ciliogenesis involving Bardet-Biedl syndrome proteins is a fundamental characteristic of adipogenic differentiation. Proc Natl Acad Sci U S A. 2009;106:1820–1825. doi: 10.1073/pnas.0812518106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May SR, Ashique AM, Karlen M, Wang B, Shen Y, Zarbalis K, Reiter J, Ericson J, Peterson AS. Loss of the retrograde motor for IFT disrupts localization of Smo to cilia and prevents the expression of both activator and repressor functions of Gli. Dev Biol. 2005;287:378–389. doi: 10.1016/j.ydbio.2005.08.050. [DOI] [PubMed] [Google Scholar]
- May-Simera HL, Ross A, Rix S, Forge A, Beales PL, Jagger DJ. Patterns of expression of Bardet-Biedl syndrome proteins in the mammalian cochlea suggest noncentrosomal functions. J Comp Neurol. 2009;514:174–188. doi: 10.1002/cne.22001. [DOI] [PubMed] [Google Scholar]
- Mazelova J, Astuto-Gribble L, Inoue H, Tam BM, Schonteich E, Prekeris R, Moritz OL, Randazzo PA, Deretic D. Ciliary targeting motif VxPx directs assembly of a trafficking module through Arf4. EMBO J. 2009;28:183–192. doi: 10.1038/emboj.2008.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MR, Angele A, Kremmer E, Kaupp UB, Muller F. A cGMP-signaling pathway in a subset of olfactory sensory neurons. Proc Natl Acad Sci U S A. 2000;97:10595–10600. doi: 10.1073/pnas.97.19.10595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milenkovic L, Scott MP. Not lost in space: trafficking in the hedgehog signaling pathway. Sci Signal. 2010;3:14. doi: 10.1126/scisignal.3117pe14. [DOI] [PubMed] [Google Scholar]
- Mirzadeh Z, Han YG, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Cilia organize ependymal planar polarity. J Neurosci. 2010;30:2600–2610. doi: 10.1523/JNEUROSCI.3744-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz OL, Tam BM, Hurd LL, Peranen J, Deretic D, Papermaster DS. Mutant rab8 Impairs docking and fusion of rhodopsin-bearing post-Golgi membranes and causes cell death of transgenic Xenopus rods. Mol Biol Cell. 2001;12:2341–2351. doi: 10.1091/mbc.12.8.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita K, Kawate T, Kakinuma N, Takeda S. Multiple primary cilia modulate the fluid transcytosis in choroid plexus epithelium. Traffic. 2009;11:287–301. doi: 10.1111/j.1600-0854.2009.01016.x. [DOI] [PubMed] [Google Scholar]
- Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- Nordman S, Abulaiti A, Hilding A, Langberg EC, Humphreys K, Ostenson CG, Efendic S, Gu HF. Genetic variation of the adenylyl cyclase 3 (AC3) locus and its influence on type 2 diabetes and obesity susceptibility in Swedish men. Int J Obes (Lond) 2008;32:407–412. doi: 10.1038/sj.ijo.0803742. [DOI] [PubMed] [Google Scholar]
- Ocbina PJ, Tuson M, Anderson KV. Primary cilia are not required for normal canonical Wnt signaling in the mouse embryo. PLoS ONE. 2009;4:e6839. doi: 10.1371/journal.pone.0006839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palay SL. The fine structure of secretory neurons in the preoptic nucleus of the goldfish. Anat. Rec. 1961;139 doi: 10.1002/ar.1091380404. [DOI] [PubMed] [Google Scholar]
- Park TJ, Mitchell BJ, Abitua PB, Kintner C, Wallingford JB. Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat Genet. 2008;40:871–879. doi: 10.1038/ng.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Baker SA, Deane JA, Cole DG, Dickert BL, Rosenbaum JL, Witman GB, Besharse JC. The intraflagellar transport protein, IFT88, is essential for vertebrate photoreceptor assembly and maintenance. J Cell Biol. 2002;157:103–113. doi: 10.1083/jcb.200107108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, Cole DG. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol. 2000;151:709–718. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen LB, Rosenbaum JL. Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr Top Dev Biol. 2008;85:23–61. doi: 10.1016/S0070-2153(08)00802-8. [DOI] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster HD. The Fine Structure of the Nervous System. Oxford: Oxford University Press; 1976. [Google Scholar]
- Peters A, Palay SL, Webster HD. The Fine Structure of the Nervous System: Neurons and Their Supporting Cells. 3rd edn. New York: Oxford University Press; 1991. [Google Scholar]
- Poretti A, Boltshauser E, Loenneker T, Valente EM, Brancati F, Il'yasov K, Huisman TA. Diffusion tensor imaging in Joubert syndrome. AJNR Am J Neuroradiol. 2007;28:1929–1933. doi: 10.3174/ajnr.A0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol. 2001;184:71–79. doi: 10.1007/s00232-001-0075-4. [DOI] [PubMed] [Google Scholar]
- Richardson TM. Cytoplasmic and ciliary connections between the inner and outer segments of mammalian visual receptors. Vision Res. 1969;9:727–731. doi: 10.1016/0042-6989(69)90010-8. [DOI] [PubMed] [Google Scholar]
- Richardson WD, Pringle NP, Yu WP, Hall AC. Origins of spinal cord oligodendrocytes: possible developmental and evolutionary relationships with motor neurons. Dev Neurosci. 1997;19:58–68. doi: 10.1159/000111186. [DOI] [PubMed] [Google Scholar]
- Rink JC, Gurley KA, Elliott SA, Sanchez Alvarado A. Planarian Hh signaling regulates regeneration polarity and links Hh pathway evolution to cilia. Science. 2009;326:1406–1410. doi: 10.1126/science.1178712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelink H, Porter JA, Chiang C, Tanabe Y, Chang DT, Beachy PA, Jessell TM. Floor plate and motor neuron induction by different concentrations of the amino-terminal cleavage product of sonic hedgehog autoproteolysis. Cell. 1995;81:445–455. doi: 10.1016/0092-8674(95)90397-6. [DOI] [PubMed] [Google Scholar]
- Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–376. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- Romano S, Milan G, Veronese C, Collin GB, Marshall JD, Centobene C, Favaretto F, Dal Pra C, Scarda A, Leandri S, et al. Regulation of Alstrom syndrome gene expression during adipogenesis and its relationship with fat cell insulin sensitivity. Int J Mol Med. 2008;21:731–736. [PubMed] [Google Scholar]
- Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002;3:813–825. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- Ross AJ, May-Simera H, Eichers ER, Kai M, Hill J, Jagger DJ, Leitch CC, Chapple JP, Munro PM, Fisher S, et al. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet. 2005;37:1135–1140. doi: 10.1038/ng1644. [DOI] [PubMed] [Google Scholar]
- Satir P, Christensen ST. Structure and function of mammalian cilia. Histochem Cell Biol. 2008;129:687–693. doi: 10.1007/s00418-008-0416-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamoto K, Wichterle H, Gonzalez-Perez O, Cholfin JA, Yamada M, Spassky N, Murcia NS, Garcia-Verdugo JM, Marin O, Rubenstein JL, et al. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science. 2006;311:629–632. doi: 10.1126/science.1119133. [DOI] [PubMed] [Google Scholar]
- Schimmang T, Lemaistre M, Vortkamp A, Ruther U. Expression of the zinc finger gene Gli3 is affected in the morphogenetic mouse mutant extra-toes (Xt) Development. 1992;116:799–804. doi: 10.1242/dev.116.3.799. [DOI] [PubMed] [Google Scholar]
- Schneider L, Cammer M, Lehman J, Nielsen SK, Guerra CF, Veland IR, Stock C, Hoffmann EK, Yoder BK, Schwab A, et al. Directional cell migration and chemotaxis in wound healing response to PDGF-AA are coordinated by the primary cilium in fibroblasts. Cell Physiol Biochem. 2010;25:279–292. doi: 10.1159/000276562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider L, Clement CA, Teilmann SC, Pazour GJ, Hoffmann EK, Satir P, Christensen ST. PDGFRalphaalpha signaling is regulated through the primary cilium in fibroblasts. Curr Biol. 2005;15:1861–1866. doi: 10.1016/j.cub.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Seeley ES, Nachury MV. The perennial organelle: assembly and disassembly of the primary cilium. J Cell Sci. 2010;123:511–518. doi: 10.1242/jcs.061093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen Gupta P, Prodromou NV, Chapple JP. Can faulty antennae increase adiposity? The link between cilia proteins and obesity. J Endocrinol. 2009;203:327–336. doi: 10.1677/JOE-09-0116. [DOI] [PubMed] [Google Scholar]
- Seo S, Guo DF, Bugge K, Morgan DA, Rahmouni K, Sheffield VC. Requirement of Bardet-Biedl syndrome proteins for leptin receptor signaling. Hum Mol Genet. 2009;18:1323–1331. doi: 10.1093/hmg/ddp031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Berbari NF, Yoder BK. Ciliary dysfunction in developmental abnormalities and diseases. Curr Top Dev Biol. 2008;85:371–427. doi: 10.1016/S0070-2153(08)00813-2. [DOI] [PubMed] [Google Scholar]
- Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, Schermer B, Benzing T, Cabello OA, Jenny A, et al. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet. 2005;37:537–543. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuller U, Heine VM, Mao J, Kho AT, Dillon AK, Han YG, Huillard E, Sun T, Ligon AH, Qian Y, et al. Acquisition of granule neuron precursor identity is a critical determinant of progenitor cell competence to form Shh-induced medulloblastoma. Cancer Cell. 2008;14:123–134. doi: 10.1016/j.ccr.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloboda RD, Rosenbaum JL. Making sense of cilia and flagella. J Cell Biol. 2007;179:575–582. doi: 10.1083/jcb.200709039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin SP. Reconstructions of centriole formation and ciliogenesis in mammalian lungs. J Cell Sci. 1968;3:207–230. doi: 10.1242/jcs.3.2.207. [DOI] [PubMed] [Google Scholar]
- Sotelo JR, Trujillo-Cenoz O. Electron microscope study on the development of ciliary components of the neural epithelium of the chick embryo. Z Zellforsch Mikrosk Anat. 1958;49:1–12. doi: 10.1007/BF00335059. [DOI] [PubMed] [Google Scholar]
- Spassky N, Merkle FT, Flames N, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J Neurosci. 2005;25:10–18. doi: 10.1523/JNEUROSCI.1108-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassky N, Han YG, Aguilar A, Strehl L, Besse L, Laclef C, Ros MR, Garcia-Verdugo JM, Alvarez-Buylla A. Primary cilia are required for cerebellar development and Shh-dependent expansion of progenitor pool. Dev Biol. 2008;317:246–259. doi: 10.1016/j.ydbio.2008.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanic D, Malmgren H, He H, Scott L, Aperia A, Hokfelt T. Developmental changes in frequency of the ciliary somatostatin receptor 3 protein. Brain Res. 2009;1249:101–112. doi: 10.1016/j.brainres.2008.10.024. [DOI] [PubMed] [Google Scholar]
- Stottmann RW, Tran PV, Turbe-Doan A, Beier DR. Ttc21b is required to restrict sonic hedgehog activity in the developing mouse forebrain. Dev Biol. 2009;335:166–178. doi: 10.1016/j.ydbio.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Kurahashi T. Photolysis of caged cyclic AMP in the ciliary cytoplasm of the newt olfactory receptor cell. J Physiol. 2002;541:825–833. doi: 10.1113/jphysiol.2002.016600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theil T, Alvarez-Bolado G, Walter A, Ruther U. Gli3 is required for Emx gene expression during dorsal telencephalon development. Development. 1999;126:3561–3571. doi: 10.1242/dev.126.16.3561. [DOI] [PubMed] [Google Scholar]
- Tissir F, Goffinet AM. Planar cell polarity signaling in neural development. Curr Opin Neurobiol. 2010;20:572–577. doi: 10.1016/j.conb.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Tissir F, Qu Y, Montcouquiol M, Zhou L, Komatsu K, Shi D, Fujimori T, Labeau J, Tyteca D, Courtoy P, et al. Lack of cadherins Celsr2 and Celsr3 impairs ependymal ciliogenesis, leading to fatal hydrocephalus. Nat Neurosci. 2010;13:700–707. doi: 10.1038/nn.2555. [DOI] [PubMed] [Google Scholar]
- Tobin D, Madsen D, Kahn-Kirby A, Peckol E, Moulder G, Barstead R, Maricq A, Bargmann C. Combinatorial expression of TRPV channel proteins defines their sensory functions and subcellular localization in C. elegans neurons. Neuron. 2002;35:307–318. doi: 10.1016/s0896-6273(02)00757-2. [DOI] [PubMed] [Google Scholar]
- Tole S, Ragsdale CW, Grove EA. Dorsoventral patterning of the telencephalon is disrupted in the mouse mutant extra-toes J. Developmental Biology. 2000;217:254–265. doi: 10.1006/dbio.1999.9509. [DOI] [PubMed] [Google Scholar]
- Town T, Breunig JJ, Sarkisian MR, Spilianakis C, Ayoub AE, Liu X, Ferrandino AF, Gallagher AR, Li MO, Rakic P, et al. The stumpy gene is required for mammalian ciliogenesis. Proc Natl Acad Sci U S A. 2008;105:2853–2858. doi: 10.1073/pnas.0712385105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran PV, Haycraft CJ, Besschetnova TY, Turbe-Doan A, Stottmann RW, Herron BJ, Chesebro AL, Qiu H, Scherz PJ, Shah JV, et al. THM1 negatively modulates mouse sonic hedgehog signal transduction and affects retrograde intraflagellar transport in cilia. Nat Genet. 2008;40:403–410. doi: 10.1038/ng.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujikawa M, Malicki J. Intraflagellar transport genes are essential for differentiation and survival of vertebrate sensory neurons. Neuron. 2004;42:703–716. doi: 10.1016/s0896-6273(04)00268-5. [DOI] [PubMed] [Google Scholar]
- Veland IR, Awan A, Pedersen LB, Yoder BK, Christensen ST. Primary cilia and signaling pathways in mammalian development, health and disease. Nephron Physiol. 2009;111:p39–p53. doi: 10.1159/000208212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace VA. Purkinje-cell-derived Sonic hedgehog regulates granule neuron precursor cell proliferation in the developing mouse cerebellum. Curr Biol. 1999;9:445–448. doi: 10.1016/s0960-9822(99)80195-x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhou Z, Walsh CT, McMahon AP. Selective translocation of intracellular Smoothened to the primary cilium in response to Hedgehog pathway modulation. Proc Natl Acad Sci U S A. 2009a;106:2623–2628. doi: 10.1073/pnas.0812110106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Li V, Chan GC, Phan T, Nudelman AS, Xia Z, Storm DR. Adult type 3 adenylyl cyclase-deficient mice are obese. PLoS ONE. 2009b;4:e6979. doi: 10.1371/journal.pone.0006979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe D, Saijoh Y, Nonaka S, Sasaki G, Ikawa Y, Yokoyama T, Hamada H. The left-right determinant Inversin is a component of node monocilia and other 9+0 cilia. Development. 2003;130:1725–1734. doi: 10.1242/dev.00407. [DOI] [PubMed] [Google Scholar]
- Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron. 1999;22:103–114. doi: 10.1016/s0896-6273(00)80682-0. [DOI] [PubMed] [Google Scholar]
- Wheatley DN. The Centriole: A Central Enigma of Cell Biology. Amsterdam: Elsevier Biomedical Press; 1982. [Google Scholar]
- Willaredt MA, Hasenpusch-Theil K, Gardner HA, Kitanovic I, Hirschfeld-Warneken VC, Gojak CP, Gorgas K, Bradford CL, Spatz J, Wolfl S, et al. A crucial role for primary cilia in cortical morphogenesis. J Neurosci. 2008;28:12887–12900. doi: 10.1523/JNEUROSCI.2084-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong ST, Trinh K, Hacker B, Chan GC, Lowe G, Gaggar A, Xia Z, Gold GH, Storm DR. Disruption of the type III adenylyl cyclase gene leads to peripheral and behavioral anosmia in transgenic mice. Neuron. 2000;27:487–497. doi: 10.1016/s0896-6273(00)00060-x. [DOI] [PubMed] [Google Scholar]
- Woodruff RH, Tekki-Kessaris N, Stiles CD, Rowitch DH, Richardson WD. Oligodendrocyte development in the spinal cord and telencephalon: common themes and new perspectives. Int J Dev Neurosci. 2001;19:379–385. doi: 10.1016/s0736-5748(00)00083-6. [DOI] [PubMed] [Google Scholar]
- Yam PT, Langlois SD, Morin S, Charron F. Sonic hedgehog guides axons through a noncanonical, Src-family-kinase-dependent signaling pathway. Neuron. 2009;62:349–362. doi: 10.1016/j.neuron.2009.03.022. [DOI] [PubMed] [Google Scholar]
- Zappaterra MD, Lisgo SN, Lindsay S, Gygi SP, Walsh CA, Ballif BA. A comparative proteomic analysis of human and rat embryonic cerebrospinal fluid. J Proteome Res. 2007;6:3537–3548. doi: 10.1021/pr070247w. [DOI] [PubMed] [Google Scholar]
- Zhang W, Zhao Y, Tong C, Wang G, Wang B, Jia J, Jiang J. Hedgehog-regulated Costal2-kinase complexes control phosphorylation and proteolytic processing of Cubitus interruptus. Dev Cell. 2005;8:267–278. doi: 10.1016/j.devcel.2005.01.001. [DOI] [PubMed] [Google Scholar]