Abstract
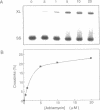
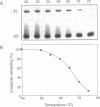
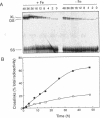
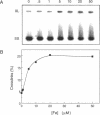
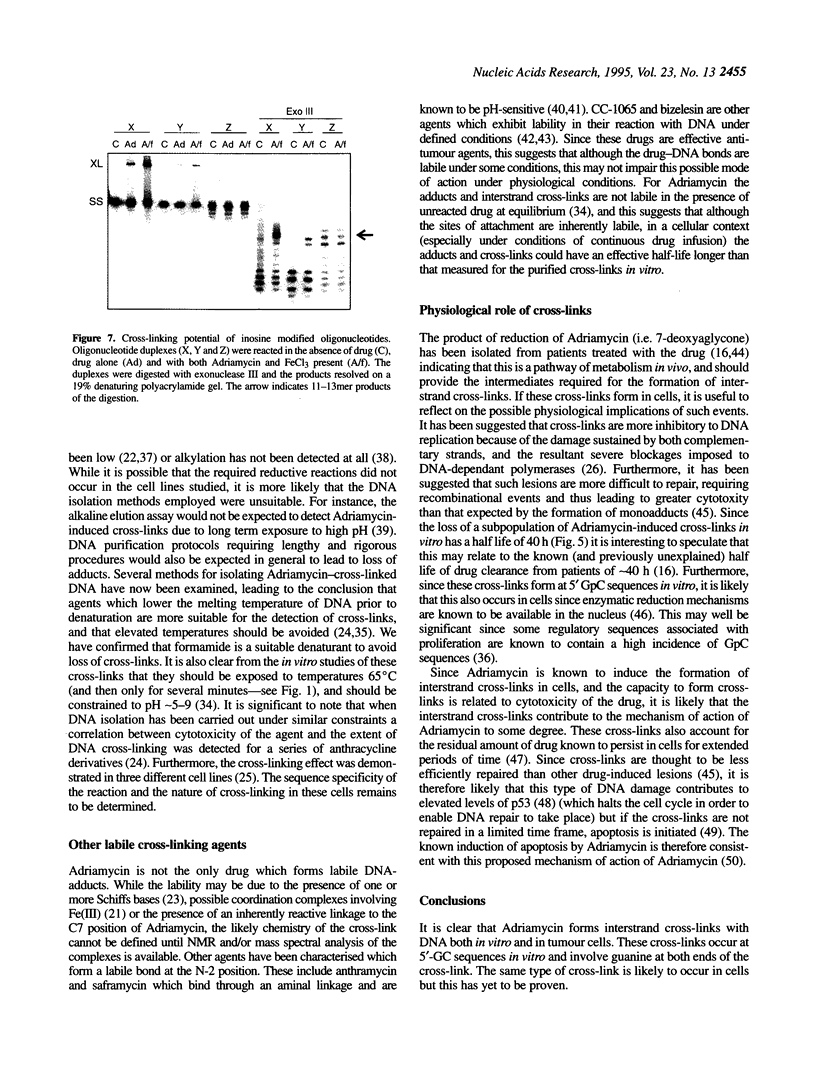
It has been known for several years that Adriamycin forms adducts and interstrand cross-links when reacted for long periods of time with bacterial and mammalian DNA in vitro, with the cross-link being restricted to 2 bp elements containing GpC sequences. The self-complementary 20mer deoxyoligonucleotide TA4T4GCA4T4A has been used in this study as a model of the apparent G-G cross-linking site at GpC sequences. The rate of formation of cross-links, as well as the dependence on both Adriamycin and Fe(III) concentration, were similar with this oligonucleotide as compared with calf thymus DNA. The cross-linking was demonstrated on both denaturing and non-denaturing sequencing gels. The half-life of the G-G cross-link was 40 h, consistent with that implied with high molecular weight, heterogeneous sequence DNA. Exonuclease III digests of adducts formed with 20mer deoxyoligonucleotides containing single, central G-G, G-I and I-I potential cross-links revealed that a guanine residue is required at both ends of the cross-link. No cross-linking was observed with a similar oligonucleotide containing only a single central (G.C) bp.
Full text
PDF

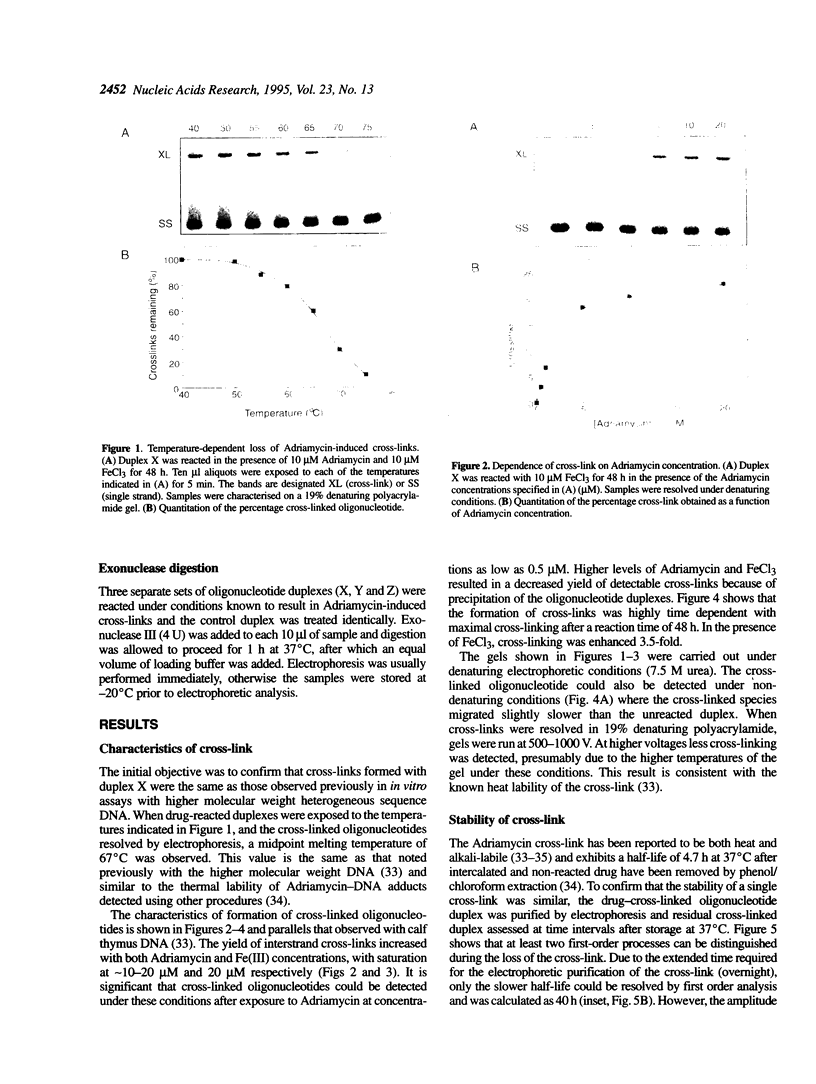

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
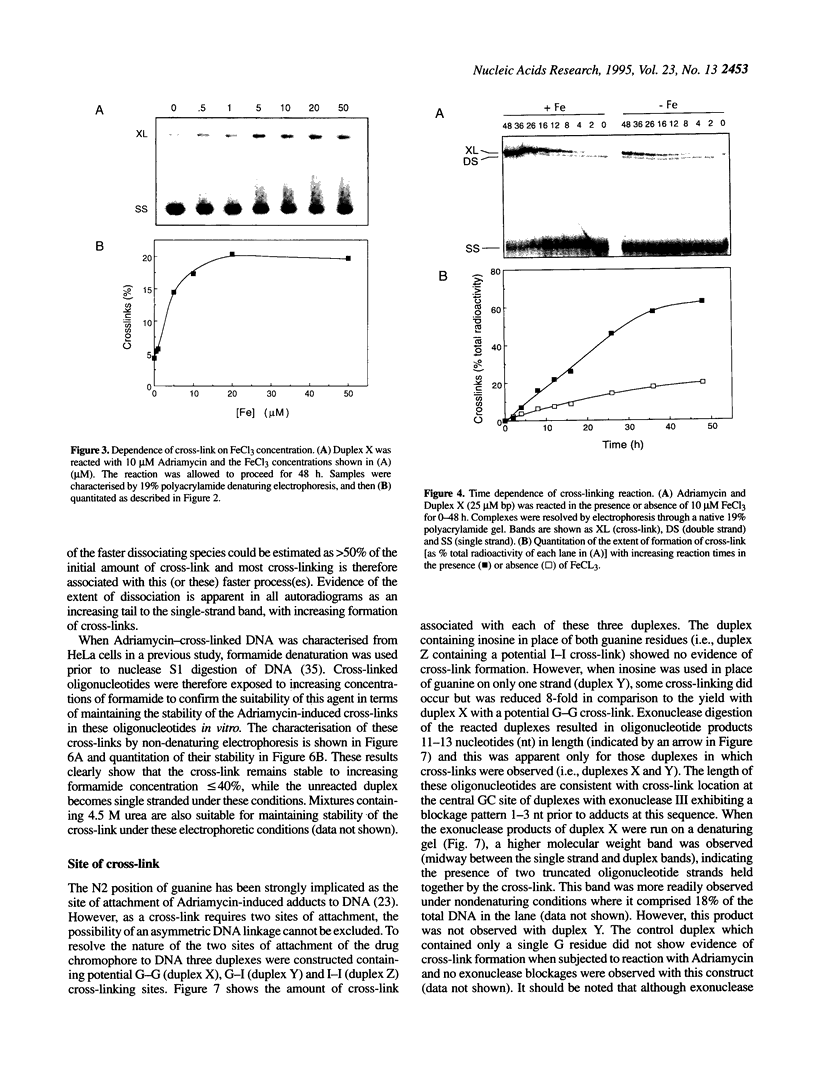
- Bachur N. R., Gordon S. L., Gee M. V., Kon H. NADPH cytochrome P-450 reductase activation of quinone anticancer agents to free radicals. Proc Natl Acad Sci U S A. 1979 Feb;76(2):954–957. doi: 10.1073/pnas.76.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoszek A., Wolf C. R. Enhancement of doxorubicin toxicity following activation by NADPH cytochrome P450 reductase. Biochem Pharmacol. 1992 Apr 1;43(7):1449–1457. doi: 10.1016/0006-2952(92)90201-s. [DOI] [PubMed] [Google Scholar]
- Binaschi M., Capranico G., De Isabella P., Mariani M., Supino R., Tinelli S., Zunino F. Comparison of DNA cleavage induced by etoposide and doxorubicin in two human small-cell lung cancer lines with different sensitivities to topoisomerase II inhibitors. Int J Cancer. 1990 Feb 15;45(2):347–352. doi: 10.1002/ijc.2910450223. [DOI] [PubMed] [Google Scholar]
- Capranico G., De Isabella P., Penco S., Tinelli S., Zunino F. Role of DNA breakage in cytotoxicity of doxorubicin, 9-deoxydoxorubicin, and 4-demethyl-6-deoxydoxorubicin in murine leukemia P388 cells. Cancer Res. 1989 Apr 15;49(8):2022–2027. [PubMed] [Google Scholar]
- Capranico G., Zunino F. DNA topoisomerase-trapping antitumour drugs. Eur J Cancer. 1992;28A(12):2055–2060. doi: 10.1016/0959-8049(92)90255-z. [DOI] [PubMed] [Google Scholar]
- Coley H. M., Amos W. B., Twentyman P. R., Workman P. Examination by laser scanning confocal fluorescence imaging microscopy of the subcellular localisation of anthracyclines in parent and multidrug resistant cell lines. Br J Cancer. 1993 Jun;67(6):1316–1323. doi: 10.1038/bjc.1993.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinane C., Cutts S. M., van Rosmalen A., Phillips D. R. Formation of adriamycin--DNA adducts in vitro. Nucleic Acids Res. 1994 Jun 25;22(12):2296–2303. doi: 10.1093/nar/22.12.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinane C., Phillips D. R. Induction of stable transcriptional blockage sites by adriamycin: GpC specificity of apparent adriamycin-DNA adducts and dependence on iron(III) ions. Biochemistry. 1990 Jun 12;29(23):5638–5646. doi: 10.1021/bi00475a032. [DOI] [PubMed] [Google Scholar]
- Cullinane C., van Rosmalen A., Phillips D. R. Does adriamycin induce interstrand cross-links in DNA? Biochemistry. 1994 Apr 19;33(15):4632–4638. doi: 10.1021/bi00181a025. [DOI] [PubMed] [Google Scholar]
- Cummings J., Allan L., Willmott N., Riley R., Workman P., Smyth J. F. The enzymology of doxorubicin quinone reduction in tumour tissue. Biochem Pharmacol. 1992 Dec 1;44(11):2175–2183. doi: 10.1016/0006-2952(92)90344-i. [DOI] [PubMed] [Google Scholar]
- Cummings J., Morrison J. G., Willmott N. Determination of anthracycline purity in patient samples and identification of in vitro chemical reduction products by application of a multi-diode array high-speed spectrophotometric detector. J Chromatogr. 1986 Sep 5;381(2):373–384. doi: 10.1016/s0378-4347(00)83603-8. [DOI] [PubMed] [Google Scholar]
- Cummings J., Willmott N., Hoey B. M., Marley E. S., Smyth J. F. The consequences of doxorubicin quinone reduction in vivo in tumour tissue. Biochem Pharmacol. 1992 Dec 1;44(11):2165–2174. doi: 10.1016/0006-2952(92)90343-h. [DOI] [PubMed] [Google Scholar]
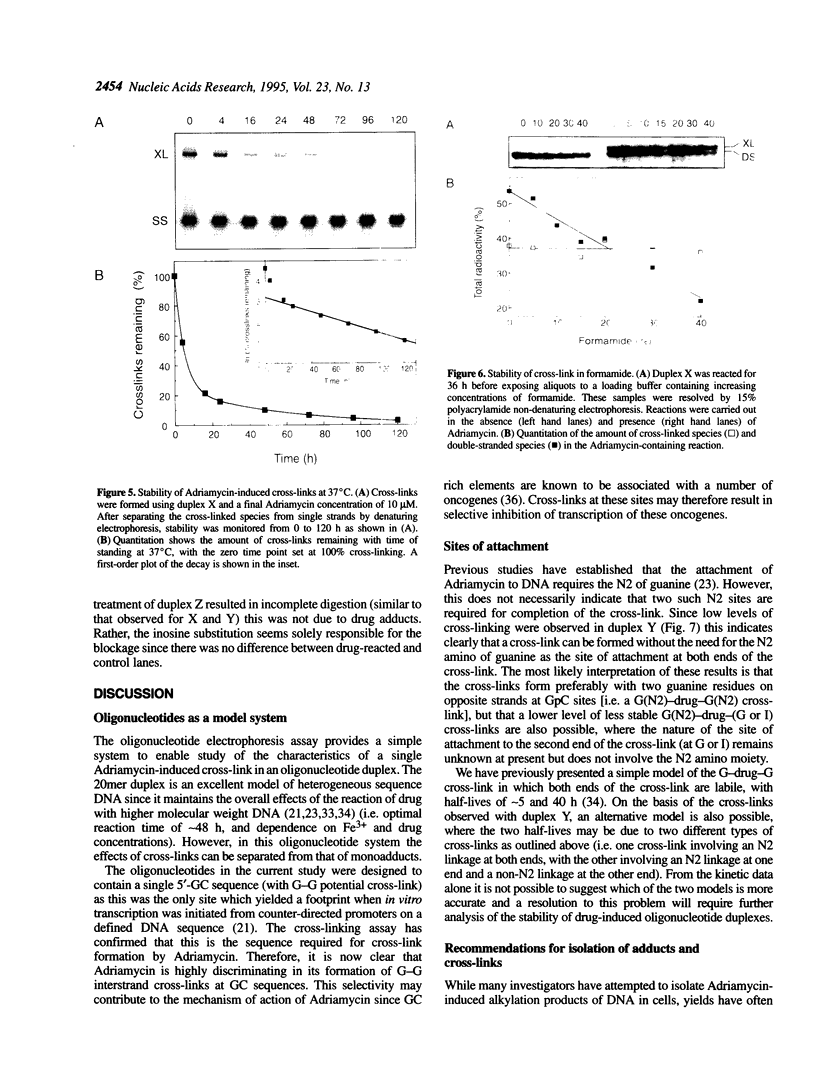
- Frezard F., Garnier-Suillerot A. DNA-containing liposomes as a model for the study of cell membrane permeation by anthracycline derivatives. Biochemistry. 1991 May 21;30(20):5038–5043. doi: 10.1021/bi00234a028. [DOI] [PubMed] [Google Scholar]
- Gigli M., Doglia S. M., Millot J. M., Valentini L., Manfait M. Quantitative study of doxorubicin in living cell nuclei by microspectrofluorometry. Biochim Biophys Acta. 1988 May 6;950(1):13–20. doi: 10.1016/0167-4781(88)90068-1. [DOI] [PubMed] [Google Scholar]
- Hall P. A., McKee P. H., Menage H. D., Dover R., Lane D. P. High levels of p53 protein in UV-irradiated normal human skin. Oncogene. 1993 Jan;8(1):203–207. [PubMed] [Google Scholar]
- Hartley J. A., Souhami R. L., Berardini M. D. Electrophoretic and chromatographic separation methods used to reveal interstrand crosslinking of nucleic acids. J Chromatogr. 1993 Aug 25;618(1-2):277–288. doi: 10.1016/0378-4347(93)80038-6. [DOI] [PubMed] [Google Scholar]
- Hurley L. H., Allen C. S., Feola J. M., Lubawy W. C. In vitro and in vivo stability of anthramycin-DNA conjugate and its potential application as an anthramycin prodrug. Cancer Res. 1979 Aug;39(8):3134–3140. [PubMed] [Google Scholar]
- Kappus H. Overview of enzyme systems involved in bio-reduction of drugs and in redox cycling. Biochem Pharmacol. 1986 Jan 1;35(1):1–6. doi: 10.1016/0006-2952(86)90544-7. [DOI] [PubMed] [Google Scholar]
- Konopa J. Adriamycin and daunomycin induce interstrand DNA crosslinks in Hela S3 Cells. Biochem Biophys Res Commun. 1983 Feb 10;110(3):819–826. doi: 10.1016/0006-291x(83)91035-5. [DOI] [PubMed] [Google Scholar]
- Lane D. P. Cancer. p53, guardian of the genome. Nature. 1992 Jul 2;358(6381):15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- Lane P., Vichi P., Bain D. L., Tritton T. R. Temperature dependence studies of adriamycin uptake and cytotoxicity. Cancer Res. 1987 Aug 1;47(15):4038–4042. [PubMed] [Google Scholar]
- Lee C. S., Gibson N. W. DNA interstrand cross-links induced by the cyclopropylpyrroloindole antitumor agent bizelesin are reversible upon exposure to alkali. Biochemistry. 1993 Sep 7;32(35):9108–9114. doi: 10.1021/bi00086a015. [DOI] [PubMed] [Google Scholar]
- Lee M., Rhodes A. L., Wyatt M. D., Forrow S., Hartley J. A. Design, synthesis, and biological evaluation of DNA sequence and minor groove selective alkylating agents. Anticancer Drug Des. 1993 Jun;8(3):173–192. [PubMed] [Google Scholar]
- Liu L. F. DNA topoisomerase poisons as antitumor drugs. Annu Rev Biochem. 1989;58:351–375. doi: 10.1146/annurev.bi.58.070189.002031. [DOI] [PubMed] [Google Scholar]
- Millard J. T., Weidner M. F., Kirchner J. J., Ribeiro S., Hopkins P. B. Sequence preferences of DNA interstrand crosslinking agents: quantitation of interstrand crosslink locations in DNA duplex fragments containing multiple crosslinkable sites. Nucleic Acids Res. 1991 Apr 25;19(8):1885–1891. doi: 10.1093/nar/19.8.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimnaugh E. G., Dusre L., Atwell J., Myers C. E. Differential oxygen radical susceptibility of adriamycin-sensitive and -resistant MCF-7 human breast tumor cells. Cancer Res. 1989 Jan 1;49(1):8–15. [PubMed] [Google Scholar]
- Moore H. W. Bioactivation as a model for drug design bioreductive alkylation. Science. 1977 Aug 5;197(4303):527–532. doi: 10.1126/science.877572. [DOI] [PubMed] [Google Scholar]
- Mustonen P., Kinnunen P. K. On the reversal by deoxyribonucleic acid of the binding of adriamycin to cardiolipin-containing liposomes. J Biol Chem. 1993 Jan 15;268(2):1074–1080. [PubMed] [Google Scholar]
- Myers C. E., McGuire W. P., Liss R. H., Ifrim I., Grotzinger K., Young R. C. Adriamycin: the role of lipid peroxidation in cardiac toxicity and tumor response. Science. 1977 Jul 8;197(4299):165–167. doi: 10.1126/science.877547. [DOI] [PubMed] [Google Scholar]
- Petrusek R. L., Anderson G. L., Garner T. F., Fannin Q. L., Kaplan D. J., Zimmer S. G., Hurley L. H. Pyrrol[1,4]benzodiazepine antibiotics. Proposed structures and characteristics of the in vitro deoxyribonucleic acid adducts of anthramycin, tomaymycin, sibiromycin, and neothramycins A and B. Biochemistry. 1981 Mar 3;20(5):1111–1119. doi: 10.1021/bi00508a011. [DOI] [PubMed] [Google Scholar]
- Ross W. E., Glaubiger D. L., Kohn K. W. Protein-associated DNA breaks in cells treated with adriamycin or ellipticine. Biochim Biophys Acta. 1978 Jun 22;519(1):23–30. doi: 10.1016/0005-2787(78)90059-x. [DOI] [PubMed] [Google Scholar]
- Sancar A., Sancar G. B. DNA repair enzymes. Annu Rev Biochem. 1988;57:29–67. doi: 10.1146/annurev.bi.57.070188.000333. [DOI] [PubMed] [Google Scholar]
- Sinha B. K., Chignell C. F. Binding mode of chemically activated semiquinone free radicals from quinone anticancer agents to DNA. Chem Biol Interact. 1979 Dec;28(2-3):301–308. doi: 10.1016/0009-2797(79)90170-4. [DOI] [PubMed] [Google Scholar]
- Sinha B. K., Gregory J. L. Role of one-electron and two-electron reduction products of adriamycin and daunomycin in deoxyribonucleic acid binding. Biochem Pharmacol. 1981 Sep 15;30(18):2626–2629. doi: 10.1016/0006-2952(81)90594-3. [DOI] [PubMed] [Google Scholar]
- Sinha B. K., Sik R. H. Binding of [14C]-adriamycin to cellular macromolecules in vivo. Biochem Pharmacol. 1980 Jun 15;29(12):1867–1868. doi: 10.1016/0006-2952(80)90156-2. [DOI] [PubMed] [Google Scholar]
- Sip M., Schwartz A., Vovelle F., Ptak M., Leng M. Distortions induced in DNA by cis-platinum interstrand adducts. Biochemistry. 1992 Mar 10;31(9):2508–2513. doi: 10.1021/bi00124a010. [DOI] [PubMed] [Google Scholar]
- Skladanowski A., Konopa J. Adriamycin and daunomycin induce programmed cell death (apoptosis) in tumour cells. Biochem Pharmacol. 1993 Aug 3;46(3):375–382. doi: 10.1016/0006-2952(93)90512-u. [DOI] [PubMed] [Google Scholar]
- Skladanowski A., Konopa J. Interstrand DNA crosslinking induced by anthracyclines in tumour cells. Biochem Pharmacol. 1994 Jun 15;47(12):2269–2278. doi: 10.1016/0006-2952(94)90265-8. [DOI] [PubMed] [Google Scholar]
- Skladanowski A., Konopa J. Relevance of interstrand DNA crosslinking induced by anthracyclines for their biological activity. Biochem Pharmacol. 1994 Jun 15;47(12):2279–2287. doi: 10.1016/0006-2952(94)90266-6. [DOI] [PubMed] [Google Scholar]
- Tewey K. M., Rowe T. C., Yang L., Halligan B. D., Liu L. F. Adriamycin-induced DNA damage mediated by mammalian DNA topoisomerase II. Science. 1984 Oct 26;226(4673):466–468. doi: 10.1126/science.6093249. [DOI] [PubMed] [Google Scholar]
- Vichi P., Tritton T. R. Adriamycin: protection from cell death by removal of extracellular drug. Cancer Res. 1992 Aug 1;52(15):4135–4138. [PubMed] [Google Scholar]
- Warpehoski M. A., Harper D. E., Mitchell M. A., Monroe T. J. Reversibility of the covalent reaction of CC-1065 and analogues with DNA. Biochemistry. 1992 Mar 10;31(9):2502–2508. doi: 10.1021/bi00124a009. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Cornwell M. M., Cardarelli C. O., Gottesman M. M., Pastan I. Single cell analysis of daunomycin uptake and efflux in multidrug-resistant and -sensitive KB cells: effects of verapamil and other drugs. Cancer Res. 1986 Nov;46(11):5941–5946. [PubMed] [Google Scholar]
- Zhen W. P., Dahl O., Buchardt O., Nielsen P. E. On the DNA bending by psoralen interstrand crosslinking. A gel electrophoretic study. Photochem Photobiol. 1988 Nov;48(5):643–646. doi: 10.1111/j.1751-1097.1988.tb02875.x. [DOI] [PubMed] [Google Scholar]
- van Rosmalen A., Cullinane C., Cutts S. M., Phillips D. R. Stability of adriamycin-induced DNA adducts and interstrand crosslinks. Nucleic Acids Res. 1995 Jan 11;23(1):42–50. doi: 10.1093/nar/23.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]