Abstract
The Elongator complex has been implicated in several cellular processes, including gene expression and tRNA modification. We investigated the biological importance of the Elp3 gene in Drosophila melanogaster. Deletion of Elp3 results in larval lethality at the pupal stage. During early development, larval growth is dramatically impaired, with progression to the third instar delayed for ∼24 hr, and pupariation occurring only at day 14 after egg laying. Melanotic nodules appear after 4 days. Microarray analysis shows that stress response genes are induced and ecdysone-induced transcription factors are severely repressed in the mutant. Interestingly, the phenotypes of Elp3 flies are similar to those of flies lacking the domino gene, encoding a SWI/SNF-like ATP-dependent chromatin-remodeling enzyme. Indeed, the gene expression profiles of these mutants are also remarkably similar. Together, these data demonstrate that Drosophila Elp3 is essential for viability, normal development, and hematopoiesis and suggest a functional overlap with the chromatin remodeler Domino.
THE Elongator complex was first identified in yeast as a protein complex associated with elongating RNA polymerase II (RNAPII) (Otero et al. 1999) and was later shown to consist of six subunits (Krogan and Greenblatt 2001; Li et al. 2001; Winkler et al. 2001). Particular attention has been paid to the function of the 60-kDa subunit, Elp3, which contains a histone acetyltransferase domain (Wittschieben et al. 1999, 2000), as well as potential SAM-binding and iron-sulfur cluster domains (Chinenov 2002; Paraskevopoulou et al. 2006; Greenwood et al. 2009). Elp3 possesses histone H3 acetylation activity in vitro (Wittschieben et al. 1999; Hawkes et al. 2002; Kim et al. 2002; Winkler et al. 2002) and affects histone acetylation levels in vivo in yeast (Kristjuhan et al. 2002; Winkler et al. 2002) and humans (Close et al. 2006; Chen et al. 2009). Yeast elp deletion mutants initially grow slowly on a variety of media, but then reach growth rates that are similar to wild type. They also show a delay in responding to stress, such as growth at 37° and a high salt environment (Otero et al. 1999; Fellows et al. 2000; Winkler et al. 2001). Furthermore, induction of genes such as INO1, PHO5, and GAL10 is delayed compared to wild type (Otero et al. 1999; Wittschieben et al. 1999). RNA immunoprecipitation has shown that Elp3 binds the nascent mRNA of active genes in yeast (Gilbert et al. 2004), and using chromatin immunoprecipitation (ChIP) Elongator has also been shown to associate with active loci in human cells, especially toward the 3′-end of genes (Metivier et al. 2003; Kouskouti and Talianidis 2005; Close et al. 2006). Together, these features suggest that Elongator participates in RNAPII transcript elongation.
Interestingly, other data have shown that Elongator is also required for modification of the wobble position in tRNA (tRNALys and tRNAGlu) in yeast (Huang et al. 2005; Esberg et al. 2006) and for tubulin acetylation in higher eukaryotes (Creppe et al. 2009; Solinger et al. 2010). Indeed, a high proportion of Elongator is found in the cytoplasm of most cell types (Hawkes et al. 2002; Kim et al. 2002; Pokholok et al. 2002; Rahl et al. 2005), all in all suggesting multiple roles for the complex (reviewed by Svejstrup 2007). Other recent data suggest a role for Elongator also in DNA demethylation (Okada et al. 2010).
The proteins of the Elongator complex are conserved from yeast to humans (Hawkes et al. 2002; Kim et al. 2002). We generated Drosophila strains that lack the highly conserved Elp3 gene (Elp3). Here, we report that Drosophila Elp3 is required for viability. Deletion of the gene thus has a profound effect on larval growth, and Elp3 mutants die at the pupal stage. Moreover, melanotic nodules are formed in third instar larvae, implicating Elp3 in Drosophila innate immune function. Interestingly, we observe that many of the characteristics of elp3 flies are shared with flies lacking the ATP-dependent chromatin remodeler Domino, suggesting functional overlap between Elongator and Domino-mediated chromatin remodeling.
MATERIALS AND METHODS
Drosophila strains:
The EP insertion line EP2367 was obtained from the Szeged Stock Center. The GAL4 driver lines, Act5C-GAL4 (y1 w*; P{Act5C6 GAL4}17bFO1/TM6B, Tb1) and hs-GAL4 (w*; P{GAL4-Hsp70.PB}2/CyO), were obtained from the Bloomington Drosophila Stock Center. The hemocyte-specific GAL4 driver lines He-GAL4 (Zettervall et al. 2004) and Pxn-GAL4 (Stramer et al. 2005) were used. UAS-Elp3 RNAi lines, CG15433R-2 and CG15433R-3, were provided by the National Institute of Genetics (NIG) stock center (Mishima, Japan). The helper transposase line used was P{ry[+t7.2] = Δ2-3}99B.
Excision of Elp3:
The P-element-containing line EP2367 contains a P element inserted 65 bp upstream of the transcription start site of Elp3 (CG15433). Excision was achieved by crossing to the helper transposase line Δ2-3. Individual males were crossed to w1118; CyO/nocSco balancer females, and progeny were assessed for homozygous lethality. Stocks of mutant lines were analyzed by PCR and sequence analysis.
Rescue:
Full-length Elp3 cDNA from the clone RE35395 (ResGen) was cloned into the vector p(UAS-HA) (Parker et al. 2001), and transgenic UAS-Elp3 lines were generated by P-element-mediated transformation. Elp3 expression was achieved by crossing to the Act5C-GAL4 driver line.
Generation of HAT-defective Elp3 strain:
Elp3 cDNA was mutated using the Stratagene QuikChange II site-directed mutagenesis kit to generate a cDNA encoding full-length Elp3 with an Y531F amino acid substitution that inactivates the HAT domain (Wittschieben et al. 2000). The resultant cDNA was cloned into p(UAS-HA), and transgenic UAS-Elp3Y531F lines were generated as above.
RT-PCR:
Wild-type and Elp3 homozygous mutant third instar larvae were homogenized in Trizol buffer, and chloroform extracted. An equal volume of 70% ethanol was added to the supernatant, which was then loaded onto an RNAeasy mini column (Qiagen), and total RNA was purified according to the manufacturer's instructions. DNAse treatment with Turbo DNAse (Ambion) was followed by cDNA synthesis using a Taqman reverse transcription kit (Applied Biosystems). Real-time PCR was performed on either an ABI 7000 or a Bio-Rad IQ real-time PCR machine. RT-PCR primers used were the following: Actin5C 5′ (agcgcggttactctttcacc), Actin5C 3′ (ggccatctcctgctcaaagtc), morgue 5′ (caagtggcagcagcagaaac), morgue 3′ (ttcactagccatccacagcg), E74A 5′ (agatcgagagcctgctgtcc), E74A 3′ (gacgctggcgaagtcatcat), Fbp1 5′ (atgctgttggtggcctttg), Fbp1 3′ (agatcctccaaggacatgcg), Dp110 5′ (cgaaccttacaccgacgaaac), Dp110 3′ (cgctgctaaagctcgttgtg), Myc 5′ (caaatcagcagggagcttcag), Myc 3′ (ttggaatgctggttgagcac), E2F 5′ (gttgataagcgcaagctgca), and E2F 3′ (ccagtcggaatttccagctct).
Northern analysis:
Total RNA was prepared every day as described above from homozygous mutant Elp3 larvae from 5 days after egg laying (AEL) to 14 days AEL. Thirty larvae of the 5- to 10-day-old larvae, and 15 of the 10- to 14-day-old larvae were harvested. RNA was separated on a 1% formaldehyde agarose gel and blotted onto a Hybond N+ membrane (GE Healthcare). The membrane was probed with a Broad Complex core region probe and Actin42A probe. Probes were generated by PCR using the following primers: Br-C 5′ (gagctaacagaggtgcccgt), Br-C 3′ (ttggcttggcgctctgtgag), Actin 5′ (cttaccctgaagtaccccatt), and Actin 3′ (ggcctccatgccgaggaacga). For antimicrobial peptide analysis, RNA was prepared from wandering stage larvae. Probes were synthesized using the following primers: TEP 5′ (ctacatttgatactgcagaaaatatagaac), TEP 3′ (ccgagctcctgtagatatttatattcgcat), DIPT 5′ (ttcttcaattgagaacaactgagatgc), DIPT 3′ (gaagtctgcctcaatgttccgggttaa), DRS 5′ (atgatgcagatcaagtacttgttcgcc), and DRS 3′ (ttagcatccttcgccaccagcacttcag).
Immunofluorescence:
Drosophila hemocytes were isolated from five wandering-stage third instar larvae of each genotype and stained for immunofluorescence as described in Kwon et al. (2008). Lamellocyte-specific mouse MAb L1b (Sinenko et al. 2004) and the pan-hemocyte mouse MAb anti-Hemese (Kurucz et al. 2003) were used at 1:60 dilution. FITC-conjugated secondary antibodies (Jackson ImmunoResearch) were used at 1:1000. Samples were mounted in Vectashield containing DAPI (Vector Laboratories) and observed using a confocal microscope (Zeiss Axiovert 100M).
Microarray analysis:
Wandering-stage wild type, Elp3, and domino (Braun et al. 1997, 1998) larvae were frozen in liquid nitrogen and then homogenized in Trizol (Invitrogen). RNA was purified on RNeasy columns (Qiagen) and analyzed on GeneChip Drosophila Genome 2.0 arrays. The domino and Elp3 data sets were processed separately, performing background correction and quartile normalization and calculating signal intensity estimates using robust multichip analysis (RMA) (Irizarry et al. 2003). Prior to statistical analysis, we preselected informative genes (>0.05 coefficient of variance). Using ANOVA, we selected Elp3-dependent and Domino-dependent changes against wild-type controls. We selected genes using a 0.05 false discovery rate (fdr) threshold. Analysis was carried out using Bioconductor 2.5 (Gentleman et al. 2004).
RESULTS
Isolation and characterization of Drosophila Elp3 mutants:
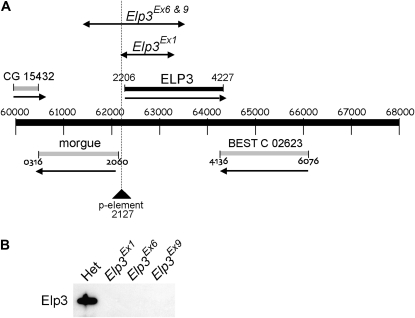
The Drosophila homolog of yeast Elp3 was identified by sequence homology searching. CG15433 (here named Drosophila Elp3) shares a 92% amino acid similarity with human Elp3, diverging mainly at the N terminus (Wittschieben et al. 1999). Elp3 is located at cytological position 24F, and its promoter overlaps that of the oppositely transcribed morgue gene. As a first step in generating an Elp3 mutant strain, we searched for P-element insertions in the vicinity of Elp3 and identified EP2367, which contains an EP element inserted 65 bp upstream of the Elp3 transcription start site. The homozygous viable EP2367 line was used as the basis for generating additional imprecise excision deletions of Elp3. Three independent homozygous lethal excision alleles were isolated: Elp3Ex1, Elp3Ex6, and Elp3Ex9. Elp3Ex1 deletes Elp3 from the P-element insertion site to the triplet encoding K277, while both Elp3Ex6 and Elp3Ex9 delete Elp3 to the triplet encoding S377, as well as a substantial part of the flanking morgue gene (Figure 1A). Western blot analysis confirmed that Elp3 protein was not detectable in imaginal disc and brain extracts of these mutants (Figure 1B). Importantly, expression of the flanking gene, morgue, was unaffected in the Elp3Ex1 mutant (Figure 3C). For this reason, Elp3Ex1 was used in all subsequent experiments.
Figure 1.—
Gene deletions in Elp3Ex1 and Elp3Ex6. (A) Map of the 24F region of chromosome 2 and regions deleted in different Elp3 lines. Lines with double arrows designate deletions. The position of the ELP3 gene (solid bar), as well as that of other genes in the region (shaded bars), is indicated. (B) Western blot of larval brain and imaginal disc extracts probed with affinity-purified yeast Elp3 antibody (JSV59).
Figure 3.—
Ecdysone-induced transcription is delayed and reduced in Elp3 mutants. Total RNA was prepared from whole larvae 4 days AEL and daily until pupariation occurred. cDNA either was synthesized for quantitative RT-PCR analysis or RNA (10 μg) was subjected to Northern analysis. (A) E74A transcription is not induced to detectable levels in Elp3 larvae (left), while Fbp1 is induced slowly from 8 days until maximal levels are reached at 12 days AEL (right). The level of transcription is very low compared to the control. Numbers on y-axes (and above bars) are in arbitrary units from RT-PCR. nd, not determined. (B) Northern analysis of Broad Complex transcript. A probe from the core region of BR-C was used. Expression is induced to a high level at 5 days AEL in wild type (lane 1), while very weak induction is observed at 12 days AEL in the mutant (lane 9). (C) Other tested genes are expressed at normal levels in the Elp3 mutant. All numbers obtained from RT-PCRs were first normalized to actin. The expression in Elp3 mutant is shown relative to the level in heterozygotes (dashed line). E74A and Fbp1 (from A) are shown for comparison.
Rescue of Elp3 lethality:
To verify that the lethal phenotype of the ElpEx1 mutant strain was indeed caused by disruption of Elp3, we attempted to rescue lethality by expression of a wild-type Elp3 cDNA using the GAL4-UAS system (Brand and Perrimon 1993). Full-length Drosophila Elp3 cDNA was cloned into the pUASHA vector (Parker et al. 2001), and UAS-Elp3 transgenic lines were generated. Ubiquitous ectopic expression of UAS-Elp3 under the control of the Act5C-GAL4 strain rescued the lethality of Elp3Ex1. However, while males showed normal fertility, the resulting female flies were sterile. It is known that the GAL4-UAS driver used here is not expressed in the female germline (Brand and Perrimon 1993). The sterility of the rescued female flies thus suggests that Elp3 plays a role in the development of the female reproductive system.
Deletion of Elp3 leads to a pronounced delay in larval development:
Homozygous Elp3 larvae showed a profound developmental delay and died during pupariation. By day 5 AEL, a marked difference in size between mutant and heterozygous larvae was apparent (Figure 2A). Progression to the third instar was delayed by ∼24 hr, and larvae remained slow growing. However, by 10 day AEL, a normal size was reached, and at day 14, pupariation occurred. Growth of the larval discs and wing discs was severely impaired (Figure 2, B and C, and data not shown). Salivary glands remained small, and squashes of polytene chromosomes showed that the chromatin was fragmented and fragile, effectively precluding analysis by polytene chromosome spreading.
Figure 2.—
Phenotype of Elp3 mutant larvae. (A) Larvae at 5 days AEL. Heterozygotes are at the wandering stage, but Elp3 larvae remain in the food. Note the presence of melanotic nodules (black spots) already at this stage. (B and C) Wandering-stage Elp3 larvae show poorly developed leg and wing discs. (D) Comparison of 5 days AEL wild-type and 12 days AEL Elp3 larvae, showing melanotic nodules in the mutant larvae.
Ecdysone-induced transcription is delayed and reduced:
The development and progression of larvae to pupae are controlled by pulses of the steroid hormone ecdysone. During the third larval instar, a major ecdysone pulse that initiates transcription of a set of genes required for metamorphosis occurs. Mutation of some of the activated genes, such as Broad Complex (BR-C) and E74, leads to a loss of viability at the pupal stage and to abnormal development of imaginal discs (Kiss et al. 1988; Fletcher and Thummel 1995). Since the Elp3 mutants died at the pupal stage and displayed a failure of disc development, the induction of these ecdysone-induced transcripts was examined. RT-PCR analysis of ecdysone target genes revealed that many were not induced correctly in Elp3 larvae. No induction was apparent until 8–9 days after egg laying, and even at maximal levels, induction was 50- to 100-fold lower than in control larvae (Figure 3A: see right panel inset for detail of delayed and reduced Fbp1 induction in the mutant). Northern analysis of the Broad Complex (BR-C), one of the primary responders to ecdysone, showed that expression of this gene (maximally expressed after 5 days in normal cells) was also delayed and greatly diminished in Elp3 larvae (Figure 3B: compare lane 9 with lane 1). In contrast to the ecdysone-induced genes, many other tested genes were expressed at levels comparable to controls (examples shown in Figure 3C; see also below).
Elp3 larvae develop melanotic nodules:
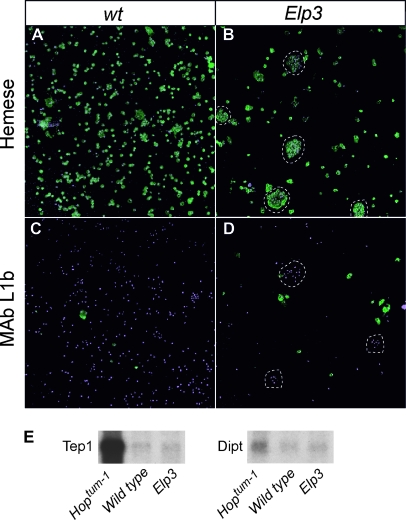
One particularly obvious feature of Elp3 mutant larvae is the appearance of melanotic nodules (Figure 2D). Melanotic nodules are often caused by dysregulated activation of either the Toll pathway (Qiu et al. 1998) or the JAK/STAT pathway, as in the Hopscotch mutant hopTum-1 (Hanratty and Dearolf 1993; Harrison et al. 1995). A number of cellular and molecular features accompany the production of melanotic nodules promoted by these pathways. First, elevated numbers of circulating hemocytes are detected. Second, many of these hemocytes differentiate into an otherwise rare hemocyte type, the lamellocyte. Finally, increases in the expression of antimicrobial peptides are detected, for example, Drosomycin (Drs) and Diptericin (Dipt) in the case of the Toll pathway and the complement-like protein Tep1 in the case of the JAK/STAT pathway (Lagueux et al. 2000). Strikingly, staining of hemocytes in Elp3 larvae with lamellocyte- and hemocyte-specific antibodies showed that neither lamellocyte nor hemocyte numbers were increased. Instead, hemocyte numbers were reduced, and the remaining hemocytes aggregated to form micro-nodules, which were in circulation (Figure 4, A–D). Crystal cell numbers were also unchanged in Elp3 larvae (our unpublished observations). It seems likely that these micro-nodules nucleate the large melanotic nodules observed in the larvae. Antimicrobial peptides do not appear to be strongly induced in Elp3 mutants (Figure 4E), suggesting that the Toll and JAK/STAT pathways are not the primary pathways induced in this case.
Figure 4.—
Hemocytes in Elp3 mutants. Hemocytes from control and Elp3 larvae were stained with (A and B) pan-hemocytic (anti-Hemese) and (C and D) lamellocyte-specific (MAb L1b) antibodies. Antibody staining is revealed in green; DNA stained with DAPI is shown in purple. (E) Northern analysis of expression of thiolester containing protein 1 (Tep1) and Diptericin (Dipt) in control (wild type), Elp3, and hopTum-1 larvae.
Expression of Elp3 RNA interference in hemocytes:
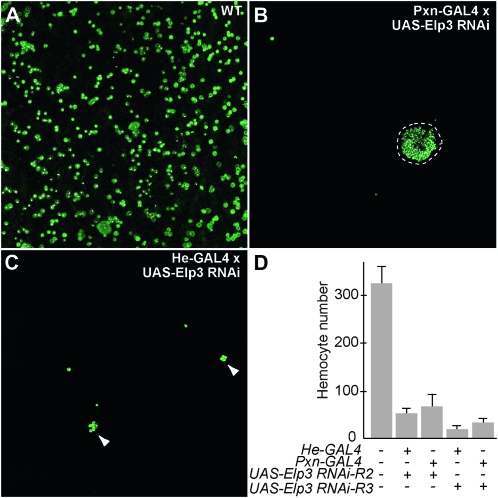
To investigate whether melanotic nodules were caused by disruption of Elp3 in hemocytes or by the loss of Elp3 in other tissues, we specifically ablated Elp3 function in hemocytes by inducible RNA interference (RNAi). UAS-RNAi lines that target Elp3 were obtained from the NIG stock center and crossed with either of two hemocyte-specific GAL4 drivers, Pxn-GAL4 and He-GAL4. Elp3 RNAi reduced hemocyte numbers between 4- and 15-fold (Figure 5D), and resulted in the aggregation of hemocytes (Figure 5, A–C), resembling the hemocyte phenotype observed in ElpEx1 homozygous mutant larvae. This suggests that Elp3 is required autonomously for hemocyte development. However, visible melanotic nodules were not observed in the progeny of the RNAi crosses. The inability of Elp3 RNAi to trigger melanotic nodule formation may be due either to incomplete knockdown of Elp3 or to a lack of expression of the RNAi in all cells involved in the formation of the nodules.
Figure 5.—
Hemocyte-specific expression of Elp3 RNAi reduces hemocyte number and induces cell aggregation. (A) Wild-type hemocytes and hemocytes after (B) Pxn-GAL4 and (C) He-GAL4 directed Elp3 RNAi. (D) Number of hemocytes in hemolymph of control and Elp3 RNAi larvae. Hemocyte numbers are unchanged in backgrounds that contain only the He-GAL4, Pxn-GAL4, or UAS-Elp3-RNAi constructs alone (Zettervall et al. 2004; Stramer et al. 2005; data not shown).
Expression of a HAT-defective Elp3:
Yeast Elp3 mutated at Y540 has dramatically reduced histone acetyltransferase activity (Wittschieben et al. 2000). To assess the contribution of the HAT activity to the Elp3 phenotype in flies, we created a mutant at the corresponding site in the Drosophila protein (Y531F). Induction of the resulting PUAS-Elp3HAT− strain in a wild-type Elp3 background with embryonic GAL4 drivers leads to embryonic lethality (Table 1), while induction by the wing-specific driver MS1096-GAL4 produces abnormal wing phenotypes (supporting information, Figure S1). Induction by a heat-shock driver resulted in delayed development and pupal lethality as in the deletion mutants. The HAT mutant Elp3 therefore acts as a dominant negative. These results indicate that the histone acetyltransferase activity of Elp3 is crucial to its role in development and survival.
TABLE 1.
Expression of an Elp3 HAT-defective mutant by embryonic GAL4 drivers
| Gal4 driver of Elp3 HAT mutant | Phenotype |
|---|---|
| PtcGal4 | Embryonic lethal |
| EngrailedGal4 | Embryonic lethal |
| RhomboidGal4 | Embryonic lethal |
| Actin5CGal4 | Embryonic lethal |
| HairyGal4 | Normal development |
| KruppelGal4 | Normal development |
| ElavGal4 | Normal development |
| MS1096Gal4 | Deformed wings |
| Heat shock | Delayed development and pupal death |
In a wild-type background, expression of an Elp3 HAT point-mutant typically results in lethality. Wing development is severely impaired when the mutant protein is expressed from a wing-specific driver (MS1096-GAL4) (see also Figure S1). Heat-shock induction of the mutant Elp3 at first and second instar stages caused a delay in development and death at late larval stages.
Whole-genome expression profiling of Elp3Ex1 mutant larvae:
To identify pathways that may be affected in Elp3 mutant larvae, microarray analysis of wandering-stage mutant larvae was performed. Although several of the changes observed in the mutant relate to the formation of melanotic nodules, expression of many stress-induced proteins, transcription factors, and neuronal genes was also affected (Table 2; a complete list of significantly affected genes can be found in Table S1). Highly overexpressed transcripts of relevance to the melanotic nodules include the integrins αPS4 and αPS5. These lamellocyte-specific integrins are also overexpressed in hopTum-l and Tl10b larvae (Irving et al. 2005) and are thought to contribute to the adhesion of lamellocytes in the encapsulation event. Similarly, the prophenoloxidase enzyme DoxA3 and serine proteases are highly overexpressed, reflecting the activation of the melanization cascade.
TABLE 2.
Examples of altered gene expression in Elp3 wandering-stage larvae
| Gene Ontology | Fold change |
|---|---|
| Defense | |
| Diphenol OxidaseA3 | 32 |
| IM2 | 28 |
| IM10 | 22 |
| IM1 | 17 |
| Scavenger Receptor Class C | 9 |
| Spheroide | 9 |
| IM23 | 7 |
| CG13422 | 6 |
| Proteolysis | |
| λ-Try | 26 |
| CG30098 | 9 |
| CG16997 | 8 |
| CG31265 | 7 |
| Serine protease immune response integrator | −3 |
| Serine protease inhibitor | −16 |
| Stress | |
| Turandot C | 94 |
| Victoria | 59 |
| Turandot B | 14 |
| Glutathione transferase D5 | 11 |
| Glutathione transferase E6 | 10 |
| Peroxiredoxin2540 | 5 |
| Methuselah-like 2 | 8 |
| Integrins | |
| αPS4 | 115 |
| αPS5 | 10 |
| Ecdysone-induced | |
| ImpE2 | −34 |
| Sgs1 | −27 |
| Eig71Ee | −12 |
| Sgs4 | −10 |
| Broad Complex | −7 |
| Sgs5 | −6 |
| Eip74EF | −5 |
| Eip78C | −3 |
| Axonal guidance | |
| Nervous fingers 1 | −3 |
| Smooth | −3 |
| SoxNeuro | −3 |
Genes are grouped into Gene Ontology functions. Whole-number fold changes compared to wild type are shown.
Several of the stress-induced Turandot peptides are also strongly induced in Elp3 mutants. These peptides are induced by the MEKK pathway (Brun et al. 2006) and are also induced in hopTum-l and Nurf301 mutants (Kwon et al. 2008). Turandot peptides are secreted into the hemolymph after stress induction such as heat shock or oxidative stress and may act in a similar way to heat-shock proteins (Ekengren and Hultmark 2001). Similarly, many glutathione transferase genes are highly induced, further implying an activation of oxidative stress pathways. Members of the immune induced molecule family are also highly induced. These transcripts have been found to be associated with the activation of Toll and JAK/STAT pathways of immunity (Kwon et al. 2008) and with salt-stressed flies (Stergiopoulos et al. 2009).
Conversely, it is interesting that some neuronal genes were downregulated in Elp3 mutants since neuronal defects are evident in the human disorder familial dysautonomia, a genetic disorder caused by a tissue-specific reduction in the amount of the Elongator Elp1 subunit. This affects the development and survival of sensory, sympathetic, and some parasympathetic neurons in the autonomic and sensory nervous system (Anderson et al. 2001; Cuajungco et al. 2001).
Comparison of Elp3 and domino microarrays:
The lack of antimicrobial peptide induction in Elp3 flies was also confirmed by the microarray analysis. Lack of antimicrobial peptide induction is rare in larvae that display a melanotic nodule phenotype. It is, however, also seen in domino (dom) mutants, in which a DNA-dependent ATPase of the SWI/SW2 family is inactivated (Braun et al. 1998). Some other characteristics of the dom mutants are also similar to those of Elp3 mutants, such as a prolonged larval stage, pupal lethality, poor formation of imaginal discs, and the formation of larval melanotic nodules. Both Elp3 and domino mutants are lethal, and the genes are on the same chromosome, complicating genetic interaction experiments. Elp3 and dom mutants do not show dominant interactions as Elp3/dom trans-heterozygous (Elp3/+, domino/+) flies are viable and indistinguishable from wild type. However, both Elp3 and dom mutants act as dominant enhancers of hopTum-l melanotic tumor mutations (data not shown). To confirm interactions between Elp3 and dom, we prepared mRNA from dom mutants, analyzed it on microarrays, and then compared it to the expression pattern of Elp3 mutants.
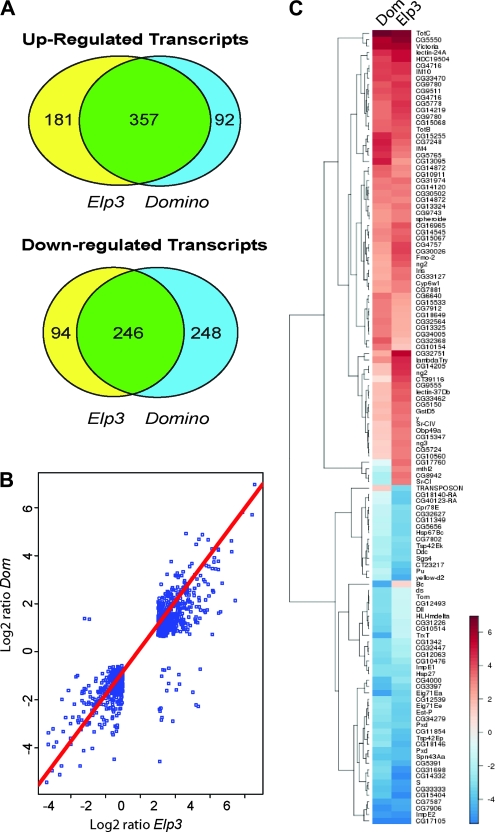
The overlap in overall gene expression levels between the two data sets was striking, both in upregulated and downregulated genes (P < 0.05 and fold change relative to wild type >2.5) (Figure 6A). Transcription of 357 genes was elevated >2.5-fold in both mutants, which represents 66% of upregulated transcripts in Elp3 and 80% of upregulated genes in the dom mutant. Of the transcripts downregulated >2.5-fold, 246 were repressed in both Elp3 and dom mutants, representing 72% of genes repressed in Elp3 and 49% of genes repressed in dom. Linear regression analysis (Figure 6B) as well as the heat map of genes whose expression was changed >3-fold in mutant relative to wild-type genes (Figure 6C) also showed that the fold changes in expression correlate extremely well between the two mutants. The overlap in gene expression profiles thus mirrors the observed striking similarity of phenotypes in the dom and Elp3 mutants.
Figure 6.—
Microarray analysis of Elp3 and dom mutants shows considerable overlap. (A) A Venn diagram of transcripts, the expression of which is increased or decreased >2.5-fold in Elp3 and dom mutants compared to wild type (wandering-stage larvae). (B) Scatter plot showing the concordance in fold change of the overlapping genes between the dom and Elp3 comparisons. The red line shows a linear regression fit across the data (r2 = 0.89). (C) Heat map showing overlap between the Elp3 and dom differential gene lists (expression changing by more than 3-fold). Genes have been clustered using euclidean distance and a complete hierarchical method.
DISCUSSION
Previous experiments on Elongator complex mostly focused on its role in transcription or tRNA modification. In this report, we examined the biological consequences of a lack of Elp3 function in Drosophila melanogaster and identified a striking similarity to those observed upon deleting the gene encoding the SWI/SNF family chromatin-remodeling enzyme Domino. In yeast, Elongator mutants are viable, but characterized by their slow-growth phenotype and delayed response to stress stimuli (Otero et al. 1999). In Arabidopsis, deletion of Elongator subunits results in a delay in germination and seedling growth (Nelissen et al. 2005). Deletion of an Elongator subunit is lethal in the mouse (Chen et al. 2009). We found that deletion of the gene encoding the Elongator subunit Elp3 in the fly results in larval lethality at the pupal stage. Moreover, larval growth is dramatically impaired during early development, with progression to the third instar significantly delayed and pupariation occurring only at day 14 after egg laying. Interestingly, melanotic nodules appear in larvae after 4 days.
To help define which pathways were altered in Elp3 larvae, a genome-wide expression experiment was carried out. The microarray analysis of late-stage Elp3 mutant larvae shows that many stress-induced genes are upregulated. For example, members of the Turandot and glutathione S-transferase gene families are highly induced. These proteins are often associated with the oxidative stress response in cells (Ekengren et al. 2001; Li et al. 2008). Recent reports suggest that the Elp3 mutation is associated with amylotrophic lateral sclerosis (Simpson et al. 2009), a neurodegenerative disorder, which may be associated with oxidative damage induced by free radicals. Elp3 contains an oxidation-sensitive iron-sulfur cluster (Greenwood et al. 2009), so it is an intriguing possibility that Elp3 itself somehow plays a role in detecting the redox state of cells.
The appearance of melanotic nodules indicates dysregulation of the innate immune system. This is confirmed by the whole-genome expression profile, which revealed the induction of many genes known to be involved in the aberrant activation of the larval immune system (Irving et al. 2005). However, it is not clear which signal transduction pathways have been activated in this case, as antimicrobial peptides such as drosomycin (Toll pathway) and TEP (JAK/STAT pathway) do not appear to be induced. It is possible that the effect on the immune system stems from the activation of the stress response described above, underlining the close connection between the stress and immune responses in Drosophila.
Our analysis also revealed that ecdysone-induced transcription is severely impaired in Elp3 mutant larvae. The delayed development of the mutant larvae might potentially postpone the ecdysone burst. However, the size of the mutant was similar to that of the wild type at the time of the microarray analysis, arguing against this possibility. Northern analysis of Broad Complex confirms that the induction is delayed and reduced, suggesting a requirement for Elp3 in the regulation of transcription. Interestingly, a failure of ecdysone induction is also observed in other known chromatin modifier mutants such as Nurf301 (Badenhorst et al. 2005) and dom (this report). The absence of these chromatin-remodeling factors results in lethality and the production of melanotic nodules, supporting the idea that, like these factors, Elongator also affects the ability of the chromatin to respond to ecdysone and other environmental and developmental cues in an appropriate manner. Indeed, comparison of Elp3 and dom gene expression profiles shows a compelling similarity in overall effects on gene expression. This is also reflected in several aspects of similarity in the mutant phenotypes, such as delayed growth and progression to third instar, poor disc formation, pupal lethality, and melanotic nodule formation. Domino is a member of the SWI2/SNF2 family of DNA-dependent ATPases and is involved in several aspects of chromatin remodelling. We suggest that the similar phenotypes arise from coordinate regulation of similar sets of target genes and imply functional collaboration between Elongator- and Domino-mediated chromatin remodeling.
Since Elongator is also known to be present in the coding region of certain human genes (Kouskouti and Talianidis 2005; Close et al. 2006), it seems possible that the effect of Elp3 deletion in the fly could also be due to alterations in chromatin regulation. In general, the Drosophila genome-wide expression data presented here complement gene expression analysis in Arabidopsis, mouse, and humans, further supporting a role for Elongator during transcript elongation and chromatin remodeling (Close et al. 2006; Chen et al. 2009; Nelissen et al. 2010). Our data also reveal important roles for Elongator in ecdysone regulation, morphogenesis, and the suppression of melanotic nodules during development.
Acknowledgments
We thank Jay Uhler, Nic Tapon, Sally Leevers, and Christophe Antoniewski for discussions and reagents; Terence Gilbank and Stephen Murray for their help with micro-injections; and Luke Alphey for supplying the pUASHA plasmid. This work was supported by grants from Cancer Research UK to J.Q.S. and H.M.
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.110.123893/DC1.
Available freely online through the author-supported open access option.
References
- Anderson, S. L., R. Coli, I. W. Daly, E. A. Kichula, M. J. Rork et al., 2001. Familial dysautonomia is caused by mutations of the IKAP gene. Am. J. Hum. Genet. 68 753–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badenhorst, P., H. Xiao, L. Cherbas, S. Y. Kwon, M. Voas et al., 2005. The Drosophila nucleosome remodeling factor NURF is required for Ecdysteroid signaling and metamorphosis. Genes Dev. 19 2540–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand, A. H., and N. Perrimon, 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118 401–415. [DOI] [PubMed] [Google Scholar]
- Braun, A., B. Lemaitre, R. Lanot, D. Zachary and M. Meister, 1997. Drosophila immunity: analysis of larval hemocytes by P-element-mediated enhancer trap. Genetics 147 623–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun, A., J. A. Hoffmann and M. Meister, 1998. Analysis of the Drosophila host defense in domino mutant larvae, which are devoid of hemocytes. Proc. Natl. Acad. Sci. USA 95 14337–14342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun, S., S. Vidal, P. Spellman, K. Takahashi, H. Tricoire et al., 2006. The MAPKKK Mekk1 regulates the expression of Turandot stress genes in response to septic injury in Drosophila. Genes Cells 11 397–407. [DOI] [PubMed] [Google Scholar]
- Chen, Y. T., M. M. Hims, R. S. Shetty, J. Mull, L. Liu et al., 2009. Loss of mouse Ikbkap, a subunit of elongator, leads to transcriptional deficits and embryonic lethality that can be rescued by human IKBKAP. Mol. Cell. Biol. 29 736–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinenov, Y., 2002. A second catalytic domain in the Elp3 histone acetyltransferases: A candidate for histone demethylase activity? Trends Biochem. Sci. 27 115–117. [DOI] [PubMed] [Google Scholar]
- Close, P., N. Hawkes, I. Cornez, C. Creppe, C. A. Lambert et al., 2006. Transcription impairment and cell migration defects in elongator-depleted cells: implication for familial dysautonomia. Mol. Cell 22 521–531. [DOI] [PubMed] [Google Scholar]
- Creppe, C., L. Malinouskaya, M. L. Volvert, M. Gillard, P. Close et al., 2009. Elongator controls the migration and differentiation of cortical neurons through acetylation of alpha-tubulin. Cell 136 551–564. [DOI] [PubMed] [Google Scholar]
- Cuajungco, M. P., M. Leyne, J. Mull, S. P. Gill, J. F. Gusella et al., 2001. Cloning, characterization, and genomic structure of the mouse Ikbkap gene. DNA Cell Biol. 20 579–586. [DOI] [PubMed] [Google Scholar]
- Ekengren, S., and D. Hultmark, 2001. A family of Turandot-related genes in the humoral stress response of Drosophila. Biochem. Biophys. Res. Commun. 284 998–1003. [DOI] [PubMed] [Google Scholar]
- Ekengren, S., Y. Tryselius, M. S. Dushay, G. Liu, H. Steiner et al., 2001. A humoral stress response in Drosophila. Curr. Biol. 11 714–718. [DOI] [PubMed] [Google Scholar]
- Esberg, A., B. Huang, M. J. Johansson and A. S. Bystrom, 2006. Elevated levels of two tRNA species bypass the requirement for elongator complex in transcription and exocytosis. Mol. Cell 24 139–148. [DOI] [PubMed] [Google Scholar]
- Fellows, J., H. Erdjument-Bromage, P. Tempst and J. Q. Svejstrup, 2000. The Elp2 subunit of elongator and elongating RNA polymerase II holoenzyme is a WD40 repeat protein. J. Biol. Chem. 275 12896–12899. [DOI] [PubMed] [Google Scholar]
- Fletcher, J. C., and C. S. Thummel, 1995. The ecdysone-inducible Broad-complex and E74 early genes interact to regulate target gene transcription and Drosophila metamorphosis. Genetics 141 1025–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentleman, R. C., V. J. Carey, D. M. Bates, B. Bolstad, M. Dettling et al., 2004. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 5 R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert, C., A. Kristjuhan, G. S. Winkler and J. Q. Svejstrup, 2004. Elongator interactions with nascent mRNA revealed by RNA immunoprecipitation. Mol. Cell 14 457–464. [DOI] [PubMed] [Google Scholar]
- Greenwood, C., L. A. Selth, A. B. Dirac-Svejstrup and J. Q. Svejstrup, 2009. An iron-sulfur cluster domain in Elp3 important for the structural integrity of elongator. J. Biol. Chem. 284 141–149. [DOI] [PubMed] [Google Scholar]
- Hanratty, W. P., and C. R. Dearolf, 1993. The Drosophila Tumorous-lethal hematopoietic oncogene is a dominant mutation in the hopscotch locus. Mol. Gen. Genet. 238 33–37. [DOI] [PubMed] [Google Scholar]
- Harrison, D. A., R. Binari, T. S. Nahreini, M. Gilman and N. Perrimon, 1995. Activation of a Drosophila Janus kinase (JAK) causes hematopoietic neoplasia and developmental defects. EMBO J. 14 2857–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes, N. A., G. Otero, G. S. Winkler, N. Marshall, M. E. Dahmus et al., 2002. Purification and characterization of the human elongator complex. J. Biol. Chem. 277 3047–3052. [DOI] [PubMed] [Google Scholar]
- Huang, B., M. J. Johansson and A. S. Bystrom, 2005. An early step in wobble uridine tRNA modification requires the Elongator complex. RNA 11 424–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry, R. A., B. M. Bolstad, F. Collin, L. M. Cope, B. Hobbs et al., 2003. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 31 e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving, P., J. M. Ubeda, D. Doucet, L. Troxler, M. Lagueux et al., 2005. New insights into Drosophila larval haemocyte functions through genome-wide analysis. Cell. Microbiol. 7 335–350. [DOI] [PubMed] [Google Scholar]
- Kim, J. H., W. S. Lane and D. Reinberg, 2002. Human Elongator facilitates RNA polymerase II transcription through chromatin. Proc. Natl. Acad. Sci. USA 99 1241–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss, I., A. H. Beaton, J. Tardiff, D. Fristrom and J. W. Fristrom, 1988. Interactions and developmental effects of mutations in the Broad-Complex of Drosophila melanogaster. Genetics 118 247–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouskouti, A., and I. Talianidis, 2005. Histone modifications defining active genes persist after transcriptional and mitotic inactivation. EMBO J. 24 347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristjuhan, A., J. Walker, N. Suka, M. Grunstein, D. Roberts et al., 2002. Transcriptional inhibition of genes with severe histone h3 hypoacetylation in the coding region. Mol. Cell 10 925–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogan, N. J., and J. F. Greenblatt, 2001. Characterization of a six-subunit Holo-Elongator complex required for the regulated expression of a group of genes in Saccharomyces cerevisiae. Mol. Cell. Biol. 21 8203–8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurucz, E., C. J. Zettervall, R. Sinka, P. Vilmos, A. Pivarcsi et al., 2003. Hemese, a hemocyte-specific transmembrane protein, affects the cellular immune response in Drosophila. Proc. Natl. Acad. Sci. USA 100 2622–2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon, S. Y., H. Xiao, B. P. Glover, R. Tjian, C. Wu et al., 2008. The nucleosome remodeling factor (NURF) regulates genes involved in Drosophila innate immunity. Dev. Biol. 316 538–547. [DOI] [PubMed] [Google Scholar]
- Lagueux, M., E. Perrodou, E. A. Levashina, M. Capovilla and J. A. Hoffmann, 2000. Constitutive expression of a complement-like protein in toll and JAK gain-of-function mutants of Drosophila. Proc. Natl. Acad. Sci. USA 97 11427–11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H. M., G. Buczkowski, O. Mittapalli, J. Xie, J. Wu et al., 2008. Transcriptomic profiles of Drosophila melanogaster third instar larval midgut and responses to oxidative stress. Insect Mol. Biol. 17 325–339. [DOI] [PubMed] [Google Scholar]
- Li, Y., Y. Takagi, Y. Jiang, M. Tokunaga, H. Erdjument-Bromage et al., 2001. A multiprotein complex that interacts with RNA polymerase II elongator. J. Biol. Chem. 276 29628–29631. [DOI] [PubMed] [Google Scholar]
- Metivier, R., G. Penot, M. R. Hubner, G. Reid, H. Brand et al., 2003. Estrogen receptor-alpha directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell 115 751–763. [DOI] [PubMed] [Google Scholar]
- Nelissen, H., D. Fleury, L. Bruno, P. Robles, L. De Veylder et al., 2005. The elongata mutants identify a functional Elongator complex in plants with a role in cell proliferation during organ growth. Proc. Natl. Acad. Sci. USA 102 7754–7759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelissen, H., S. De Groeve, D. Fleury, P. Neyt, L. Bruno et al., 2010. Plant Elongator regulates auxin-related genes during RNA polymerase II transcription elongation. Proc. Natl. Acad. Sci. USA 107 1678–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada, Y., K. Yamagata, K. Hong, T. Wakayama and Y. Zhang, 2010. A role for the elongator complex in zygotic paternal genome demethylation. Nature 463 554–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero, G., J. Fellows, Y. Li, T. de Bizemont, A. M. G. Dirac et al., 1999. Elongator, a multisubunit component of a novel RNA polymerase II holoenzyme for transcriptional elongation. Mol. Cell 3 109–118. [DOI] [PubMed] [Google Scholar]
- Paraskevopoulou, C., S. A. Fairhurst, D. J. Lowe, P. Brick and S. Onesti, 2006. The Elongator subunit Elp3 contains a Fe4S4 cluster and binds S-adenosylmethionine. Mol. Microbiol. 59 795–806. [DOI] [PubMed] [Google Scholar]
- Parker, L., S. Gross and L. Alphey, 2001. Vectors for the expression of tagged proteins in Drosophila. Biotechniques 31 1280–1282, 1284, 1286. [DOI] [PubMed] [Google Scholar]
- Pokholok, D. K., N. M. Hannett and R. A. Young, 2002. Exchange of RNA polymerase II initiation and elongation factors during gene expression in vivo. Mol. Cell 9 799–809. [DOI] [PubMed] [Google Scholar]
- Qiu, P., P. C. Pan and S. Govind, 1998. A role for the Drosophila Toll/Cactus pathway in larval hematopoiesis. Development 125 1909–1920. [DOI] [PubMed] [Google Scholar]
- Rahl, P. B., C. Z. Chen and R. N. Collins, 2005. Elp1p, the yeast homolog of the FD disease syndrome protein, negatively regulates exocytosis independently of transcriptional elongation. Mol. Cell 17 841–853. [DOI] [PubMed] [Google Scholar]
- Simpson, C. L., R. Lemmens, K. Miskiewicz, W. J. Broom, V. K. Hansen et al., 2009. Variants of the elongator protein 3 (ELP3) gene are associated with motor neuron degeneration. Hum. Mol. Genet. 18 472–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinenko, S. A., E. K. Kim, R. Wynn, P. Manfruelli, I. Ando et al., 2004. Yantar, a conserved arginine-rich protein is involved in Drosophila hemocyte development. Dev. Biol. 273 48–62. [DOI] [PubMed] [Google Scholar]
- Solinger, J. A., R. Paolinelli, H. Kloss, F. B. Scorza, S. Marchesi et al., 2010. The Caenorhabditis elegans Elongator complex regulates neuronal alpha-tubulin acetylation. PLoS Genet. 6 e1000820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stergiopoulos, K., P. Cabrero, S. A. Davies and J. A. Dow, 2009. Salty dog, an SLC5 symporter, modulates Drosophila response to salt stress. Physiol. Genomics 37 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stramer, B., W. Wood, M. J. Galko, M. J. Redd, A. Jacinto et al., 2005. Live imaging of wound inflammation in Drosophila embryos reveals key roles for small GTPases during in vivo cell migration. J. Cell Biol. 168 567–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svejstrup, J. Q., 2007. Elongator complex: How many roles does it play? Curr. Opin. Cell Biol. 19 331–336. [DOI] [PubMed] [Google Scholar]
- Winkler, G. S., T. G. Petrakis, S. Ethelberg, M. Tokunaga, H. Erdjument-Bromage et al., 2001. RNA polymerase II Elongator holoenzyme is composed of two discrete subcomplexes. J. Biol. Chem. 276 32743–32749. [DOI] [PubMed] [Google Scholar]
- Winkler, G. S., A. Kristjuhan, H. Erdjument-Bromage, P. Tempst and J. Q. Svejstrup, 2002. Elongator is a histone H3 and H4 acetyltransferase important for normal histone acetylation levels in vivo. Proc. Natl. Acad. Sci. USA 99 3517–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittschieben, B. O., G. Otero, T. de Bizemont, J. Fellows, H. Erdjument-Bromage et al., 1999. A novel histone acetyltransferase is an integral subunit of elongating RNA polymerase II holoenzyme. Mol. Cell 4 123–128. [DOI] [PubMed] [Google Scholar]
- Wittschieben, B. O., J. Fellows, W. Du, D. J. Stillman and J. Q. Svejstrup, 2000. Overlapping roles for the histone acetyltransferase activities of SAGA and Elongator in Vivo. EMBO J. 19 3060–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zettervall, C. J., I. Anderl, M. J. Williams, R. Palmer, E. Kurucz et al., 2004. A directed screen for genes involved in Drosophila blood cell activation. Proc. Natl. Acad. Sci. USA 101 14192–14197. [DOI] [PMC free article] [PubMed] [Google Scholar]