Abstract
Many higher eukaryotes have evolved strategies for the maternal control of growth and development of their offspring. In higher plants this is achieved in part by postmeiotic gene activity controlling the development of the haploid female gametophyte. stunter1 (stt1) is a novel, recessive, maternal effect mutant in maize that displays viable, miniature kernels. Maternal inheritance of stt1 results in seeds with reduced but otherwise normal endosperms and embryos. The stt1 mutation displays reduced transmission through the male and female parents and causes significant changes in the sizes of both male and female gametophytes. stt1 pollen grains are smaller than wild type, have reduced germination efficiency, and reduced pollen tube growth. stt1 embryo sacs have smaller central cells and abnormal antipodal cells that are larger, more vacuolated, and fewer in number than wild type. Embryos and endosperms produced by fertilization of stt1 embryo sacs develop and grow more slowly than wild type. The data suggest that the morphology of mutant embryo sacs influences endosperm development, leading to the production of miniature kernels in stt1. Analysis of seeds carrying a mutant maternal allele of stt1 over a deletion of the paternal allele demonstrates that both parental alleles are active after fertilization in both the endosperm and embryo. This analysis also indicates that embryo development until the globular stage in maize can proceed without endosperm development and is likely supported directly by the diploid mother plant.
THE maize female gametophyte (embryo sac) follows the Polygonum-type pattern of development, which results in an eight-nucleate, seven-celled megagametophyte (Reiser and Fischer 1993; Christensen et al. 1997). Differentiation of the cells produces a mature embryo sac consisting of antipodal cells at the chalazal end, two synergid cells and the egg cell at the micropylar end, and the homodiploid central cell in the middle. In maize, the antipodal cells proliferate after cellularization, producing a cluster of 30–100 cells (Bedinger and Russell 1994). Seed production is accomplished through double fertilization, during which the male gametophyte (pollen) contributes two sperm cells that fuse with the female gametes, the egg cell and central cell to give rise to the sporophytic tissues of the seed—the diploid embryo and the triploid endosperm, respectively (for reviews see Sheridan and Clark 1994; Walbot and Evans 2003).
Following fertilization, the maize embryo develops through three distinct phases (for reviews see Sheridan and Clark 1994; Vernoud et al. 2005): formation of the proembryo; establishment of radial symmetry, the embryonic axis, and the shoot and root meristems; and maturation and dehydration of the embryonic structures. Maize endosperm development begins just after fertilization and occurs in four stages. First there is a period of free nuclear divisions to produce a syncytium consisting of >250 nuclei [1–2 days after pollination (DAP)] (Randolph 1936). Following syncytial development, the endosperm cellularizes, starting at the periphery and finishing in the center of the endosperm (3–4 DAP). The endosperm then begins differentiating into four distinct domains: the aleurone, the basal endosperm transfer layer (BETL), the central starchy endosperm (CSE), and the embryo-surrounding region (ESR) (6 DAP) (Olsen et al. 1999; Olsen 2004). The CSE undergoes rapid mitotic growth followed by endoreduplication before undergoing programmed cell death (PCD), beginning near the crown and finally encompassing all of the CSE (16–40 DAP) (Young et al. 1997).
Several large mutant studies have been conducted in maize to try to understand the number and types of genes involved in seed development in general (Neuffer and Sheridan 1980; Sheridan and Neuffer 1980; Clark and Sheridan 1991; Scanlon et al. 1994; McCarty et al. 2005; Settles et al. 2007) and endosperm development in particular (for reviews see Bommert and Werr 2001; Olsen 2001, 2004). Additionally, many studies have been done in an attempt to elucidate the genetic makeup of the female haploid plant in maize and Arabidopsis (Patterson 1978; Cordts et al. 2001; Le et al. 2005; Pagnussat et al. 2005; Sprunck et al. 2005; Yu et al. 2005; Yang et al. 2006; Jones-Rhoades et al. 2007; Steffen et al. 2007; Wuest et al. 2010). Many of these mutant and gene expression studies helped determine that early seed development is largely under the control of the maternal genome (Vielle-Calzada et al. 2000). Mutations in genes required in the embryo sac for proper seed development can be identified by mode of inheritance (Grossniklaus and Schneitz 1998; Evans and Kermicle 2001). Although required in the female but not male gametophyte for normal seed development, many gametophytic maternal effect mutations also influence development of the male gametophyte, reducing male transmission without paternal effects on seed development (Pagnussat et al. 2005; Boavida et al. 2009).
To date, several gametophytic maternal effect mutants have been identified in both Arabidopsis and maize (Olsen 2004; Kohler and Grossniklaus 2005; Pagnussat et al. 2005; Pien and Grossniklaus 2007). Studies of these mutants and others have revealed several causes for maternal effects. Possible mechanisms include: changes in functional gene dosage in the endosperm (e.g., floury3; Ma and Nelson 1975), abnormal embryo sac morphology (e.g., baseless1; Gutierrez-Marcos et al. 2006), loss of embryo sac proteins normally stored cytoplasmically to act after fertilization (perdurance) (e.g., PROLIFERA; Springer et al. 2000), or imprinting (e.g., Polycomb group and other genes in maize and Arabidopsis (recently reviewed in Huh et al. 2008; Johnson and Bender 2009; Jullien and Berger 2009). Known maternal gametophyte effect mutants are involved in basic cellular process, such as DNA demethylation, DNA repair, and cell cycle regulation, as well as seed specific processes such as the suppression of autonomous endosperm development (Golden et al. 2002; Holding and Springer 2002; Ngo et al. 2007; Andreuzza et al. 2009).
Maternal effect mutants have been described in maize that affect the endosperm and embryo [e.g., maternal effect lethal1 (mel1)] (Evans and Kermicle 2001), bsl1, (Gutierrez-Marcos et al. 2006), and Dappled (Dap) (Gavazzi et al. 1997). Despite lacking a paternal effect on seed development, these mutations often have a gametophytic effect on pollen development (e.g., mel1, bsl1, and Dap). To better understand how maternal gene expression and embryo sac morphology can affect seed development, we have identified and characterized a novel maternal effect mutant in maize, named stunter1 (stt1). The stt1 mutation reduces the size of both male and female gametophytes, and the defective embryo sacs in turn produce seeds with smaller embryos and endosperms.
MATERIALS AND METHODS
Plant material and growth conditions:
The stt1 mutation arose spontaneously in a standard W23 inbred maize (Zea mays) plant. The stt1 mutation was propagated as a heterozygote by transmission through the female and selection for miniature stt1/+ kernels. stt1 was backcrossed by the M14 inbred maize stock through at least four generations prior to characterization of the effects of stt1 on seed and gametophyte development. Mutants and wild-type controls were grown side by side for each experiment either in summer field conditions or in greenhouses under long-day conditions (16 hr light:8 hr dark cycles).
Male and female transmission of the stt1 mutation and penetrance of the miniature kernel phenotype were partially assessed using plants carrying stt1 linked in repulsion phase to the waxy1 (wx1)-marked T2-9d reciprocal translocation in the M14 inbred background (Maize Genetics Cooperation Stock Center). Reciprocal crosses between F1 plants and the wx1 T2-9d tester were made to calculate transmission rates. The genetic distance between stt1 and wx1 on T2-9d was determined using the pollen phenotypes of wx1 and stt1. Female transmission (Ft) of stt1 was calculated on the basis of linkage to and transmission of the wx1 marker. Transmission of wx1 observed after crossing T2-9d/Normal chromosomes (N) +/stt1 wx1/+ as a female equals the number of nonrecombinant wx1 Stt1+ kernels and recombinant wx1 stt1 kernels divided by the total number of kernels. This relationship was used to calculate the Ft of stt1 and therefore the percentage of embryo sacs carrying stt1 that produced a detectable kernel. To test for male transmission of stt1, Wx1+ kernels resulting from the cross of wx1 T2-9d females by T2-9d/N +/stt1 wx1/+ were progeny tested to determine whether they carried stt1 or Stt1+.
Endosperms and embryos lacking maternal and paternal stt1 function were produced by crossing plants carrying stt1 and anthocyaninless2 (a2) as females by the TB-5Sc-2L015-3 (TB-5S-2L) compound translocation carrying a segment of the short arm of chromosome 5 with A2+ and the long arm of chromosome 2 with Stt1+ translocated onto the supernumerary B chromosome (Bill Sheridan, University of North Dakota). Inheritance of the TB-5S-2L chromosome was assessed using the expression of anthocyanin in the endosperm or embryo as conferred by A2+. Nondisjunction was inferred if one of the endosperm or embryo was pigmented while the other was colorless. Kernels expressing anthocyanin in the endosperm were further analyzed in testcrosses with a2/a2 to verify the presence or absence of A2+ (and hence B-5S-2L) in the embryo. The rate of nondisjunction for the TB-5S-2L stock was determined by crossing white3/+ heterozygotes as females by the TB-5S-2L stock, which carries the wild-type allele of white3 (w3) on the long arm of chromosome 2. w3 homozygous seeds have white endosperms and viviparous embryos. Seeds with w3 hypoploid endosperms and hyperploid embryos have white endosperms and dormant embryos, and seeds with w3 hypoploid embryos and hyperploid endosperms have viviparous embryos and yellow endosperms.
A +/+/stt1, +/a2/a2, triB-5S-2L/N/N tertiary trisomic stock was identified by making reciprocal crosses between a2 homozygotes and plants from the purple kernels of the above cross carrying stt1 (verified by production of miniature kernels when crossed as a female) and the TB-5S-2L translocation (verified by nondisjunction when crossed as a male). Trisomic individuals carrying stt1 were identified from these crosses by growing plants from the purple kernels (those inheriting B-5S-2L) of this cross and making reciprocal crosses with a2. Trisomic plants carrying stt1 segregate miniatures as females, do not undergo nondisjunction, and have reduced, nonidentical male and female transmission of A2+ (Auger and Birchler 2002).
Mapping with simple sequence repeat and insertion/deletion markers:
stt1 mapping populations were generated by crossing stt1W23/+M14 or stt1W23/+B73 hybrid females by wild-type M14 or B73 males, respectively. DNA was extracted from seedlings by minor modification of the method of Saghai-Maroof et al. (1984), and PCR reactions were performed as described (Evans and Kermicle 2001). Twenty simple sequence repeat (SSR) markers from bin locations across the genome were tested against 48 miniature kernels to determine which markers cosegregated with the kernel phenotype, revealing linkage only on the long arm of chromosome 2. Markers that demonstrated linkage were then tested on a larger population of miniature and normal kernels, and progeny testing was conducted to verify whether recombinant individuals were stt1/+ or wild type. Map position was refined using additional SSR and insertion/deletion markers designed for genes within the chromosomal region that showed polymorphisms between M14 or B73 and the original stt1 chromosome.
Pollen staining and germination and tube growth assays:
Pollen was analyzed from stt1/+ heterozygotes grown in field conditions or in the greenhouse under long-day conditions. Old anthers were removed 2 hr before pollen was collected in tassel bags and pollen was stained or spread on pollen germination medium (Walden 1994). Pollen was allowed to germinate at 28° for 0 or 60 min before fixation with FAA (50% ethanol, 10% formalin, 5% glacial acetic acid) and staining with I2/KI (0.1% I2, 1% KI reagent). Digital images were acquired using a Leica DFC320 camera attached to a Leica MZ125 stereomicroscope and analyzed using Image Pro Express 6.0. For 4′-6-diamidino-2-phenylindole (DAPI) staining, pollen coats were digested according to Jewell and Islam-Faridi (1994). Pollen was collected by centrifugation, resuspended in 100% EtOH, placed on a microscope slide, and allowed to dry. Pollen was stained with 1 μg/ml of DAPI (Sigma) in H2O for 5 min before visualization and imaging on a Nikon Eclipse E600 UV fluorescent microscope equipped with a Fujifilm FinePix S5 Pro camera.
Confocal microscopy and histology:
Embryo sacs were analyzed from stt1/+ heterozygous and wild-type M14 ears collected at similar stages of development. Endosperms and embryos from developing kernels were analyzed from stt1/+ heterozygotes crossed as females by M14 or TB-5S-2L males, and ears were fixed at multiple DAP. Samples were processed according to Gutierrez-Marcos et al. (2006) and visualized on a Leica SP5 (Wetzlar, Germany) laser scanning confocal microscope. Excitation was performed at 405 nm, 488 nm, and 561 nm and emission was collected at 410–480 nm, 495–555 nm, and 565–730 nm for the merged images. Images were analyzed and processed using ImageJ and Adobe Photoshop CS3.
For the reporter assays, stt1/+ heterozygous plants were crossed as females by males carrying either ProBet1∷B-glucuronidase (GUS) or ProVP1∷GUS transgenic reporters (Hueros et al. 1999; Cao et al. 2007). Normal and miniature kernels were cut along the longitudinal axis and either stained for GUS activity as previously described (Costa et al. 2003) or stained with 0.1% Evans Blue for 2 min and washed two times with water to visualize programmed cell death. Images were collected as described above for the pollen germination assay.
RESULTS
stunter1 is a novel recessive maternal effect mutation in maize:
The stt1 mutation was originally isolated from a single cross (standard W23 inbred female crossed by a W22 inbred male) that was segregating miniature kernels. The miniature kernels were viable, and, in reciprocal crosses with wild type, miniature kernels were only seen when mutants were used as females, suggesting a maternal effect mutant. The stt1 seeds display reduced but otherwise normal endosperms and embryos (Figure 1) that produce normal seedlings and mature plants. stt1 was mapped to the long arm of chromosome 2 in Bin 2.08, 1.2% (14/1141) away from bnlg1233 and 1.0% (3/304) away from GRMZM2g077823 (annotation based on the Maize Genome Sequencing Project, Release 4a.53, http://maizesequence.org/). The mutation was crossed twice to several inbred lines and found to exhibit strong phenotypic expression in M14. Therefore the mutation was backcrossed for two more generations to M14 before further analysis.
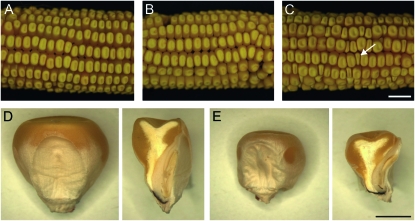
Figure 1.—
Seed phenotypes of stt1. (A) Ear from a homozygous wild-type female pollinated by a homozygous wild-type male. (B) Ear from a homozygous wild-type female pollinated by a stt1/+ heterozygous male. (C) Ear from a stt1/+ heterozygous female pollinated by a homozygous wild-type male (arrow indicates a stt1/+ miniature kernel). Bar, (A–C) 1 cm. (D and E) Germinal (embryo) face (left) and a median longitudinal section (right) of wild-type (D) and stt1-affected (E) kernels at the same scale. Bar, (D and E) 0.25 cm.
The percentage of miniature seeds that result by pollinating stt1/+ plants with wild-type pollen ranges from 4 to 29% (Figure 1 and Table 1), indicating incomplete penetrance of the miniature kernel phenotype or failure of a subset of stt1 embryo sacs to produce a seed. To aid in the assessment of male and female transmission rates of stt1, the wx1 T2-9d reciprocal translocation stock was crossed to stt1 to link wx1 as a visible endosperm and pollen marker in repulsion to stt1. F1 plants were subsequently crossed reciprocally with the wx1 T2-9d tester. Progeny testing of the normal kernels and/or scoring for wx1 in crosses using stt1/+ as the female revealed that 3–25% of the phenotypically normal kernels were carrying the stt1 mutation. Total stt1/+ mutant progeny (miniature plus normal kernels carrying stt1) comprises <50% of all progeny from a stt1/+ female, indicating that a subset of stt1 embryo sacs are never fertilized or produce seeds that abort at a stage too early to identify by visual examination. Using the wx1 female transmission rate in the kernels and the wx1 stt1 recombination rate determined from the pollen (below), we calculated that approximately half (47%) of stt1 embryo sacs made an identifiable seed while the other half (53%) did not.
TABLE 1.
Male and female transmission of the stt1 mutation
| Seed phenotype after female transmission of the mutation |
Seed genotype after male transmission of the mutation |
|||||
|---|---|---|---|---|---|---|
| Mutant stt1/+ (♀) × wild type (♂) |
Wild type (♀) × mutant stt1/+ (♂) |
|||||
| Cross no. (N) | Miniature (stt1) (%) | Normal (stt1) (%) | Normal (WT) (%) | Cross no. (N) | Heterozygous stt1/+ (%) | Homozygous WT (%) |
| 1 (177) | 4 | 15 | 81 | 1 (77) | 0 | 100 |
| 2 (203) | 29 | 3 | 68 | 2–13 (466) | 0 | 100 |
| 3–27 (1127) | 8 | 25a | 67a | |||
When stt1 is crossed as a heterozygous mutant female by a wild-type male <50% of the progeny are abnormal, demonstrating incomplete penetrance of the maternal effect phenotype. Transmission of the mutation through the pollen was not detected, suggesting stt1 is required for normal pollen development and/or function.
Calculations made on the basis of transmission of wx1 on T2-9d in repulsion to stt1.
To determine whether the maternal effect of the stt1 mutation is recessive and therefore more likely to be a loss of function, we generated a stt1 trisomic stock (+/+/stt1, +/a2/a2, triB-5S-2L/N/N; see materials and methods), which will produce some disomic stt1/+ embryo sacs. We crossed miniature, purple individuals (carrying stt1 and the B-5S-2L chromosome) by a2/a2. From this cross, four seed phenotypes are expected in the progeny: normal, purple; normal, yellow; miniature (mn), purple; and mn, yellow. Because the unpaired, triB-5S-2L chromosome is not transmitted as efficiently as the normal chromosomes 2 and 5, only ∼40% instead of 50% of the seeds are expected to inherit B-5S-2L and thus be purple. Table 2 lists the expected ratios of each seed phenotype and the causal genotypes for dominant or recessive action of stt1. From this cross, we observed 162 normal, purple seeds (43%); 124 normal, yellow seeds (33%); 4 mn, purple seeds (1%); and 88 mn, yellow seeds (23%). This ratio is not consistent with stt1 being dominant (χ2 = 108.2, P < 0.0001), but is consistent with stt1 being recessive (χ2 = 2.69, P = 0.44). The key frequency is that of miniature purple seeds (i.e., those inheriting both stt1 and A2+). If stt1 were dominant, these would be almost as common as the yellow miniatures that lack the trisome. Instead, this class was very rare, which is consistent with stt1 being recessive, and likely resulted from rare recombination between B-5S-2L and one of the normal chromosomes.
TABLE 2.
stt1 is a recessive maternal effect mutation
stunter1 affects the size and germination efficiency of pollen:
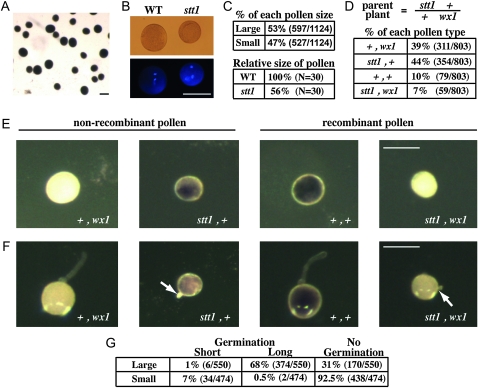
Segregation distortion of wx1 and progeny testing in crosses using stt1/+ as the male revealed a strong defect in stt1 pollen transmission (Table 1). Consistent with this observation, stt1/+ heterozygotes produce ∼50% small pollen grains consistent with a heterozygous gametophytic mutation (Figure 2, A–C, χ2 = 0.36, P = 0.55, n = 1124). The smaller grains are approximately half the size of the larger grains. As visualized by DAPI staining, the small pollen had normal vegetative and sperm nuclei, suggesting mitosis I and II proceeded normally. I2/KI staining of the pollen from a stt1/+ heterozygote revealed that the small pollen grains were also fully filled with starch like wild type.
Figure 2.—
Effect of stt1 on pollen. (A) I2/KI -stained pollen from a stt1/+ heterozygote. (B) DAPI staining to show nuclei of pollen from a stt1/+ heterozygote. (C) The percentage of pollen grains of each size and their relative sizes (to large pollen) are indicated. (D–F) Pollen from T2-9/N wx1/+ +/stt1 heterozygotes with wx1 and stt1 in repulsion phase (parent plant). (D) The percentage of recombinant and nonrecombinant pollen classes (n = 803). (E and F) Pollen from T2-9/N wx1/+ +/stt1 plants stained with I2/KI without germination (E) or after 60-min incubation on pollen germination medium (F). Arrows point to arrested pollen tubes. (G) The percentage of nongerminated pollen and germinated pollen with long and short pollen tubes. Bars, (A, B, E, and F) 100 μm.
To determine whether the smaller grains are a consequence of the stt1 mutation, we utilized the T2-9/N wx1/+ +/stt1 translocation stock described above. Pollen grains carrying the Wx1+ allele dye purple when stained with I2/KI reagent, while pollen carrying the wx1 allele stain red (Figure 2, D–F, purple vs. yellow appearance with transmitted light) (Brink and MacGillivray 1924; Demerec 1924). After staining, 80% of the large pollen grains were red and 86% of the small pollen grains were purple, which confirms that the small pollen phenotype is linked to wx1 (∼17 cM away) and so likely results from the stt1 mutation.
Despite having normal nuclei and starch filling, the small pollen was not functional. In vitro germination of pollen from stt1/+ heterozygotes revealed that the small stt1 pollen grains had reduced germination (8%) compared to the large wild-type grains (69%) (Figure 2G), and, of the small stt1 pollen grains that did germinate, only 6% extended a long pollen tube while 94% had a short arrested pollen tube. This is in contrast to the germinated, large pollen grains that extended long pollen tubes 98% of the time and short pollen tubes only 2% of the time (n = 1024).
To see whether competition with wild-type pollen caused the reduced stt1 pollen transmission, we crossed wx1 T2-9d ears by T2-9/N wx1/+ +/stt1 heterozygous males using varying amounts of pollen. In six paired crosses, ears were pollinated either sparsely or heavily creating less and more competition between wild-type and stt1 sibling pollen grains, respectively. Male transmission rates of wx1 in repulsion to stt1 were not significantly different between the paired sparse (8.9%) and heavy (12.3%) pollinations (χ2 = 1.07, P = 0.30; supporting information, Table S1). Taken together, these data support the hypothesis that stt1 pollen is never functional.
stunter1 embryo sacs are smaller than wild type:
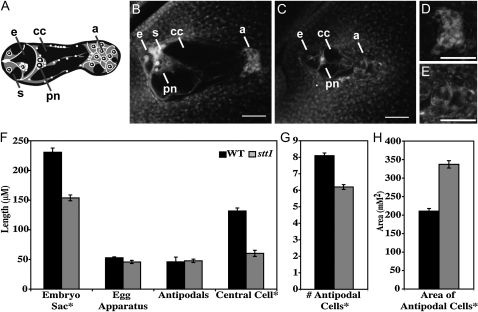
In stt1/+ heterozygotes, two types of embryo sacs (Figure 3) segregated ∼1:1 (40/75 mutant:35/75 normal), consistent with a gametophytic mutation with high penetrance (χ2 = 0.44, P = 0.51). Mutant embryo sacs (Figure 3C) are significantly smaller than wild type (Figure 3B) with abnormal antipodal cells and synergids.
Figure 3.—
Effect of stt1 on embryo sac morphology. (A) Diagram of a mature normal embryo sac. (B–E) Confocal micrographs of embryo sacs of wild type (B and D) and stt1 (C and E). (B and C) Whole embryo sacs with the micropyle to the left. a, antipodal cells; cc, central cell; pn, polar nuclei; e, egg cell; s, synergid. (D and E) Close up view of the antipodal regions. Bars, 50 μm. (F) The length (μm) of the whole embryo sac, egg apparatus, central cell, and antipodal cell region of normal and mutant embryo sacs (N = 21 for WT and N = 25 for stt1). (G) The number of antipodal cells in wild-type and mutant embryo sacs (N = 21 for WT and N = 25 for stt1). (H) The size of individual antipodal cells in wild-type and mutant embryo sacs (μm2 in cross-section) (N = 60). For F, G, and H comparisons are made between sibling embryo sacs from stt1/+ heterozygotes. Error bars ± 1 SEM. *Significantly different from WT (P < 0.0001).
The difference in size between the two types of embryo sacs is largely due to a smaller central cell in stt1—approximately half that of wild type (Figure 3F). Both the egg apparatus (measured from the micropyle to the polar nuclei) and antipodal cell region are similar in size in the mutant embryo sacs and wild type, but mutant antipodal cells appear larger, less cytoplasmically dense, and fewer in number than wild type (Figure 3, D–F). Additionally, the mutant synergids are less distinguishable than wild-type synergids, which fluoresce brightly under these fixation conditions since they degenerate at maturity (Figure 3, B and C).
Maize ovules are initiated at the base of the ear first, so the tip of the ear has the least mature ovules, and ovules at the base of the ear are the most mature. Because of this progressive development, adjacent ovules should be at the same developmental stage and close in size. In stt1/+ heterozygotes, we were able to identify neighboring embryo sacs that were either two distinct sizes or at two different stages of development at least as early as the two-nucleate stage (Figure S1). These results indicate that the stt1 mutation acts very early in development prior to embryo sac maturation to affect the size of the embryo sac.
Fertilization of stt1 embryo sacs:
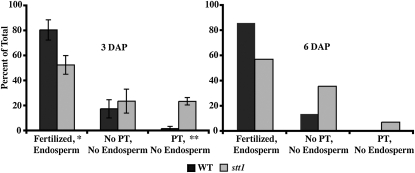
Since fewer than half of the seeds of a stt1/+ heterozygote inherit stt1, some stt1 embryo sacs are never fertilized or produce seeds that arrest so early they are not distinguishable from unfertilized ovules. To distinguish between these two scenarios, we examined embryo sacs from stt1/+ heterozygotes crossed by wild type at 3 DAP (Figure 4, left graph). At 3 DAP, wild type and stt1 can be distinguished by size and antipodal cell morphology. Fertilization was inferred by syncytial endosperm development. At 3 DAP, a ring of cortical nuclei is evident in cross-section of both stt1 and wild type with significantly fewer nuclei in stt1 (Figure 5A). Pollen tube entry into the embryo sac was inferred either by intense fluorescence of the penetrated synergid (Figure S2) or by endosperm growth. We imaged and categorized 310 total ovules at 3 DAP from four independent crosses (Figure 4, left graph). Of the wild-type ovules, 81% had attracted a pollen tube and been fertilized, 18% were unfertilized without evidence of pollen tube (PT) entry, and 2% showed evidence of PT entry without endosperm growth. For stt1 ovules, we observed that 53% were fertilized, 24% remained unfertilized without evidence of PT entry, and the remaining 23% showed PT entry into the synergid with no visible signs of endosperm growth, making it unclear whether these ovules remained unfertilized or were fertilized but developing slowly. These results confirm that while PT attraction to stt1 embryo sacs is normal, a significant percentage of stt1 embryo sacs are either never fertilized or initiate development very slowly.
Figure 4.—
Fertilization rate of stt1 embryo sacs. Heterozygous stt1/+ ears were pollinated by wild type and fixed 3 or 6 DAP. Embryo sacs were classified as WT or stt1 and as fertilized with endosperm proliferation, lacking both PT entry and endosperm proliferation, or having PT entry without endosperm proliferation. For 3 DAP, average ratios ± SEM are shown, N = 4 independent crosses for a total of 162 WT individuals and 148 stt1 individuals. For 6 DAP, ratios are shown for 1 cross (N = 36 for WT, N = 42 for stt1). Significantly different from WT (*P < 0.05 or **P < 0.001).
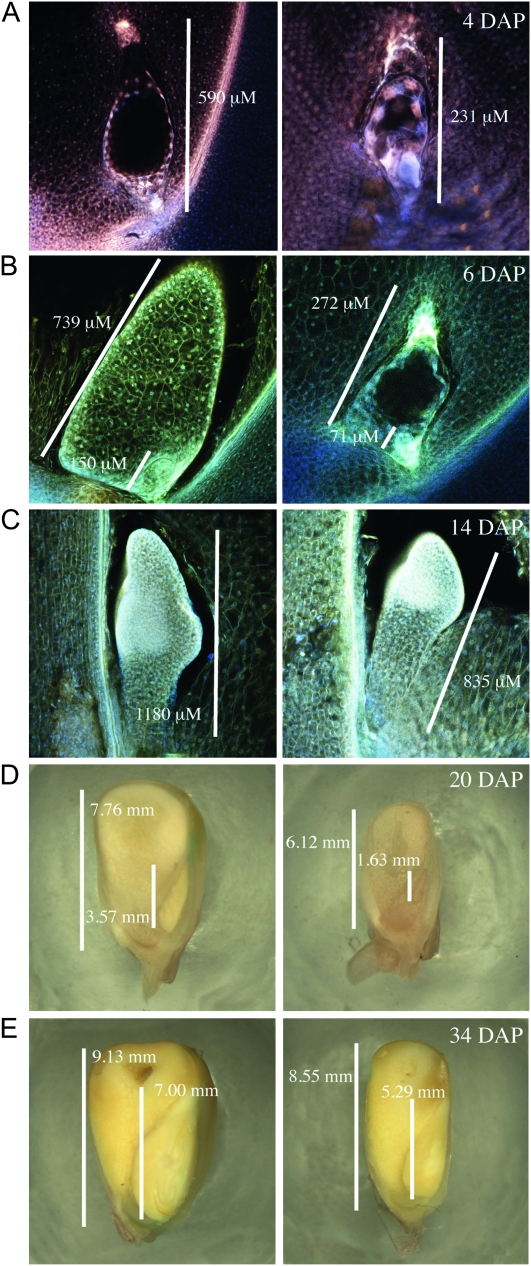
Figure 5.—
stt1 seeds are developmentally delayed compared to wild type. Embryos and/or endosperm 4 DAP (A), 6 DAP (B), 14 DAP (C), 20 DAP (D), and 34 DAP (E). Left panels depict wild type-like kernels and right panels depict mutant kernels. Bars, length of each embryo or endosperm.
To determine whether the ovules that had evidence of PT entry without endosperm development at 3 DAP were fertilized but delayed in initiation of seed development, we examined 78 embryo sacs from a stt1/+ heterozygote crossed by wild type at 6 DAP (Figure 4, right graph). At 6 DAP, it was rare to detect PT entry without endosperm development when compared to 3 DAP, possibly because the distinctive bright fluorescence of the penetrated synergid had faded. For wild-type embryo sacs at 6 DAP, there were no significant changes in the percentage of ovules for each class when compared to 3 DAP. The percentage of stt1 embryo sacs that had initiated endosperm development at 6 DAP was also unchanged from 3 DAP, suggesting that some stt1 embryo sacs never initiate seed development despite pollen tube entry. That this phenotype is due to a lack of fertilization of these stt1 embryo sacs is consistent with these observations, but alternate models are also possible.
Immaturity of stt1 embryo sacs at the time of pollination could explain a reduced fertilization rate. To test this hypothesis, pollination of the T2-9/N wx1/+ +/stt1 females by wx1 T2-9d males was performed on ears at different stages of maturity. Allowing embryo sacs more time to mature did not increase the transmission rate of stt1, making it less likely that the reduced female transmission of stt1 is a simple consequence of delayed maturation (Table S2).
stunter1 embryos and endosperms are developmentally delayed:
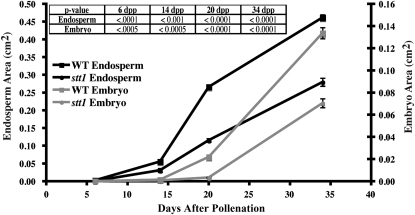
We examined developing kernels from crosses of stt1/+ heterozygous females by homozygous wild-type males at 3, 4, 6, 14, 20, and 34 DAP (Figures 4 and 5). At all time points we could clearly identify two classes of kernels, where one class had significantly smaller embryos and endosperms (Figure 6). The endosperms in the stt1/+ kernels were 30–66% smaller than in the wild-type kernels, while the embryos were 45–85% smaller in stt1/+. Retarded development of stt1 embryos likely caused their reduced size (e.g., 14 DAP wild-type embryos were at the coleoptile stage of development while the stt1/+ embryos were still in transition stage; Figure 5C). Though developmentally delayed, no gross morphological differences in the embryo or endosperm of the stt1/+ seeds were apparent. All regions of the differentiated endosperm (aleurone, CSE, BETL, and ESR) developed typical cell shapes and sizes (Scanlon and Takacs 2009).
Figure 6.—
Growth rate and size of stt1 seeds. The area of the endosperm and embryo at the median longitudinal cut face for WT and miniature kernels from stt1/+ ears at 6, 14, 20, and 34 DAP. Error bars are ±1 SEM, N ≥ 10 for each data point. The table inset lists the P values for comparisons of stt1 and wild type at each pair of data points.
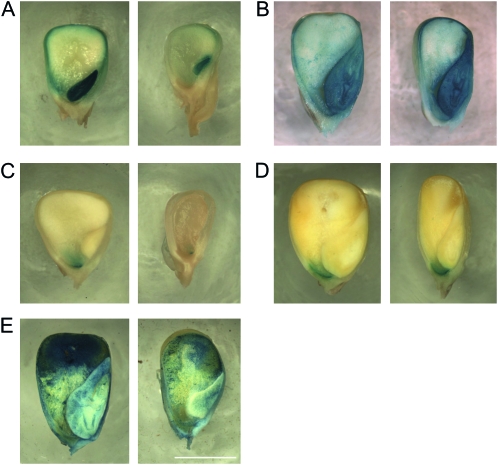
To determine the effects of stt1 in more detail we examined several markers of endosperm development. Since some maternal effect mutants cause kernels to develop patches lacking aleurone (Gavazzi et al. 1997) or BETL (Gutierrez-Marcos et al. 2006), and some zygotic mutants ectopically express aleurone tissue (Costa et al. 2003), we examined the expression of markers for these two tissues. For aleurone, we crossed stt1/+ females by males carrying ProVp1∷GUS and stained for GUS activity in mutant and wild-type endosperms at 20 and 34 DAP (Figure 7) (Cao et al. 2007). At 20 DAP, while the larger wild-type kernels showed GUS activity in the entire aleurone layer and embryo, the miniature kernels only showed expression in the embryo and in the aleurone layer at the very crown of the seed where VP1 expression begins. By 34 DAP, both large and miniature seeds showed VP1 expression throughout the aleurone layer, indicating that VP1 expression in the aleurone is delayed in the mutant kernels but eventually develops the same pattern as wild type.
Figure 7.—
stt1 effects on cell type differentiation and maturation. (A and C) 20 DAP; (B, D, and E) 34 DAP. Kernels from a stt1/+ ear expressing (A and B) ProVP1∷GUS in the aleurone layer and embryo or (C and D) ProBet1∷GUS in the BETL. (E) Kernels from a stt1/+ ear stained with Evans Blue to indicate the extent of PCD in the CSE. Left panels depict wild type-like kernels and right panels depict stt1 kernels. Bar, 5 mm.
For the BETL, we crossed stt1/+ females by males carrying ProBET1∷GUS, which should be active throughout the basal domain of the endosperm (Hueros et al. 1999). At 34 DAP, the area of GUS staining in the kernels was similar between normal and miniature kernels, but the area was smaller in the miniature kernels at 20 DAP, suggesting that the development of the BETL, like that of the aleurone, is delayed in stt1 kernels (Figure 7).
Programmed cell death of the CSE, which typically begins at the crown of the kernel and progresses to the base, was examined using Evans Blue staining, which stains dead cells (Young et al. 1997; Young and Gallie 2000). At 34 DAP, a smaller region of less intense staining was seen in the miniature kernels compared to the normal kernels (Figure 7), suggesting that PCD progression was delayed by stt1. Taken together, these data suggest that the stt1 mutation causes significant delays in all aspects of endosperm and embryo development beginning at fertilization.
Embryos and endosperms lacking both maternal and paternal wild-type stt1 activity arrest early:
Lack of stt1 pollen transmission precluded an analysis of stt1 homozygotes for possible mutant phenotypes. To test for sporophytic roles of stt1, we crossed stt1 females with plants carrying a reciprocal translocation between the supernumerary B chromosome and the long arm of chromosome 2, carrying the wild-type allele of stt1. Nondisjunction of the B-A chromosome during the second pollen mitosis produces a pollen grain carrying one sperm cell with zero doses of the long arm of chromosome 2 (i.e., no copies of wild-type paternal Stt1+) and another sperm carrying two copies of the long arm of chromosome 2. Fertilization of stt1 embryo sacs by such a pollen grain produces one progeny (either the embryo or endosperm) with no paternal copies of Stt1+ (hypoploid) and the other progeny (either the endosperm or embryo) with two copies (hyperploid). The hypoploid embryos and endosperms produced by fertilization of stt1 embryo sacs consequently have no functional stt1 alleles.
To identify stt1/− hypoploid individuals, we crossed stt1/+; a2/+ heterozygotes as females by males carrying the compound B-A chromosome, TB-5S-2L, which is carrying A2+ and Stt1+. Fertilization of a2 embryo sacs by pollen grains that undergo nondisjunction would produce seeds that either contain a hypoploid embryo (anthocyaninless with no paternal A2+ or Stt1+) with a hyperploid endosperm (purple with two paternal copies of A2+ and Stt1+) or a hyperploid embryo and a hypoploid endosperm. Fertilization of embryo sacs by pollen grains in which the chromosomes disjoin normally produces seeds with one paternal dose of both A2+ and Stt1+ in the endosperm and the embryo causing both to be purple, a balanced individual similar to pollination by stocks without B-A translocations.
Within the class of seed with A2+ in the endosperm, seeds potentially carrying stt1 hypoploid embryos were identified by an aborted embryo/germless kernel phenotype that was more severe than the miniature stt1/+ seed phenotype. Since identification of seeds lacking anthocyanin in the embryo was difficult when the endosperm was purple, other candidate seeds for stt1 hypoploid embryos having miniature, purple (i.e., A2+) endosperms and normal embryos were progeny tested for stt1 and a2 in the embryo. Fourteen plants from seeds with purple miniature endosperms segregated miniature seed when crossed by a2, but none were stt1/− hypoploids, on the basis of the lack of the spindly growth habit characteristic of B-5S-2L hypoploids and the segregation of A2+ in the progeny (Beckett 1994; Birchler and Guo 1997). For comparison, 23 normal, purple seeds were tested and 7/23 exhibited the hypoploid syndrome. The difference in the percentages of hypoploid individuals in these two classes of seed is statistically significant (Fisher's exact test; P = 0.01), demonstrating that there is a deficit of stt1 kernels with hypoploid embryos.
Due to the potential lethality of stt1/− hypoploid embryos, we tested for a similar phenotype for stt1/− hypoploid endosperms (rare miniature seeds with yellow endosperms and purple embryos). Of the eight miniature seeds tested, all had hyperploid embryos producing >90% A2+ progeny on the resultant ears, but none segregated stt1. The lack of stt1/+ ears from the eight miniature seeds tested is statistically significant (likelihood that stt1/+ plants were missed by random chance P = 0.04), indicating that stt1 hypoploids in the endosperm are not recoverable as mature seeds. The small seed size in the kernels tested was likely caused by the variable effects of hypoploidy for the B-5S-2L chromosome in the endosperm rather than by maternal inheritance of the stt1 mutation.
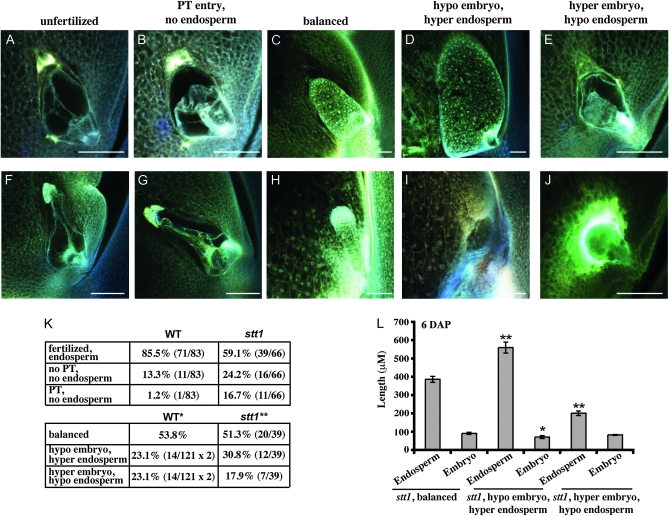
To identify the phenotype of stt1/− embryos and endosperm, we examined ovules of stt1/+ females crossed by TB-5S-2L males at 6 DAP using confocal microscopy. The ratios of wild-type and stt1 ovules fertilized (Figure 8, C and H), unfertilized without evidence of PT entry (Figure 8, A and F), or with evidence of PT entry but without endosperm development (Figure 8, B and G) were similar to the ratios observed at 6 DAP when stt1/+ was crossed by the standard diploid stocks (Figures 4 and 8K), indicating that sperm cells lacking stt1 function can fertilize stt1 mutant female gametes as efficiently as wild-type sperm. However, within the class of fertilized ovules we observed several types of developing seeds that differed from what we observed in the standard cross (Figure 5B). In addition to the typical wild-type and stt1 seeds (presumably genetically balanced) (Figure 8, C and H), we also observed ovules that had well-developed stt1 endosperms with very small, aborted, or absent embryos (Figure 8D) and ovules that had developing embryos with little or no endosperm proliferation (Figure 8E). These novel phenotypes likely represent individuals with hypoploid stt1/− embryos (31% hypoploid embryos) and hypoploid stt1/− endosperms (18% hypoploid endosperms), respectively (Figure 8K). We calculated the expected rates of nondisjunction from a cross of a w3/+ female by the same TB-5S-2L plants and found that the rates of nondisjunction as measured by the frequency of hypoploidy of w3 in the endosperm and embryo, identified as described in materials and methods, were not statistically different from the observed rates of hypoploidy of stt1 (χ2 = 4.04, P = 0.13).
Figure 8.—
Lethality of hypoploid stt1/− embryos and endosperms. Confocal micrographs of embryo sacs and developing seeds from stt1/+ crossed as a female by TB-5S-2L males at 6 DAP (A–H) or 10 DAP (I and J). (A–E and I and J) stt1. (F–H) WT. Bars, 100 μm (A–J). (K) Fertilization rates (top) and frequency of kernels with each genetic constitution (bottom) resulting from stt1/+ crossed as a female by TB-5S-2L as a male 6 DAP. Rates of hypoploidy for *WT calculated from a cross of w3/+ females by the same TB-5S-2L males and **stt1 calculated from the frequency of novel stt1 phenotypes. (L) Embryo and endosperm length (±SEM) of stt1 individuals from stt1/+ by TB-5S-2L for each genetic constitution. *Significantly different from balanced stt1 individuals (*P < 0.05 or **P < 0.001).
At 10 DAP, the individuals with stt1/− hypoploid embryos were not very different from 6 DAP and would likely form seeds that were germless or had aborted embryos (as supported by the novel mature seed phenotype) (Figure 8I). However, those individuals with stt1/− hypoploid endosperms had already begun to abort at 10 DAP and would likely never form an identifiable seed (in agreement with the absence of these individuals at ear maturity) (Figure 8J).
Interestingly, despite abortion of stt1/− seeds at 10 DAP, the stt1 hyperploid embryos could develop until 6 DAP without the support of a developing endosperm (Figure 8E). At 6 DAP, the globular-stage embryos of stt1 seeds with nonproliferating stt1/− endosperms are not significantly different in size from balanced stt1 individuals (Figure 8L). This phenotype suggests that early embryo development in maize is supported directly by the mother plant but later embryo development requires a functional endosperm.
DISCUSSION
stunter1 is a new maternal effect mutant in maize:
Seed development in angiosperms is a highly regulated process requiring gene activity not only in the genomes of the embryo and endosperm but also that of the female gametophyte (for reviews see Olsen 2004; Kohler and Grossniklaus 2005; Pien and Grossniklaus 2007; Jullien and Berger 2009). Analyses of maternal effect mutants, such as baseless1 in maize, have demonstrated that the morphology of the embryo sac can have a significant effect on seed development (Gutierrez-Marcos et al. 2006). Our studies have identified a new maternal effect gene, stt1, which is required in the female gametophyte for proper embryo sac development. Similar to baseless1, improper development of the female gametophyte is likely the primary cause for the maternal effects of stt1. stt1 mutant seeds are developmentally delayed and reduced in size but are patterned correctly with the typical constitution of cell types.
stunter1 affects female gametophyte development:
The stt1 mutation causes abnormal and delayed development of the female gametophyte prior to fertilization such that the mature embryo sac is smaller, most notably in the size of the central cell. Additionally, the antipodal cells in stt1 embryo sacs are larger and more vacuolated than in wild type. To date, little is known about the functions of the antipodal cells in fertilization and seed development. It has been speculated that in maize these cells play a specialized role in nutrient transfer from the nucellus of the mother plant into the female gametophyte, because they possess papillate cell walls (Diboll and Larson 1966; Huang and Sheridan 1994). Consequently, changes in the morphology of the antipodal cells and thus the ability to transfer nutrients into the female gametophyte could result in the smaller size of the stt1 embryo sacs. However, stt1 embryo sacs are smaller than wild-type embryo sacs prior to cellularization making it likely that factors acting early in megagametogenesis lead to the reduced size of stt1 embryo sacs. Whether or not these factors are involved in nutrient transport into embryo sacs at the syncytial stage and later into antipodal cells remains to be determined. Despite these abnormalities, stt1 embryo sacs attract pollen tubes at a normal frequency but some may fail to be fertilized despite pollen tube entry.
stunter1 affects male gametophyte development:
Like mutant embryo sacs, stt1 pollen grains are smaller than wild type and also germinate poorly and extend small, stunted pollen tubes. These mutant pollen grains are likely nonfunctional, as we are unable to detect stt1 male transmission even when competition with wild-type pollen grains is reduced. It is possible that, similar to megagametogenesis, microgametogenesis in stt1 is slower and mutant pollen grains never fully mature, despite having normal starch filling and sperm morphology.
Of the known pollen mutants in maize and Arabidopsis, many are affected during pollen development and a number have been identified as playing a role in the progamic phase, including mutants in maize and Arabidopsis pollen specific Rop GTPases (reviewed in Bedinger and Fowler 2009). Mutations in maize rop2 or Arabidopsis ROP1 result in defective pollen tube growth, reduced ability to compete with wild-type pollen, and shorter, broader pollen tubes (Li et al. 1999; Arthur et al. 2003). These phenotypes are distinct from the pollen tubes of stt1 both in their appearance and in their ability to function when competition from wild-type pollen is reduced.
Vesicle fusion is very dynamic in the growing pollen tube (Hicks et al. 2004), and vesicle and vacuole formation play significant roles in development of both the embryo sac and pollen and may contribute to the size of both gametophytes (for reviews see Bedinger and Fowler 2009; Evans and Grossniklaus 2009). The SABRE-like proteins encoded by aberrant pollen transmission1 (apt1) in maize and KINKY POLLEN (KIP) in Arabidopsis are critical for membrane trafficking in the pollen tube tip (Procissi et al. 2003; Xu and Dooner 2006). Mutations in apt1 and KIP result in pollen grains that germinate, but extend short, twisted, or kinky pollen tubes, which, like pollen of rop mutants, are distinct from the effects of stt1 in appearance and their ability to achieve fertilization. However, a more severe impairment in membrane trafficking could explain the arrested pollen tubes, smaller pollen grains, and smaller central cells in stt1 mutants. In addition to identification of the stt1 gene, a more detailed characterization of the developing pollen and the individual cell types of the embryo sac will help to determine the causes of these phenotypes.
Role of stunter1 in the developing seed:
We have shown that stt1 continues to have an effect during development of the seed after fertilization. stt1 endosperms develop more slowly than wild type in growth, in onset of expression of Vp1 and Bet1, and in initiation of PCD. Although Vp1 and Bet1 show delayed expression, the aleurone and BETL do eventually develop completely in stt1 seeds. The reduced size of stt1 seeds likely results from the slower growth and delayed development of the endosperm. The maternal effects of stt1 on the embryo may be an indirect effect of an abnormal endosperm; alternatively, maternal stt1 may be required in both the embryo and the endosperm independently.
Since the defects in pollen function precluded generation of stt1 homozygotes, we took advantage of nondisjunction of maize B-A chromosomes to produce embryos and endosperms lacking the paternal allele of stt1. Our data from these crosses indicates that postfertilization expression of stt1 is essential for seed development and that both maternal and paternal alleles are active in the embryo and endosperm. In these crosses we saw novel phenotypes in the endosperms or embryos of different seeds, consistent with the absence of maternal and paternal wild-type stt1 function (from maternal inheritance of the stt1 mutation and paternal inheritance of a chromosome arm deletion). Seeds with cellularized endosperms and arrested or degenerating embryos suggest that there is postfertilization expression of both stt1 alleles in the embryo. Similarly, seeds with globular-stage embryos developing with no endosperm proliferation suggest that there is postfertilization expression of both stt1 alleles in the endosperm. Since a subset of these endosperms undergo one or two rounds of free-nuclear divisions, it is likely that arrest of stt1/− endosperm development produces this class of seeds rather than failure of stt1-deficient sperm to fertilize stt1 central cells. These phenotypes also demonstrate that requirements for postfertilization stt1 function in the embryo and endosperm are partially independent of each other.
It is striking to note how much the embryo will develop without the support of a developing endosperm, even endosperms that undergo no free nuclear divisions. Our findings in maize are similar to those seen in several Arabidopsis gametophyte mutants, which produce embryos without producing endosperm (e.g., retinoblastoma related1, cdka-1, and msi1 mutants) (Iwakawa et al. 2006; Chen et al. 2008; Ingouff et al. 2009). In all cases of embryo development without endosperm formation in Arabidopsis, the embryos arrest at the globular stage, similar to stt1/+/+ embryos with a hypoploid stt1/− endosperm, suggesting that in monocots and dicots the early stages of embryo development are supported directly by the mother plant and that postglobular stage development is supported by the developing endosperm.
A parsimonious model for stt1 action in seed development is that the abnormal morphology of the embryo sacs is responsible for the slower development of the seed. However, we cannot at this time rule out direct, postfertilization effects on seed size caused by inheritance of stt1 rather than an indirect effect of stt1 embryo sac abnormalities. stt1 can be classified as a new maternal effect miniature seed mutant with novel gametophyte phenotypes. Of known maize mutants, only Mn∷Uq bears any resemblance to stt1 (Pan and Peterson 1989). The Mn∷Uq mutation is only transmitted maternally, and heterozygotes segregate large and small, nonfunctional pollen, also like stt1, but Mn∷Uq seeds are more severely abnormal than stt1. Further investigation is necessary to determine whether stt1 and Mn∷Uq are allelic or affect the same genetic pathway.
Acknowledgments
We thank Bill Sheridan, Phil Becraft, and Richard Thompson for stocks of the TB-5S-2L B-A-A translocation, the ProVp1∷GUS reporter, and the ProBet1∷GUS reporter, respectively, and Antony Chettoor, Clayton Coker, Enrico Magnani, Yongxian Lu, Anisha Patel, Sejal Parekh, and Kathy Barton for technical assistance and helpful discussions. This project was supported by a postdoctoral fellowship from the National Research Initiative of the Department of Agriculture Cooperative State Research, Education, and Extension Service (2008-35304-04620) to A.R.P.
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.110.125286/DC1.
References
- Andreuzza, S., J. Li, A. E. Guitton, J. E. Faure, S. Casanova et al., 2009. DNA LIGASE I exerts a maternal effect on seed development in Arabidopsis thaliana. Development 137 73–81. [DOI] [PubMed] [Google Scholar]
- Arthur, K. M., Z. Vejlupkova, R. B. Meeley and J. E. Fowler, 2003. Maize ROP2 GTPase provides a competitive advantage to the male gametophyte. Genetics 165 2137–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger, D. L., and J. A. Birchler, 2002. Maize tertiary trisomic stocks derived from B-A translocations. J. Hered. 93 42–47. [DOI] [PubMed] [Google Scholar]
- Beckett, J. B., 1994. Locating recessive genes to chromosome arms with B-A translocations, pp. 315-–327 in The Maize Handbook, edited by M. Freeling and V. Walbot. Springer-Verlag, New York.
- Bedinger, P., and S. D. Russell, 1994. Gametogenesis in maize, pp. 48–61 in The Maize Handbook, edited by M. Freeling and V. Walbot. Springer-Verlag, New York.
- Bedinger, P. A., and J. E. Fowler, 2009. The maize male gametophyte, pp. 57–77 in Handbook of Maize: Its Biology, edited by J. L. Bennetzen and S. C. Hake. Springer-Verlag, New York.
- Birchler, J. A., and M. Guo, 1997. Marker systems for the phenotypic recognition of maternally derived trisomics in maize. J. Hered. 88 27–30. [Google Scholar]
- Boavida, L. C., B. Shuai, H. J. Yu, G. C. Pagnussat, V. Sundaresan et al., 2009. A collection of Ds insertional mutants associated with defects in male gametophyte development and function in Arabidopsis thaliana. Genetics 181 1369–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bommert, P., and W. Werr, 2001. Gene expression patterns in the maize caryopsis: clues to decisions in embryo and endosperm development. Gene 271 131–142. [DOI] [PubMed] [Google Scholar]
- Brink, R. A., and J. H. MacGillivray, 1924. Segregation for the waxy character in maize pollen and differential development of the male gametophyte. Am. J. Bot. 11 465–469. [Google Scholar]
- Cao, X., L. M. Costa, C. Biderre-Petit, B. Kbhaya, N. Dey et al., 2007. Abscisic acid and stress signals induce Viviparous1 expression in seed and vegetative tissues of maize. Plant Physiol. 143 720–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Z., J. L. Tan, M. Ingouff, V. Sundaresan and F. Berger, 2008. Chromatin assembly factor 1 regulates the cell cycle but not cell fate during male gametogenesis in Arabidopsis thaliana. Development 135 65–73. [DOI] [PubMed] [Google Scholar]
- Christensen, C. A., E. J. King, J. R. Jordan and G. N. Drews, 1997. Megagametogenesis in Arabidopsis wild type and the Gf mutant. Sex. Plant Reprod. 10 49–64. [Google Scholar]
- Clark, J. K., and W. F. Sheridan, 1991. Isolation and characterization of 51 embryo-specific mutations of maize. Plant Cell 3 935–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordts, S., J. Bantin, P. E. Wittich, E. Kranz, H. Lorz et al., 2001. ZmES genes encode peptides with structural homology to defensins and are specifically expressed in the female gametophyte of maize. Plant J. 25 103–114. [DOI] [PubMed] [Google Scholar]
- Costa, L. M., J. F. Gutierrez-Marcos, T. P. Brutnell, A. J. Greenland and H. G. Dickinson, 2003. The globby1–1 (glo1–1) mutation disrupts nuclear and cell division in the developing maize seed causing alterations in endosperm cell fate and tissue differentiation. Development 130 5009–5017. [DOI] [PubMed] [Google Scholar]
- Demerec, M., 1924. A case of pollen dimorphism in maize. Am. J. Bot. 11 461–464. [Google Scholar]
- Diboll, A. G., and D. A. Larson, 1966. An electron microscopic study of the mature megagametophyte in Zea mays. Am. J. Bot. 53 391–402. [PubMed] [Google Scholar]
- Evans, M. M. S., and U. Grossniklaus, 2009. The maize megagametophyte, pp. 79–104 in Handbook of Maize: Its Biology, edited by J. L. Bennetzen and S. Hake. Springer-Verlag, New York.
- Evans, M. M. S., and J. L. Kermicle, 2001. Interaction between maternal effect and zygotic effect mutations during maize seed development. Genetics 159 303–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavazzi, G., S. Dolfini, D. Allegra, P. Castiglioni, G. Todesco et al., 1997. Dap (Defective aleurone pigmentation) mutations affect maize aleurone development. Mol. Gen. Genet. 256 223–230. [DOI] [PubMed] [Google Scholar]
- Golden, T. A., S. E. Schauer, J. D. Lang, S. Pien, A. R. Mushegian et al., 2002. SHORT INTEGUMENTS1/SUSPENSOR1/CARPEL FACTORY, a Dicer homolog, is a maternal effect gene required for embryo development in Arabidopsis. Plant Physiol. 130 808–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossniklaus, U., and K. Schneitz, 1998. The molecular and genetic basis of ovule and megagametophyte development. Semin. Cell Dev. Biol. 9 227–238. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Marcos, J. F., L. M. Costa and M. M. S. Evans, 2006. Maternal gametophytic baseless1 is required for development of the central cell and early endosperm patterning in maize (Zea mays). Genetics 174 317–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks, G. R., E. Rojo, S. Hong, D. G. Carter and N. V. Raikhel, 2004. Germinating pollen has tubular vacuoles, displays highly dynamic vacuole biogenesis, and requires VACUOLESS1 for proper function. Plant Physiol. 134 1227–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holding, D. R., and P. S. Springer, 2002. The Arabidopsis gene PROLIFERA is required for proper cytokinesis during seed development. Planta 214 373–382. [DOI] [PubMed] [Google Scholar]
- Huang, B. Q., and W. F. Sheridan, 1994. Female gametophyte development in maize: microtubular organization and embryo sac polarity. Plant Cell 6 845–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hueros, G., E. Gomez, N. Cheikh, J. Edwards, M. Weldon et al., 1999. Identification of a promoter sequence from the BETL1 gene cluster able to confer transfer-cell-specific expression in transgenic maize. Plant Physiol. 121 1143–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh, J. H., M. J. Bauer, T. F. Hsieh and R. L. Fischer, 2008. Cellular programming of plant gene imprinting. Cell 132 735–744. [DOI] [PubMed] [Google Scholar]
- Ingouff, M., T. Sakata, J. Li, S. Sprunck, T. Dresselhaus et al., 2009. The two male gametes share equal ability to fertilize the egg cell in Arabidopsis thaliana. Curr. Biol. 19 R19–R20. [DOI] [PubMed] [Google Scholar]
- Iwakawa, H., A. Shinmyo and M. Sekine, 2006. Arabidopsis CDKA;1, a cdc2 homologue, controls proliferation of generative cells in male gametogenesis. Plant J. 45 819–831. [DOI] [PubMed] [Google Scholar]
- Jewell, D. C., and N. Islam-Faridi, 1994. A technique for somatic chromosome preparation and C-banding of maize, pp. 484–493 in The Maize Handbook, edited by M. Freeling and V. Walbot. Springer-Verlag, New York.
- Johnson, M. A., and J. Bender, 2009. Reprogramming the epigenome during germline and seed development. Genome Biol. 10 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Rhoades, M. W., J. O. Borevitz and D. Preuss, 2007. Genome-wide expression profiling of the Arabidopsis female gametophyte identifies families of small, secreted proteins. PLoS Genet. 3 1848–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jullien, P. E., and F. Berger, 2009. Gamete-specific epigenetic mechanisms shape genomic imprinting. Curr. Opin. Plant Biol. 12 637–642. [DOI] [PubMed] [Google Scholar]
- Kohler, C., and U. Grossniklaus, 2005. eed development and genomic imprinting in plants. Prog. Mol. Subcell. Biol. 38 237–262. [DOI] [PubMed] [Google Scholar]
- Le, Q., J. F. Gutierrez-Marcos, L. M. Costa, S. Meyer, H. G. Dickinson et al., 2005. Construction and screening of subtracted cDNA libraries from limited populations of plant cells: a comparative analysis of gene expression between maize egg cells and central cells. Plant J. 44 167–178. [DOI] [PubMed] [Google Scholar]
- Li, H., Y. Lin, R. M. Heath, M. X. Zhu and Z. Yang, 1999. Control of pollen tube tip growth by a Rop GTPase-dependent pathway that leads to tip-localized calcium influx. Plant Cell 11 1731–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, Y., and O. E. Nelson, 1975. Amino acid composition and storage proteins in two high lysine mutants in maize. Cereal Chem. 52 412–419. [Google Scholar]
- McCarty, D. R., A. M. Settles, M. Suzuki, B. C. Tan, S. Latshaw et al., 2005. Steady-state transposon mutagenesis in inbred maize. Plant J. 44 52–61. [DOI] [PubMed] [Google Scholar]
- Neuffer, M. G., and W. F. Sheridan, 1980. Defective kernel mutants of maize. I. Genetic and lethality studies. Genetics 95 929–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo, Q. A., J. M. Moore, R. Baskar, U. Grossniklaus and V. Sundaresan, 2007. Arabidopsis GLAUCE promotes fertilization-independent endosperm development and expression of paternally inherited alleles. Development 134 4107–4117. [DOI] [PubMed] [Google Scholar]
- Olsen, O. A., 2001. ENDOSPERM DEVELOPMENT: cellularization and cell fate specification. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52 233–267. [DOI] [PubMed] [Google Scholar]
- Olsen, O. A., 2004. Nuclear endosperm development in cereals and Arabidopsis thaliana. Plant Cell 16(Suppl): S214–S227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen, O. A., C. Linnestad and S. E. Nichols, 1999. Developmental biology of the cereal endosperm. Trends Plant Sci. 4 253–257. [DOI] [PubMed] [Google Scholar]
- Pagnussat, G. C., H. J. Yu, Q. A. Ngo, S. Rajani, S. Mayalagu et al., 2005. Genetic and molecular identification of genes required for female gametophyte development and function in Arabidopsis. Development 132 603–614. [DOI] [PubMed] [Google Scholar]
- Pan, Y. B., and P. A. Peterson, 1989. Tagging of a maize gene involved in kernel development by an activated Uq transposable element. Mol. Gen. Genet. 219 324–327. [DOI] [PubMed] [Google Scholar]
- Patterson, E. B., 1978. Properties and uses of duplicate-deficient chromosome complements in maize, pp. 693–710 in Maize Breeding and Genetics, edited by D. B. Walden. John Wiley & Sons, New York.
- Pien, S., and U. Grossniklaus, 2007. Polycomb group and trithorax group proteins in Arabidopsis. Biochim. Biophys. Acta 1769 375–382. [DOI] [PubMed] [Google Scholar]
- Procissi, A., A. Guyon, E. S. Pierson, A. Giritch, B. Knuiman et al., 2003. KINKY POLLEN encodes a SABRE-like protein required for tip growth in Arabidopsis and conserved among eukaryotes. Plant J. 36 894–904. [DOI] [PubMed] [Google Scholar]
- Randolph, L. F., 1936. Developmental morphology of the caryopsis in maize. J. Agric. Res. 53 881–916. [Google Scholar]
- Reiser, L., and R. L. Fischer, 1993. The ovule and the embryo sac. Plant Cell 5 1291–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghai-Maroof, M. A., K. M. Soliman, R. A. Jorgensen and R. W. Allard, 1984. Ribosomal DNA spacer-length polymorphisms in barley: mendelian inheritance, chromosomal location, and population dynamics. Proc. Natl. Acad. Sci. USA 81 8014–8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlon, M. J., P. S. Stinard, M. G. James, A. M. Myers and D. S. Robertson, 1994. Genetic analysis of 63 mutations affecting maize kernel development isolated from Mutator stocks. Genetics 136 281–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlon, M. J., and E. M. Takacs, 2009. Kernel biology, pp. 121–143 in The Handbook of Maize: Its Biology, edited by J. L. Bennetzen and S. Hake. Springer-Verlag, New York.
- Settles, A. M., D. R. Holding, B. C. Tan, S. P. Latshaw, J. Liu et al., 2007. Sequence-indexed mutations in maize using the UniformMu transposon-tagging population. BMC Genomics 8 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan, W. F., and M. G. Neuffer, 1980. Defective kernel mutants of maize II. Morphological and embryo culture studies. Genetics 95 945–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan, W. F., and J. K. Clark, 1994. Fertilization and embryogeny in maize, pp. 1–10 in The Maize Handbook, edited by M. Freeling and V. Walbot. Springer-Verlag, New York.
- Springer, P. S., D. R. Holding, A. Groover, C. Yordan and R. A. Martienssen, 2000. The essential Mcm7 protein PROLIFERA is localized to the nucleus of dividing cells during the G(1) phase and is required maternally for early Arabidopsis development. Development 127 1815–1822. [DOI] [PubMed] [Google Scholar]
- Sprunck, S., U. Baumann, K. Edwards, P. Langridge and T. Dresselhaus, 2005. The transcript composition of egg cells changes significantly following fertilization in wheat (Triticum aestivum L.). Plant J. 41 660–672. [DOI] [PubMed] [Google Scholar]
- Steffen, J. G., I. H. Kang, J. Macfarlane and G. N. Drews, 2007. Identification of genes expressed in the Arabidopsis female gametophyte. Plant J. 51 281–292. [DOI] [PubMed] [Google Scholar]
- Vernoud, V., M. Hajduch, A.-S. Khaled, N. Depege and P. M. Rogowsky, 2005. Maize embryogenesis. Maydica 50 469–483. [Google Scholar]
- Vielle-Calzada, J. P., R. Baskar and U. Grossniklaus, 2000. Delayed activation of the paternal genome during seed development. Nature 404 91–94. [DOI] [PubMed] [Google Scholar]
- Walbot, V., and M. M. S. Evans, 2003. Unique features of the plant life cycle and their consequences. Nat. Rev. Genet. 4 369–379. [DOI] [PubMed] [Google Scholar]
- Walden, D. B., 1994. In vitro pollen germination, pp. 723–724 in The Maize Handbook, edited by M. Freeling and V. Walbot. Springer-Verlag, New York.
- Wuest, S. E., K. Vijverberg, A. Schmidt, M. Weiss, J. Gheyselinck et al., 2010. Arabidopsis female gametophyte gene expression map reveals similarities between plant and animal gametes. Curr. Biol. 20 506–512. [DOI] [PubMed] [Google Scholar]
- Xu, Z., and H. K. Dooner, 2006. The maize aberrant pollen transmission 1 gene is a SABRE/KIP homolog required for pollen tube growth. Genetics 172 1251–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, H., N. Kaur, S. Kiriakopolos and S. McCormick, 2006. EST generation and analyses towards identifying female gametophyte-specific genes in Zea mays L. Planta 224 1004–1014. [DOI] [PubMed] [Google Scholar]
- Young, T. E., and D. R. Gallie, 2000. Regulation of programmed cell death in maize endosperm by abscisic acid. Plant Mol. Biol. 42 397–414. [DOI] [PubMed] [Google Scholar]
- Young, T. E., D. R. Gallie and D. A. Demason, 1997. Ethylene mediated programmed cell death during maize endosperm development of Su and sh2 genotypes. Plant Physiol. 115 737–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, H. J., P. Hogan and V. Sundaresan, 2005. Analysis of the female gametophyte transcriptome of Arabidopsis by comparative expression profiling. Plant Physiol. 139 1853–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]