Abstract
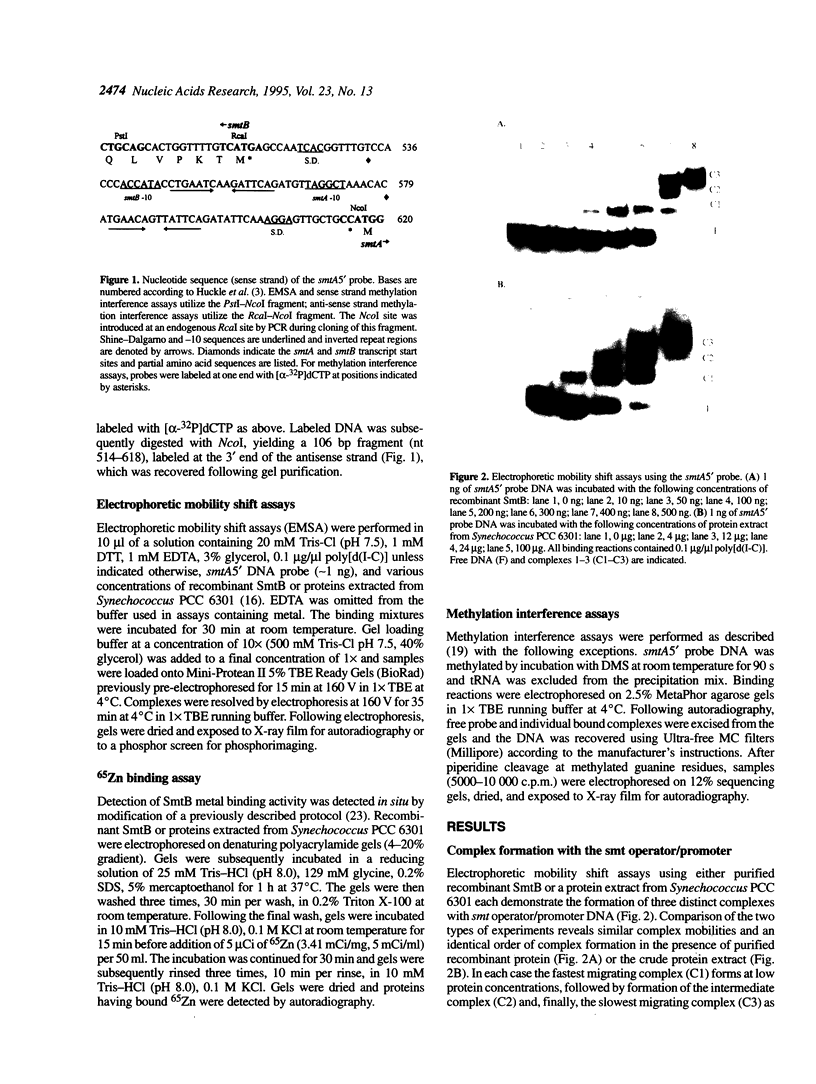
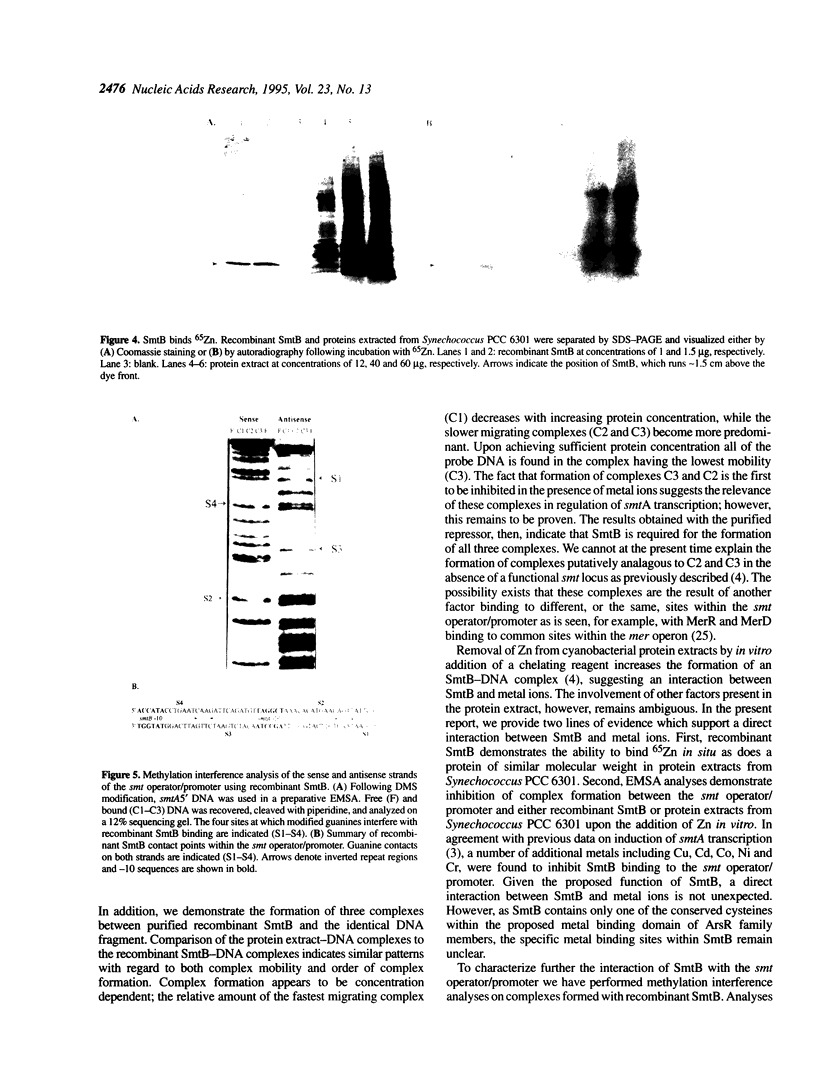
The smtB gene of Synechococcus PCC 7942 encodes a trans-acting repressor of the metal-regulated smtA gene that encodes a class II metallothionein. Recombinant SmtB has been expressed in Escherichia coli and purified. Electrophoretic mobility shift assays using recombinant SmtB or a protein extract from Synechococcus PCC 6301 reveal the concentration-dependent formation of three specific complexes with the smt operator/promoter. SmtB is also capable of direct interaction with metals as evidenced by 65Zn binding to the SmtB protein as well as the inhibition of repressor-DNA complex formation in the presence of various metal ions. Methylation interference analysis of such complexes identifies four protein contact points within the smt operator/promoter DNA. The points of contact appear to represent two pairs of binding sites, one pair in each of two inverted repeats (nt 548-563, 589-602). The contact points within each pair lie on opposing DNA strands and are separated by 10 bp, placing the repressor binding sites on opposite sides of the DNA helix. Based on electrophoretic mobility shift assays, methylation interference and molecular size calculations we propose that recombinant SmtB binds to the smt operator/promoter in multimeric fashion.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gupta A., Whitton B. A., Morby A. P., Huckle J. W., Robinson N. J. Amplification and rearrangement of a prokaryotic metallothionein locus smt in Synechococcus PCC 6301 selected for tolerance to cadmium. Proc Biol Sci. 1992 Jun 22;248(1323):273–281. doi: 10.1098/rspb.1992.0072. [DOI] [PubMed] [Google Scholar]
- Harrison S. C., Aggarwal A. K. DNA recognition by proteins with the helix-turn-helix motif. Annu Rev Biochem. 1990;59:933–969. doi: 10.1146/annurev.bi.59.070190.004441. [DOI] [PubMed] [Google Scholar]
- Huckle J. W., Morby A. P., Turner J. S., Robinson N. J. Isolation of a prokaryotic metallothionein locus and analysis of transcriptional control by trace metal ions. Mol Microbiol. 1993 Jan;7(2):177–187. doi: 10.1111/j.1365-2958.1993.tb01109.x. [DOI] [PubMed] [Google Scholar]
- Ivey D. M., Guffanti A. A., Shen Z., Kudyan N., Krulwich T. A. The cadC gene product of alkaliphilic Bacillus firmus OF4 partially restores Na+ resistance to an Escherichia coli strain lacking an Na+/H+ antiporter (NhaA). J Bacteriol. 1992 Aug;174(15):4878–4884. doi: 10.1128/jb.174.15.4878-4884.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G., Silver S. Regulation and expression of the arsenic resistance operon from Staphylococcus aureus plasmid pI258. J Bacteriol. 1992 Jun;174(11):3684–3694. doi: 10.1128/jb.174.11.3684-3694.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondorosi E., Pierre M., Cren M., Haumann U., Buiré M., Hoffmann B., Schell J., Kondorosi A. Identification of NolR, a negative transacting factor controlling the nod regulon in Rhizobium meliloti. J Mol Biol. 1991 Dec 20;222(4):885–896. doi: 10.1016/0022-2836(91)90583-r. [DOI] [PubMed] [Google Scholar]
- Morby A. P., Turner J. S., Huckle J. W., Robinson N. J. SmtB is a metal-dependent repressor of the cyanobacterial metallothionein gene smtA: identification of a Zn inhibited DNA-protein complex. Nucleic Acids Res. 1993 Feb 25;21(4):921–925. doi: 10.1093/nar/21.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay D., Yu H. R., Nucifora G., Misra T. K. Purification and functional characterization of MerD. A coregulator of the mercury resistance operon in gram-negative bacteria. J Biol Chem. 1991 Oct 5;266(28):18538–18542. [PubMed] [Google Scholar]
- Olafson R. W., McCubbin W. D., Kay C. M. Primary- and secondary-structural analysis of a unique prokaryotic metallothionein from a Synechococcus sp. cyanobacterium. Biochem J. 1988 May 1;251(3):691–699. doi: 10.1042/bj2510691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson N. J., Gupta A., Fordham-Skelton A. P., Croy R. R., Whitton B. A., Huckle J. W. Prokaryotic metallothionein gene characterication and expression: chromosome crawling by ligation-mediated PCR. Proc Biol Sci. 1990 Dec 22;242(1305):241–247. doi: 10.1098/rspb.1990.0130. [DOI] [PubMed] [Google Scholar]
- Rosenstein R., Nikoleit K., Götz F. Binding of ArsR, the repressor of the Staphylococcus xylosus (pSX267) arsenic resistance operon to a sequence with dyad symmetry within the ars promoter. Mol Gen Genet. 1994 Mar;242(5):566–572. doi: 10.1007/BF00285280. [DOI] [PubMed] [Google Scholar]
- Rosenstein R., Peschel A., Wieland B., Götz F. Expression and regulation of the antimonite, arsenite, and arsenate resistance operon of Staphylococcus xylosus plasmid pSX267. J Bacteriol. 1992 Jun;174(11):3676–3683. doi: 10.1128/jb.174.11.3676-3683.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Francisco M. J., Hope C. L., Owolabi J. B., Tisa L. S., Rosen B. P. Identification of the metalloregulatory element of the plasmid-encoded arsenical resistance operon. Nucleic Acids Res. 1990 Feb 11;18(3):619–624. doi: 10.1093/nar/18.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J., Lindsay W. P., Huckle J. W., Morby A. P., Robinson N. J. Cyanobacterial metallothionein gene expressed in Escherichia coli. Metal-binding properties of the expressed protein. FEBS Lett. 1992 Jun 1;303(2-3):159–163. doi: 10.1016/0014-5793(92)80509-f. [DOI] [PubMed] [Google Scholar]
- Shi W., Wu J., Rosen B. P. Identification of a putative metal binding site in a new family of metalloregulatory proteins. J Biol Chem. 1994 Aug 5;269(31):19826–19829. [PubMed] [Google Scholar]
- Turner J. S., Morby A. P., Whitton B. A., Gupta A., Robinson N. J. Construction of Zn2+/Cd2+ hypersensitive cyanobacterial mutants lacking a functional metallothionein locus. J Biol Chem. 1993 Feb 25;268(6):4494–4498. [PubMed] [Google Scholar]
- Williams S. G., Attridge S. R., Manning P. A. The transcriptional activator HlyU of Vibrio cholerae: nucleotide sequence and role in virulence gene expression. Mol Microbiol. 1993 Aug;9(4):751–760. doi: 10.1111/j.1365-2958.1993.tb01735.x. [DOI] [PubMed] [Google Scholar]
- Williams S. G., Manning P. A. Transcription of the Vibrio cholerae haemolysin gene, hlyA, and cloning of a positive regulatory locus, hlyU. Mol Microbiol. 1991 Aug;5(8):2031–2038. doi: 10.1111/j.1365-2958.1991.tb00825.x. [DOI] [PubMed] [Google Scholar]
- Wu J., Rosen B. P. Metalloregulated expression of the ars operon. J Biol Chem. 1993 Jan 5;268(1):52–58. [PubMed] [Google Scholar]
- Wu J., Rosen B. P. The ArsR protein is a trans-acting regulatory protein. Mol Microbiol. 1991 Jun;5(6):1331–1336. doi: 10.1111/j.1365-2958.1991.tb00779.x. [DOI] [PubMed] [Google Scholar]