Abstract
In eukaryotic cells, the ubiquitin/proteasome system (UPS) is a key determinant of proteostasis as it regulates the turnover of damaged proteins. However, it is still unclear how the UPS integrates intrinsic and environmental challenges to promote organismal development and survival. Here, we set up an in vivo degradation assay to facilitate the genetic identification of ubiquitin-dependent proteolysis pathways in the multicellular organism Caenorhabditis elegans. Using this assay, we found that mild induction of protein-folding stress, which is nontoxic for wild-type worms, strongly reduces ubiquitin-dependent protein turnover. Ubiquitin-mediated degradation is also reduced by metabolic stress, which correlates with life-span extension. Unlike other stress conditions, however, acute heat stress results in enhanced rather than reduced proteolysis. Intriguingly, our study provides the first evidence for the existence of tissue-specific degradation requirements because loss of key regulators of the UPS, such as proteasomal subunits, causes accumulation of the model substrate, depending on the tissue type. Thus, here we establish a screenable degradation assay that allows diverse genetic screening approaches for the identification of novel cell-type-specific proteostasis networks important for developmental processes, stress response, and aging, thereby substantially extending the work on recently described mechanistic UPS reporter studies.
ENVIRONMENTAL conditions and developmental processes challenge the integrity of the proteome in every eukaryotic cell. The maintenance of proteostasis is a fundamental process orchestrated by refolding and degradation of unfolded proteins supporting organismal development and longevity (Powers et al. 2009). The ability to sustain protein quality control (PQC) is a long-term challenge for individual cells and entire organisms since high levels of damaged proteins accumulate during stress and aging. Defects in PQC affect proteostasis and often result in aggregation of unfolded proteins that can be toxic for cells. However, not all tissues are equally susceptible to the toxicity of protein aggregates (Gidalevitz et al. 2009), suggesting that there are tissue-specific differences in proteostasis pathways.
As part of the cellular PQC network, the ubiquitin/proteasome system (UPS) supports proteostasis by degradation of damaged proteins (Ciechanover et al. 2000; Kerscher et al. 2006). Substrates of the UPS are earmarked by covalent attachment of multiple ubiquitin molecules to an internal lysine residue. Typically, a chain of about four to six ubiquitin moieties is necessary and sufficient to target a substrate for degradation by the 26S proteasome, a multi-catalytic protease complex (Richly et al. 2005; Zhang et al. 2009). Polyubiquitylation of substrate proteins is usually mediated by an enzymatic cascade based on ubiquitin-activating enzymes (E1), ubiquitin-conjugating enzymes (E2), and ubiquitin protein ligases (E3). However, recently it was shown that in some cases polyubiquitylation requires the additional activity of a ubiquitin-chain elongation factor, called E4 enzyme (Hoppe 2005). In Saccharomyces cerevisiae, Ufd2p is the first E4 originally discovered in a genetic screen for mutants that are impaired in the turnover of ubiquitin fusion degradation (UFD) substrates (Johnson et al. 1995). Other so-called UFD proteins required for UFD substrate turnover in yeast are Ufd1p, Npl4p, and Cdc48p (CDC-48.1 and CDC-48.2 in Caenorhabditis elegans, p97 in mammals) (Richly et al. 2005). Together with the cofactors Ufd1p and Npl4p, Cdc48p forms a complex that functions as a ubiquitin-selective chaperone in substrate recruitment and ubiquitin chain assembly (Rape et al. 2001).
Detailed mechanistic insights in UPS regulation have been obtained in extensive studies during the past decade. However, those studies in yeast and tissue culture experiments are limited in their approaches in gaining further insight into the physiological relevance of ubiquitin-dependent degradation during development and survival of multicellular organisms. Here, we established an in vivo degradation assay to study protein turnover during development and in differentiated tissues in response to intrinsic and environmental challenges. In contrast to already existing degradation assays in tissue culture, invertebrates, or mice, applicable to mechanistic studies but not to high-throughput screening approaches (Johnson et al. 1995; Dantuma et al. 2000; Lindsten et al. 2003; Hamer et al. 2010), our in vivo degradation assay is suitable for the genetic identification of novel proteolysis factors and pathways important for developmental processes and longevity. Using this approach, we demonstrate cell-type-specific requirements for degradation factors of the UPS, which intriguingly suggest the existence of tissue-dependent proteostasis pathways rather than similar requirements in all tissues. Moreover, low doses of different stresses have an influence on the UPS, suggesting that different PQC pathways are activated through the existence of specialized stress sensors. In summary, we established an in vivo degradation assay that can serve to monitor and compare proteolytic networks, which are important in maintaining proteostasis during physiological and developmental challenges of whole organisms.
An in vivo degradation assay in C. elegans:
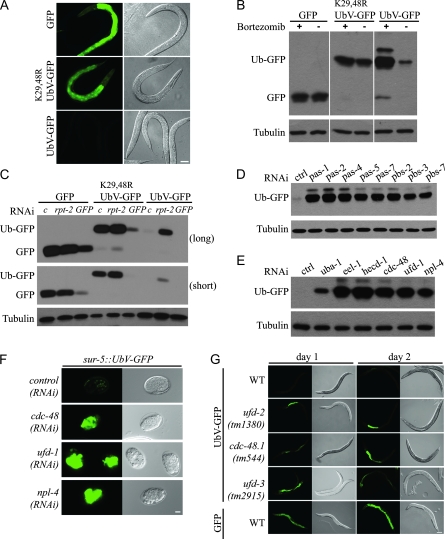
To establish an in vivo degradation assay in a multicellular organism, we engineered a UFD model substrate in which a noncleavable ubiquitin is N-terminally fused to GFP (UbV-GFP) and expressed under control of the sur-5 promoter that is active in most tissues of C. elegans (Gu et al. 1998). To rule out differences based on the expression of the transgene influencing the amount of substrate protein, GFP alone or mCherry were similarly expressed under control of the same promoter, and the constructs were stably integrated into the genome of unc-119(ed4) worms (Maduro and Pilgrim, 1995; Praitis et al. 2001). All strains used display comparable transgene expression as revealed by quantification of the mRNA levels (supporting information, Figure S1). Whereas GFP alone accumulates in several tissues including the hypodermis that surrounds the pharynx, the tail, vulva, and the intestine (Gu et al. 1998), no fluorescent signal is detectable in worms carrying the UbV-GFP fusion construct (Figure 1A). However, replacement of lysine (K) residues 29 and 48 with arginine (K29, 48R) in the ubiquitin moiety results in stabilization of UbV-GFP, corroborating previous findings in budding yeast (Johnson et al. 1995). Given the lack of GFP signal in worms expressing UbV-GFP, ubiquitylation of the UFD substrate at K29 and K48 is likely to cause its proteasomal degradation. Indeed, incubation of transgenic worms with the proteasome inhibitor bortezomib specifically stabilizes UbV-GFP but does not affect the protein level of GFP or K29,48RUbV-GFP (Figure 1B). To confirm the importance of the 26S proteasome for degradation of the UFD substrate, we depleted individual proteasomal subunits by RNA interference (RNAi). Similar to bortezomib treatment, rpt-2(RNAi) or depletion of other proteasomal subunits stabilized UbV-GFP (Figure 1, C and D). The downregulation of GFP or K29,48RUbV-GFP by GFP(RNAi) shows that all tissues expressing sur-5 can be efficiently targeted by RNAi treatment (Figure 1C and Figure 3A). Intriguingly, stabilization of UbV-GFP in animals lacking both p97 homologs CDC-48.1 and CDC-48.2 [hereafter referred to as CDC-48 depletion or cdc-48.1/cdc-48.2(RNAi)], UFD-1, NPL-4, UFD-2, or UFD-3 suggests the existence of a conserved UFD pathway in C. elegans (Figure 1E, E and G). To identify the corresponding E3 enzyme, we performed sequence comparison of the C. elegans genome to yeast Ufd4p and screened the potential candidates in our degradation assay. Interestingly, we found HECD-1 and EEL-1 to be important for degradation of the UFD substrate (Figure 1E). Thus, expression of the UPS model substrate UbV-GFP helped us to identify novel endogenous degradation factors in C. elegans.
Figure 1.—
In vivo detection of UPS activity. (A) Fluorescence and Nomarski images of L4-staged hermaphrodite worms expressing GFP, UbV-GFP, or K29,48RUbV-GFP from integrated transgenes under control of the sur-5 promoter. Fluorescence images were taken with the same exposure time. Scale bar, 50 μm. (B) Protein extracts of the strains used in A that were treated with 10 nm bortezomib (+) or DMSO as control (−) were analyzed by immunoblotting with GFP antibodies. Tubulin was used as loading control. (C) Lysates of the strains used in A were RNAi-depleted for control (c, empty RNAi vector), rpt-2, or GFP and analyzed by immunoblotting as in B. Long and short exposure is shown for comparison. (D and E) Lysates of worms expressing UbV-GFP and lacking the indicated genes were analyzed as in B. (F) Embryos carrying the sur-5∷UbV-GFP transgene and depleted for the indicated genes by RNAi were imaged with fluorescence and Nomarski optics as in A. Scale bar, 10 μm. (G) Fluorescence (same exposure) and Nomarski images of adult worms carrying the integrated transgenes sur-5∷UbV-GFP (UbV-GFP) or sur-5∷GFP (GFP) in N2 (WT, wild type) and the indicated deletion mutants at day 1 and day 2 of adulthood. Scale bar, 100 μm.
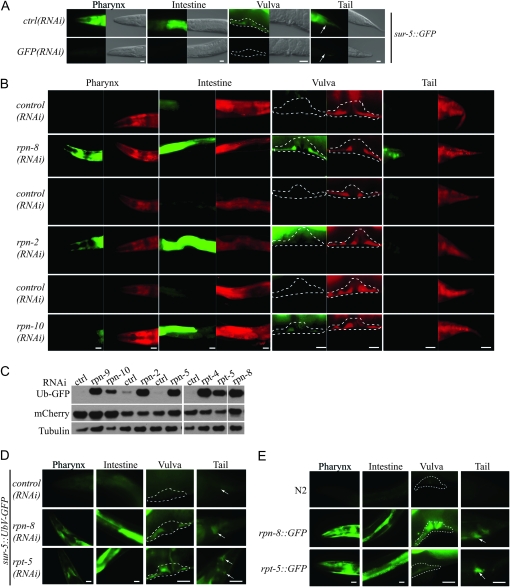
Figure 3.—
Cell-type-specific requirements for degradation factors of the UPS. (A) Fluorescence and Nomarski images of worms expressing sur-5∷GFP were grown on control plates [ctrl(RNAi)] or depleted for GFP by RNAi. Scale bars, 20 μm. Fluorescence images were taken with the same exposure time. (B) Hermaphrodites expressing sur-5∷UbV-GFP and sur-5∷mCherry were RNAi-depleted for rpn-8, rpn-2, or rpn-10 as compared to the empty vector control and fluorescence images taken of GFP (green) and mCherry (red) at the same exposure times, respectively. Dashed outlines highlight the regions of interest. Scale bars, 20 μm. Note that the RNAi was applied for different time points (see Table S2), so for each RNAi experiment the corresponding control is depicted at the top. (C) Western blot of extracts from hermaphrodites containing the sur-5∷UbV-GFP and sur-5∷mCherry transgenes after control RNAi (ctrl, empty vector) or RNAi against the indicated proteasomal subunits. Because worms were analyzed at different time points (see Table S2), the corresponding controls are always depicted at the left. The blots were probed against GFP, mCherry, and Tubulin. (D) UbV-GFP is stabilized in worms that were RNAi-depleted for rpn-8 or rpt-5. Hermaphrodite worms were imaged during early adulthood as in A. Scale bars, 20 μm. (E) GFP expression driven by rpn-8 and rpt-5 promoters. Hermaphrodite worms were imaged during early adulthood. Arrows and dashed outlines highlight the regions of interest. Scale bars, 20 μm.
Following the GFP signal during differentiation and development demonstrated that the UFD substrate accumulates in arrested embryos lacking CDC-48, UFD-1, or NPL-4, which is consistent with the fact that the CDC-48UFD-1/NPL-4 complex is essential for cell cycle progression during embryogenesis (Figure 1F) (Mouysset et al. 2008). Thus, the UFD reporter substrate can be used to identify essential genes that regulate proteostasis during embryogenesis. The existence of null mutations of the UFD pathway that accumulate the substrate post-embryonically during adulthood suggests that defects in proteostasis are not necessarily lethal (Figure 1G). Therefore, forward genetic screens can identify mutants that abrogate proteolysis without affecting development.
UPS function is affected by protein-folding stress:
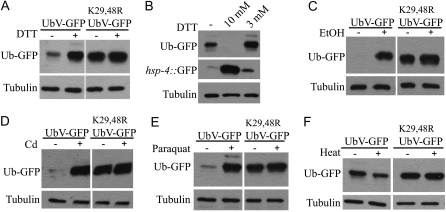
Previous work in S. cerevisiae showed that certain mutants of the UPS are hypersensitive to elevated stress conditions, causing an accumulation of unfolded proteins in the cell (Jungmann et al. 1993; Kerscher et al. 2006). To determine whether protein-folding stress influences ubiquitin-dependent protein degradation in animals with an unaffected UPS, we challenged wild-type worms expressing the UFD model substrate with different stressors. Intriguingly, worms treated with low levels of DTT (3 mm) specifically stabilized the UbV-GFP substrate expressed under the sur-5 promoter (Figure 2, A and B). This low dose of DTT had only a mild effect on ER stress as shown by the use of the inducible hsp-4∷GFP reporter that monitors ER stress (Urano et al. 2002). In contrast, treatment with a high dose of DTT (10 mm) caused a high level of ER stress and led to a lack of the UbV-GFP substrate, presumably by affecting its expression (Figure 2, A and B). Similarly, exposing worms to low doses of ethanol, the heavy metal cadmium, or the oxidative stress-inducing agent paraquat resulted in strong stabilization of UbV-GFP (Figure 2, C–E). In contrast, however, acute heat stress enhanced rather than reduced turnover of UbV-GFP (Figure 2F). Since this heat-induced degradation depends on K29 and K48 of the N-terminal ubiquitin moiety, it seems to require polyubiquitylation of the UFD substrate. These results indicate that the UPS differently integrates diverse protein-folding stress pathways in C. elegans. Moreover, the reporter assay is able to serve as a specific biomarker to monitor environmental stress conditions that influence proteostasis of multicellular organisms.
Figure 2.—
Modulation of ubiquitin-dependent proteolysis by proteotoxic stress conditions. (A) Lysates of adult worms carrying sur-5∷UbV-GFP treated with (+) or without (−) 3 mm DTT were analyzed by immunoblotting with GFP and tubulin antibodies. (B) Worms carrying sur-5∷UbV-GFP or hsp-4∷GFP were treated without (−) or with 10 mm or 3 mm DTT and analyzed as in A. (C) As in A, but worms were treated with or without 1% ethanol. (D) As A, but worms were treated with or without 100 μm cadmium. (E) As A, but worms were treated with or without 2 mm paraquat. (F) As A, but worms were grown to day 1 of adulthood and subjected to 37° for 2 hr (+) or as control (−) were kept at 20°.
Tissue-specific protein degradation pathways:
The 26S proteasome is composed of one proteolytically active 20S core particle attached to two 19S regulatory particles (RPs) that are important for unfolding and translocation of ubiquitylated substrates into the central 20S core (Finley 2009). In multicellular organisms, the proteolytic function of the 26S proteasome is expected to occur similarly in all tissues. However, whether the expression or composition of certain subunits varies in individual cell types is presently unknown. Having proven by GFP depletion that RNAi is similarly efficient in all sur-5-expressing tissues (Figure 1C and Figure 3A), we further addressed the importance of different proteasomal subunits for degradation of the UbV-GFP substrate in various tissues of the worm categorized into hypodermal cells surrounding the pharynx, the intestine, vulva, and tail. As shown before, UbV-GFP is stabilized in lysates of worms depleted for proteasomal subunits (Figure 1, C and D). Given the essential role of the 20S subunits, their depletion resulted in lethality of the worms (data not shown), and these worms displayed only a weak GFP signal in the intestine, most likely due to reduced transcript levels of UbV-GFP (Figure S2 and Table S1). In contrast, knockdown of most subunits of the 19S RPs showed stabilization in different tissues (Figure 3, B, C, and D; Figure S2; Figure S3; Table S1). Surprisingly, some RP subunits support degradation of the UFD substrate in the hypodermis surrounding the pharynx and in the vulva, tail, and intestine (i.e., rpn-8, rpt-5), whereas other subunits were not required in most tissues examined (i.e., rpn-10) (Figure 3, B, C, and D; Figure S3B; Table S1). Since RNAi can efficiently downregulate GFP in the whole worm, it is unlikely that the tissue-specific differences in UFD substrate turnover between worms lacking the proteasomal subunits are due to incomplete RNAi depletion (Figure 1C and Figure 3A). To rule out that transcriptional or translational regulation contributes to changes in UFD substrate levels, we co-expressed both UbV-GFP and mCherry under the same promoter and compared the GFP and mCherry levels (Figure 3, B and C). In contrast to the UFD substrate, we did not detect any changes in mCherry amounts upon RNAi depletion of individual 19S subunits, indicating that the enhanced UbV-GFP levels are due to differential degradation rather than transcriptional changes. Thus, although the proteasome is required in all cell types, certain subunits of the 26S proteasome seem to be differently required or expressed in individual tissues of the worm.
In support of this idea, a comparison of the expression level of a subunit and its effect on protein stability revealed a strong correlation. For example, the tissue-specific stabilization of UbV-GFP upon RNAi depletion is related to the expression of the proteasomal subunits RPN-8 and RPT-5 (Figure 3, D and E; Table S3). Similarly, UFD pathway regulators required for ubiquitylation of the UFD substrate also demonstrate cell-type-specific activities (Figure 1G; Figure S3A; Table S1), which was confirmed by both RNAi treatment and loss-of-function mutants (Figure S3A; Table S1). These surprising observations suggest the existence of tissue-specific proteostasis networks and open completely new screening strategies. Genome-wide screening approaches based on fluorescent worm sorting will help to decipher proteolytic alterations between various tissues and how they are established during development.
Food source-dependent effects on proteolysis and longevity:
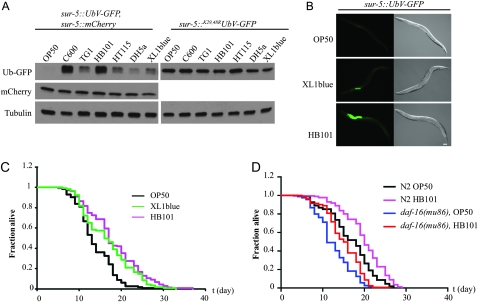
Using different bacteria as a food source for the UPS reporter worms, we noted a direct influence of food on substrate turnover at day 1 of adulthood, which varied substantially with the Escherichia coli strains (Figure 4, A and B). In contrast to OP50 bacteria, XL1blue causes a modest and HB101 a strong stabilization of the UFD substrate preferentially in the intestine. Thus, depending on the food source, ubiquitin-dependent protein degradation is tissue-specifically perturbed in young adult worms. Recently, by using metastable proteins, the transition from larval development to adulthood was shown to be a critical time frame for the maintenance of proteostasis. Given that overexpression of the life-span-regulating transcription factor DAF-16 is able to override this critical event, a link between the regulation of proteostasis early in life and aging has been proposed (Ben-Zvi et al. 2009). Thus, we analyzed the influence of the different E. coli strains on life span in regard to the diverse effects on ubiquitin-dependent proteolysis. Interestingly, wild-type worms grown on HB101 showed an increased life span compared to OP50 bacteria, whereas XL1blue bacteria caused intermediate stabilization and life-span extension. (Figure 4C, Table S4). The increased life span of HB101-fed worms is only partially suppressed by loss of daf-16, suggesting an alternative pathway that exists to link protein turnover with life span in the worm (Figure 4D, Table S5). Interestingly, the influence of nutrients on life-span extension was recently shown to depend on neuronal signaling (Maier et al. 2010). Altogether, these data suggest that metabolic changes are able to affect tissue-specific degradation pathways and longevity. It will thus be interesting to determine if neuronal signaling mutants that affect food source-dependent life-span regulation show defects in ubiquitin-dependent protein degradation.
Figure 4.—
The UFD reporter detects physiological changes. (A) Protein extracts of adult worms carrying the transgenes as indicated were fed with different E. coli strains and analyzed by immunoblotting with GFP, mCherry, or tubulin antibodies. (B) Fluorescence and Nomarski images of hermaphrodites grown on different E. coli. (C) Life-span analysis of N2 wild-type worms grown on plates containing the designated E. coli strains. (D) Life-span analysis of N2 wild-type or daf-16(mu86) mutant worms grown on plates containing the designated E. coli strains.
In summary, our work reveals the existence of cell-type- and stress-specific degradation pathways required to differently integrate proteostasis networks throughout the development and aging of multicellular organisms. Our in vivo degradation assay provides a powerful tool for combining genetic screens based on sorting GFP fluorescence in worms and next-generation sequencing of the mutants discovered (Doitsidou et al. 2008; Sarin et al. 2008). This assay thereby offers unexplored avenues for the future investigation of key regulators and pathways central to cellular differentiation, stress response, and longevity. Similarly, it can serve as an economical approach for compound screens to design specific inhibitors of central UPS regulators. Furthermore, this screening assay can be easily adapted using alternative degradation signals to investigate additional proteolytic pathways such as the N-end rule pathway (Varshavsky 1992), ER-associated protein degradation (Meusser et al. 2005), or autophagy (Komatsu and Ichimura 2010).
Acknowledgments
We thank Y. Kohara, C. Riedel, the Caenorhabditis Genetics Center (funded by the National Institutes of Health National Center for Research Resources), the Dana-Farber Cancer Institute, and Geneservice for antibodies, plasmids, cDNAs, and strains. We are grateful for comments on the manuscript from M. Ermolaeva, A. Franz, W. Pokrzywa, U. Resch, and B. Schumacher. This work is supported by grants of the Deutsche Forschungsgemeinschaft (especially the Cologne Excellence Cluster on Cellular Stress Responses in Aging-Associated Diseases and the Research Unit FOR885) and the Rubicon European Union Network of Excellence (to T.H.). T.H. is a European Molecular Biology Organization Young Investigator.
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.111.126797/DC1.
References
- Ben-Zvi, A., E. A. Miller and R. I. Morimoto, 2009. Collapse of proteostasis represents an early molecular event in Caenorhabditis elegans aging. Proc. Natl. Acad. Sci. USA 106 14914–14919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciechanover, A., A. Orian and A. L. Schwartz, 2000. Ubiquitin-mediated proteolysis: biological regulation via destruction. Bioessays 22 442–451. [DOI] [PubMed] [Google Scholar]
- Dantuma, N. P., K. Lindsten, R. Glas, M. Jellne and M. G. Masucci, 2000. Short-lived green fluorescent proteins for quantifying ubiquitin/proteasome-dependent proteolysis in living cells. Nat. Biotechnol. 18 538–543. [DOI] [PubMed] [Google Scholar]
- Doitsidou, M., N. Flames, A. C. Lee, A. Boyanov and O. Hobert, 2008. Automated screening for mutants affecting dopaminergic-neuron specification in C. elegans. Nat. Methods 5 869–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley, D., 2009. Recognition and processing of ubiquitin-protein conjugates by the proteasome. Annu. Rev. Biochem. 78 477–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidalevitz, T., E. A. Kikis and R. I. Morimoto, 2009. A cellular perspective on conformational disease: the role of genetic background and proteostasis networks. Curr. Opin. Struct. Biol. 20 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, T., S. Orita and M. Han, 1998. Caenorhabditis elegans SUR-5, a novel but conserved protein, negatively regulates LET-60 Ras activity during vulval induction. Mol. Cell. Biol. 18 4556–4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer, G., O. Matilainen and C. I. Holmberg, 2010. A photoconvertible reporter of the ubiquitin-proteasome system in vivo. Nat. Methods 7 473–478. [DOI] [PubMed] [Google Scholar]
- Hoppe, T., 2005. Multiubiquitylation by E4 enzymes: ‘one size’ doesn't fit all. Trends Biochem. Sci. 30 183–187. [DOI] [PubMed] [Google Scholar]
- Johnson, E. S., P. C. Ma, I. M. Ota and A. Varshavsky, 1995. A proteolytic pathway that recognizes ubiquitin as a degradation signal. J. Biol. Chem. 270 17442–17456. [DOI] [PubMed] [Google Scholar]
- Jungmann, J., H. A. Reins, C. Schobert and S. Jentsch, 1993. Resistance to cadmium mediated by ubiquitin-dependent proteolysis. Nature 361 369–371. [DOI] [PubMed] [Google Scholar]
- Kerscher, O., R. Felberbaum and M. Hochstrasser, 2006. Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu. Rev. Cell Dev. Biol. 22 159–180. [DOI] [PubMed] [Google Scholar]
- Komatsu, M., and Y. Ichimura, 2010. Selective autophagy regulates various cellular functions. Genes Cells 15 923–933. [DOI] [PubMed] [Google Scholar]
- Lindsten, K., V. Menendez-Benito, M. G. Masucci and N. P. Dantuma, 2003. A transgenic mouse model of the ubiquitin/proteasome system. Nat. Biotechnol. 21 897–902. [DOI] [PubMed] [Google Scholar]
- Maduro, M., and D. Pilgrim, 1995. Identification and cloning of unc-119, a gene expressed in the Caenorhabditis elegans nervous system. Genetics 141 997–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier, W., B. Adilov, M. Regenass and J. Alcedo, 2010. A neuromedin U receptor acts with the sensory system to modulate food type-dependent effects on C. elegans lifespan. PLoS Biol. 8 e1000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meusser, B., C. Hirsch, E. Jarosch and T. Sommer, 2005. ERAD: the long road to destruction. Nat. Cell Biol. 7 766–772. [DOI] [PubMed] [Google Scholar]
- Mouysset, J., A. Deichsel, S. Moser, C. Hoege, A. A. Hyman et al., 2008. Cell cycle progression requires the CDC-48UFD-1/NPL-4 complex for efficient DNA replication. Proc. Natl. Acad. Sci. USA 105 12879–12884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers, E. T., R. I. Morimoto, A. Dillin, J. W. Kelly and W. E. Balch, 2009. Biological and chemical approaches to diseases of proteostasis deficiency. Annu. Rev. Biochem. 78 959–991. [DOI] [PubMed] [Google Scholar]
- Praitis, V., E. Casey, D. Collar and J. Austin, 2001. Creation of low-copy integrated transgenic lines in Caenorhabditis elegans. Genetics 157 1217–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rape, M., T. Hoppe, I. Gorr, M. Kalocay, H. Richly et al., 2001. Mobilization of processed, membrane-tethered SPT23 transcription factor by CDC48(UFD1/NPL-4), a ubiquitin-selective chaperone. Cell 107 667–677. [DOI] [PubMed] [Google Scholar]
- Richly, H., M. Rape, S. Braun, S. Rumpf, C. Hoege et al., 2005. A series of ubiquitin binding factors connects CDC48/p97 to substrate multiubiquitylation and proteasomal targeting. Cell 120 73–84. [DOI] [PubMed] [Google Scholar]
- Sarin, S., S. Prabhu, M. M. O'Meara, I. Pe'er and O. Hobert, 2008. Caenorhabditis elegans mutant allele identification by whole-genome sequencing. Nat. Methods 5 865–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urano, F., M. Calfon, T. Yoneda, C. Yun, M. Kiraly et al., 2002. A survival pathway for Caenorhabditis elegans with a blocked unfolded protein response. J. Cell Biol. 158 639–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshavsky, A., 1992. The N-end rule. Cell 69 725–735. [DOI] [PubMed] [Google Scholar]
- Zhang, D., T. Chen, I. Ziv, R. Rosenzweig, Y. Matiuhin et al., 2009. Together, Rpn10 and Dsk2 can serve as a polyubiquitin chain-length sensor. Mol. Cell 36 1018–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]