Abstract
Bone Morphogenetic Protein (BMP) activity has been implicated as a key regulator of multiple aspects of dorsal neural tube development. BMP signaling in the dorsalmost neuroepithelial cells presumably plays a critical role. We use tissue-specific gene ablation to probe the roles of BMPR1A, the type 1 BMP receptor that is seemingly the best candidate to mediate the activities of BMPs on early dorsal neural development. We use two different Cre lines expressed in the dorsal neural folds, one prior to spinal neurulation and one shortly afterward, together with a Bmpr1a conditional null mutation. Our findings indicate that BMPR1A signaling in the dorsal neural folds is important for hindbrain neural tube closure, but suggest it is dispensable for spinal neurulation. Our results also demonstrate a requirement for BMP signaling in patterning of dorsal neural tube cell fate and in neural crest cell formation, and imply a critical period shortly before neural tube closure.
Keywords: BMP, BMPR1A, neural crest, neurulation, dorsal spinal cord neurons
INTRODUCTION
The mammalian central nervous system forms from a single layer of neuroepithelial cells, rapidly developing into a closed neural tube with remarkable regional specialization. Local failures of neurulation give rise to specific neural tube defects, such as spina bifida or exencephaly (open brain); collectively, neural tube defects are among the most common and significant of human congenital malformations (Botto et al., 1999; Copp et al., 2003). Beyond overt morphology, the mature neural tube has both dorsal and ventral character. Axially distinct neuronal differentiation is essential for proper function of the nervous system (Jessell and Sanes, 2000).
Concomitant with neurulation and neural patterning, neural crest cells (NCCs) are formed in the lateral edges of the neural plate as they bend toward each other and merge dorsally (Knecht and Bronner-Fraser, 2002). Following induction, NCCs delaminate from the dorsal neural folds and follow stereotypic migration patterns to their destinations. They form multiple cell populations within the embryo, including elements of the peripheral nervous system, craniofacial bone and cartilage, melanocytes, and portions of the cardiovascular system.
Bone morphogenetic proteins (BMPs) are a family of secreted ligands that are important regulators of dorsal neural tube development (Liu and Niswander, 2005). Several BMPs and BMP target genes are expressed in and around the forming dorsal neural tube of vertebrate embryos, and studies with an antibody against phosphorylated Smad1/5/8 proteins to mark areas of active BMP signaling show significant pSmad1 accumulation in the chick dorsal neural tube (Faure et al., 2002). Elevated pSMAD1/5/8 staining is also seen in the dorsal neural folds and tube of mouse embryos, further increased in embryos lacking the BMP antagonists Noggin and Chordin (Yang and Klingensmith, 2006; Mine et al., 2008). Despite its being an inhibitor of BMP signaling, Noggin is expressed in the closing dorsal neural folds and in the dorsal neural tube after closure – coincident with areas of elevated BMP expression and activity, and indeed, Noggin expression is itself a positive target of BMP signaling (Stottmann et al., 2001). Such considerations imply that BMP signal transduction must be precisely regulated in these tissues for appropriate dorsal development.
Morphogenesis of the dorsal neural tube is disrupted when BMP levels are either too high or low. Noggin mutants have neural tube defects, specifically lumbar spina bifida and exencephaly (Stottmann et al., 2006). Phenotypic analysis suggested that dorsal BMP is a negative regulator of the bending of dorsal neural folds toward the midline. Indeed, BMP2 inhibits dorsal bending in culture, and Bmp2 mutants have premature and exaggerated bending of the caudal neuropore (Ybot-Gonzalez et al., 2007), as well as variable cranial neurulation anomalies (Castranio and Mishina, 2009).
BMPs are also involved in dorsoventral patterning of the neural tube. In chick, recombinant BMP or transgenic BMP overexpression can recapitulate inductive activity of the surface ectoderm (Liem et al., 1995, 1997; Lee et al., 1998; Timmer et al., 2002; Arkell and Beddington, 1999). Loss-of-function BMP mutations in zebrafish are further consistent with a requirement for proper BMP signaling to generate normal dorsal neural tube pattern (Barth et al., 1999; Nguyen et al., 2000). The roof plate is a key source of dorsalizing signals in the neural tube (Lee et al., 2000), expressing multiple BMP family members. Mice lacking Gdf7 (a TGFβ ligand related to BMPs) lack the dI1 classes of dorsal interneurons (Lee et al., 1998), which are specifically expanded upon transgenic overexpression of Bmp4/7 or activated BMP type 1 receptors in chick (Chizhikof and Millen, 2004; Liu et al., 2004).
BMP signaling is also involved in multiple steps of NCC biology. The border between neural and surface ectoderm is the source of NCCs, and can be relocated along the medio-lateral axis in response to the addition of BMP protein (Streit and Stern, 1999). Zebrafish mutants in BMP2/4 signaling show a decrease in NCC production (Barth et al., 1999; Nguyen et al., 1998). In mouse Bmp2 mutants, NCCs are induced but fail to migrate properly from the neural tube (Correia et al., 2007). Bmp5/Bmp7 double mutant embryos show reduced pharyngeal arches, structures heavily populated by NCC derivatives (Solloway and Robertson, 1999). In contrast, embryos lacking Noggin show excess formation of NCCs, further increased in embryos also lacking Chordin; these BMP antagonists are also required for normal migration of NCCs as well as their survival (Anderson et al., 2006). Collectively, these results suggest that potential NCC cells need to respond appropriately to local BMP signals.
The activities of BMP in regulating dorsal development suggest that BMP signal transduction in dorsal neurepithelial cells is essential for normal embryogenesis. Reception of BMP signals is mediated by a complex consisting of a type 2 receptor (BMPR2) and a type 1 receptor. Of the three known BMP type 1 receptors, BMPR1A (Alk3) seems particularly likely to be playing an important role in early dorsal neural tube development, for several reasons. First, the Bmpr1a gene is expressed throughout the neural folds from the earliest stages, in contrast to the other two BMP type 1 receptors, Bmpr1b (Alk6) and Alk2 (Panchision et al., 2001; Dewulf et al., 1995; Gu et al., 1999; Dudas et al., 2004). Second, BMPR1A transduces BMP4 and BMP2 signals (Massague and Chen, 2000), which are very active in dorsal neural assays (Liem et al., 1997; Ybot-Gonzalez et al., 2007). Third, overexpression of Bmpr1a in the neural tube resulted in increased dorsal neurons (Panchision et al., 2001; Timmer et al, 2002; Yamauchi et al., 2008), showing that it is biologically active in this context. Ablation of Bmpr1a after E9.75 in the dorsal neural tube caused no detected defect, while the same ablation in a Bmpr1b mutant background resulted in a failure to maintain Math1 expression, reflecting a loss of the dorsal most neuronal fates (Wine-Lee et al., 2004). Thus there is a demonstrated requirement for BMP type I receptors (in which BMPR1A and BMPR1B are interchangeable) after approximately the 25 somite stage, when the neural tube is completely closed and neurogenesis is underway, to maintain the dorsal differentiation program.
Despite the evidence that BMP signaling is important for multiple aspects of early dorsal neural tube formation, which if any BMP receptor is necessary within this domain for its early development has yet to be determined. In that it appears to be the most likely candidate, we sought to assess the spatiotemporal requirements for Bmpr1a in dorsal neural cells for the morphogenesis, patterning and differentiation of the early neural tube and neural crest. We use the Cre-LoxP system to ablate a conditional null allele of Bmpr1a (Mishina et al., 2002) in the dorsal neural folds at two timepoints: prior to spinal cord closure or shortly afterward. Comparison of the two resulting phenotypes defines a period during early neural development in which Bmpr1a is uniquely required for dorsal neural tube closure and patterning, as well as for NCC development.
RESULTS
Deletion of a conditional allele of Bmpr1a in two distinct spatio-temporal domains within the dorsal neural tube
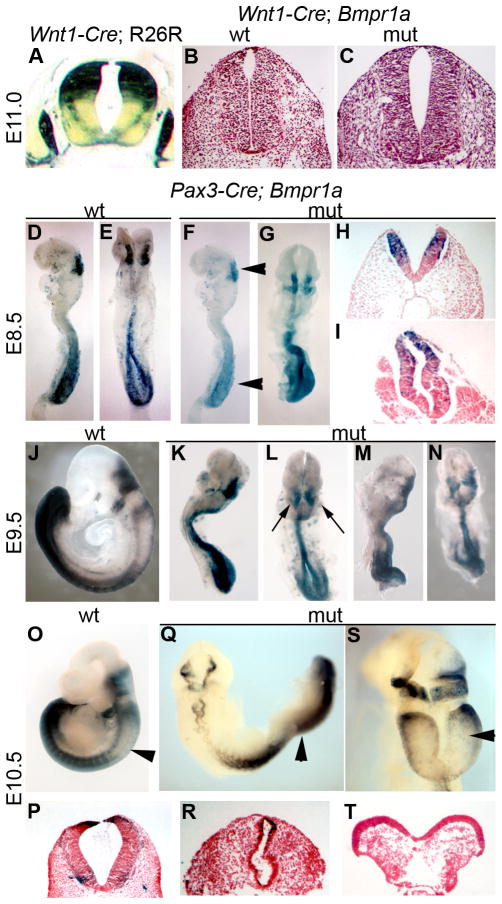
We used two different Cre recombinase transgenes to remove Bmpr1a from the dorsal neural tube in closely related, yet distinct spatiotemporal domains during early neural tube development. Reflecting the expression of the endogenous genes from which their promoters are derived, Pax3-Cre (Li et al., 2000) and Wnt1-Cre (Danielian et al., 1998) both drive recombination of sequences flanked by loxP sites specifically in the dorsal neural epithelium. We assessed the location and timing of Cre recombinase activity using the reporter line, R26R (Soriano, 1999); we generated Pax3-Cre;R26R and Wnt1-Cre;R26R embryos, which express a β-galactosidase transgene only in cells expressing Cre recombinase, and in the descendents of such cells. No signs of Pax3-Cre recombination were detected in multiple embryonic day (E) 7 embryos through the early head fold stage (data not shown). A few positive cells were seen in the neural folds of the presomite embryo (data not shown), and were much more plentiful by the two-somite (2s) stage (Fig. 1A,B). These cells were distributed in the dorsal neural folds throughout the anterior-posterior (A–P) axis caudal to the midbrain, with the same pattern evident at the 6s stage (Fig. 1E,F). By E9.5 and E10.5, Pax3-Cre;R26R embryos showed β-galactosidase positive cells in the dorsal neural tube and migrating neural crest (Fig. 1K–Q). Pax3-Cre;R26R cells heavily populated the second pharyngeal arch (Fig. 1K) and also contributed to the more posterior arches, but not the first arch (arrow in Fig. 1K,O). Pax3-Cre activity was detected from the hindbrain, at its most rostral extent, to the caudal extreme of the embryo at E9.5 (Fig. 1K,L) but was more intense in the neural tube posterior to the forelimb (Fig. 1O,P). In the extreme posterior of the embryo, Pax3-Cre; R26R showed limited recombination outside the neural tube (e.g. Fig. 1I). To further confirm our results with the R26R reporter, we repeated a portion of this analysis using a second Cre reporter: Z/AP expresses alkaline phosphatase in the presence of Cre (Lobe et al., 1999). This reporter yielded very similar results (data not shown).
Figure 1. Spatiotemporal recombination patterns of Pax3-Cre and Wnt1-Cre.
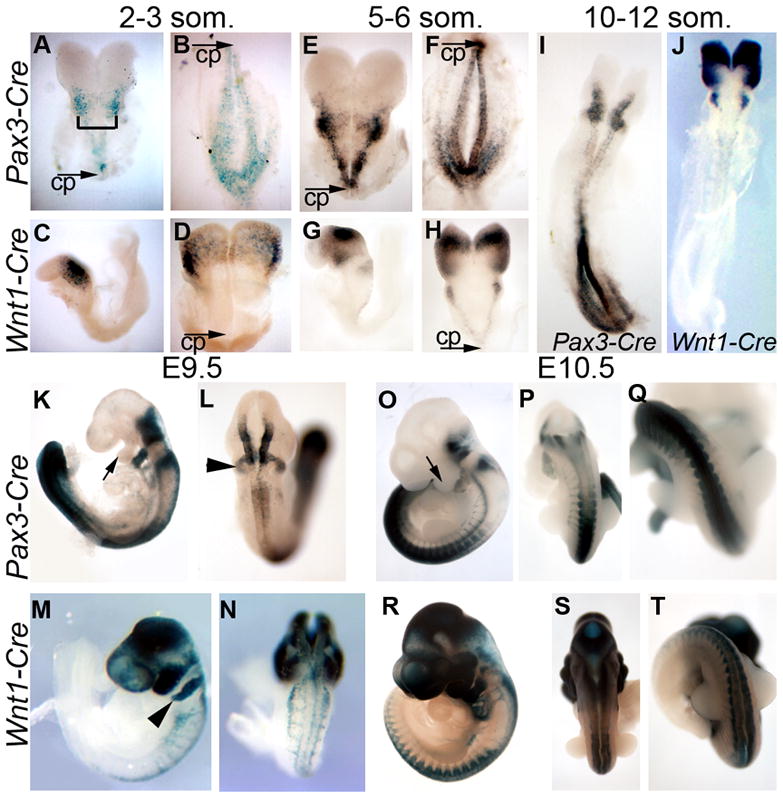
Embryos carrying the R26R Cre reporter and either Pax3-Cre (A,B,E,F,I,K,L,O–Q) or Wnt1-Cre (C,D,G,H,J,M,N,R–T) were stained for β-galactosidase activity to mark cells which have undergone Cre-mediated recombination. Embryos at 2–3 somites (A–D) and 5–6 somites (E–H) were bisected at the initial closure point (CP) to allow visualization of the entire dorsal neural epithelium (bracket in A indicates hindbrain). At E9.5 (K–N) and E10.5 (O–T), embryos are shown in lateral (K, M,O,R) as well as dorsal views at the level of the thorax (L,N,P,S) or posterior to the forelimb bud (Q,T). Arrows (K,O) highlight the first pharyngeal arch, arrowheads (L,M) indicate the second pharyngeal arch.
In contrast to the Pax3-Cre recombination pattern, Wnt1-Cre;R26R embryos first showed β-galactosidase positive cells in the neural tube at the 4s stage and were plentiful by the 6s stage (Fig. 1C,D,G,H; Stottmann et al., 2004), but only at relatively anterior positions in the neural tube. At the 10–12s stage (E9.0), Wnt1-Cre activity was strong in brain regions and weak in the rostral section of the developing spinal cord (Fig. 1J) and was not present in the caudal-most neural tube until at least E10 (Fig. 1T). Although Wnt1-Cre begins to be expressed at later stages than Pax3-Cre in the spinal cord, activity is seen at equal intensity along the spinal cord once it is initiated. This is in contrast to the markedly increased activity of the Pax3-Cre transgene in posterior regions of the embryo. In addition to the spinal cord, Wnt1-Cre activity in the rostral neural tube was initially present in the midbrain (Fig. 1C,D) and spread anteriorly in more mature embryos, both within the brain and in the NCC population of the craniofacial region (Fig. 1M,R). Indeed, Wnt1-Cre positive cells also populated the pharyngeal arches, consistent with previous results that Wnt1-Cre activity occurs throughout the cranial NCC progenitor region (Chai et al., 2000; Jiang et al., 2000; Stottmann et al., 2004).
Thus, while both transgenes are active in the dorsal neural tube, the Pax3-Cre transgene drives loxP recombination in the spinal neural folds significantly earlier than Wnt1-Cre. In Pax-3 Cre transgenic embryos, the spinal regions of the embryo show robust Cre activity long before closure begins. In contrast, Wnt1-Cre activity in dorsal spinal cord is absent or insignificant until shortly after closure. Both drive recombination in the hindbrain dorsal neural folds before closure, though Pax3-Cre is much more active. Wnt1-Cre is expressed well before neurulation in the midbrain, where Pax3-Cre is not expressed. Closure initiates at the level of somite 3, at the hindrain/cervical junction, at the 6–7 somite stage (E8.25) and proceeds rostrally and caudally from there (Copp et al., 2003). Thus, the use of these Cre transgenes allows different timing of Bmpr1a ablation at several rostrocaudal points along the dorsal neural epithelium.
Both Cre transgenes were used in identical mating schemes in conjunction with a conditional allele of Bmpr1a, which results in deletion of the second exon upon Cre recombination (Mishina et al., 2002). Mice carrying the Pax3-Cre transgene and a null allele of Bmpr1a (Bmpr1anull) were crossed to mice homozygous for a conditional allele of Bmpr1a (Bmpr1aflox). One quarter of the resulting embryos are of the genotype Pax3-Cre/+;Bmpr1aflox/null (hereafter called Pax3-Cre;Bmpr1a mutant) and are unable to signal through BMPRIA in the cells of the neural tube expressing the Cre transgene. Similarly, Wnt1-Cre/+;Bmpr1aflox/null embryos (hereafter called Wnt1-Cre;Bmpr1a mutant) will be unable to signal through BMPRIA in the Wnt1-Cre domain.
Bmpr1a functions in the hindbrain and spinal dorsal neural folds for neural tube morphogenesis and closure
Transverse sections of Wnt1-Cre;R26R embryos at midgestation indicated that cells derived from the dorsalmost neural tube populate the dorsal half of the spinal cord, as well as neural crest derived peripheral structures (Fig. 2A). In spite of this significant Wnt1-Cre recombinase activity early in neural tube development, Wnt1-Cre;Bmpr1a embryos showed no neurulation phenotypes at any axial level (Fig. 2B). Given the strong Cre expression in the putative dorsal midbrain well before closure, our data suggest that Bmpr1a is not required in the dorsal neural folds of the presumptive midbrain for closure, from at least the 5-somite stage.
Figure 2. Neural tube phenotypes in Wnt1-Cre;Bmpr1a and Pax3-Cre;Bmpr1a mutants.
(A) Transverse section of E11.0 Wnt1-Cre;R26R embryo stained for β-galactosidase activity. Transverse sections of control (B) and Wnt1-Cre;Bmpr1a (C) neural tubes were stained with haematoxylin and eosin and show no neurulation defects in the Wnt1-Cre;Bmpr1a mutants. (D–T) Pax3-Cre ablation of Bmpr1a. (D–I; E8.5) Pax3-Cre; R26R control embryos (D lateral view, E dorsal view) have initiated neurulation in the spinal cord. Pax3-Cre;Bmpr1a;R26R embryos also initiate spinal neurulation but the neural tube is dysmorphic (F lateral, G, dorsal: arrowheads in F indicate regions shown in section in H,I). (J–N; E9.5) Pax3-Cre;R26R control embryo (J) has completed neurulation, whereas Pax3-Cre; Bmpr1a; R26R littermates fail to turn and often show hindbrain exencephaly (K,M lateral; L,N dorsal; arrows highlight hindbrain exencephaly). (O–T; E10.5) Pax3-Cre; R26R control embryo (O); Pax3-Cre; Bmpr1a; R26R mutant embryos show a failure to turn and mid-hindbrain exencephaly (Q,S). Arrowheads in O, Q, S indicate regions shown in section in P,R,T respectively.
In contrast to Wnt1-Cre;Bmpr1a embryos, Pax3-Cre;Bmpr1a embryos were identifiable at E8.5 by their dysmorphic neural tubes (Fig. 2F–I). Pax3-Cre;Bmpr1a embryos at E9.5 and E10.5 had increasingly more dramatic phenotypes, including smaller pharyngeal arches, frequent pericardial edema and decreased body size (Fig. 2K–N,Q–T), as compared to their littermates. In addition to these phenotypes, approximately 40% of the Pax3-Cre;Bmpr1a embryos also had hindbrain exencephaly (Fig. 2L,N,Q,S,T; 3B,L,M). The vast majority of Pax3-Cre;Bmpr1a embryos dissected at E11.5 were severely necrotic and thus unsuitable for further analysis (data not shown). These data show Bmpr1a is required in the Pax3-Cre domain for embryonic survival past E10.5. With the caveat that recombination in the uppermost spinal cord is not robust at early somite stages, these results reveal that ablation of Bmpr1a in the dorsal neural folds before neurulation does not alter neural tube closure in the caudal and mid-spinal regions. By contrast, absence of Bmpr1a from the hindbrain prior to closure often leads to exencephaly.
Figure 3. Dorsal cell fate in neural tubes of Bmpr1a mutants.
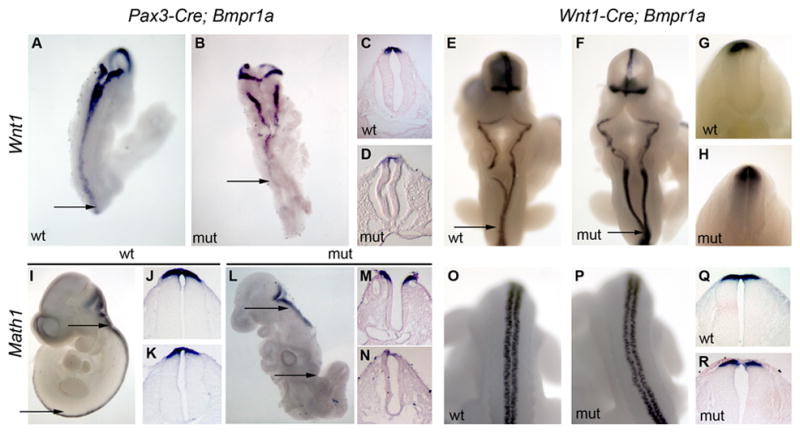
In situ hybridization of Wnt1 (A–H) and Math1 (I–R) expression in Pax3-Cre;Bmpr1a (A–D, I–N) and Wnt1-Cre; Bmpr1a (E–H, O–R) embryos. (C,D) Transverse sections of E9.5 wildtype (C) and mutant (D) embryos from the presumptive lumbar region (as approximated by the arrows in A,B) highlight the reduced expression of Wnt1 in the posterior of the mutant embryos. (E–H) No such differences are seen at any axial level between wildtype and Wnt1-Cre; Bmpr1a embryos. All sections from Wnt1-Cre; Bmpr1a embryos are from the thoracic region, as indicated by the arrows in E,F. (I–N) The expression of Math1 in the posterior of the Pax3-Cre;Bmpr1a mutant is reduced compared to wildtype, or more anterior positions in the mutant. Math1 sections are from the hindbrain region (J,M) and posterior neural tube neural tube (K,N) as indicated by the arrows in I,L. Note also the exencephaly present in M. Math1 expression is very similar in mutant and Wnt1-Cre;Bmpr1a embryos. O,P show the posterior neural tube at the level between the limbs, and Q,R are thoracic sections (see E,F).
Some Pax3-Cre;Bmpr1a embryos at E9.5 also showed much more severe phenotypes, which closely resembled the phenotype seen in an ablation of Bmpr1a throughout the entire epiblast using Mox2-Cre (data not shown; Davis et al., 2004). This suggested ectopic recombination was occurring in some embryos, as has been shown for other Cre transgenes in some genetic backgrounds (e.g., Hebert and McConnell, 2000). In order to confirm which phenotypes we observed were the result of dorsal neural tube restricted Pax3-Cre expression, we added the R26R reporter allele to our ablation of Bmpr1a. In this modified scheme, embryos of the genotype Pax3-Cre;Bmpr1aflox/nullP;R26R/+ were generated in which cells lacking BMPRIA were also expressing β-galactosidase. This confirmed that the more severe phenotypes were the result of excision of Bmpr1a outside the neural tissue, in that the highly dysmorphic embryos showed a much broader extent of recombination (data not shown). In contrast, embryos in which Pax3-Cre recombined the R26R reporter in the expected pattern had defects consistent with ablation of Bmpr1a specifically in the dorsal neural tube (e.g. Fig 2F–I,K–N,Q,S). Thus, we were able to determine the phenotype resulting from Bmpr1a ablation in the dorsal neural tube specifically, as opposed to more general deletion, and the analysis reported here is limited to those phenotypes.
Bmpr1a is required for patterning of the dorsal spinal cord before neurulation
We next examined dorsal-ventral patterning in the neural tubes of embryos lacking dorsal Bmpr1a activity. Wnt1 is both a factor promoting dorsal cell proliferation as well as a marker of the most dorsal tissue in the neural tube (Megason and McMahon, 2002). Pax3-Cre;Bmpr1a embryos at E9.5 showed a significant decrease in Wnt1 expression in the posterior neural tube (Fig. 3B), markedly more severe in more posterior portions of the embryo. Math1 is a proneural gene bHLH protein marking a distinct class of neurons in the extreme dorsal portion of the neural tube (Helms and Johnson, 1998). Similar to Wnt1, Math1 expression was decreased in Pax3-Cre;Bmpr1a embryos, with lowest signal at more posterior levels of the embryo (Fig. 3L–N). The changes in expression patterns were not due simply to a loss of tissue or a general defect in posterior gene expression. Embryos with similar phenotypes produced dorsal β–galactosidase protein (e.g. Fig. 2P) and expressed other genes in their appropriate patterns in posterior tissues, such as Hoxb9 (data not shown). Furthermore, when Bmpr1a was ablated throughout the embryo using Mox1-Cre, gene expression was not globally reduced in the caudal portion of the embryo (Davis et al., 2004). As opposed to the Pax3-Cre;Bmpr1a mutants, Wnt1-Cre;Bmpr1a mutants showed no loss of Wnt1 (Fig. 3E–H) or Math1 (Fig. 3O–R) expression at any point along the anterior-posterior axis. Thus, ablation of Bmpr1a in the dorsal neural folds shortly after closure of the spinal cord (via Wnt1-Cre) doesn’t alter dorsal patterning. In contrast, deletion in the same domain before closure (via Pax3-Cre) results in decreased expression of key regulators of dorsal neural tube development.
Bmpr1a is required before spinal neurulation for normal neural crest cell production and maturation
In the mouse, the epithelial-to-mesenchymal transformation resulting in NCC formation is initiated in the rostral hindbrain around the five-somite (5s) stage (Nichols, 1981), prior to closure, and subsequently extends bi-directionally along the A-P axis. At any given level, active NCC generation lasts about 9 hours (Serbedzija et al., 1992); i.e. generation lasts until well after closure of the neural folds. We have previously shown that ablation of Bmpr1a by Wnt1-Cre has no effect on the initial production, migration and differentiation of NCCs (Stottmann et al., 2004), raising the alternative possibilities that BMPR1A is not required for these events, or that the Bmpr1afx conditional allele is recombined too late by Wnt1-Cre to reflect such requirement(s).
Whereas anteriormost NCCs were generated at approximately normal levels in the Pax3-Cre;Bmpr1a embryos, considerable NCC deficiencies were seen in the hindbrain and spinal regions of these mutants. Because Pax3-Cre;Bmpr1a embryos were somewhat delayed relative to littermate controls, we stage matched mutant and control embryos by counting somites. This confirmed that there were many fewer NCCs produced at comparable caudal positions along the A-P axis in the mutants. NCCs were greatly reduced as highlighted by expression of crabp1 (Fig. 4B,C), tcfap2a (Fig. 4E), and cadherin-6 (data not shown). In rostral regions, we observed that NCC marker expression is not changed in the first pharyngeal arch (PA1), but PA2 shows little or no NCC marker expression (arrow in Fig. 4B). This finding corresponds exactly to the domains populated by β-galactosidase positive cells in the Pax3-Cre;R26R recombination mapping experiments (Fig. 1K,O).
Figure 4. Neural crest cell development in Pax3-Cre;Bmpr1a mutants.
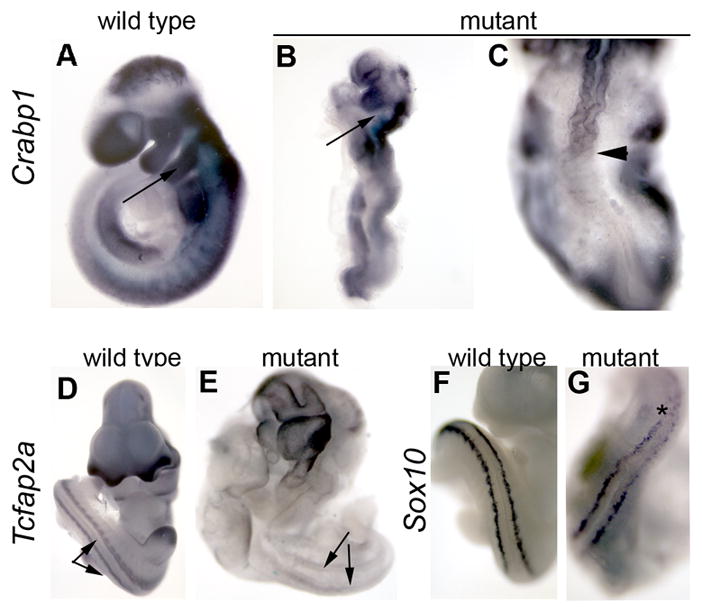
Whole mount in situ hybridization for expression of Crabp1 (A–C), Tcfap2a (D,E), and Sox10 (F,G) in wildtype (A,D,F) and Pax3-Cre;Bmpr1a mutants (B,C,E,G). Arrows in A,B indicate the second pharyngeal arch, which is devoid of NCCs in mutants. (C) Dorsal view of the posterior of same embryo shown in B. Arrow head shows caudal extent of Crabp1 expression. (D,E) Paired arrows indicate columns of NCC derivatives along the spinal cord. Note their deficiency in the mutant, as reflected by lack of Tcfap2a expression. (F,G) Dorsal view of E10.5 embryo just posterior to the forelimb bud. Note reduction of posterior Sox10 expression in mutant in the area indicated by the asterisk.
We also assessed the spatiotemporal expression of several markers to evaluate NCC differentiation in mutant embryos. First, we analyzed Sox10 expression, which has roles both in production and development of NCCs (Honore et al., 2003). Initially, Sox10 is expressed in all neural crest progenitors, but later becomes restricted to melanocytes and glial cells of the peripheral nervous system (Kuhlbrodt et al., 1998). We observed a marked decrease in expression in posterior regions of Pax3-Cre; Bmpr1a embryos (Fig. 4G) as compared to controls (Fig. 4F). We performed immunohistochemistry for the 2H3 neurofilament antibody to identify peripheral nervous system elements derived from the NCCs. Pax3-Cre;Bmpr1a embryos show almost no 2H3 immunoreactive cells peripheral to the caudal spinal cord in either whole mount or section analysis (data not shown). Conveniently, the Pax3-Cre;Bmpr1a;R26R embryos can themselves be used to trace the NCC lineage in mutant embryos, because recombination of the R26R reporter allele by Cre provides an indelible marker of cells derived from the dorsal neural folds. Such embryos showed significantly less distribution of β galactosidasepositive NCCs away from the neural tube and differentiation into structures of the peripheral nervous system (e.g. Fig. 2K–N, Q,S). Taken together, these data reveal that Bmpr1a function is required in dorsal neural tissue for both the proper specification and early differentation of NCCs.
BMPRIA activity is required for the continued development of NCC derivatives
The lack of defects in NCC production and initial migration in Wnt1-Cre;Bmpr1a embryos allowed us to assess later stages of NCC maturation. These embryos survive to E11.5, when they succumb to cardiac dysfunction (Stottmann et al., 2004), but appear normal until that point. To address the specialization of NCC derivatives in these mutants, we assessed expression of Dct (dopachrome tautomerase). Dct is specifically expressed in and required for melanocyte development (Jackson et al., 1992; Steel et al., 2002), a differentiated NCC derivative cell type. Whole mount in situ hybridization for Dct revealed a significant decrease in the expression in Wnt1-Cre; Bmpr1a embryos (Fig. 5D–F). The reduction in melanocytes was more significant in the forelimb region of the embryo (Fig. 5E) than in the area of the hindlimb (Fig. 5F). This may reflect the earlier expression of the Wnt1-Cre transgene in the anterior region of the embryo as compared to more caudal regions (see Fig. 1). These embryos appeared healthy upon fixation, though their destiny would have been death within a day. Although we cannot exclude that the pending death of these mutants is contributing to the decrease in Dct expression, these data suggest that Bmpr1a continues to be required in NCC derivatives during maturation stages of development.
Figure 5. Dct expression and NCC maturation in Wnt1-Cre;Bmpr1a mutants.
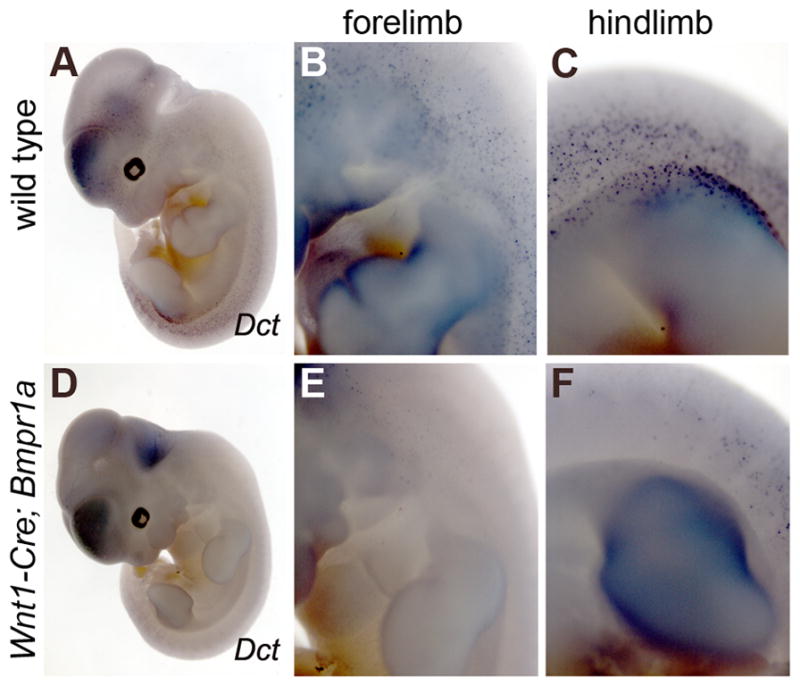
Whole mount in situ hybridization for Dct expression in wild type (A–C) and Wnt1-Cre; Bmpr1a mutants (D–F) at E11.5. B,E,C,F are higher magnification, lateral views of embryos at the level of the forelimbs (B,E) and hindlimbs (C,F). Dct expression in differentiated melanocytes is seen throughout the wildtype embryo on the dorso-lateral surface, especially in the region of the hindlimb (C).. Significantly fewer Dct-expressing cells are seen in the mutant embryos, with a more pronounced difference seen at the level of the forelimb in mutants (E) as compared to wildtype.
BMPRIB expression is not dependent on BMPRIA activity in the neural tube
Previous work has suggested that BMPRIA activity in the dorsal neural tube induces the expression of a second type I BMP receptor, BMPRIB at E9.0 (Panchision et al., 2001). This led to a model in which BMPR1A activity promotes neuronal precursor proliferation, until the expression of BMPR1B causes their mitotic arrest. Using data from constitutively active Bmpr1a/b transgenic embryos and explant studies, the authors proposed a feed-forward mechanism in which the sequential actions of BMPR1A and BMPR1B control the production and fate of dorsal neuronal precursor cells. Our tissue-specific ablation of Bmpr1a in the dorsal neural presented a loss-of-function test of this model. We assayed the expression of Bmpr1b in the neural tube of Wnt1-Cre;Bmpr1a embryos by whole mount in situ hybridization from E9.0–10.5. We detected no difference in the extent or level of expression of Bmpr1b in these mutant embryos (Fig. 6C,D). Thus, Bmpr1a is not required for the onset, localization or gross magnitude of Bmpr1b expression in the dorsal neural tube.
Figure 6. Expression of Bmpr1b in Wnt1-Cre;Bmpr1a mutants.
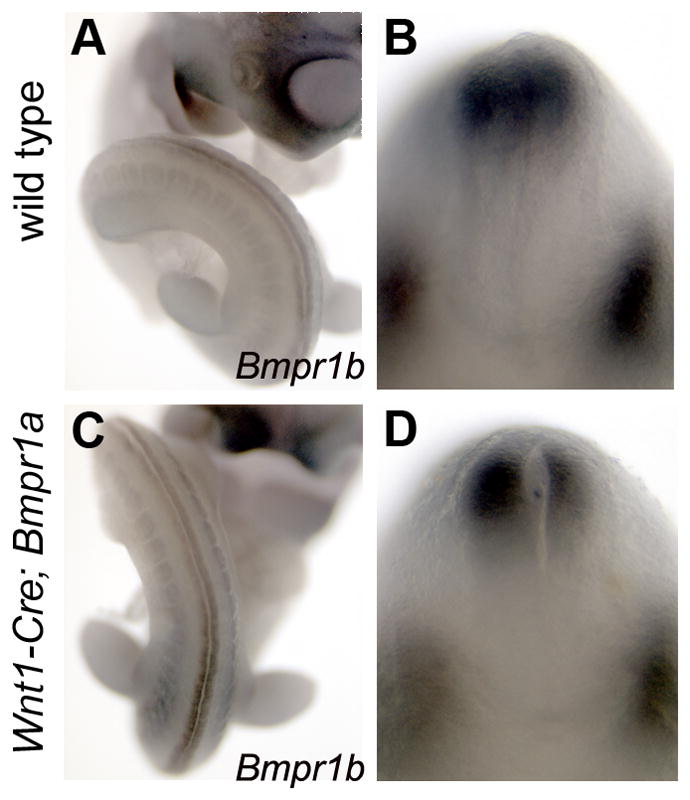
Whole mount in situ hybridization for Bmpr1b in wildtype (A,C) and Wnt1-Cre;Bmpr1a mutants (C,D), at E10.5. (A,C) Wildtype and mutant littermate imaged to show Bmpr1b expression caudal to the hindlimb bud. (B,D) Hybridized embryos were grossly sectioned in the transverse plane to show expression of Bmpr1b in the dorsal spinal cord (top) and in the paired dorsal root ganglia (bottom part of each image). Note that the lack of dorsal Bmpr1a does not preclude robust Bmpr1b expression.
DISCUSSION
In this work, we have shown that BMPR1A signaling is essential in the spinal portion of the dorsal neural folds for acquisition of dorsal neuronal identity, as well as for the generation of NCCs. We also show that neural tube closure is sensitive to reduced BMPR1A signaling at the level of the hindbrain. By comparing the results of two distinct spatiotemporal deletions of Bmpr1a in the dorsal neural epithelium, we show that this requirement for BMPR1A signaling in spinal cord and NCC development is prior to neural fold closure. In contrast, removing BMPRIA function shortly after spinal neurulation had no overt affects on neural tube formation or dorsal patterning, though it did result in failed differentiation of spinal NCC derivatives. These phenotypes are in addition to previously documented defects in heart development and sympathetic nervous system development resulting from ablation of Bmpr1a in the dorsal neural tube (Stottmann et al., 2004; Morikawa et al., 2009; Nomura-Kitabayashi et al., 2009).
BMPR1A signaling is required before neural tube closure for patterning of dorsal spinal neurons
Previous studies have demonstrated a general requirement for BMP signal transduction activity in patterning the dorsal-most spinal cord neurons after approximately E9.75 (~25 somites) in mouse, in which Bmpr1a and Bmpr1b are redundant (Wine-Lee et al., 2004). A general requirement for BMP signaling from stages 12–15 (16–26 somites) in chick is implied by the loss of dorsal-most neuronal markers upon Noggin overexpression or expression of siRNA against Smad4 (Chesnutt et al., 2004). These studies manipulated BMP reception too late to address potential earlier requirements.
We used the Pax3-Cre and Wnt1-Cre to ablate Bmpr1a in the forming or nascent dorsal neural tube. This allowed us to assess the potential requirement for BMPR1A well before or shortly after neural tube closure in the future spinal region. We saw no spinal cord formation defects upon ablation of Bmpr1a via Wnt1-Cre, expressed after closure. In contrast, we observed severe and fully penetrant spinal cord defects when Pax3-Cre was the driver. In these embryos, expression of early dorsal patterning genes, such as the proneural gene Math1, was reduced or absent in the posterior of the embryo. Whereas Wnt1-Cre is not expressed in the spinal neural tube until shortly after dorsal closure, the Pax3-Cre transgene is active from the 2–3s stage along the future spinal cord. Closure begins at the 5s stage at the level of the third somite, progressing in both directions from there (Copp et al., 2003). For most of the spinal region, with the exception of the uppermost spinal cord, Pax3-Cre recombination is robust well before closure occurs. Taken together, these considerations indicate a critical role for BMPR1A signaling in patterning the precursors of dorsal spinal neurons, and suggest that it is largely fulfilled prior to spinal cord closure.
We consistently saw that the most dramatic effects on dorsal neural gene expression were in the posterior half of the spinal cord. This may reflect an elevated need for BMPR1A signaling in this region. However, we think a more likely explanation is that BMPR1A may be ablated at a higher efficiency in posterior than anterior presumptive spinal cord in our experimental design. Pax3-Cre initially recombines the R26R reporter equally well anterior and posterior to the initial closure point, but by 10 somites, while closure is ongoing, recombination is much more robust in the dorsal neural folds of the posterior neuropore. Subsequently, the posterior spinal region has a much greater degree of marked neuroepithelial and neural crest cells than the anterior spinal region. Meanwhile, Wnt1-Cre also fails to recombine R26R efficiently in the anterior dorsal spinal cord until well after closure. We were not able to assess Bmpr1a recombination per se with single cell resolution, and R26R recombination is likely an approximate rather than precise spatiotemporal indicator of Bmpr1a recombination (as a result of different recombination efficiencies of different floxed sequences). Nevertheless, these considerations raise the possibility that there may be sufficient unrecombined Bmpr1afx alleles in the anterior spinal neural folds to mask any early requirements for Bmpr1a in this region.
Bmpr1a is expressed in the dorsal neural tissue from the earliest stages, while Bmpr1b normally is expressed from as early as E9.0 (Panchision et al., 2001). Transgenic overexpression of Bmpr1a has been shown to increase Bmpr1b transcription in the neural tube, leading to a model in which BMPR1A activity promotes proliferation and initiates Bmpr1b expression, which in turn limits further proliferation by inducing mitotic arrest (Panchision et al., 2001). Our study provides a test of this model; however, the data show no dependence of Bmpr1b expression on Bmpr1a, at least in the dorsal neural tube. Nevertheless, a potential explanation for the lack of more severe neural tube phenotypes in Wnt1-Cre;Bmpr1a mutants is functional compensation for Bmpr1a by Bmpr1b after E9.0. This is supported by the results of Wine-Lee et al., (2004), whose relatively late ablation of Bmpr1a in a Bmpr1b mutant background revealed functional redundancy between these receptors as early as E9.75. The Pax3-Cre and Wnt1-Cre transgenes, however, ablate Bmpr1a signaling earlier in neural development, leading to numerous defects. We suggest these defects reflect roles for BMP signaling that occur before Bmpr1b can compensate, due to lack of expression; we saw no evidence of precocious Bmpr1b in the absence of Bmpr1a.
Roles of Bmpr1a in neural crest cell development
Our results show that BMPRIA activity within the dorsal neural tube is necessary for early stages of NCC development in the hindbrain and trunk. Loss of BMPRIA before neurulation in the posterior region of Pax3-Cre;Bmpr1a embryos led to a dramatic decrease in NCCs. This included not only a lack of differentiating NCC derivatives but also a lack of early NCC markers in the dorsal neural tube. The Bmpr1a ablation was most effective in precluding generation of NCCs in the posterior half of the embryo. Although this may reflect a differential requirement for BMP signaling, at least in spinal regions it is likely a result of the more robust early expression of Pax3-Cre in the trunk region, relative to that observed in the spinal segment anterior to the forelimb buds. Thus there may be a time-window during which spinal NCCs are especially dependent on BMP signals, becoming less so after neurulation. Embryonic sensitivity to the timing of BMP signaling perturbation has recently been reported in the chicken embryo (Yamauchi et al., 2008). In these experiments, misexpression of a constitutively active BMPR led to a change in cell fate at an early stage, but not at later stages.
In the brain, the role of BMPR1A in NCC development may be variable at any given time across the rostrocaudal axis. In the hindbrain, where there is an early domain of strong Pax3-Cre recombination activity, there was a striking reduction in the amount of NCCs entering the second pharyngeal arch in Pax3-Cre;Bmpr1a embryos. This affected PA2 but not PA1, exactly mirroring the activity of the Pax3-Cre transgene, and thus, the removal of BMPRIA activity. By contrast, Bmpr1a is ablated early and robustly in the midbrain by Wnt1-Cre, yet NCCs are generated normally in the cranial region of Wnt1-Cre;Bmpr1a mutants. NCCs not only formed normally in this region in the absence of Bmpr1a, but migrated successfully into PA1. Thus, there appear to be significant regional differences in the requirements for BMPR1A signaling in early stages of NCC development.
BMPs have been implicated in the differentiation of neural crest derivatives toward particular cell types. The Pax3-Cre; Bmpr1a embryos die too early to address this issue. In contrast, Wnt1-Cre; Bmpr1a mutant embryos do not show early NCC defects, and their migration to target tissues appears largely normal; thus, these embryos provide a useful model to assess the requirements of BMPR1A signaling in the contribution of NCC derivatives toward specific tissues and cell types. Loss of either Bmpr1a or Alk2, encoding a third type 1 BMP receptor, in the Wnt1-Cre domain leads to outflow tract defects during heart development (Stottmann et al., 2004; Kaartinen et al., 2004), with this manipulation of Alk2 also resulting in cleft palate and micrognathia (Dudas et al., 2004). These defects reflect a paucity of correctly differentiating NCC derivatives at their final organ destinations. BMP reception by melanocytes and their immediate precursors is likely to play an important role in their normal and pathological development. BMP activity is thought to negatively regulate FoxD3, which functions to promote the melanocyte lineage (Thomas and Erickson, 2008), suggesting a negative role for BMP signaling in melanocyte differentiation. On the other hand, BMPs are up-regulated in melanoma cell lines, and promote their migratory and invasive properties (Rothhammer et al., 2005). In Wnt1-Cre; Bmpr1a embryos, we observed a normal distribution of antibody 2H3 staining, suggesting that at least the early differentiation and migration of peripheral neurons is normal (Stottmann et al., 2004). Nevertheless, Wnt1-Cre;Bmpr1a embryos lack the cells of the sympathetic nervous system (Morikawa et al., 2009), which are derived from NCC. Here we observed that the number of cells expressing an early melanocyte marker, Dif, was greatly reduced, particularly in the forelimb region. This indicates that BMPR1A plays a critical, positive role in melanocyte differentiation or survival from pluripotent NCCs. Thus, the data indicate that cell-autonomous BMP signaling via BMPR1A is required for the post-migratory development of a subset of NCC derivative cell types.
Neurulation in the absence of dorsal BMPR1A
A recent study by Castraino and Mishina (2009) demonstrated that in some genetic backgrounds, embryos that lacked Bmp2, or were moderate-level chimeras for Bmp2−/− cells, displayed partially penetrant and variable cranial neurulation defects. The locations of such malformations suggested that forebrain closure was most sensitive to reduced BMP2 levels. It is unclear from these studies whether the observed NTDs resulted from a specific role for BMP signaling in dorsal neural tube precursors, or were a secondary consequence of the many other structural defects present in the mutants. Our results indicate that there is a positive role for BMP signaling in this tissue for cranial closure. We found that loss of Bmpr1a function in the developing hindbrain in the Pax3-Cre domain leads to an incompletely penetrant exencephaly phenotype. In contrast, we saw no neurulation defects as a result of loss of Bmpr1a in the Wnt1-Cre domain, which includes the midbrain from the 2 somite stage (long before local closure). Thus, BMP signaling has a positive role in cranial neurulation that varies regionally within the future brain ventricles. It is possible that the role of BMP signal transduction in the dorsal neural tube is not an absolute requirement, or that some other unknown receptor(s) can partially compensate for the lack of BMPR1A.
Spinal neurulation also involves BMP signaling, but with different requirements than in the brain. In contrast to the rostral neural tube, the spinal cord may not require BMPR1A for successful closure; although Pax3-Cre drove the loss of Bmpr1a in the spinal dorsal neural folds well before closure, none of the mutants had spinal NTDs. A caveat, however, is that our genetic manipulation may have been either too late or too inefficient to remove all BMPR1A activity prior to spinal neurulation. In any case, too much BMP does disrupt spinal neurulation. In Nog−/− mutants, the spinal cord closed, but closure was not maintained in the lumbar region (Stottmann et al., 2006). Genetic reduction of Bmp4 (by heterozygosity for a null allele) completely rescued this spina bifida. At the caudal neuropore, Bmp2−/− mutants were found to have exaggerated bending at the dorsolateral hingepoints, while Nog−/− mutants had reduced bending (Ybot-Gonzalez et al., 2007). This is consistent with the hypothesis based on the exencephaly in Nog−/− mutants that BMP inhibits DLHP formation in the mid/hindbrain (Stottmann et al., 2006). At least in the caudal region, BMP beads were able to repress bending at the putative DLHP in wildtype embryos (Ybot-Gonzalez et al., 2007). Altogether, it appears that within both the spinal cord and brain, the role of BMP signaling in neurulation varies by region.
EXPERIMENTAL PROCEDURES
Mouse maintenance and genotyping
The Wnt1-Cre transgenic strain and its genotyping are as described (Danielian et al., 1998). The Pax3-Cre driver used in this work is a transgene in which Cre recombinase expression is driven by the Pax3 regulatory sequences for neural tube expression (Li et al., 2000). All Bmpr1a alleles and their genotyping are as published (Mishina et al., 2002). The genetic background used was mixed 129S6 and C57Bl/6. Embryos were generated by mating Pax3-Cre/+;Bmpr1anull/+ or Wnt1-Cre/+;Bmpr1anull/+ mice with mice homozygous for the Bmpr1aflox allele and/or homozygous for the R26R (Soriano, 1999) and Z/AP reporters (Lobe et al., 1999). Mutant embryos were provisionally identified by phenotype and confirmed by PCR genotyping for presence of the Cre transgene and the null allele of Bmpr1a as described (Stottmann et al., 2004). Embryos were staged by somite count or comparison to established references (Downs and Davies, 1993).
Gene expression assays, immunohistochemistry and histology
Whole mount in situ hybridization with digoxygenin-labeled (Roche) cRNA probes was conducted as described (Anderson et al., 2002), with a hybridization temperature of 70 degrees. All probes used in this study are published: AP2/Tcfap2a (Mitchell et al., 1991), Cadherin 6 (Inoue et al., 1997), Crabp1 (Stoner and Gudas, 1989), Sox10 (Kuhlbrodt et al., 1998), Isl1(Pfaff et al., 1996), Math1(Ben-Arie et al., 1996), and Wnt1 (McMahon and Bradley, 1990), Bmpr1b (Panchision et al., 2001) and Trp2/Dct (Steel et al., 1992). LacZ transgene expression was visualized by fixing embryos for 10–15 minutes at room temperature with 4% paraformaldehyde, rinsed and stained overnight with X-gal stain as described (Stottmann et al., 2001). Histological analysis was conducted on 8m paraffin sections using established protocols (Hogan et al., 1994). Whole mount and section immunohistochemistry was done with 2H3 (1:200) and Pax6 (1:200) concentrated supernatants from the Developmental Studies Hybridoma Bank at the University of Iowa. Confocal microscopy was on a Zeiss LSM 510. Assays were performed on at least five mutants of each genotype (Pax3Cre and Wnt1Cre), along with at least eight wildtype, stage-matched littermates.
Acknowledgments
We thank E. Meyers and M. Choi for insightful discussions. M. Berrong, J. Goodwin, and A. Lawrence provided technical assistance. Previously published mouse strains were generously provided by Y. Mishina, J. Epstein, A. McMahon, P. Soriano and C. Lobe. We are grateful to D. Panchision, I. Jackson, T. Jessell, H. Zhogbi, A. McMahon, M. Wegner, L. Gudas, P. Mitchell, and M. Takeichi for plasmids to make riboprobes. The 2H3 antibody, developed by J. Dodd and T. Jessell, was obtained from the Developmental Studies Hybridoma Bank maintained by the Univ. of Iowa, Department of Biological Sciences, Iowa City, IA 52242.
Grant sponsor:
NIH/NINDS RC1-NS068932
NIH/NIDCR R01-DE13674
NIH/NINDS F31-NS42486
References
- Anderson RM, Lawrence AR, Stottmann RW, Bachiller D, Klingensmith J. Chordin and noggin promote organizing centers of forebrain development in the mouse. Development. 2002;129:4975–4987. doi: 10.1242/dev.129.21.4975. [DOI] [PubMed] [Google Scholar]
- Anderson RM, Stottmann RW, Choi M, Klingensmith J. Endogenous BMP antagonists regulate mammalian neural crest generation and survival. Developmental Dynamics. 2006;235:2507–2520. doi: 10.1002/dvdy.20891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkell R, Beddington RSP. BMP-7 influences pattern and growth of the developing hindbrain of mouse embryos. Development. 1997;124:1–12. doi: 10.1242/dev.124.1.1. [DOI] [PubMed] [Google Scholar]
- Barth KA, Kishimoto Y, Rohr KB, Seydler C, Schulte-Merker S, Wilson SW. Bmp activity establishes a gradient of positional information throughout the entire neural plate. Development. 1999;126:4977–87. doi: 10.1242/dev.126.22.4977. [DOI] [PubMed] [Google Scholar]
- Ben-Arie N, McCall AE, Berkman S, Eichele G, Bellen HJ, Zoghbi HY. Evolutionary conservation of sequence and expression of the bHLH protein Atonal suggests a conserved role in neurogenesis. Hum Mol Genet. 1996;5:1207–16. doi: 10.1093/hmg/5.9.1207. [DOI] [PubMed] [Google Scholar]
- Botto LD, Moore CA, Khoury MJ, Erickson JD. Neural-tube defects. N Engl J Med. 1999;341:1509–19. doi: 10.1056/NEJM199911113412006. [DOI] [PubMed] [Google Scholar]
- Castranio T, Mishina Y. Bmp2 is required for cephalic neural tube closure in the mouse. Dev Dyn. 2009;238:110–22. doi: 10.1002/dvdy.21829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai Y, Jiang X, Ito Y, Bringas P, Jr, Han J, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–9. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- Chesnutt C, Burrus LW, Brown AM, Niswander L. Coordinate regulation of neural tube patterning and proliferation by TGFbeta and WNT activity. Dev Biol. 2004;274:334–47. doi: 10.1016/j.ydbio.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Chizhikov VV, Millen KJ. Control of roof plate development and signaling by Lmx1b in the caudal vertebrate CNS. J Neurosci. 2004;24:5694–5703. doi: 10.1523/JNEUROSCI.0758-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizhikov VV, Millen KJ. Roof plate-dependent patterning of the vertebrate dorsal central nervous system. Dev Biol. 2005;277:287–95. doi: 10.1016/j.ydbio.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Colas JF, Schoenwolf GC. Towards a cellular and molecular understanding of neurulation. Dev Dyn. 2001;221:117–45. doi: 10.1002/dvdy.1144. [DOI] [PubMed] [Google Scholar]
- Copp AJ, Greene ND, Murdoch JN. The genetic basis of mammalian neurulation. Nat Rev Genet. 2003;4:784–93. doi: 10.1038/nrg1181. [DOI] [PubMed] [Google Scholar]
- Correia AC, Costa M, Moraes F, Bom J, Nóvoa A, Mallo M. Bmp2 is required for migration but not for induction of neural crest cells in the mouse. Dev Dyn. 2007;236:2493–501. doi: 10.1002/dvdy.21256. [DOI] [PubMed] [Google Scholar]
- Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998;8:1323–6. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- Davis S, Miura S, Hill C, Mishina Y, Klingensmith J. BMP receptor IA is required in the mammalian embryo for endodermal morphogenesis and ectodermal patterning. Dev Biol. 2004;270:47–63. doi: 10.1016/j.ydbio.2004.01.048. [DOI] [PubMed] [Google Scholar]
- Dewulf N, Verschueren K, Lonnoy O, Morén A, Grimsby S, Vande Spiegle K, Miyazono K, Huylebroeck D, Ten Dijke P. Distinct spatial and temporal expression patterns of two type I receptors for bone morphogenetic proteins during mouse embryogenesis. Endocrinology. 1995;136:2652–63. doi: 10.1210/endo.136.6.7750489. [DOI] [PubMed] [Google Scholar]
- Downs KM, Davies T. Staging of gastrulating mouse embryos by morphological landmarks in the dissecting microscope. Development. 1993;118:1255–1266. doi: 10.1242/dev.118.4.1255. [DOI] [PubMed] [Google Scholar]
- Dudas M, Sridurongrit S, Nagy A, Okazaki K, Kaartinen V. Craniofacial defects in mice lacking BMP type I receptor Alk2 in neural crest cells. Mech Dev. 2004;121:173–82. doi: 10.1016/j.mod.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Faure S, de Santa Barbara P, Roberts DJ, Whitman M. Endogenous patterns of BMP signaling during early chick development. Dev Biol. 2002;244:44–65. doi: 10.1006/dbio.2002.0579. [DOI] [PubMed] [Google Scholar]
- Gu Z, Reynolds EM, Song J, Lei H, Feijen A, Yu L, He W, Maclaughlin DT, van den Eijnden-van Raaij J, Donahoe PK, Li E. The type I serine/threonine kinase receptor ActRIA (ALK2) is required for gastrulation of the mouse embryo. Development. 1999;126:2551–61. doi: 10.1242/dev.126.11.2551. [DOI] [PubMed] [Google Scholar]
- Hebert JM, McConnell SK. Targeting of cre to the Foxg1 (BF-1) locus mediates loxP recombination in the telencephalon and other developing head structures. Dev Biol. 2000;222:296–306. doi: 10.1006/dbio.2000.9732. [DOI] [PubMed] [Google Scholar]
- Hebert JM, Mishina Y, McConnell SK. BMP signaling is required locally to pattern the dorsal telencephalic midline. Neuron. 2002;35:1029–41. doi: 10.1016/s0896-6273(02)00900-5. [DOI] [PubMed] [Google Scholar]
- Helms AW, Johnson JE. Progenitors of dorsal commissural interneurons are defined by MATH1 expression. Development. 1998;125:919–28. doi: 10.1242/dev.125.5.919. [DOI] [PubMed] [Google Scholar]
- Hogan B, Beddington R, Costantini F, Lacy E. Manipulating the Mouse Embryo. Cold Spring Harbor Press; New York: 1994. [Google Scholar]
- Honore SM, Aybar MJ, Mayor R. Sox10 is required for the early development of the prospective neural crest in Xenopus embryos. Dev Biol. 2003;260:79–96. doi: 10.1016/s0012-1606(03)00247-1. [DOI] [PubMed] [Google Scholar]
- Inoue T, Chisaka O, Matsunami H, Takeichi M. Cadherin-6 expression transiently delineates specific rhombomeres, other neural tube subdivisions, and neural crest subpopulations in mouse embryos. Dev Biol. 1997;183:183–94. doi: 10.1006/dbio.1996.8501. [DOI] [PubMed] [Google Scholar]
- Ikeya M, Lee SM, Johnson JE, McMahon AP, Takada S. Wnt signalling required for expansion of neural crest and CNS progenitors. Nature. 1997;389:966–70. doi: 10.1038/40146. [DOI] [PubMed] [Google Scholar]
- Jackson IJ, Chambers DM, Tsukamoto K, Copeland NG, Gilbert DJ, Jenkins NA, Hearing V. A second tyrosinase-related protein, TRP-2, maps to and is mutated at the mouse slaty locus. EMBO J. 1992;11:527–35. doi: 10.1002/j.1460-2075.1992.tb05083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessell TM, Sanes JR. Development. The decade of the developing brain. Curr Opin Neurobiol. 2000;10:599–611. doi: 10.1016/s0959-4388(00)00136-7. [DOI] [PubMed] [Google Scholar]
- Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cardiac neural crest. Development. 2000;127:1607–16. doi: 10.1242/dev.127.8.1607. [DOI] [PubMed] [Google Scholar]
- Kanzler B, Foreman RK, Labosky PA, Mallo M. BMP signaling is essential for development of skeletogenic and neurogenic cranial neural crest. Development. 2000;127:1095–104. doi: 10.1242/dev.127.5.1095. [DOI] [PubMed] [Google Scholar]
- Kaartinen V, Dudas M, Nagy A, Sridurongrit S, Lu MM, Epstein JA. Cardiac outflow tract defects in mice lacking ALK2 in neural crest cells. Development. 2004;131:3481–90. doi: 10.1242/dev.01214. [DOI] [PubMed] [Google Scholar]
- Knecht AK, Bronner-Fraser M. Induction of the neural crest: a multigene process. Nat Rev Genet. 2002;3:453–61. doi: 10.1038/nrg819. [DOI] [PubMed] [Google Scholar]
- Kuhlbrodt K, Herbarth B, Sock E, Hermans-Borgmeyer I, Wegner M. Sox10, a novel transcriptional modulator in glial cells. J Neurosci. 1998;18:237–50. doi: 10.1523/JNEUROSCI.18-01-00237.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KJ, Mendelsohn M, Jessell TM. Neuronal patterning by BMPs: a requirement for GDF7 in the generation of a discrete class of commissural interneurons in the mouse spinal cord. Genes Dev. 1998;12:3394–407. doi: 10.1101/gad.12.21.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Chen F, Epstein JA. Neural crest expression of Cre recombinase directed by the proximal Pax3 promoter in transgenic mice. Genesis. 2000;26:162–4. doi: 10.1002/(sici)1526-968x(200002)26:2<162::aid-gene21>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Liem KF, Jr, Tremml G, Roelink H, Jessell TM. Dorsal differentiation of neural plate cells induced by BMP-mediated signals from epidermal ectoderm. Cell. 1995;82:969–979. doi: 10.1016/0092-8674(95)90276-7. [DOI] [PubMed] [Google Scholar]
- Liem KF, Tremml G, Jessell TM. A role for the roof plate and its resident TGFbeta-related proteins in neuronal patterning in the dorsal spinal cord. Cell. 1997;91:127–138. doi: 10.1016/s0092-8674(01)80015-5. [DOI] [PubMed] [Google Scholar]
- Liu A, Niswander L. Bone morphogenetic protein signalling and vertebrate nervous system development. Nature Rev Neuroscience. 2005;6:945–954. doi: 10.1038/nrn1805. [DOI] [PubMed] [Google Scholar]
- Liu Y, Helms AW, Johnson JE. Distinct activities of Msx1 and Msx3 in dorsal neural tube development. Development. 2004;131:1017–1028. doi: 10.1242/dev.00994. [DOI] [PubMed] [Google Scholar]
- Lobe CG, Koop KE, Kreppner W, Lomeli H, Gertsenstein M, Nagy A. Z/AP, a double reporter for cre-mediated recombination. Dev Biol. 1999;208:281–92. doi: 10.1006/dbio.1999.9209. [DOI] [PubMed] [Google Scholar]
- Massagué J, Chen YG. Controlling TGF-beta signaling. Genes Dev. 2000;14:627–44. [PubMed] [Google Scholar]
- McMahon AP, Bradley A. The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell. 1990;62:1073–85. doi: 10.1016/0092-8674(90)90385-r. [DOI] [PubMed] [Google Scholar]
- McMahon JA, Takada S, Zimmerman LB, Fan CM, Harland RM, McMahon AP. Noggin-mediated antagonism of BMP signaling is required for growth and patterning of the neural tube somite. Genes Dev. 1998;12:1438–1452. doi: 10.1101/gad.12.10.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megason SG, McMahon AP. A mitogen gradient of dorsal midline Wnts organizes growth in the CNS. Development. 2002;129:2087–98. doi: 10.1242/dev.129.9.2087. [DOI] [PubMed] [Google Scholar]
- Mine N, Anderson R, Klingensmith J. BMP antagonism is required in both the node and lateral plate mesoderm for mammalian left-right axis establishment. Development. 2008;135:2425–2434. doi: 10.1242/dev.018986. [DOI] [PubMed] [Google Scholar]
- Mishina Y, Hanks MC, Miura S, Tallquist MD, Behringer RR. Generation of Bmpr/Alk3 conditional knockout mice. Genesis. 2002;32:69–72. doi: 10.1002/gene.10038. [DOI] [PubMed] [Google Scholar]
- Mitchell PJ, Timmons PM, Hebert JM, Rigby PW, Tjian R. Transcription factor AP-2 is expressed in neural crest cell lineages during mouse embryogenesis. Genes Dev. 1991;5:105–19. doi: 10.1101/gad.5.1.105. [DOI] [PubMed] [Google Scholar]
- Morikawa Y, Zehir A, Maska E, Deng C, Schneider MD, Mishina Y, Cserjesi P. BMP signaling regulates sympathetic nervous system development through Smad4-dependent and -independent pathways. Development. 2009;136:3575–84. doi: 10.1242/dev.038133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen VH, Schmid B, Trout J, Connors SA, Ekker M, Mullins MC. Ventral and lateral regions of the zebrafish gastrula, including the neural crest progenitors, are established by a bmp2b/swirl pathway of genes. Dev Biol. 1998;199:93–110. doi: 10.1006/dbio.1998.8927. [DOI] [PubMed] [Google Scholar]
- Nguyen VH, Trout J, Connors SA, Andermann P, Weinberg E, Mullins MC. Dorsal and intermediate neuronal cell types of the spinal cord are established by a BMP signaling pathway. Development. 2000;127:1209–1220. doi: 10.1242/dev.127.6.1209. [DOI] [PubMed] [Google Scholar]
- Nichols DH. Neural crest formation in the head of the mouse embryo as observed using a new histological technique. J Embryol Exp Morphol. 1981;64:105–120. [PubMed] [Google Scholar]
- Nomura-Kitabayashi A, Phoon CK, Kishigami S, Rosenthal J, Yamauchi Y, Abe K, Yamamura K, Samtani R, Lo CW, Mishina Y. Outflow tract cushions perform a critical valve-like function in the early embryonic heart requiring BMPRIA-mediated signaling in cardiac neural crest. Am J Physiol Heart Circ Physiol. 2009;297:H1617–28. doi: 10.1152/ajpheart.00304.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchision DM, Pickel JM, Studer L, Lee SH, Turner PA, Hazel TG, McKay RD. Sequential actions of BMP receptors control neural precursor cell production and fate. Genes Dev. 2001;15:2094–110. doi: 10.1101/gad.894701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff SL, Mendelsohn M, Stewart CL, Edlund T, Jessell TM. Requirement for LIM homeobox gene Isl1 in motor neuron generation reveals a motor neuron-dependent step in interneuron differentiation. Cell. 1996;84:309–20. doi: 10.1016/s0092-8674(00)80985-x. [DOI] [PubMed] [Google Scholar]
- Rothhammer T, Poser I, Soncin F, Bataille F, Moser M, Bosserhoff AK. Bone morphogenic proteins are overexpressed in malignant melanoma and promote cell invasion and migration. Cancer Res. 2005;65:448–56. [PubMed] [Google Scholar]
- Serbedzija GN, Bronner-Fraser M, Fraser SE. Vital dye analysis of cranial neural crest cell migration in the mouse embryo. Development. 1992;116:297–307. doi: 10.1242/dev.116.2.297. [DOI] [PubMed] [Google Scholar]
- Solloway MJ, Robertson EJ. Early embryonic lethality in Bmp5; Bmp7 double mutant mice suggests functional redundancy within the 60A subgroup. Development. 1999;126:1753–1768. doi: 10.1242/dev.126.8.1753. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–1. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Steel KP, Davidson DR, Jackson IJ. TRP-2/DT, a new early melanoblast marker, shows that steel growth factor (c-kit ligand) is a survival factor. Development. 2002;115:1111–9. doi: 10.1242/dev.115.4.1111. [DOI] [PubMed] [Google Scholar]
- Stoner CM, Gudas LJ. Mouse cellular retinoic acid binding protein: cloning, complementary DNA sequence, and messenger RNA expression during the retinoic acid- induced differentiation of F9 wild type and RA-3–10 mutant teratocarcinoma cells. Cancer Res. 1989;49:1497–504. [PubMed] [Google Scholar]
- Stottmann RW, Anderson RM, Klingensmith J. The BMP antagonists Chordin and Noggin have essential but redundant roles in mouse mandibular outgrowth. Dev Biol. 2001;240:457–473. doi: 10.1006/dbio.2001.0479. [DOI] [PubMed] [Google Scholar]
- Stottmann RW, Choi M, Mishina Y, Meyers EN, Klingensmith J. BMP receptor IA is required in mammalian neural crest cells for development of the cardiac outflow tract and ventricular myocardium. Development. 2004;131:2205–18. doi: 10.1242/dev.01086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stottmann RW, Berrong M, Matta K, Choi M, Klingensmith J. The BMP antagonist Noggin promotes cranial and spinal neurulation by distinct mechanisms. Dev Biol. 2006;295:647–63. doi: 10.1016/j.ydbio.2006.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit A, Stern CD. Establishment and maintenance of the border of the neural plate in the chick: involvement of FGF and BMP activity. Mech Dev. 1999;82:51–66. doi: 10.1016/s0925-4773(99)00013-1. [DOI] [PubMed] [Google Scholar]
- Thomas AJ, Erickson CA. The making of a melanocyte: the specification of melanoblasts from the neural crest. Pigment Cell Melanoma Res. 2008;21:598–610. doi: 10.1111/j.1755-148X.2008.00506.x. [DOI] [PubMed] [Google Scholar]
- Timmer JR, Wang C, Niswander L. BMP signaling patterns the dorsal and intermediate neural tube via regulation of homeobox and helix-loop-helix transcription factors. Development. 2002;129:2459–72. doi: 10.1242/dev.129.10.2459. [DOI] [PubMed] [Google Scholar]
- Ulloa F, Briscoe J. Morphogens and the control of cell proliferation and patterning in the spinal cord. Cell Cycle. 2007;6:2640–9. doi: 10.4161/cc.6.21.4822. [DOI] [PubMed] [Google Scholar]
- Wine-Lee L, Ahn KJ, Richardson RD, Mishina Y, Lyons KM, Crenshaw EB., III Signaling through BMP type 1 receptors is required for development of interneuron cell types in the dorsal spinal cord. Development. 2004;131:5393–5403. doi: 10.1242/dev.01379. [DOI] [PubMed] [Google Scholar]
- Yamauchi K, Phan KD, Butler SJ. BMP type I receptor complexes have distinct activities mediating cell fate and axon guidance decisions. Development. 2008;135:1119–28. doi: 10.1242/dev.012989. [DOI] [PubMed] [Google Scholar]
- Ybot-Gonzalez P, Gaston-Massuet C, Girdler G, Klingensmith J, Arkell R, Greene ND, Copp AJ. Neural plate morphogenesis during mouse neurulation is regulated by antagonism of Bmp signalling. Development. 2007;134:3203–11. doi: 10.1242/dev.008177. [DOI] [PubMed] [Google Scholar]
- Yang YP, Klingensmith J. Roles of organizer factors and BMP antagonism in mammalian forebrain establishment. Dev Biol. 2006;296:458–475. doi: 10.1016/j.ydbio.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Zhang H, Bradley A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development. 1996;122:2977–2986. doi: 10.1242/dev.122.10.2977. [DOI] [PubMed] [Google Scholar]